Abstract
Antibiotic-loaded cement (ABLC) has been widely utilized as an adjuvant treatment for patients with periprosthetic joint infection (PJI) but has also evolved to play a prophylactic role against infection in primary total joint arthroplasties (TJA). Nevertheless, there is currently a paucity of studies that systematically investigated this concept. This review aimed at answering the following questions: (I) Can routine use of ABLC help reduce the current infection rates in primary TJA? (II) What are the risks associated with this approach? And (III) can routine use be justified in primary TJA from an economic standpoint? Multiple databases were queried including PubMed, EMBASE, EBSCO Host, and SCOPUS. Studies published between January 1, 1990 and March 31, 2018 were reviewed. Inclusion criteria were studies reporting: (I) clinical outcomes of routine use of ABLC in primary hip and knee arthroplasty with 2-year minimum follow-up, (II) complications related to the use of ABLC, (III) cost of using ABLC. The final analysis included 24 studies. Data from multiple studies demonstrate contradictory results for infection rates when ABLC is used in all primary procedures with a majority of studies showing similar infection rates between ABLC and plain cement. The main concerns associated with routine use of ABLC are negative effects on the mechanical stability of cement, possible systemic and local toxicity of the absorbed antibiotic, and development of resistant bacterial strains. However, current literature has not clinically validated these concerns. Lastly, with an estimated increase in 117 million dollars with the routine use of ABLC in only 50% of TJAs performed each year, it is difficult to justify the use of ABLC without clear superiority in reducing infection. The use of ABLC has undeniably changed the way orthopaedic surgeons deal with PJI today. However, the large-scale, prophylactic use of ABLC in primary TJAs requires further research and justification.
Keywords: Antibiotic-loaded bone cement, total joint arthroplasty, adjuvant, infection
Introduction
Periprosthetic joint infection (PJI) can be a devastating complication following primary hip or knee arthroplasties leading to poor functional outcomes, quality of life, or even mortality (1). In addition, a significant cost to the healthcare system is often associated with treating these infections, with many patients requiring multiple staged revisions, hardware exchanges, and repeated readmissions (1-4). With the current growth of total joints utilization, it is estimated that more than half a million primary total hip arthroplasty (THA) and more than three millions primary total knee arthroplasty (TKA) are expected to be performed by the year 2030 (2,3). Current PJI rates have been reported at 1% to 7% of primary total joints arthroplasties. Therefore, it is projected that between 38,000 and 270,000 PJIs can be expected by the same year (2,3). Furthermore, the annual cost of infected revisions is expected to exceed $1.6 billion by year 2020 (3). It is clear that when addressing this challenge, reducing cost should also be considered as one of the primary aims undertaken by orthopaedic surgeons.
Antibiotic-loaded cement (ABLC) has been widely utilized as an adjuvant treatment for patients who have PJIs (5,6). Several studies have demonstrated its efficacy in septic revisions and correlate reduction in re-infection rates to its use (1,4,6-8). Multiple authors have also advocated its use as an essential step in the one-stage revision approach to PJIs (9-13). With wider utilization, the use of ABLC evolved to also play a prophylactic role against infection in primary total joint arthroplasties (TJA) (14). In recent studies, the adoption for routine use in primary TJA has witnessed substantial growth, and in some cases, some surgeons reportedly used it in 90% or more of their primary total knee and hip patients (15). This approach although aiming at protecting the patients against the catastrophic complication of PJIs, may not take into account factors such as efficacy, antibiotic resistance, and cost. Additionally, it has not been validated by clinical trials and clear superiority of its usage in primary TJAs is far from established (8).
PJI remains a major concern, and advocators of routine use of ABLC suggest that it can offer additional advantages protecting patients and saving the cost of revisions on the healthcare system. Nevertheless, there is currently a paucity of studies that systematically investigated this concept. Therefore, the aim of this review was to answer the following questions: (I) Can routine use of ABLC help reduce the current infection rates in primary TJA? (II) What are the risks associated with this approach? And (III) can routine use be justified in primary TJA from an economic standpoint?
Methods
Literature search
A comprehensive literature search of the following databases was performed; PubMed, EMBASE, EBSCO Host, and SCOPUS. Studies published between January 1, 1990 and March 31, 2018 were reviewed. The following key words were used in combination with Boolean operators AND or OR for the literature search; “antibiotic loaded cement”, “laden cement”, “total knee arthroplasty”, “total hip arthroplasty”, “periprosthetic”, “infection”, “advantages”, “risks”, “disadvantages”, “cost”, “complications,” “septic”, and “revisions”. Inclusion criteria for studies to be included in this review were: (I) studies reporting clinical outcomes of routine use of ABLC in primary hip and knee arthroplasty with 2-year minimum follow-up; (II) studies that reported on complications related to the use of ABLC; (III) studies that reported on the cost of using ABLC. In addition, we employed the following exclusion criteria: (I) basic science and purely biomechanical studies; (II) case reports; (III) previous reports; (IV) duplicate studies across databases, (V) studies not in English language. These inclusion criteria were applied by two independent researchers: a board-certified orthopaedic surgeon and an orthopaedic surgery clinical research fellow. If disagreement was encountered, a third independent reviewer, a senior board-certified orthopaedic surgeon was consulted.
Data acquisition
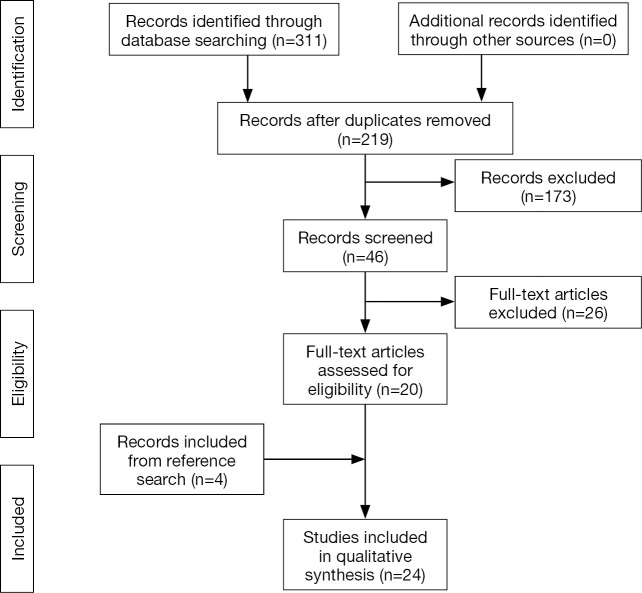
The initial search yielded 312 reports that were screened for relevant studies. This yielded 220 reports whose abstracts were thoroughly reviewed for eligibility according to the inclusion and exclusion criteria, which in turn yielded 47 studies. Next, the full text of these 47 studies were obtained and reviewed for further analysis. All available electronic copies of the reports were collected. In the event that a report was not electronically available, a digitally-scanned hard copy was requested and provided through our inter-library loan service. After thorough evaluation of the full-texts, a total of 21 studies met all our criteria. The reference lists of these studies were also reviewed for any other relevant reports, which yielded an additional four report. Therefore, our final analysis included 24 studies (5,8,16-37). The study selection process is summarized in Figure 1.
Figure 1.
Flow chart of selected studies in this review.
Results
Can routine use of ABLC help reduce the current infection rates in primary TJA?
The answer is: unclear. Data from multiple studies demonstrate contradictory results for infection rates when ABLC is used in all primary procedures (see Table 1). In primary THA, Lynch et al. (17) reviewed 1,542 patients who received plain vs. ABLC and found a statistically significant difference between the cohorts in terms of PJIs (1.7% vs. 1.6%). Engesaeter et al. (18) reported on 22,170 primary THA with up to 14 years of follow-up from the Norwegian Arthroplasty Register. The combined used of systemic antibiotics and ABLC was prevalent among 71% of patients and their results demonstrated that patients who received systemic antibiotics only had 1.8 higher risk of revision due to infection (P=0.01). Results from smaller studies showed variable results.
Table 1. Studies that reported PJI rate following the use of antibiotic-loaded cement in primary total joint arthroplasties.
| Study | Number of primary PJIs | Lower rates of PJIs? (plain vs. antibiotic-loaded cement) |
|---|---|---|
| Lynch et al. [1987] | 1,542 THAs | Yes (1.7% vs. 1.65%) |
| Josefsson and Kolmert [1993] | 1,688 THAs | Yes (1.6% vs. 0.4%) |
| Espehaug [2003] Norwegian registry | 22,170 THAs | No if used without systemic antibiotic; yes with adjuvant systemic prophylaxis |
| Chiu et al. [2002] | 340 TKAs | Yes (3% vs. 0%) (5 patients in the plain cement group, all of which were diabetic) |
| Namba et al. [2009] | 22,889 TKAs | No (0.7% vs. 1.7%, P=0.002) |
| Hinarejos et al. [2013] | 2,948 TKAs (RCT) | No (1.3% vs. 1.4%, P=0.96) |
| Bohm et al. [2014] | 36,681 TKAs | No (1.40% vs. 1.51%, P=0.41) |
PJI, periprosthetic joint infection; THA, total hip arthroplasty; TKA, total knee arthroplasty; RCT, randomized controlled trial.
The use of bone cement in primary THA has witnessed a sharp decline over the last few decades with a practice shift towards cementless prosthesis and therefore, the question may be more relevant to primary TKA, where the majority of patients still receive cemented implants. However, in studies that assessed the use of ABLC in primary TKAs, infection rates were not reduced. Namba et al. (19) reviewed a large community-based total joints registry and reported on 2030 patients who received ABLC during primary TKA. Deep infection rate was 1.4% for the ABLC cohort and 0.7% with the use of regular cement (P=0.002). Similarly, Hinarejos et al. (20) conducted a prospective randomized control trial and compared 1,465 primary TKAs in which plain cement was used to 1,483 TKAs in which ABLC was used. Patients were followed-up for a minimum of 12 months. Final results showed similar infection rate between the two cohorts with deep infection rates at 1.4% in the control cohort and 1.3% in the study cohort and P=0.96. Results from the Canadian Joint Replacement Registry and Canada’s Hospital Morbidity Database reported by Bohm et al. (21) also showed that there was no difference in PJI rates. Chiu et al. (22) did show a reduced infection rate in their study. However, their sample size included only 340 TKAs. Other studies showed statistically insignificant differences without a clear superiority of using ABLC in primary TKA (23-28).
What are the risks associated with this approach?
The main concerns associated with routine use of ABLC are the negative effect on the mechanical stability of cement implicated by adding antibiotic material to the chemical structure of polymethylmethacrylate, possible systemic and local toxicity of the absorbed antibiotic, and the development of antibiotic resistant bacterial strains. Mechanical strength of acrylic cement has been shown to decrease by higher doses of antibiotics. For gentamicin for example this has been correlated with doses >4.5 g of antibiotic (29). In addition, Moran et al. (32) demonstrated that adding Gentamicin in concentrations of 0.5, 1.0, and 2.0 g per 40 g of Palacos cement was linked to decreasing the shear strength of cement. However, commercially available ABLC for use in primary TJA comes with much lower doses of antibiotics (<2 g of antibiotic per 40 g of cement) (38). In addition, to date there has been no study that clinically validated this effect (38). Preparation techniques can also play a role. Hand mixing the antibiotic with cement has been shown to be associated with approximately 40% reduction in the mechanical strength of cement compared to commercially available products (30,31). Therefore, better understanding of this effect is yet to be uncovered by further research.
Systemic toxicity from the local ABLC use has not been demonstrated by the current evidence. Also, the local toxic effect of antibiotics on osteoblastic and osteoclastic activity has not been clinically validated with only in vitro studies suggesting negative cellular effect (33-35). However, the clinical significance of such findings is unclear.
Emergence of bacterial resistance to the low-dose antibiotics in ABLC is another concern. Studies have shown that the acrylic cement surface can be ideal for bacterial colonization and prolonged sub-therapeutic exposure to the antibiotic in the cement can allow mutational resistance to develop (15,36,39). In the event that a patient who had a primary procedure with ABLC developed a PJI, a different class of antibiotic may be necessary during a planned staged revision (36,37). This can be difficult to achieve, and an organism-specific antibiotic spacer may not be available.
Can routine use be justified in primary TJA from an economic standpoint?
Gutowski et al. (40) calculated the cost of using pre-mixed ALBC in TKA routinely to be $120,000 per prevented infection in their cohort of 4,826 knees. Taking this into account, it is reasonable to believe that this cost increase should be less than the cost to treat a PJI. In a study by Parvizi et al. (41) the cost to treat a PJI caused by a methicillin-resistant organism was calculated to be $107,000 while a PJI caused by methicillin-sensitive organisms was calculated to be $68,000. Just looking at these gross figures, one could argue that the increase cost incurred by using pre-mixed ALBC is not sustainable
Based on an estimated cost between $284 to $349 per 40-gram packet of ABLC, Jiranek et al. (8) estimated an increase in overall health-care costs of $117,000,000 with the routine use of ABLC for 50% of 500,000 primary TJA performed annually, assuming the use of two packets per case. Therefore, this estimated increase in cost must be balanced by a reduction of infection rate among primary TJA to be justifiable. At an approximately $50,000 cost for the treatment of one PJI, the authors estimated that there would have to be 2,340 fewer infected patients among the additional 195,000 patients for the routine use of ABLC to maintain the current cost figures. In their analysis, they estimated that the infection rate, currently around 1.5%, must be brought down to 0.3% to balance the cost by using ABLC routinely in only 50% of patients, which can be clinically challenging especially given the current data showing no clear superiority if ABLC in reducing infection.
Discussion
The utilization of antibiotic loaded cement has notably increased, spreading to primary TJAs as a prophylactic strategy to decrease the occurrence of PJI. Currently in the United States ABLC has not received FDA approval for use in primary procedures, with most studies reporting on its usage originating mainly from European countries. However, the clear benefit of routine prophylactic use cannot be currently justified. Current evidence shows contradictory result, at best, for PJI rates with the use of ABLC. In THA, clinical practice guidelines have shifted from using cemented implants altogether, and more recently, cementless implants have also been gaining popularity in primary TKA, which further questions the benefits of routine ABLC use given the cost. Its use is not without risks, and despite the lack of evidence, large-scale adoption should be preceded by further research to truly estimate the impact of adverse effects.
This review is not without limitations. The conclusions drawn from the literature are as good as the included studies with many of them being retrospective studies. However, we have adopted a comprehensive approach to answer very specific and clinically-relevant questions. In addition, multiple studies included in this review were conducted in different countries and therefore, the results may not account for differences in patient demographics, implant and drug manufacturing and surgical techniques, which can all be potential confounders. Nevertheless, the consistent findings across these multiple studies points to a degree of internal validity of our pooled analysis.
In conclusion, the use of ABLC has certainly changed and affected the way orthopaedic surgeons deal with PJI today. However, this impact continues to be unclear for primary TJA and large-scale, prophylactic use in primary procedures requires further research and justification points. In this systematic analysis of the literature we aimed to provide an updated reference to the orthopaedic community and provide an impetus for future work. Large, prospective, and preferably multi-center studies are needed to establish a clear and substantial benefit that would justify the prophylactic use of ABLC in primary TJAs.
Acknowledgements
None.
Footnotes
Conflicts of Interest: MA Mont: board or committee member of AAOS; paid consultant of Abbott, Cymedica, Mallinckrodt Pharmaceuticals, Pacira, Performance Dynamics Inc., Sage; paid consultant and research support of DJ Orthopaedics, Johnson & Johnson, Ongoing Care Solutions, Orthosensor, TissueGene; editorial or governing board of Journal of Arthroplasty, Journal of Knee Surgery; IP royalties of Microport; research support of National Institutes of Health (NIAMS & NICHD); editorial or governing board of Orthopedics, Surgical Techniques International; stock or stock options of Peerwell; IP royalties, paid consultant and research support of Stryker. The other authors have no conflicts of interest to declare.
References
- 1.Best JT. Revision total hip and total knee arthroplasty. Orthop Nurs 2005;24:174-9; quiz 180-1. 10.1097/00006416-200505000-00003 [DOI] [PubMed] [Google Scholar]
- 2.Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 2007;89:780-5. [DOI] [PubMed] [Google Scholar]
- 3.Bozic KJ, Kurtz SM, Lau E, et al. The Epidemiology of Revision Total Hip Arthroplasty in the United States. J Bone Joint Surg Am 2009;91:128-33. 10.2106/JBJS.H.00155 [DOI] [PubMed] [Google Scholar]
- 4.Lentino JR. Prosthetic joint infections: bane of orthopedists, challenge for infectious disease specialists. Clin Infect Dis 2003;36:1157-61. 10.1086/374554 [DOI] [PubMed] [Google Scholar]
- 5.Davies JP, O’Connor DO, Burke DW, et al. Influence of antibiotic impregnation on the fatigue life of Simplex P and Palacos R acrylic bone cements, with and without centrifugation. J Biomed Mater Res 1989;23:379-97. 10.1002/jbm.820230402 [DOI] [PubMed] [Google Scholar]
- 6.Buchholz HW, Elson RA, Engelbrecht E, et al. Management of deep infection of total hip replacement. J Bone Joint Surg Br 1981;63-B:342-53. 10.1302/0301-620X.63B3.7021561 [DOI] [PubMed] [Google Scholar]
- 7.Adalberth G, Nilsson KG, Kärrholm J, et al. Fixation of the tibial component using CMW-1 or Palacos bone cement with gentamicin: similar outcome in a randomized radiostereometric study of 51 total knee arthroplasties. Acta Orthop. Scand 2002;73:531-8. 10.1080/000164702321022802 [DOI] [PubMed] [Google Scholar]
- 8.Jiranek WA, Hanssen AD, Greenwald AS. Antibiotic-loaded bone cement for infection prophylaxis in total joint replacement. J Bone Joint Surg Am 2006;88:2487-500. 10.2106/JBJS.E.01126 [DOI] [PubMed] [Google Scholar]
- 9.Selmon GP, Slater RN, Shepperd JA, et al. Successful 1-stage exchange total knee arthroplasty for fungal infection. J Arthroplasty 1998;13:114-5. 10.1016/S0883-5403(98)90086-9 [DOI] [PubMed] [Google Scholar]
- 10.Buechel FF, Femino FP, D’Alessio J. Primary exchange revision arthroplasty for infected total knee replacement: a long-term study. Am J Orthop 2004;33:190-8; discussion 198. [PubMed] [Google Scholar]
- 11.Hansen E, Tetreault M, Zmistowski B, et al. Outcome of One-stage Cementless Exchange for Acute Postoperative Periprosthetic Hip Infection. Clin Orthop Relat Res 2013;471:3214-22. 10.1007/s11999-013-3079-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gehrke T, Zahar A, Kendoff D. One-stage exchange. Bone Joint J 2013;95-B:77-83. 10.1302/0301-620X.95B11.32646 [DOI] [PubMed] [Google Scholar]
- 13.Haddad FS, Sukeik M, Alazzawi S. Is Single-stage Revision According to a Strict Protocol Effective in Treatment of Chronic Knee Arthroplasty Infections? Clin Orthop Relat Res 2015;473:8-14. 10.1007/s11999-014-3721-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bourne RB. Prophylactic use of antibiotic bone cement: an emerging standard--in the affirmative. J Arthroplasty 2004;19:69-72. 10.1016/j.arth.2004.03.005 [DOI] [PubMed] [Google Scholar]
- 15.Mayer M, Naylor J, Harris I, et al. Evidence base and practice variation in acute care processes for knee and hip arthroplasty surgeries. PLoS One 2017;12:e0180090. 10.1371/journal.pone.0180090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen EN, Adeli B, Kenyon R, et al. Routine Use of Antibiotic Laden Bone Cement for Primary Total Knee Arthroplasty: Impact on Infecting Microbial Patterns and Resistance Profiles. J Arthroplasty 2014;29:1123-7. 10.1016/j.arth.2013.12.004 [DOI] [PubMed] [Google Scholar]
- 17.Lynch M, Esser MP, Shelley P, et al. Deep infection in Charnley low-friction arthroplasty. Comparison of plain and gentamicin-loaded cement. J Bone Joint Surg Br 1987;69:355-60. 10.1302/0301-620X.69B3.3584184 [DOI] [PubMed] [Google Scholar]
- 18.Engesaeter LB, Lie SA, Espehaug B, et al. Antibiotic prophylaxis in total hip arthroplasty: effects of antibiotic prophylaxis systemically and in bone cement on the revision rate of 22,170 primary hip replacements followed 0-14 years in the Norwegian Arthroplasty Register. Acta Orthop Scand 2003;74:644-51. 10.1080/00016470310018135 [DOI] [PubMed] [Google Scholar]
- 19.Namba RS, Chen Y, Paxton EW, et al. Outcomes of Routine Use of Antibiotic-Loaded Cement in Primary Total Knee Arthroplasty. J Arthroplasty 2009;24:44-7. 10.1016/j.arth.2009.05.007 [DOI] [PubMed] [Google Scholar]
- 20.Hinarejos P, Guirro P, Leal J, et al. The Use of Erythromycin and Colistin-Loaded Cement in Total Knee Arthroplasty Does Not Reduce the Incidence of Infection. J Bone Joint Surg Am 2013;95:769-74. 10.2106/JBJS.L.00901 [DOI] [PubMed] [Google Scholar]
- 21.Bohm E, Zhu N, Gu J, et al. Does Adding Antibiotics to Cement Reduce the Need for Early Revision in Total Knee Arthroplasty? Clin Orthop Relat Res 2014;472:162-8. 10.1007/s11999-013-3186-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiu FY, Chen CM, Lin CF, et al. Cefuroxime-impregnated cement in primary total knee arthroplasty: a prospective, randomized study of three hundred and forty knees. J Bone Joint Surg Am 2002;84-A:759-62. 10.2106/00004623-200205000-00009 [DOI] [PubMed] [Google Scholar]
- 23.McQueen MM, Hughes SP, May P, et al. Cefuroxime in total joint arthroplasty. Intravenous or in bone cement. J Arthroplasty 1990;5:169-72. 10.1016/S0883-5403(06)80236-6 [DOI] [PubMed] [Google Scholar]
- 24.Bercovy M, Beldame J, Lefebvre B, et al. A prospective clinical and radiological study comparing hydroxyapatite-coated with cemented tibial components in total knee replacement. J Bone Joint Surg Br 2012;94:497-503. 10.1302/0301-620X.94B4.27496 [DOI] [PubMed] [Google Scholar]
- 25.Nilsson KG, Kärrholm J, Carlsson L, et al. Hydroxyapatite coating versus cemented fixation of the tibial component in total knee arthroplasty: prospective randomized comparison of hydroxyapatite-coated and cemented tibial components with 5-year follow-up using radiostereometry. J Arthroplasty 1999;14:9-20. 10.1016/S0883-5403(99)90196-1 [DOI] [PubMed] [Google Scholar]
- 26.Lizaur-Utrilla A, Miralles-Muñoz FA, Lopez-Prats FA. Similar survival between screw cementless and cemented tibial components in young patients with osteoarthritis. Knee Surg Sports Traumatol Arthrosc 2014;22:1585-90. 10.1007/s00167-012-2291-0 [DOI] [PubMed] [Google Scholar]
- 27.Vrabec G, Stevenson W, Elguizaoui S, et al. What is the Intraarticular Concentration of Tobramycin Using Low-dose Tobramycin Bone Cement in TKA: An In Vivo Analysis? Clin Orthop Relat Res 2016;474:2441-7. 10.1007/s11999-016-5006-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McQueen M, Littlejohn A, Hughes SP. A comparison of systemic cefuroxime and cefuroxime loaded bone cement in the prevention of early infection after total joint replacement. Int Orthop 1987;11:241-3. 10.1007/BF00271455 [DOI] [PubMed] [Google Scholar]
- 29.Lautenschlager EP, Jacobs JJ, Marshall GW, et al. Mechanical properties of bone cements containing large doses of antibiotic powders. J Biomed Mater Res 1976;10:929-38. 10.1002/jbm.820100610 [DOI] [PubMed] [Google Scholar]
- 30.Seldes RM, Winiarsky R, Jordan LC, et al. Liquid gentamicin in bone cement: a laboratory study of a potentially more cost-effective cement spacer. J Bone Joint Surg Am 2005;87:268-72. [DOI] [PubMed] [Google Scholar]
- 31.DeLuise M, Scott CP. Addition of hand-blended generic tobramycin in bone cement: effect on mechanical strength. Orthopedics 2004;27:1289-91. [DOI] [PubMed] [Google Scholar]
- 32.Moran JM, Greenwald AS, Matejczyk MB. Effect of gentamicin on shear and interface strengths of bone cement. Clin Orthop Relat Res 1979;(141):96-101. [PubMed] [Google Scholar]
- 33.McLaren AC. Alternative materials to acrylic bone cement for delivery of depot antibiotics in orthopaedic infections. Clin Orthop Relat Res 2004;(427):101-6. 10.1097/01.blo.0000143554.56897.26 [DOI] [PubMed] [Google Scholar]
- 34.Isefuku S, Joyner CJ, Simpson AH. Gentamicin may have an adverse effect on osteogenesis. J Orthop Trauma 2003;17:212-6. 10.1097/00005131-200303000-00010 [DOI] [PubMed] [Google Scholar]
- 35.Miclau T, Edin ML, Lester GE, et al. Bone toxicity of locally applied aminoglycosides. J Orthop Trauma 1995;9:401-6. 10.1097/00005131-199505000-00007 [DOI] [PubMed] [Google Scholar]
- 36.Thomes B, Murray P, Bouchier-Hayes D. Development of resistant strains of Staphylococcus epidermidis on gentamicin-loaded bone cement in vivo. J Bone Joint Surg Br 2002;84:758-60. 10.1302/0301-620X.84B5.11907 [DOI] [PubMed] [Google Scholar]
- 37.Hope PG, Kristinsson KG, Norman P, et al. Deep infection of cemented total hip arthroplasties caused by coagulase-negative staphylococci. J Bone Joint Surg Br 1989;71:851-5. 10.1302/0301-620X.71B5.2584258 [DOI] [PubMed] [Google Scholar]
- 38.Hanssen AD. Prophylactic use of antibiotic bone cement: an emerging standard--in opposition. J Arthroplasty 2004;19:73-7. 10.1016/j.arth.2004.04.006 [DOI] [PubMed] [Google Scholar]
- 39.van de Belt H, Neut D, Schenk W, et al. Gentamicin release from polymethylmethacrylate bone cements and Staphylococcus aureus biofilm formation. Acta Orthop Scand 2000;71:625-9. 10.1080/000164700317362280 [DOI] [PubMed] [Google Scholar]
- 40.Gutowski CJ, Zmistowski BM, Clyde CT, et al. The economics of using prophylactic antibiotic-loaded bone cement in total knee replacement. Bone Joint J 2014;96-B:65-9. 10.1302/0301-620X.96B1.31428 [DOI] [PubMed] [Google Scholar]
- 41.Parvizi J, Pawasarat IM, Azzam KA, et al. Periprosthetic Joint Infection. J Arthroplasty 2010;25:103-7. 10.1016/j.arth.2010.04.011 [DOI] [PubMed] [Google Scholar]