Abstract
Since 2010, the Thailand Ministry of Public Health has recommended that men who have sex with men (MSM) have an HIV test at least two times a year. We calculated the proportion of, and factors associated with, testing adherence among the HIV-uninfected MSM clients attending Silom Community Clinic @TropMed. We defined testing adherence as repeating at least one HIV test within six months of an initial HIV-negative test, and used log-binomial regression to test for associated factors. We included 1927 clients during 2011–2014; 362 (19%) were adherent with an increased trend (p<0.01), from 16% to 24%. Clients aged 18–24 years and those having a history of HIV testing were more likely to adhere (aRR: 1.3, 95% CI: 1.1–1.6; and aRR: 1.3, 95% CI: 1.0–1.5, respectively). One-fifth adhered to the recommendation; older clients or naïve testees were less likely to adhere. We need to impress on clients the importance of repeat HIV testing.
Keywords: Asia, human immunodeficiency virus, homosexual, location, prevention, viral disease
Introduction
During 2007–2015, human immunodeficiency virus (HIV) prevalence has been estimated to be 30% among men who have sex with men (MSM) in Bangkok, Thailand.1 HIV incidence has remained high during this period, especially among young MSM.1–4 Since 2007, policies for HIV prevention among MSM have focused on encouraging HIV testing, promotion of safer sex, and education on HIV risk factors.5,6 Previous studies of HIV testing among Thai MSM reported that only half had a history of HIV testing prior to participation in research studies,2,7 and many were still reluctant to get tested for HIV.8
Despite recent advances in HIV treatment and prevention, comprehensive HIV services that aim to prevent HIV acquisition and improve the health of persons infected with HIV remain dependent on HIV voluntary counseling and testing (HCT) services as an entry point for the HIV care continuum. Repeat HCT, especially during periods of risk, is important to identify HIV infection early.9 Since 2006, the United States (U.S.) Centers for Disease Control and Prevention (CDC) has recommended that persons at increased risk for HIV infection, including MSM and those presenting for sexually transmitted infection testing, be tested at least annually for HIV;10 men at highest risk are recommended to receive testing every three to six months.11 According to the 2010 and 2014 Thailand National Guidelines on HIV/AIDS Diagnosis and Treatment and Prevention (hereafter referred to as the ‘2010/2014 Guidelines’), MSM are recommended to have an HIV test at least two times per year (every six months). The 2010/2014 Guidelines regarded all MSM as people at high risk of HIV acquisition.12,13
There are few data describing the characteristics of Thai HIV-uninfected MSM who have repeat HCT, as well as the factors associated with repeat HCT. Based on anecdotal observations, we hypothesized that few HIV-uninfected MSM who present for HCT would repeat the test regularly, even those at highest risk. Knowing the factors associated with repeat HCT could lead to policies and practices to encourage more MSM to get repeat HCT and engage in the HIV care continuum. In this analysis, among HIV-uninfected MSM clients attending Silom Community Clinic @TropMed (SCC) from 2011 to 2015, we determined the proportion of MSM clients who repeated HCT within six months of their initial HCT and evaluated factors associated with this behavior.
Methods
Study setting
As previously reported,3 SCC provided free-of-charge HCT services to MSM. We considered all clients to be at risk of HIV acquisition given that they presented themselves for HIV testing. At each HCT, SCC staff members provide a CDC-standard comprehensive testing package including pretest and posttest counseling,14 risk behavior reduction counseling, and packages of condoms and sexual lubricant. In addition to HCT, SCC provided an evaluation for syphilis infection and other STIs, such as gonococcal and non-gonococcal infections, genital ulcers, and warts. SCC provided treatment for those diagnosed with an STI. SCC provided CD4+ cell count monitoring every four to six months, as well as referral to antiretroviral therapy programs consistent with 2010/2014 Guidelines,12,13 for those testing positive for HIV infection.
If a client tested HIV negative during the initial HCT visit, SCC staff recommended that they repeat the HCT every six months, and whenever they suspected having symptoms of an STI or HIV infection. We also recommended that our clients repeat the HCT during peak visit months, the months that are immediately following the Western, Chinese, and Thai New Year celebrations (i.e. February, March, and May). These months of celebration, with long holidays and parties, were commonly known in the Thai MSM community as months of potential increased risk of HIV acquisition. Outreach workers asked clients to also visit our clinic for testing during their birth month based on Buddhist birthday merit beliefs. Clients could use their birth month as an extra reminder to repeat additional HCT in each calendar year.
At SCC, we recommended our HIV-negative clients to schedule their next appointments within 180 days as a simpler version of six months. Hereafter, we shall refer to within six months as within 180 days.
Study participants
For our analysis, we included clients who had initial HCT from January 2011 to December 2014, were HIV-negative, and had repeat HCT through December 2015. Clients who participated in clinical research conducted at SCC were excluded, as their follow-up HCT visits were based on schedules in those studies. Prior to 2015, MSM aged 18 years or older were regarded as the HIV risk group;1–8 therefore, SCC focused our free-of-charge HCT services on MSM who were at least 18 years old or older. Although some MSM aged less than 18 years attended our clinic, we only provided the service on the need-to-have basis and did not focus our effort on the retention of these young MSM and therefore excluded them from this analysis. Although the Thai 2014 Guidelines recommended providing pre-exposure prophylaxis (PrEP) to MSM, PrEP was in early stages of implementation in 2014 and 201515,16 and SCC did not start providing PrEP until March 2016. Therefore, repeating HCT under the PrEP service was not part of this analysis.
Data collection
We collected basic demographic information, such as age, current residency, nationality, birthplace, and history of HIV testing at the time of the initial visit. Our system collected test results and testing months of all visits. As this was a clinical service, we did not collect behavioral risk factor data. Although this analysis was not designed to demonstrate the high rate of HIV acquisition, previous analysis3 provided such evidence (during 2005–2011, prevalence of HIV at first visit was 28.3% while HIV incidence was 6.3 per 100 person-years of follow-up) with an update currently under review.
Data analysis
The authors defined repeat HIV testers as those with initial HIV-negative test results and one or more subsequent HCT, regardless of the subsequent test results. Among those repeating HCT, we defined adherence to the 2010/2014 Guidelines as having repeat HCT within 180 days of their initial HCT.
The authors also identified the number of MSM who had HCT that year and the proportion of those who had repeat HCT within 180 days. We used binomial distribution to calculate 95% confidence intervals (95% CIs), and the Chi square test for trend to assess changes in the proportion of MSM who had repeat HCT within 180 days, by year of their initial HCT. We used log-binomial regression to investigate factors associated with repeat HCT within 180 days, and included those with p ≤ 0.1 in the multivariable model; statistical significance was evaluated using a two-sided p<0.05. We used the Chi square to test the difference between two sample proportions. We performed all analyses using STATA® (Version 13, 2013; Stata Corp., College Station, Texas, USA).
Results
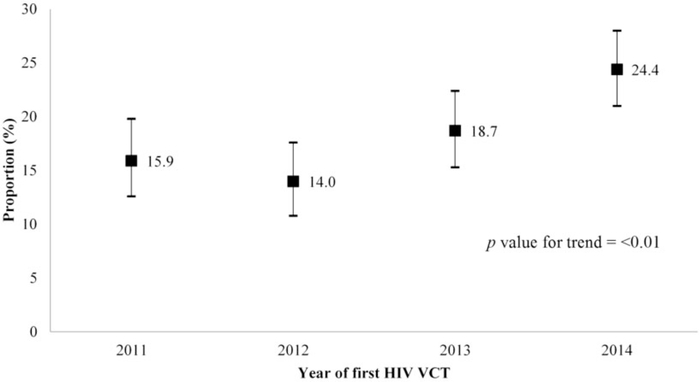
A total of 4529 first-time clients had an initial HCT visit from January 2011 to December 2014, of which 3067 (67.7%) tested HIV negative. Of these, 1927 (62.8%) had at least one repeat HCT (median number of subsequent HCTs was two, interquartile range [IQR] 1–4). Overall, there were 701 (36.4%) clients aged 18–24 years, and 1123 (58.3%) had a history of HIV testing prior to their initial HCT at SCC (Table 1). Of the 1927 initially testing HIV negative, 362 (18.8%) had a repeat HCT within 180 days (≤180 days) of the initial HCT, with the proportion increasing from 15.9% in 2011 to 24.4% in 2014 (p for trend by year <0.01, Figure 1). There were 511 (26.5%) clients who had repeat HCT during the peak visit months, and only 89 (4.6%) did so within 180 days after their initial HCT. We calculated the HIV incidence among those who had an initial HCT and repeated HCT during 2011–2014 as 5.0 per 100 person-years (95% CI: 4.2–5.8).
Table 1.
Characteristics of men who have sex with men who repeated HIV voluntary counseling and testing (HCT) at the Silom Community Clinic @TropMed, Bangkok, Thailand, 2011–2014 (n = 1927).
| Characteristics | Number | % | Median (IQR) |
|---|---|---|---|
| Number of subsequent HCTa | 2(1,4) | ||
| Second HCT ≤180 days | 362 | 18.8 | |
| Second HCT > 180 days | 1565 | 81.2 | |
| Year of initial HCT | |||
| 2011 | 421 | 21.8 | |
| 2012 | 429 | 22.3 | |
| 2013 | 482 | 25.0 | |
| 2014 | 595 | 30.9 | |
| Aged 18–24 years at initial HCT | 701 | 36.4 | |
| Thai nationality | 1786 | 92.7 | |
| Born in Bangkok or peripheral provinces | 627 | 32.5 | |
| Currently residing in Bangkok or peripheral provinces | 1719 | 89.2 | |
| Had history of HIV test prior to initial HCT | 1123 | 58.3 | |
| Diagnosed with STI at initial HCT | 268 | 13.9 | |
| Had initial HCT during one’s birth month | 149 | 7.7 | |
| Had initial HCT during peak visit monthsb | 519 | 26.9 |
Visit with HIV testing.
February, March, or May.
HCT: HIV voluntary counseling and testing; STI: sexually transmitted infection.
Figure 1.
Proportion of clients repeating HIV testing during Voluntary Counseling and Testing (HCT) within 180 days, among men who have sex with men attending the Silom Community Clinic @TropMed, Bangkok, Thailand, 2011–2014 (n=1927).
Factors associated with repeated testing within 180 days
In bivariable analysis, we found clients who had their initial HCT in 2011, 2012, or 2013 were less likely to repeat HCT within 180 days (relative risk [RR] 0.7, p<0.01; RR 0.6, p<0.01; and RR 0.8, p=0.03, respectively), when compared to clients who had their initial HCT in 2014. Clients who had their initial HCT during their birth month were also less likely to repeat HCT within 180 days (RR 0.7, p=0.09) compared to those who did not. We found that clients aged 18–24 years and those who reported a history of prior HIV testing were more likely to repeat HCT within 180 days (RR 1.3, p=0.01; RR 1.2, p=0.08; respectively). The proportion having repeat HCT within 180 days did not differ by nationality, birthplace, current residency, being diagnosed with an STI at the initial HCT, repeating HCT during their birth month, or having the initial or repeat HCT during three peak visit months (Table 2).
Table 2.
Factors associated with repeat HIV voluntary counseling and testing (HCT) within 180 days among men who have sex with men attending the Silom Community Clinic @TropMed, Bangkok, Thailand, 2011–2014 (n = 1927).
| Factors | Second HCT (Number, %) | Bivariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|---|
| ≤ 180 days (n = 362) | > 180 days (n = 1565) | RR | p | Adjusted RR | 95% CI | p | |
| Year of initial HCTa | |||||||
| 2011 | 67, 15.9 | 354, 84.1 | 0.7 | <0.01 | 0.7 | 0.5–0.9 | <0.01 |
| 2012 | 60, 14.0 | 369, 86.0 | 0.6 | <0.01 | 0.6 | 0.4–0.8 | <0.01 |
| 2013 | 90, 18.7 | 392, 81.3 | 0.8 | .03 | 0.8 | 0.6–0.97 | 0.05 |
| 2014 | 145, 24.4 | 450, 75.6 | 1.0 | 1.0 | |||
| Age (years) at initial HCT | |||||||
| 18–24 | 152, 21.7 | 549, 78.3 | 1.3 | .01 | 1.3 | 1.1–1.6 | <0.01 |
| >25 | 210, 17.1 | 1016, 82.9 | 1.0 | 1.0 | |||
| Nationality | |||||||
| Thai | 334, 18.7 | 1452, 81.3 | 0.9 | .73 | |||
| Others | 28, 19.9 | 113, 80.1 | 1.0 | ||||
| Birthplace | |||||||
| Bangkok or suburbs | 118, 18.8 | 509, 81.2 | 1.0 | .98 | |||
| Elsewhere | 244, 18.8 | 1056, 81.2 | 1.0 | ||||
| Current residency at initial HCT | |||||||
| Bangkok or suburbs | 320, 18.6 | 1399, 81.4 | 0.9 | .58 | |||
| Elsewhere | 42, 20.2 | 166, 79.8 | 1.0 | ||||
| History of HIV testing at initial HCT | |||||||
| Ever | 226, 20.1 | 897, 79.9 | 1.2 | .08 | 1.3 | 1.04–1.5 | 0.02 |
| Never | 136, 16.9 | 668, 83.1 | 1.0 | 1.0 | |||
| Clinical STI diagnosisb at initial HCT | |||||||
| Yes | 43, 16.0 | 225, 84.0 | 0.8 | .22 | |||
| No | 319, 19.2 | 1340, 80.8 | 1.0 | ||||
| Had initial HCT during one’s birth month | |||||||
| Yes | 20, 13.4 | 129, 86.6 | 0.7 | .09 | NSc | ||
| No | 342, 19.2 | 1436, 80.8 | 1.0 | 1.0 | |||
| Had second HCT visit during one’s birth month | |||||||
| Yes | 31, 19.0 | 132, 81.0 | 1.0 | .94 | |||
| No | 331, 18.8 | 1433, 81.2 | 1.0 | ||||
| Had initial HCT during peak visit monthsd | |||||||
| Yes | 92, 17.7 | 427, 82.3 | 0.9 | .47 | |||
| No | 270, 19.2 | 1138, 80.8 | 1.0 | ||||
| Had second HCT during peak visit months | |||||||
| Yes | 89, 17.4 | 422, 82.6 | 0.9 | .36 | |||
| No | 273, 19.3 | 1143, 80.7 | 1.0 | ||||
HCT: HIV voluntary counseling and testing; STI: sexually transmitted infection.
Visit with HIV testing.
Physician diagnosis.
NS=Non significant (p > 0.05).
February, March, and May.
In multivariable analysis, when adjusted for calendar year of initial HCT and having initial HCT during the birth month, clients aged 18–24 years (adjusted relative risk [aRR]: 1.3, 95% CI: 1.1–1.6) and those who reported a history of prior HIV testing (aRR: 1.3, 95% CI: 1.04–1.5) were more likely to repeat HCT within 180 days (Table 2).
In addition, among 3067 clients who tested HIV negative at their initial HCT, we found that the proportion of clients aged 18–24 years among 1927 clients who had at least one repeat HCT visit (701, 36.4%) did not differ from the proportion (414, 36.3%) among 1140 clients who did not repeat HCT visit (p=0.04). However, the proportion of clients who had history of HIV testing among 1927 clients who had at least one repeat HCT visit (1123, 58.3%) significantly differed from the proportion (621, 54.5%) among 1140 clients who did not have a repeat HCT visit (p=0.04).
Discussion
The 2010/2014 Guidelines regarded all MSM as people at high risk of HIV acquisition. Overall, less than one-fifth of our clients who were HIV-uninfected at their initial HCT repeated HCT within 180 days; however, this proportion increased over the four-year study period, reaching 24.4% in 2014. Two factors associated with a higher likelihood of repeating HCT within 180 days, namely, age <25 years and prior experience with HIV testing, suggest that, while the history of prior HIV testing was a crucial factor for repeating HCT, targeting HIV testing messages toward youth could further improve testing behaviors later in life. Although we saw a high volume of HCT during the peak visit months, we did not find an association between having HCT during this period, either as the initial or 180-day HCT and having a repeat HCT. Only about one-quarter of repeat HCTs occurred during this period.
Few analyses have evaluated repeat HCT among MSM in Thailand. The proportion having repeat HCT, especially with the definition of having two HCTs within a year, was lower than the proportion of MSM who repeated HIV testing in high-income countries like Croatia (Zagreb), Netherlands (Amsterdam), and New Zealand (Auckland) where the proportion was more than 30%.17–19 However, the proportion of our clients (24.4%) having frequent repeat HIV testing in 2014 was similar to estimates (25.6%) used for the 2014–2016 Thai National AIDS Strategy. Unfortunately, this is far from the goal of 90% set for 2016.20
The Thailand Department of Disease Control, Ministry of Public Health, documented unfamiliarity with the 2010/2014 Guidelines recommending people at high risk of HIV acquisition get at least two HIV tests per year (only 43.5% of high-risk individuals who were interviewed in 24 Thai provinces knew about this recommendation).21 In our study, a history of prior HIV testing was clearly associated with repeat HCT within 180 days. Overall, the proportion of clients reporting prior HIV testing (58.3%) was higher than in other studies among MSM in Bangkok and in Thailand,2,6 and also higher than in upper middle-income countries like China (Tianjin) where the proportion was 43%.22 However, they were lower than the proportions reporting a history of HIV testing found among urban MSM in high-income countries like Japan (Yokohama), United Kingdom (London), and United States (several cities) where proportions were more than 60%.23–25 We anticipate the proportion of MSM with repeat HCT will continue to increase as HCT is further promoted, hopefully resulting in an increase in the proportion of MSM who repeat HCT within 180 days.
We found younger clients were more likely to repeat HCT within 180 days. Drawing from experiences of our own and others, young clients may have had fewer obstacles, such as workload and family matters, to allow for more frequent clinic visits. Alternatively, it may reflect the young client’s perceived higher risk,26 or more frequent HIV testing services at locations where higher risk sexual activity occurs (e.g. bathhouses).22
Data from this real-world setting of clients attending HCT, rather than study participants, could be more useful for public health policy. Nevertheless, our findings have limitations. The proportion of clients having repeat HCT within 180 days in our study might be underestimated as clients might have repeated HIV testing elsewhere. Secondly, we were unable to assess HIV risk factors leading to repeating HCT as limited data were collected during HCT. Although we respected our clients’ self-assessment of their HIV risk and considered them all to be individuals at risk of HIV acquisition, the lack of behavioral data to identify those at highest risk is still a major limitation of this study. Measures to assess the client’s preference for different HCT methods, content, and aspects of the counseling session, as well as the clinic facility and location, were not collected. Furthermore, MSM who attended our clinic might not be representative of all MSM in Bangkok. The 2010/2014 Guidelines recommend twice yearly testing, and we interpreted this to mean 180-day windows for testing, as this is how counseling messaging occurred in practice. It is possible that the arbitrary cut-off of 180 days may not be relevant to some HCT or HIV prevention programs. As we focused primarily on the comparison between clients who ever repeated HCT and who repeated within 180 days, it is possible that we did not take fully into account the association between the history of HIV testing and ever repeating HCT. Lastly, although our analysis included data from 2014, the 2014 Thailand National Guidelines on HIV/AIDS Diagnosis and Treatment and Prevention were not released until October 2014 and as such, we have very limited data to assess the adherence to these HIV testing recommendations, separately.
Our data showed that a low proportion of MSM attending our HCT clinic during 2011–2014 adhered to the 2010/2014 Guidelines on repeat testing, although this improved with time. Data from the Bangkok MSM Cohort Study, a behavioral study that asked participants to be followed up every four months for behavioral survey and HIV testing, demonstrated that participants with a higher number of visits tended to lower their risk behaviors over time.27 Therefore, repeat HCT, especially among men with high-risk behavior for HIV, could be used as an opportunity to: (1) help reduce their HIV-risk behaviors; (2) provide HIV prevention interventions including PrEP, post-exposure prophylaxis, condoms, and counseling; or (3) detect early HIV infection, facilitating rapid referrals for HIV treatment. Counseling during HCT can emphasize the importance of regular and repeat HCT. A systematic and regular strategy to retain at-risk clients to repeat HCT, such as personalized and confidential reminder systems, should also be implemented. Further investigations will focus on the relationship between: the likelihood of repeat HCT and the level of understanding of HIV infection, prevention, and treatment; awareness of individual HIV risk level; and support from family, partners, and friends. Engaging men at risk for HIV infection in regular HCT settings is critical and approaches to retain them for repeat HCT are needed. MSM undergoing regular HCT might be considered as core messengers to others at risk on the importance of HCT.
Acknowledgments
We thank the staff and volunteers of the Rainbow Sky Association of Thailand, Bangkok Rainbow Organization, Service Workers In Group, the Poz Home Center, HIV Foundation, GayBKK Online Club, TestBKK Campaign by APCOM, Thai Red Cross Society AIDS Research Centre, Thai Puen Magazine, and the Multiple-sexuality persons Community Advisory Board: M-CAB for referring our services to the community. We greatly thank the clients of the SCC who continue to use our services as well as refer our services to their friends. We appreciate the scientific writing support provided by Dorothy L Southern and her critical review of this manuscript. Disclaimer: The findings and conclusions reported in this paper are those of the authors and do not represent the views of the U.S. CDC.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The services at SCC and this study were supported by the Thailand MOPH – U.S. CDC Collaboration.
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Sirinirund P, Limanond B, Saonuam P, et al. (eds) 2012. Thailand AIDS response progress report. 617 [Google Scholar]
- 2.van Griensven F, Thienkrua W, McNicholl J, et al. Evidence of an explosive epidemic of HIV infection in a cohort of men who have sex with men in Thailand. AIDS 2013; 27: 825–832. [DOI] [PubMed] [Google Scholar]
- 3.Ananworanich J, Chitwarakorn A, Wimonsate W, et al. HIV and syphilis infection among men who have sex with men – Bangkok, Thailand, 2005–2011. MMWR 2013; 62: 518–520. [PMC free article] [PubMed] [Google Scholar]
- 4.van Griensven F, Holtz TH, Theinkrua W, et al. Temporal trends in HIV-1 incidence and risk behaviours in men who have sex with men in Bangkok, Thailand, 2006–13: an observational study. Lancet HIV 2015; 2: e64–e70. [DOI] [PubMed] [Google Scholar]
- 5.The National Committee for HIV and AIDS Prevention and Alleviation. The National Plan for Strategic and Integrated HIV and AIDS Prevention and Alleviation 2007–2011: key contents. The Agricultural Co-operative Federation of Thailand Publishing, 2007. [Google Scholar]
- 6.The Global Fund. Thailand grant performance report, www.theglobalfund.org/ProgramDocuments/THA/THA-809-G10-H/THA-809-G10-H_GPR_0_en/ (2011, accessed 18 May 2016).
- 7.Wimonsate W, Naorat S, Varangrat A, et al. Factors associated with HIV testing history and returning for HIV test results among men who have sex with men in Thailand. AIDS Behav 2011; 15: 693–701. [DOI] [PubMed] [Google Scholar]
- 8.Chemnasiri T, Varangrat A, Todd CS, et al. Barriers to HIV testing among young men who have sex with men in Bangkok, Thailand: a qualitative exploration. In: 11th International Congress on AIDS in Asia and the Pacific (E-Poster Presentation), Bangkok, Thailand 18–22 December 2013. [Google Scholar]
- 9.WHO. Consolidated guidelines on HIV testing services. Geneva: World Health Organization, 2015. [PubMed] [Google Scholar]
- 10.Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006; 55: 1–17. [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Announcements: National Gay Men’s HIV/AIDS Awareness Day: focus on HIV testing – September 27, 2011. MMWR 2011; 60: 1283. [Google Scholar]
- 12.Center for Development of Antiretroviral Treatment Service System for HIV-infected People. National Guidelines on HIV/AIDS Diagnosis and Treatment: Thailand 2010 Thailand: The Agricultural Co-operative Federation of Thailand Publishing, 2010. (Thai) [Google Scholar]
- 13.Department of Disease Control and Prevention. Thailand National Guidelines on HIV/AIDS Treatment and Prevention 2014. Thailand: The Agricultural Co-operative Federation of Thailand Publishing, 2014. (Thai) [Google Scholar]
- 14.Divine BT, Greby SM, Hunt KV, et al. Revised guidelines for HIV counseling, testing, and referral. MMWR 2001; 50: 1–58. [PubMed] [Google Scholar]
- 15.Colby D, Srithanaviboonchai K, Vanichseni S, et al. HIV pre-exposure prophylaxis and health and community systems in the Global South: Thailand case study. J Int Aids Soc 2015; 18: 19953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zablotska I, Grulich AE, Phanuphak N, et al. PrEP implementation in the Asia-Pacific region: opportunities, implementation and barriers. J Int Aids Soc 2016; 19: 21119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matkovic PV, Kosanovic LML, Kavic M, et al. Repeat HIV testing at voluntary testing and counseling centers in Croatia: successful HIV prevention or failure to modify risk behaviors? PloS One 2014; 9: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vriend HJ, Stolte IG, Heijne JCM, et al. Repeated STI and HIV testing among HIV-negative men who have sex with men attending a large STI clinic in Amsterdam: a longitudinal study. Sex Transm Infect 2014; 9: 1–6. [DOI] [PubMed] [Google Scholar]
- 19.Saxton PJ, Dickson NP and Hughes AJ. Location-based HIV behavioral surveillance among MSM in Auckland, New Zealand 2002–2011: condom use stable and more HIV testing. Sex Transm Infect 2014; 90: 133–138. [DOI] [PubMed] [Google Scholar]
- 20.National AIDS Committee. 2013–2015 National AIDS Strategy. Thailand: The Agricultural Co-operative Federation of Thailand Publishing; 2012. (Thai) [Google Scholar]
- 21.Bureau of Risk Communication and Health Behavior Development, Department of Disease Control. Department of Disease Control Revealed Results of DDC Poll: 48.9% of Thai People are still afraid, stigmatize, and discriminate people with HIV. http://www.riskcomthai.org/en/news/mass-media-detail.php?id=31988 (accessed 3 July 2018).
- 22.Bai X, Xu J, Yang J, et al. HIV prevalence and high-risk sexual behaviors among MSM repeat and first-time testers in China: implications for HIV prevention. J Int Aids Soc 2014; 17: 18848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Itoda I, Hoshino S, Sawada T, et al. Prevalence of HIV and sexually transmitted infections and characteristics of men who have sex with men at a community-based center in Yokohama, Japan. (Niho Koshu Eisei Zasshi). Japanese J Public Health 2013; 60: 253–261. [PubMed] [Google Scholar]
- 24.Leaity S, Sherr L, Wells H, et al. Repeat HIV testing: high-risk behavior or risk reduction strategy? AIDS 2000; 14: 547–552. [DOI] [PubMed] [Google Scholar]
- 25.Fisher HH, Habarta N, Hardnett F, et al. Characteristics of first-time and repeat HIV tests among men who have sex with men who test at CDC-supported sites, 2007. AIDS Educ Prev 2011; 23: 17–29. [DOI] [PubMed] [Google Scholar]
- 26.Fernyak SE, Page-Shafer K, Kellogg TA, et al. Risk behaviors and HIV incidence among repeat testers at publicly funded HIV testing sites in San Francisco. JAIDS 2002; 31: 63–70. [DOI] [PubMed] [Google Scholar]
- 27.Holtz TH, Pattanasin S, Chonwattana W, et al. Longitudinal analysis of key HIV-risk behavior patterns and predictors in men who have sex with men, Bangkok, Thailand. Arch Sex Behav 2015; 44: 341–348. [DOI] [PubMed] [Google Scholar]