Abstract
Pain, tobacco cigarette smoking, and prescription opioid misuse are all highly prevalent among persons living with HIV (PLWH). Smoking and pain medication misuse can lead to deleterious outcomes, including more severe pain and physical impairment. However, we are not aware of any interventions that have attempted to address these issues in an integrated manner.Participants (N = 68) were recruited from an outpatient infectious disease clinic and randomized to either a computer-based personalized feedback intervention (Integrated PFI) that aimed to increase motivation, confidence, and intention to quit smoking, and decrease intentions to misuse prescription analgesic medications, or a Control PFI. Results indicated that PLWH who received the Integrated PFI (vs. Control PFI) evinced greater post-treatment knowledge of interrelations between pain and tobacco smoking. Moreover, participants who received the Integrated PFI and smoked at least 10 cigarettes per day (but not < 10 CPD) reported greater confidence and readiness/intention to quit smoking. Effects of the Integrated PFI on knowledge of pain and opioid misuse, and attitudes/intentions regarding prescription pain medication misuse were not statistically-significant. Taken together, these results indicate that this novel intervention strategy may offer promise for addressing a critical public health need in a population that is generally underrepresented in clinical research.
Rates of cigarette smoking among individuals with chronic pain (~24–68%; Michna et al., 2004; Orhurhu, Pittelkow, & Hooten, 2015) and persons living with HIV (PLWH; 45–74%; Vidrine, 2009) are substantially higher than those observed in the general population (14%; Norris, Schiller, & Clarke, 2018). Pain is the second most common symptom reported among PLWH (Carr, 2004), and the point prevalence of pain among PLWH ranges from 54% to 83% using a three-month recall period (Parker, Stein, & Jelsma, 2014). Pain among PLWH is often frequent and persistent (Breitbart et al., 1996), and can be attributed to various causes, including HIV/AIDS, side effects of medication, and living conditions (Miaskowski et al., 2011). Notably, there is evidence that pain is more intense among PLWH who smoke cigarettes (Patel et al., 2006; Turner et al., 2001), possibly via smoking-related dysregulation of the endogenous opioid system (Shi, Weingarten, Mantilla, Hooten, & Warner, 2010).
Consistent with a reciprocal model of pain and tobacco smoking (Ditre, Brandon, Zale, & Meagher, 2011; Zale, Maisto, & Ditre, 2016), pain has been shown to motivate smoking (Ditre & Brandon, 2008), pain patients endorse the use of cigarettes to cope with pain (Hooten et al., 2011b; Patterson et al., 2012), and cigarette smoking has been implicated in the onset and exacerbation of painful conditions (Shiri, Karppinen, Leino-Arjas, Solovieva, & Viikari-Juntura, 2010; Sugiyama et al., 2010). Furthermore, cross-sectional evidence suggests that perceived interrelations between pain and tobacco smoking (e.g., that smoking can help one cope with pain) are associated with more severe pain and functional impairment (i.e., interference with daily activities, work, and recreational/social activities) among individuals with chronic pain and PLWH (Ditre, Zale, Heckman, & Hendricks, 2017; Weinberger, Seng, Ditre, Willoughby, & Shuter, 2018).
Given the ubiquity of pain among PLWH, it is not surprising that they tend to be prescribed more analgesic medications than persons in the general population (Silverberg et al., 2012). There is also evidence that opioid misuse behaviors (e.g., taking more medication than prescribed, seeking multiple prescriptions) are especially prevalent among PLWH (Hansen et al., 2011; Tsao, Dobalian, & Stein, 2005), with rates of misuse as high as 62% (Robinson-Papp, Elliott, Simpson, Morgello, & Bank, 2012). Cigarette smoking has been associated with an elevated risk for engaging in aberrant drug-related behaviors (Michna et al., 2004), and chronic pain patients using opioids have reported that opioid consumption stimulates smoking (Hooten et al., 2011a). Moreover, those who endorse smoking for pain-coping have demonstrated greater reliance on analgesic medications (Jamison, Stetson, & Parris, 1991), and there is reason to suspect that complex nicotine-opioid interactions may lead to cross-tolerance (Shi et al., 2010) and diminished pain relief (Zevin & Benowitz, 1999).
Previous research has demonstrated that interventions for PLWH can enhance motivation/success in quitting smoking (e.g., Moadel et al., 2012; Vidrine, Arduino, Lazev, & Gritz, 2006; Wewers, Neidig, & Kihm, 2000), and improve pain outcomes (e.g., Trafton et al., 2012). However, despite the high prevalence and deleterious effects of co-occurring pain, tobacco smoking, and prescription analgesic misuse among PLWH, we are not aware of any interventions that have attempted to address these issues in an integrated manner. Integrated treatments are often preferable to traditional approaches for treating co-occurring disorders (e.g., sequential and parallel treatment), because they are more efficient, cost-effective, and do not require that one condition takes precedent over another (e.g., Mueser, 2003). One promising therapeutic format is the computer-based personalized feedback intervention (PFI; e.g., Noar, 2011; Riper et al., 2009). Drawing on motivational and social psychology perspectives (Bandura, 1994; Miller & Rollnick, 2002), PFIs rely on the presentation of discrepant information, including personalized profiles (e.g., regarding smoking rate and chronic pain status), risk factors (e.g., cigarette smoking as a risk factor for chronic pain), and normative comparisons (e.g., perceptions regarding others’ misuse of prescription pain medications). Computer-based PFIs are portable, adaptable, easy to implement, and can be delivered to a large number of patients by non-specialized care providers, thereby reducing patient burden and making intervention participation more feasible (Cunningham, 2007; Hasin et al., 2013). Computer-based PFIs have been recommended for HIV care settings (Noar, 2011), and they enhance standardization of treatment in terms of format, contact time, and focus of content.
The goal of this study was to pilot test a newly developed computer-based PFI for PLWH who are prescribed analgesic medication aimed at: (1) increasing motivation, confidence, and intention to quit smoking, and (2) decreasing positive attitudes and intentions toward the misuse of analgesic medications (e.g., prescription opioids). The integrated intervention included a novel psychoeducation component that was informed by several theories of health behavior change, including the transtheoretical model (Prochaska, Redding, & Evers, 2008), the health belief model (Champion & Skinner, 2008), and the theory of planned behavior (Ajzen, 1991). In accordance with these models, it is our contention that PLWH may become more motivated to quit smoking and less motivated to misuse prescription pain medication as they develop discrepancy between the continuation of these behaviors and their desired pain outcomes. Indeed, developing discrepancy between continued substance use and desired outcomes is a core component of evidence-based motivational substance use interventions, including PFIs (e.g., Abrams & Niaura, 2003; Larimer et al., 2007). Moreover, psychoeducation regarding interrelations between pain, smoking, and analgesic misuse may increase motivation for behavior change because individuals may begin to perceive themselves as immediately susceptible to the negative pain-related effects of smoking and medication misuse and may come to understand the pain-related benefits of quitting smoking and taking pain medication as prescribed. Finally, in accordance with the theory of planned behavior, providing personalized feedback regarding interrelations between pain, smoking, and analgesic misuse may change attitudes towards these health behaviors, perceived norms regarding the consequences of these behaviors, and perceived behavioral control, which may, in turn, alter behavioral intentions.
Participants were randomized to receive either the Integrated PFI that addressed pain, smoking, and analgesic misuse, or a Control PFI, which addressed health behaviors that were not related to pain or substance use (e.g., nutrition, physical activity). We hypothesized that participants randomized to the Integrated PFI intervention would evince greater post-treatment knowledge regarding pain-smoking interrelations, as well as greater motivation, confidence, and intention to quit smoking. We also hypothesized that participants in the PFI condition would evince greater knowledge regarding pain and the misuse of analgesic medications, as well as fewer maladaptive attitudes regarding prescription opioid misuse, and reduced intention to misuse prescription pain medications in the future.
Method
Participants
Participants were recruited from a university hospital-based outpatient infectious disease clinic in central New York. Medical records were screened for the following inclusion criteria: (1) current cigarette smoking, and (2) current use of prescription analgesic medication. Participants aged ≤ 30 years and those who self-reported a current attempt to quit smoking were excluded. A total of 68 participants met these criteria and completed the single study session.
Procedure
Prospective participants were identified via medical chart review of patients presenting to a university hospital-based outpatient infectious disease clinic in central New York. During routine clinic visits, a designated health care provider informed patients about the opportunity to participate in the study and obtained verbal consent regarding their willingness to be introduced to a trained research assistant who worked in collaboration with clinic staff. Patients who provided permission were then introduced to the research assistant, who described the study, assessed additional inclusion/exclusion criteria, and scheduled a study appointment. Of 140 patients who completed the initial screening, 68 met all inclusion criteria. Upon arrival to the study appointment, informed consent was obtained, and participants were assured that all information collected for the study would be kept confidential and would not be shared with their health care providers. Participants were then administered a brief computer-based baseline questionnaire that would be used to collect sociodemographic data and generate personalized content for the PFI. Participants were then randomized to either PFI or Control computer-based PFI conditions. Responses to baseline questionnaires were immediately transmitted into the personalized feedback interventions, so there was no delay between completion of the baseline measures and commencement of the intervention. Primary outcome measures were administered on the same computer immediately following the intervention. All participants completed the same pre-and post-intervention questionnaires, regardless of condition assignment. Study sessions typically lasted approximately 1.5 hours, and participants were compensated $45 for the completion of the visit.
Intervention Conditions
All participants received a brief computer-based intervention. Both interventions included visual and audio components (presented at a 5th grade reading level) and took approximately 20–30 minutes to complete. All feedback was presented in a non-confrontational tone, and incorporated personalized information collected during the baseline assessment.
Integrated PFI.
The Integrated PFI included content relevant to interrelations between pain, tobacco smoking, and analgesic medication use/misuse in the context of HIV and aging. Components of this intervention included personalized profiles for chronic pain (e.g., intensity, duration, goals for future functioning), smoking (e.g., cigarettes smoked per day, past quit attempts, motivation to quit), analgesic medication use/misuse (e.g., prescription compliance), and perceptions of pain-smoking interrelations. The Integrated PFI also incorporated pain-smoking psychoeducation (e.g., reciprocal relations between pain and smoking; risks of continued smoking vs. benefits of quitting in the context of chronic pain, HIV, and aging), and feedback regarding analgesic medication use/misuse (e.g., use vs. misuse; relations between pain, smoking, and the efficacy of prescription opioid medications in the context of HIV and aging). In addition, participants were given normative feedback regarding the percentage of pain patients that increase their dose of pain medication on their own, and the percentage of pain patients that seek more than one doctor for pain medications. Participants were presented with responses regarding perceived prevalence of these behaviors, and then received feedback regarding the perceptions of others, as well as the actual percentage of pain patients who engage in these maladaptive health behaviors. Reference group data was derived from the empirical literature (e.g., McDonald & Carlson, 2013). Finally, the Integrated PFI addressed the utility of employing more adaptive (i.e., non-substance related) strategies for coping with pain (e.g.,distraction, deep breathing), and prompted participants to consider alternatives to cigarette smoking and the misuse of prescription opioids.
Control PFI.
The Control PFI included content relevant to the importance of nutrition, exercise, and medication adherence in the context of HIV and aging. The Control PFI content was selected to clearly distinguish it from the PFI (i.e., there was no mention of smoking or opioid misuse), while also providing benefit to PLWH. Components of this intervention included psychoeducation regarding nutrition and exercise (e.g., health consequences of poor nutrition and physical inactivity), and personalized feedback regarding perceptions of interrelations between nutrition, exercise, and HIV and aging (e.g., using nutrition as part of HIV symptom management, using exercise to reduce the negative effects of HIV and aging). Control PFI components were approximately equivalent to the PFI in terms of personalization, duration, graphic design, and focus on health-related behaviors.
Primary Outcome Measures
Knowledge of pain-smoking interrelations.
The 8-item Pain and Smoking Questionnaire (PSQ) was developed for this study to assess knowledge of pain-smoking interrelations, including whether cigarette smoking can cause chronic pain, contribute to pain-related functional impairment, reduce the effectiveness of prescription analgesics, or aid in efforts to cope with pain. Items also assessed knowledge of whether pain can motivate smoking behavior and whether quitting smoking may be associated with improved pain and physical functioning. Response options included yes, no, or not sure/don’t know. The PSQ was administered before and after intervention delivery, and total scores reflect the number of items that were answered correctly (range 0–8). This measure demonstrated good internal consistency in the current sample (Cronbach’s α = .82).
Motivation, intention, and confidence to quit smoking.
The Rulers for Smoking Cessation (RSC; Boudreaux et al., 2012) is a widely-used measure that includes three items to assess: perceived importance of quitting (“How important is stopping smoking to you?” 0 = Not important at all; 10 = Most important goal of my life); readiness/intention to quit smoking (“How ready are you to quit smoking within the next month?” 0 = Not at all; 10 = 100% ready); and confidence in quitting (“How confident are you that you will quit smoking within the next month?” 0 = Not at all; 10 = 100% confident).
Knowledge of pain-prescription analgesic interrelations.
The 6-item Pain and Analgesic Medication Questionnaire (PAMQ) was developed for this study to assess knowledge of interrelations between pain and use/misuse of analgesic medication, including whether misusing opioids can make pain worse over time, reduce their effectiveness, or lead to tolerance. Items also assessed knowledge of what constitutes misuse (e.g., taking more than prescribed, using another person’s medication), and whether taking opioids as prescribed can improve pain/functioning and reduce the risk of dependence. Response options included yes, no, or not sure/don’t know. The PAMQ was administered before and after intervention delivery, and total scores reflect the number of items that were answered correctly (range 0–6). This measure demonstrated acceptable internal consistency (Cronbach’s α = .79).
Attitudes and beliefs regarding prescription analgesic use.
Attitudes and beliefs regarding prescription analgesic use were assessed pre-and post-treatment using an adapted version of the Attitudes and Beliefs Questionnaire (ABQ; Passik et al., 2000). The ABQ includes items that query a range of aberrant drug-related attitudes and behaviors in the context of cancer and HIV, and we adapted this questionnaire for a more general population (i.e., so items were not specific to cancer or HIV). Participants indicated their agreement with 15 statements (e.g.,”People should consider taking any prescription pain medication that relieves their pain, even if their primary doctor would not approve”), using a scale ranging from 0 (strongly disagree) to 4 (strongly agree). Two additional items assessed the percentage of patients they believe either increase their dose of pain medicine on their own or seek out more than one physician for prescription pain medications (1 = 0–25%, 2 = 26–50%, 3 = 51–75%, 4 = 76–100%). Items were summed to generate a total ABQ score, with higher scores representing a greater number of maladaptive attitudes and beliefs regarding the use/misuse of prescription pain medications. The ABQ demonstrated excellent internal consistency (α = .91).
Intention to misuse prescription analgesics.
Intention to misuse opioids and other prescription pain medications in the next 30 days was assessed using an adapted version of the Current Opioid Misuse Measure (COMM; Butler et al., 2007), which consisted of 10 items using a 5-point Likert scale ranging from 0 (extremely unlikely) to 4 (extremely likely). Seven items from the 17-item COMM were excluded from the COMM-Intention measure because they could not be adapted for a future orientation (e.g., “In the past 30 days, how often have you had trouble with thinking clearly or had memory problems?”). The COMM-Intention items were administered before and after intervention delivery, and higher scores reflect greater intentions to misuse prescription analgesic medications in future (range: 10–50). The COMM-Intention demonstrated excellent internal consistency (α = .94).
Other Measures
Smoking characteristics.
Smoking characteristics (e.g., number of cigarettes smoked per day, number of years smoking) were assessed via self-report.
Pain characteristics.
Pain characteristics were all assessed via self-report. First, participants were asked to indicate their primary pain location (i.e., location that hurt the most over the past three months). Response options consisted of back, head, face, neck, shoulders, arms, hands, chest, breast, stomach, abdomen, hips, legs, and feet. Second, a single item was used to assess pain duration (“How long have you been experiencing this type of pain?”; CDC, 2007). Third, pain intensity was assessed using the characteristic pain intensity subscale of the Graded Chronic Pain Scale (GCPS; Von Korff, Ormel, Keefe, & Dworkin, 1992). The GCPS provides a reliable and valid method of assessing global pain severity across a range of chronically painful conditions. Participants rated the intensity of their pain right now, their worst pain in the past 24 hours, and their pain on average during the past 24 hours using separate 0–10 numerical rating scales. Consistent with GCPS scoring instructions (Von Korff, 2011), these items were then averaged and multiplied by 10 to yield a continuous composite score (range 0–100) of characteristic pain intensity/severity.
HIV/AIDS symptom count.
The AIDS Clinical Trial Group Symptom Distress Module (SDM) is a 20-item measure used to assess HIV/AIDS symptoms (Justice et al., 2001).Participants indicated whether they have experienced any of 20 different symptoms (e.g., fatigue or loss of energy, feeling dizzy or lightheaded) over the past two weeks, and subsequently rated the degree to which they have been bothered by each symptom on a scale ranging from 1 (doesn’t bother me) to 4 (bothers me a lot). The SDM has been shown to have excellent construct validity (Justice et al., 2001), and demonstrated good internal consistency in the current sample (α = .89).
Data Analytic Plan
Analyses were conducted using SPSS Version 22 (IBM Corp, 2013). First, group differences in baseline variables (e.g., demographics, smoking characteristics, pain characteristics) were examined using t-tests and chi-square analyses to verify that randomization was successful. No differences in baseline characteristics were observed between intervention conditions (all ps > .05). Second, the distributions of all outcome variables were examined for normality, and skewness and kurtosis fell within acceptable ranges (George & Mallery, 2003). Third, we tested the effects of the intervention on each outcome measure (PSQ, RSC, PAMQ, ABQ, and COMM-Intention) using analysis of covariance (ANCOVA), with the baseline score entered as a covariate for each outcome. This approach to analyzing pretest-posttest outcomes has been recommended by researchers, and has shown to be a more powerful technique compared to repeated measures ANOVAs and gain scores (e.g., Huck & McLean, 1975). Given the observed variability in number of cigarettes smoked per day (SD = 10.97, with approximately 40% of participants smoking fewer than 10 CPD), we conducted exploratory analyses to test the effects of the interaction between treatment condition assignment and CPD (dichotomized using a median split as < or ≥ 10 CPD) on all smoking outcomes. Finally, the magnitude of group differences was examined using partial eta squared (ηp2), with values of .01, .09, and .25 characterizing effects as small, medium, or large.
Results
Participant Characteristics
Participants included 68 PLWH (39.7% female; Mage = 51.35 years, SD = 7.94, range: 32–70), who reported smoking an average of approximately 13 CPD (SD = 10.97). Nearly half of the sample (47%) identified as black or African American. All participants reported an annual income below $50,000, more than half reported earning less than $10,000 per year, and only 3 participants completed four years of college. Participants reported experiencing an average of 12.78 HIV/AIDS symptoms over the past two weeks (SD = 6.63), with “fatigue or loss of energy”, “pain, numbness, or tingling in the hands or feet”, “difficulty falling or staying asleep”, and “muscle aches or joint pain” representing the most frequently endorsed symptoms. Over 95% of the sample endorsed pain lasting longer than 3 months, which is a commonly used cutoff for indexing chronic pain (e.g., Merskey, 1986). Half of all participants reported neck/back as primary source of pain, and over one-third reported lower extremity pain. The mean characteristic pain intensity score was high (M = 54.41, SD = 31.61; Von Korff, Dworkin, & Le Resche, 1990). Finally, participants reported using a variety of prescription analgesics, including both opioid (e.g., Oxycodone, Hydrocodone, Percocet) and non-opioid (e.g., Gabapentin, Lyrica, Naproxen) pain medications. Complete sociodemographic and clinical data are presented in Table 1.
Table 1.
Sociodemographic, smoking, and pain characteristics
| PFI n = 32 |
Control n = 36 |
Total N = 68 |
|
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Gender | |||
| Female | 14 (43.8%) | 13 (36.1%) | 27 (39.7%) |
| Race | |||
| White | 12 (37.5%) | 16 (44.4%) | 28 (41.2%) |
| Black or African American | 16 (50.0%) | 16 (44.4%) | 32 (47.1%) |
| American Indian/Alaska Native | 4 (12.5%) | 4 (11.1%) | 8 (11.8%) |
| Ethnicity | |||
| Hispanic | 2 (6.3%) | 6 (16.7%) | 8 (11.8%) |
| Income | |||
| < $10,000 | 16 (50.0%) | 19 (52.8%) | 35 (51.5%) |
| $10,000 - $19,999 | 11 (34.4%) | 8 (22.2%) | 19 (27.9%) |
| $20,000 - $29,999 | 3 (9.4%) | 3 (8.3%) | 6 (8.8%) |
| $30,000 - $39,999 | 1 (3.1%) | 5 (13.9%) | 6 (8.8%) |
| $40,000 - $49,999 | 1 (3.1%) | 1 (2.8%) | 2 (2.9%) |
| Education | |||
| Did not graduate high school | 15 (46.9%) | 10 (27.8%) | 25 (36.8%) |
| High school graduate | 7 (21.9%) | 9 (25.0%) | 16 (23.5%) |
| Some college | 4 (12.5%) | 9 (25.0%) | 13 (19.1%) |
| Technical school/Associate’s degree | 6 (18.8%) | 5 (13.9%) | 11 (16.2%) |
| 4-year college degree | 0 (0.0%) | 2 (5.6%) | 2 (2.9%) |
| School beyond 4-year college degree | 0 (0.0%) | 1 (2.8%) | 1 (1.5%) |
| Primary Pain Location | |||
| Neck/Back | 14 (43.8%) | 19 (52.8%) | 33 (48.6%) |
| Lower Extremity | 14 (43.8%) | 11 (30.6%) | 25 (36.8%) |
| Upper Extremity | 2 (6.3%) | 3 (8.3%) | 5 (7.4%) |
| Chest | 1 (3.1%) | 0 (0.0%) | 1 (1.5%) |
| Stomach | 0 (0.0%) | 1 (2.8%) | 1 (1.5%) |
| Head | 1 (3.1%) | 2 (5.6%) | 3 (4.4%) |
| Pain Duration | |||
| < 3 months | 3 (9.4%) | 1 (2.8%) | 3 (5.9%) |
| 3–12 months | 7 (21.9%) | 5 (13.9%) | 12 (17.6%) |
| > 12 months | 22 (68.8%) | 30 (83.3%) | 52 (76.5%) |
| Pain Medication | |||
| Opioid | 15 (46.9%) | 23 (63.9%) | 38 (55.9%) |
| Non-opioid | 17 (53.1%) | 13 (36.1%) | 30 (44.1%) |
| M (SD) | M (SD) | M (SD) | |
| Age | 51.91 (8.23) | 50.86 (7.74) | 51.35 (7.94) |
| Characteristic pain intensity a | 57.60 (32.41) | 51.57 (31.07) | 54.41 (31.61) |
| Cigarettes per day | 10.75 (7.55) | 15.06 (13.07) | 13.03 (10.97) |
| Years smoking | 28.13 (10.69) | 29.00 (10.17) | 28.59 (10.35) |
| ACTG Symptom Count b | 13.91 (6.82) | 12.00 (6.48) | 12.78 (6.63) |
Note.
Graded Chronic Pain Scale-Characteristic Pain Intensity Subscale
AIDS Clinical Trial Group Symptom Distress Module – Symptom Count.
Smoking-Relevant Outcomes
Pain-smoking knowledge.
Participants randomized to the Integrated PFI demonstrated greater knowledge regarding pain-smoking interrelations at post-treatment, when compared to those randomized to the Control PFI (M = 6.26, SE = .33 vs. M = 4.91, SE = .31; F[1, 65] = 8.84, p < .01, ηp2 = .12; see Figure 1), with an observed effect that may be characterized as medium-to-large in magnitude.
Figure 1.
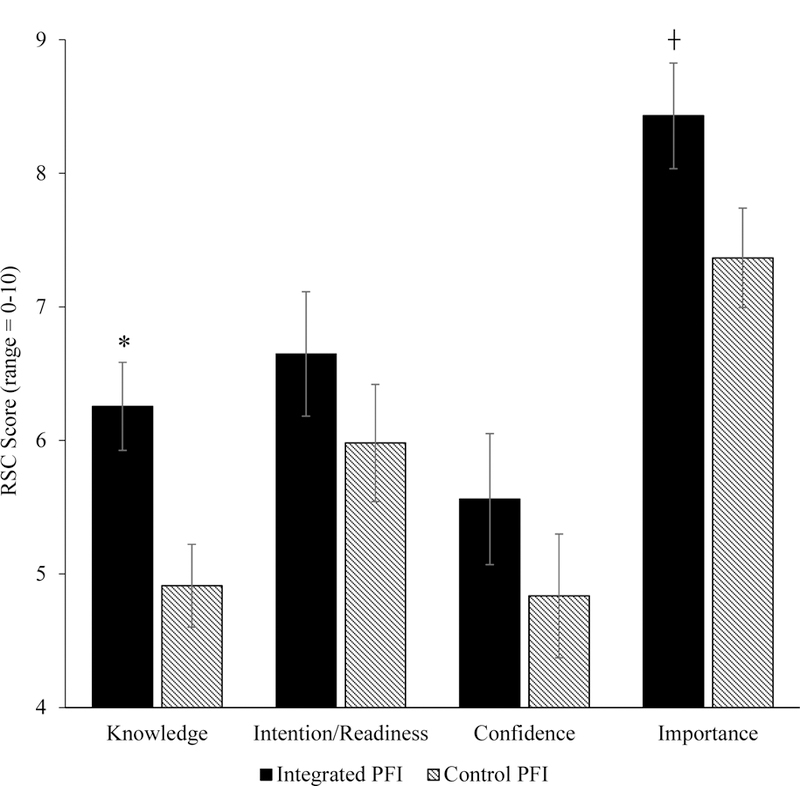
Smoking-Relevant Outcomes as a Function of Intervention Condition Assignment. Note. *p < .05, ┼ p < .10. RSC = Rulers for Smoking Cessation. Knowledge = knowledge regarding pain-smoking interrelations; Importance = importance of stopping smoking; Readiness = Intention/readiness to quit smoking within the next month; Confidence = Confidence in quitting smoking within the next month.
Readiness/Intention to quit smoking.
Although we did not observe a main effect for post-treatment readiness/intention to quit smoking (F[1, 65] = 1.08, p = .30, ηp2 = .02), adjusted means were in the expected direction (PFI M = 6.65, SE = .47 vs. Control PFI M = 5.98, SE =.44; see Figure 1). The intervention condition x CPD interaction term, however, was statistically-significant (F[1, 63] = 7.46, p < .01, ηp2 = .11; see Figure 2), indicating that the Integrated PFI resulted in greater readiness/intention to engage a quit attempt among participants who smoked at least 10 CPD (but not among those who smoked less than 10 CPD), with partial eta squared indicating a medium-to-large effect size.
Figure 2.

Readiness, Confidence, and Importance of Quitting Smoking as a Function of Interaction between Intervention Condition and Cigarettes Smoked Per Day. Note. *p < .05. RSC= Rulers for Smoking Cessation. CPD = Cigarettes smoked per day; Importance = importance of stopping smoking; Readiness = Intention/readiness to quit smoking within the next month; Confidence = Confidence in quitting smoking within the next month.
Confidence in quitting smoking.
Although we did not observe a main effect for post-treatment confidence in quitting over the next month (F[1, 65] = 1.15, p = .29, ηp2 = .02), adjusted means were in the expected direction (PFI M = 5.56, SE = .49 vs. Control PFI M = 4.84, SE = .46; see Figure 1). Again, however, the condition x CPD interaction term was statistically-significant (F[1, 63] = 4.62, p = .04, ηp2 = .07), indicating that the Integrated PFI resulted in greater confidence to engage a quit attempt only among participants who smoked at least 10 CPD, with an effect that may be characterized as medium in magnitude.
Perceived importance of quitting.
A trend-level main-effect was observed for perceived importance of quitting smoking (F[1, 65] = 3.73, p = .06, ηp2 = .05), such that post-treatment ratings of quitting importance were higher among participants randomized to the (M = 8.43, SE =.40) vs. Control (M = 7.37, SE = .37) PFI. Partial eta squared indicates a medium sized main effect, and the intervention condition x CPD interaction did not reach statistical significance (F[1, 63] = .10, p = .76, ηp2 = .00).
Prescription Opioid/Pain Medication-Relevant Outcomes
Pain-prescription analgesic knowledge.
Although adjusted means were in the expected direction (Integrated PFI M = 5.00, SE = .29 vs. Control PFI M = 4.42, SE = .28), group differences in post-treatment knowledge regarding interrelations between pain and the use/misuse of analgesic medication did not reach statistical significance (F[1, 65] = 2.05, p = .16, ηp2 = .03).
Attitudes and beliefs regarding prescription opioid/analgesic misuse.
Although participants randomized to the Integrated PFI (vs. Control PFI) endorsed fewer post-treatment maladaptive attitudes and beliefs regarding the misuse of prescription pain medications (M =, SE = 1.90 vs. M = 18.41, SE = 1.79), this difference was not statistically-significant (F[1, 65] = .87, p = .35, ηp2 = .01).
Intention to misuse prescription pain medications in the future.
No main effect was observed regarding intention to misuse opioids and other prescription pain medications in the next 30 days as a function of condition assignment (Integrated PFI M = 16.57, SE = 1.27 vs. Control PFI M = 16.42, SE = 1.20; F[1, 65] = .01, p = .93, ηp2 < .001).
Discussion
This is the first study to examine the effects of a brief integrated computer-based PFI that was developed to increase motivation to quit smoking and decrease intention to misuse prescription opioid medications among people living with HIV. In terms of smoking-related variables, results indicated that participants randomized to the Integrated PFI intervention (vs. Control PFI) evinced greater post-treatment knowledge of interrelations between pain and tobacco smoking (e.g., that smoking is a unique risk factor in the onset/progression of chronic pain, and that quitting smoking may improve pain and physical impairment). Two significant interactions further indicated that the Integrated PFI resulted in greater confidence in quitting and readiness/intention to quit only among heavier smoking PLWH (i.e., those who reported smoking at least 10 cigarettes per day). Finally, we observed a trend-level main effect, such that Integrated PFI participants reported greater perceived importance of quitting smoking. Effect sizes ranged from medium to medium-large in magnitude, and these findings are generally consistent with evidence that providing individuals with explicit links between their smoking and health may increase motivation to quit smoking (McCaul et al., 2006).
In terms of pain medication misuse outcomes, results were less robust. Specifically, although adjusted means were generally consistent with hypotheses, we observed no statistically-significant differences as a function of treatment condition assignment regarding pain-opioid misuse knowledge, prescription medication misuse beliefs/attitudes, or intention to misuse opioids in the next 30 days. Collectively, effects ranged from small to less than small in magnitude. One possible explanation for these null findings is that participants were not selected based on current misuse or risk for future misuse of prescription pain medications. Nearly 1/3 of participants in this study endorsed zero intention for misuse at baseline, which may have limited our ability to detect change in related outcomes. Future research may benefit from recruiting smokers with PLWH who are determined to be at risk for aberrant use of prescription opioids.
Tobacco smoking, chronic pain, and prescription analgesic misuse are all highly prevalent among PLWH, and unique interrelations between pain, smoking, and the misuse of prescription analgesia represent a prime target for novel intervention in this population. Consistent with this perspective, smokers who are not yet ready to quit should receive brief interventions to increase motivation to quit and promote future quit attempts (Fiore et al., 2008). In addition, the authors of a seminal review of smoking cessation among PLWH observed that approximately 80% of HIV+ smokers had not considered quitting and suggested that new interventions for this population should be more accessible and specifically target motivation to quit (Niaura et al., 2000). Single-session PFIs (without therapeutic guidance) have been shown to be both efficacious and cost-effective in reducing problematic substance use (Riper et al., 2009), and these data suggest that a brief computer-based PFI may function as a “first line of defense” in addressing confidence and readiness to quit smoking in HIV care settings.
Despite novel and encouraging aspects of this approach, several important limitations should be considered. First, the current sample was relatively small, and although these data provide preliminary support for a larger efficacy study (perhaps focusing on smoking-related outcomes), indices of statistical significance and effect size should be interpreted with caution. Future work should recruit a larger sample of PLWH, which would result in greater power to detect statistically significant effects and would allow for the inclusion of additional theoretically relevant covariates (e.g., pain intensity). Second, because primary outcomes were assessed immediately following treatment delivery, there is some potential for demand, and the extent to which these effects are maintained beyond the initial assessment period remains unclear. Similarly, although these data provide support for intervention-related change in motivation to quit smoking and intention to misuse prescription pain medication, the effects of the intervention on behavior are unknown. Future work would benefit from employing longer-follow up periods, with an emphasis on rates of engagement and subsequent outcomes of post-treatment smoking cessation attempts. Third, given that the Integrated PFI was compared to a control intervention that did not address pain-smoking relations or motivation to quit smoking, additional research is needed to determine the incremental utility of incorporating tailored/integrated pain-smoking content into brief motivational smoking treatments. Finally, participants were not selected based on current misuse or risk for future misuse of prescription pain medications at baseline, and future work should recruit a sample of patients who engage in aberrant medication-related behaviors.
In summary, results of this pilot study indicate that a brief (20–30 minute) computer-based personalized feedback intervention may result in greater knowledge regarding pain-smoking interrelations and enhance confidence/intention to quit smoking, particularly among heavier cigarette smokers living with HIV and chronic pain. There is an emerging global emphasis on the adaptation of behavioral interventions for PLWH that can be easily disseminated (Collins et al., 2011), and by developing a portable/integrated smoking intervention for PLWH with chronic pain, our novel intervention strategy addresses a critical public health need in a population that is generally underrepresented in clinical research.
Highlights.
A brief PFI was developed to address cigarette smoking and prescription pain medication misuse
The computer-based Integrated PFI content was tailored for older PLWH who were prescribed analgesic medication
The Integrated PFI (vs. Control PFI) resulted in greater knowledge of pain-smoking interrelations
The Integrated PFI also resulted in greater confidence/readiness to quit, but only among heavier smokers
Future research should examine the efficacy of this portable integrated treatment
Acknowledgments
This work was supported by NIDA grant R21-DA038204 awarded to Joseph W. Ditre. We have no conflicts of interest to declare.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrams DB, & Niaura R (2003). The tobacco dependence treatment handbook: A guide to best practices: Guilford Press. [Google Scholar]
- Ajzen I (1991). The theory of planned behavior. Organizational Behavior and Human Decision Processes, 50(2), 179–211. [Google Scholar]
- Bandura A (1994). Social cognitive theory and exercise of control over HIV infection Preventing AIDS (pp. 25–59): Springer. [Google Scholar]
- Boudreaux ED, Sullivan A, Abar B, Bernstein SL, Ginde AA, & Camargo CA (2012). Motivation rulers for smoking cessation: a prospective observational examination of construct and predictive validity. Addiction Science & Clinical Practice, 7(1), 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitbart W, McDonald MV, Rosenfeld B, Passik SD, Hewitt D, Thaler H, & Portenoy RK (1996). Pain in ambulatory AIDS patients. I: Pain characteristics and medical correlates. Pain, 68(2–3), 315–321. [DOI] [PubMed] [Google Scholar]
- Butler SF, Budman SH, Fernandez KC, Houle B, Benoit C, Katz N, & Jamison RN (2007). Development and validation of the current opioid misuse measure. Pain, 130(1), 144–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB (2004). Pain In Hiv/Aids A Major Global Healthcare Problem [Press release] Retrieved from http://www.iasp-pain.org/AM/Template.cfm?Section=Press_Release&Template=/CM/ContentDisplay.cfm&ContentID=2910
- Champion VL, & Skinner CS (2008). The health belief model. Health Behavior and Health Education: Theory, Research, and Practice, 4, 45–65. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Behavioral Risk Factor Surveillance System Survey Questionnaire Atlanta, Georgia: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2007. [Google Scholar]
- Collins PY, Patel V, Joestl SS, March D, Insel TR, Daar AS,… Fairburn C (2011). Grand challenges in global mental health. Nature, 475(7354), 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham JA (2007). Internet-based interventions for alcohol, tobacco and other substances of abuse Translation of Addictions Science into Practice (pp. 399–416): Elsevier. [Google Scholar]
- Ditre JW, & Brandon TH (2008). Pain as a motivator of smoking: Effects of pain induction on smoking urge and behavior. Journal of Abnormal Psychology, 117(2), 467–472. doi: 10.1037/0021-843X.117.2.467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditre JW, Brandon TH, Zale EL, & Meagher MM (2011). Pain, nicotine, and smoking: Research findings and mechanistic considerations. Psychological Bulletin, 137(6), 1065–1093. doi: 10.1037/a0025544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditre JW, Zale EL, Heckman BW, & Hendricks PS (2017). A measure of perceived pain and tobacco smoking interrelations: pilot validation of the pain and smoking inventory. Cognitive Behaviour Therapy, 46(4), 339–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, & Dorfman SF (2008). Treating Tobacco Use and Dependence: 2008 Update Clinical Practice Guideline Rockville, MD: U.S. Department of Health and Human Services. Public Health Service; May 2008. [Google Scholar]
- George D, & Mallery M (2003). Using SPSS for Windows step by step: a simple guide and reference
- Hansen L, Penko J, Guzman D, Bangsberg DR, Miaskowski C, & Kushel MB (2011). Aberrant behaviors with prescription opioids and problem drug use history in a community-based cohort of HIV-infected individuals. Journal of Pain and Symptom Management, 42(6), 893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Aharonovich E, O’leary A, Greenstein E, Pavlicova M, Arunajadai S,…Johnston B (2013). Reducing heavy drinking in HIV primary care: a randomized trial of brief intervention, with and without technological enhancement. Addiction, 108(7), 1230–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooten WM, Vickers KS, Shi Y, Ebnet KL, Townsend CO, Patten CA, & Warner DO (2011a). Smoking cessation and chronic pain: patient and pain medicine physician attitudes. Pain Practice, 11(6), 552–563. doi: 10.1111/j.1533-2500.2011.00462.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooten WM, Vickers KS, Shi Y, Ebnet KL, Townsend CO, Patten CA, & Warner DO (2011b). Smoking cessation and chronic pain: patient and pain medicine physician attitudes. Pain Practice, 11(6), 552–563. doi: 10.1111/j.1533-2500.2011.00462.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huck SW, & McLean RA (1975). Using a repeated measures ANOVA to analyze the data from a pretest-posttest design: A potentially confusing task. Psychological Bulletin, 82(4), 511. [Google Scholar]
- IBM Corp, N. (2013). IBM SPSS statistics for windows Version, 22.
- Jamison RN, Stetson BA, & Parris WC (1991). The relationship between cigarette smoking and chronic low back pain. Addictive Behaviors, 16(3–4), 103–110. doi: 10.1016/0306-4603(91)90002-Y [DOI] [PubMed] [Google Scholar]
- Justice AC, Holmes W, Gifford AL, Rabeneck L, Zackin R, Sinclair G,… Wu AW (2001). Development and validation of a self-completed HIV symptom index. Journal of Clinical Epidemiology, 54 Suppl 1, S77–90. [DOI] [PubMed] [Google Scholar]
- Larimer ME, Lee CM, Kilmer JR, Fabiano PM, Stark CB, Geisner IM,… Feeney M (2007). Personalized mailed feedback for college drinking prevention: A randomized clinical trial. Journal of Consulting and Clinical Psychology, 75(2), 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchikanti L (2006). Prescription drug abuse: what is being done to address this new drug epidemic? Testimony before the Subcommittee on Criminal Justice, Drug Policy and Human Resources. Pain Physician, 9(4), 287. [PubMed] [Google Scholar]
- McCaul KD, Hockemeyer JR, Johnson RJ, Zetocha K, Quinlan K, & Glasgow RE (2006). Motivation to quit using cigarettes: a review. Addictive Behaviors, 31(1), 42–56. [DOI] [PubMed] [Google Scholar]
- McDonald DC, & Carlson KE (2013). Estimating the prevalence of opioid diversion by “doctor shoppers” in the United States. PloS One, 8(7), e69241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merskey HE (1986). Classification of chronic pain: descriptions of chronic pain syndromes and definitions of pain terms. Pain [PubMed]
- Miaskowski C, Penko JM, Guzman D, Mattson JE, Bangsberg DR, & Kushel MB (2011). Occurrence and characteristics of chronic pain in a community-based cohort of indigent adults living with HIV infection. The Journal of Pain, 12(9), 1004–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michna E, Ross EL, Hynes WL, Nedeljkovic SS, Soumekh S, Janfaza D,…Jamison RN (2004). Predicting aberrant drug behavior in patients treated for chronic pain: importance of abuse history. Journal of Pain and Symptom Management, 28(3), 250–258. doi: 10.1016/j.jpainsymman.2004.04.007 [DOI] [PubMed] [Google Scholar]
- Miller W, & Rollnick S (2002). Preparing people for change. Motivational Interviewing
- Moadel AB, Bernstein SL, Mermelstein RJ, Arnsten JH, Dolce EH, & Shuter J (2012). A randomized controlled trial of a tailored group smoking cessation intervention for HIV-infected smokers. Journal of Acquired Immune Deficiency Syndromes (1999), 61(2), 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueser KT (2003). Integrated treatment for dual disorders: A guide to effective practice: Guilford Press. [Google Scholar]
- Niaura R, Shadel WG, Morrow K, Tashima K, Flanigan T, & Abrams DB (2000). Human immunodeficiency virus infection, AIDS, and smoking cessation: the time is now: The University of Chicago Press. [DOI] [PubMed] [Google Scholar]
- Noar SM (2011). Computer technology-based interventions in HIV prevention: state of the evidence and future directions for research. AIDS Care, 23(5), 525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris T, Schiller J, & Clarke T (2018). Early release of selected estimates based on data from the National Health Interview Survey Retrieved from https://www.cdc.gov/nchs/nhis.htm.
- Orhurhu VJ, Pittelkow TP, & Hooten WM (2015). Prevalence of smoking in adults with chronic pain. Tobacco Induced Diseases, 13(1), 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R, Stein DJ, & Jelsma J (2014). Pain in people living with HIV/AIDS: a systematic review. Journal of the International AIDS Society, 17(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passik SD, Kirsh KL, McDonald MV, Ahn S, Russak SM, Martin L,…Portenoy K (2000). A pilot survey of aberrant drug-taking attitudes and behaviors in samples of cancer and AIDS patients. Journal of Pain and Symptom Management, 19(4), 274–286. [DOI] [PubMed] [Google Scholar]
- Patel N, Talwar A, Reichert VC, Brady T, Jain M, & Kaplan MH (2006). Tobacco and HIV. Clinics in Occupational and Environmental Medicine, 5(1), 193–207, xi Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16446262 [DOI] [PubMed] [Google Scholar]
- Patterson AL, Gritzner S, Resnick MP, Dobscha SK, Turk DC, & Morasco BJ (2012). Smoking cigarettes as a coping strategy for chronic pain is associated with greater pain intensity and poorer pain-related function. Journal of Pain doi: 10.1016/j.jpain.2011.11.008 [DOI] [PMC free article] [PubMed]
- Prochaska J, Redding C, & Evers K (2008). The transtheoretical model and stagesof change. Chapter 5. Health Behavior and Health Education: Theory, Research and Practice, fourth ed. Jossey-Bass, Inc, San Francisco, CA, 170–222. [Google Scholar]
- Riper H, van Straten A, Keuken M, Smit F, Schippers G, & Cuijpers P (2009). Curbing problem drinking with personalized-feedback interventions: a meta-analysis. American Journal of Preventive Medicine, 36(3), 247–255. [DOI] [PubMed] [Google Scholar]
- Robinson-Papp J, Elliott K, Simpson DM, Morgello S, & Bank MHB (2012). Problematic prescription opioid use in an HIV-infected cohort: The importance of universal toxicology testing. Journal of Acquired Immune Deficiency Syndromes (1999), 61(2), 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Weingarten TN, Mantilla CB, Hooten WM, & Warner DO (2010). Smoking and pain: Pathophysiology and clinical implications. Anesthesiology, 113(4), 977–992. doi: 10.1097/ALN.0b013e3181ebdaf900000542-201010000-00033 [pii] [DOI] [PubMed] [Google Scholar]
- Shiri R, Karppinen J, Leino-Arjas P, Solovieva S, & Viikari-Juntura E (2010). The association between smoking and low back pain: a meta-analysis. American Journal of Medicine, 123(1), 87 e87–35. doi: 10.1016/j.amjmed.2009.05.028 [DOI] [PubMed] [Google Scholar]
- Silverberg MJ, Ray GT, Saunders K, Rutter CM, Campbell CI, Merrill JO,… Weisner C (2012). Prescription long-term opioid use in HIV-infected patients. The Clinical Journal of Pain, 28(1), 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama D, Nishimura K, Tamaki K, Tsuji G, Nakazawa T, Morinobu A, & Kumagai, (2010). Impact of smoking as a risk factor for developing rheumatoid arthritis: a meta-analysis of observational studies. Annals of the Rheumatic Diseases, 69(1), 70–81. doi: 10.1136/ard.2008.096487 [DOI] [PubMed] [Google Scholar]
- Trafton JA, Sorrell JT, Holodniy M, Pierson H, Link P, Combs A, & Israelski D (2012). Outcomes associated with a cognitive-behavioral chronic pain management program implemented in three public HIV primary care clinics. The Journal of Behavioral Health Services & Research, 39(2), 158–173. [DOI] [PubMed] [Google Scholar]
- Tsao JC, Dobalian A, & Stein JA (2005). Illness burden mediates the relationship between pain and illicit drug use in persons living with HIV. Pain, 119(1), 124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J, Page-Shafer K, Chin DP, Osmond D, Mossar M, Markstein L,… Chesney M (2001). Adverse impact of cigarette smoking on dimensions of health-related quality of life in persons with HIV infection. AIDS Patient Care and STDS, 15(12), 615–624. doi: 10.1089/108729101753354617 [DOI] [PubMed] [Google Scholar]
- Vidrine DJ (2009). Cigarette smoking and HIV/AIDS: health implications, smoker characteristics and cessation strategies. AIDS Education and Prevention, 21(3_supplement), 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidrine DJ, Arduino RC, Lazev AB, & Gritz ER (2006). A randomized trial of a proactive cellular telephone intervention for smokers living with HIV/AIDS. AIDS, 20(2), 253–260. [DOI] [PubMed] [Google Scholar]
- Von Korff M (2011). Assessment of chronic pain in epidemiological and health services research. In Turk DC & Melzack R (Eds.), Handbook of Pain Assessment: Third Edition. New York, NY: The Guilford Press. [Google Scholar]
- Von Korff M, Dworkin SF, & Le Resche L (1990). Graded chronic pain status: an epidemiologic evaluation. Pain, 40(3), 279–291. [DOI] [PubMed] [Google Scholar]
- Von Korff M, Ormel J, Keefe FJ, & Dworkin SF (1992). Grading the severity of chronic pain. Pain, 50(2), 133–149. [DOI] [PubMed] [Google Scholar]
- Weinberger AH, Seng EK, Ditre JW, Willoughby M, & Shuter J (2018). Perceived interrelations of pain and cigarette smoking in a sample of adult smokers living with HIV/AIDS. Nicotine and Tobacco Research doi: 10.1093/ntr/nty021 [DOI] [PMC free article] [PubMed]
- Wewers ME, Neidig JL, & Kihm KE (2000). The feasibility of a nurse-managed, peer-led tobacco cessation intervention among HIV-positive smokers. Journal of the Association of Nurses in AIDS Care, 11(6), 37–44. [DOI] [PubMed] [Google Scholar]
- Zale EL, Maisto SA, & Ditre JW (2016). Anxiety and depression in bidirectional relations between pain and smoking: implications for smoking cessation. Behavior Modification, 40(1–2), 7–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zevin S, & Benowitz NL (1999). Drug interactions with tobacco smoking. Clinical Pharmacokinetics, 36(6), 425–438. [DOI] [PubMed] [Google Scholar]


