Abstract
Background
Because poverty is a multifaceted concept with a complex definition, this concept may not be useful when formulating economic policy. Thus, most governments use the relative poverty line to identify poor participants who may receive economic support. The aim of this study was to investigate the association between living below the relative poverty line and the prevalence of chronic obstructive pulmonary disease (COPD).
Methods
We retrospectively analyzed data from 3,223 individuals included in South Korea. Living below the poverty line was defined as receiving a monthly income less than the minimum cost of living.
Results
Of the 3,223 participants included in this analysis, 832 (25.8%) met the definition of living below the relative poverty line and 384 (11.9%) had COPD. Of the 3,223 participants, 161 of the 832 (19.4%) living below the poverty line and 223 of the 2,391 (9.3%) living above the poverty line had COPD. In our study, participants living below the poverty line had a 1.4-time higher risk of COPD development compared with those living above the poverty line (OR =1.4; P=0.012). Elderly people living below the poverty line were 1.5-time more likely to be at risk of COPD development than those living above the poverty line (OR =1.5; P=0.021).
Conclusions
People living below the relative poverty line have an increased prevalence of COPD, especially older people with COPD. From the perspective of COPD disease control, policy makers should consider providing national economic support for the early detection and management of COPD in people living below the relative poverty line.
Keywords: Poverty, chronic obstructive pulmonary disease (COPD), epidemiology, spirometry, economics
Introduction
Poverty is a serious health issue. People living in poverty have difficulty gaining access to the health-care system and are at increased risk of various diseases because of their environment. Support of the poor is a major goal of economic policy in many countries (1-7). Because poverty is a multifaceted concept with a complex definition, this abstract concept may not be useful when formulating economic policy (3-7). In most nations, poverty is usually measured using either the absolute or relative poverty line to determine who may receive economic support (6-9). The poverty line is the official level of income needed to achieve a basic standard of living with enough money for things such as food, clothing, and a place to live. According to the World Health Organization recommendations, poverty can be often defined in absolute terms as a low income of less than 2 US dollar per day (6). However, the absolute poverty line is not an optimal indicator of the need for national economic support in middle- or high-income countries because, very few people in these countries, live below the absolute poverty line. Thus, the relative poverty line is used to identify poor people in need of support in these countries. The relative poverty line is the official standard for determining who will receive national financial support. A measure of income inequality, the relative poverty line is determined by social consensus and can differ between countries (6,8,9).
Poverty is known risk factor for chronic obstructive pulmonary disease (COPD) (10-18). Many COPD patients live in poverty and, because of the cost, have limited access to hospital services, which may increase their risk of disease progression, acute exacerbations, and death. Because some nations cannot financially support their entire population, the prevalence rates of COPD and COPD mortality are higher in low-income countries than high-income countries (12,19). These relationships have been shown by many epidemiological studies (12,16,19). Therefore, each country must find a way to determine which COPD patients should receive national financial support to maximize the benefits for these people. The definition of poverty is not always clear in previous studies of the relationship between COPD and poverty. In some previous studies, the definition of poverty was replaced by socioeconomic status, which is a metaphysical concept based on occupation, education, and income (10-17,20). The use of socioeconomic status is not always helpful in determining who should receive national support to reduce the prevalence and progression of COPD. We have found no studies of the association between living below the relative poverty line and the prevalence of COPD. In this study, we used the relative poverty line to define poverty and to provide a clear practical criterion for identifying COPD patients who should be provided with national economic support.
The aim of this study was to investigate the association between living below the relative poverty line and the prevalence of COPD, and whether this association could be used to determine which patients should be provided national economic support to maximize its efficacy for COPD patients.
Methods
Overview
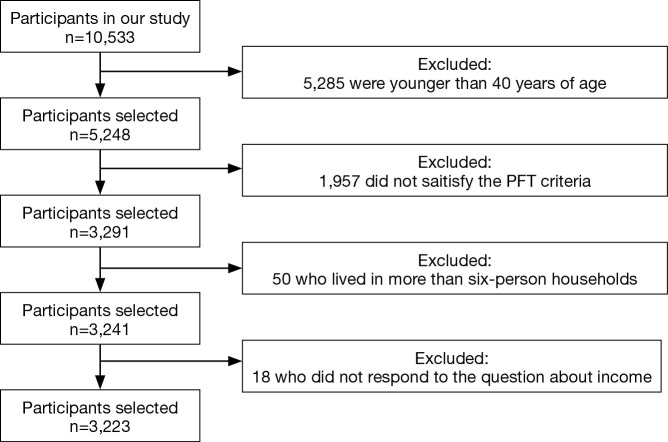
We retrospectively analyzed the data collected in the fourth Korea National Health and Nutrition Examination Survey (KNHANES IV-3), a prospectively administered survey performed from January 2009 to December 2009 by Korea Centers for Disease Control and Prevention within the Ministry of Health and Welfare (21-24). In the KNHANES IV-3, nationwide stratified random sampling was performed based on data from the Korean Statistical Office census. The 600 survey areas were drawn from a census of the population and housing by considering the proportion of each subgroup. Spirometry was performed and trained interviewers administered standardized questionnaires to determine the participants’ health statuses, education, incomes, smoking statuses, and work environments (21-24). A total of 10,533 people were investigated by the KNHANES IV-3 (response rate, 83%). Participants younger than 40 years (n=5,285), those whose pulmonary function test (PFT) results did not meet the necessary acceptability and reproducibility standards (n=1,957), those who resided in households with more than six people (n=50), and those who did not respond to questions about income (n=18) were excluded from the data analysis. The remaining 3,223 participants were included in the statistical analyses (Figure 1).
Figure 1.
Flowchart of study participant selection.
The study was approved by the Institutional Review Board of the Korea Centers for Disease Control and Prevention (approval number: 2009-01CON-03-2C) and informed consent was obtained from all participants prior to initial data collection.
Definition of poverty and COPD
A participant with COPD was defined as a person aged ≥40 years with a forced expiratory volume in 1 second (FEV1) divided by a forced vital capacity (FVC) <0.7 (22,23). Living below the poverty line was defined as receiving a monthly income less than the minimum cost of living. Living above the poverty line was defined as receiving a monthly income more than the minimum cost of living.
In Korea, the government publicly announces the monthly minimum cost of living according to the yearly number of people per household. This value is used to define the relative poverty line and to identify individuals who will receive support because their income is below this line (25). In 2009, the monthly minimum cost of living was $374 for one-person households, $634 for two-person households, $824 for three-person households, $1,008 for four-person households, $1,198 for five-person households, and $1,382 for six-person households (25).
Definition and categorization of other variables
The analytical variables used in this study were age, sex, smoking history, body mass index (BMI), educational level, stress level, and the presence of comorbid disease. Age was defined as the age, in years, at the subject’s last birthday. Being elderly was defined as age 65 years or older. Smoking history was categorized as “current smoker”, “ex-smoker”, or “never smoker”. Current-smoker was defined as a person who had smoked more than 100 cigarettes in their lifetime, and ex-smoker was defined as one who had not smoked in the last 28 days. BMI was calculated as the weight divided by height squared and is expressed as kg/m2. Educational level was categorized as elementary school, middle school, high school, or university. The level of stress was defined as subjective stress felt by the person in his/her daily life and was categorized as “little”, “some”, “much”, or “very much.” Comorbid diseases were ascertained from answers to the question: “Were you diagnosed with a comorbid disease by a doctor?” For example, stroke was ascertained by the question: “Were you diagnosed with stroke by a doctor?”
Spirometry
Spirometry was performed 3 to 8 times by one of the four well-trained technicians using dry rolling-seal spirometers (Model 2130; SensorMedics, Yorba Linda, CA, USA), thus controlling for PFT quality, as recommended by American Thoracic Society/European Respiratory Society criteria for the standardization of PFTs (26). Spirometric data obtained on site by technicians were transferred to the review center through the internet (23). Another trained technician assessed whether the data met the criteria for acceptability and reproducibility and provided quality control feedback to the technicians, which improved the performance of spirometry (23,26). Data were finally confirmed by the principal investigator and saved in the data management system of the Korea Center for Disease Control and Prevention (23).
Statistical analyses
Continuous values are expressed as mean ± standard deviation (SD), and categorical data are expressed as numbers (percentage). Univariate analyses were performed to identify potential associations between COPD and seven variables based on two national guidelines: age, sex, smoking amount, BMI, educational status, history of pulmonary tuberculosis, and living below the poverty line (10,27). We performed multivariate logistic regression analysis using backward elimination to evaluate the relationship between COPD and living below the poverty line after adjusting for other variables with P≤0.05 in the univariate analysis. The results are shown as adjusted odds ratios (ORs), 95% confidence intervals (CIs), and P. P≤0.05 were considered to be significant. All statistical analyses were performed using SPSS version 21.0 (IBM Corp., Armonk, NY, USA).
Results
Characteristics of participants living below the relative poverty line
The clinical characteristics of the participants are shown in Table 1. Of the 3,223 participants included in this analysis, 832 (25.8%) met the definition of living below the relative poverty line. Those living below the relative poverty line were older than those living above the poverty line. Although the smoking pattern did not differ between these two groups, people living below the poverty line had lower lung function than those living above the poverty line. People living below the poverty line also had a lower educational level and were more likely to have experienced stroke, ischemic heart disease, hypertension, osteoporosis, diabetes mellitus, or COPD than those living above the poverty line. Of the 3,223 total participants, 161 of the 832 people (19.4%) living below the poverty line and 223 of the 2,391 people (9.3%) living above the poverty line had COPD (P<0.001).
Table 1. Clinical characteristics of participants living below the poverty line (n=3,223).
| Variable | Living below poverty line (n=832) | Living above poverty line (n=2,391) | P |
|---|---|---|---|
| Age (years)* | 64±11 | 54±10 | <0.001 |
| Male, n (%) | 323 (38.8) | 1,090 (45.6) | 0.001 |
| Smoking history§, n (%) | 0.192 | ||
| Never smokers | 495 (59.5) | 1,365 (57.1) | |
| Ex-smokers | 173 (20.8) | 530 (22.2) | |
| Current-smokers | 159 (19.1) | 485 (20.3) | |
| Missing | 5 (0.6) | 11 (0.4) | |
| Smoking amount (pack-year)* | 14±22 | 12±20 | 0.233 |
| BMI (kg/m2)* | 24±3 | 24±3 | 0.243 |
| PFT* | |||
| FEV1 (L) | 2.3±0.6 | 2.8±0.7 | <0.001 |
| FEV1 (% predicted value) | 91±16 | 93±13 | 0.026 |
| FVC (L) | 3.1±0.8 | 3.5±0.9 | <0.001 |
| FVC (% predicted value) | 90±13 | 92±12 | <0.001 |
| Educational level, n (%) | <0.001 | ||
| Elementary school | 530 (63.7) | 681 (28.5) | |
| Middle school | 132 (15.9) | 405 (16.9) | |
| High school | 122 (14.7) | 755 (31.6) | |
| University | 44 (5.3) | 543 (22.7) | |
| Missing | 4 (0.4) | 7 (0.3) | |
| Level of stress, n (%) | 0.553 | ||
| Little | 193 (23.2) | 359 (15.0) | |
| Some | 388 (46.6) | 1402 (58.6) | |
| Much | 192 (23.1) | 530 (22.2) | |
| Very much | 55 (6.6) | 93 (3.9) | |
| Missing | 4 (0.5) | 4 (0.3) | |
| Comorbidity#, n (%) | |||
| Stroke | 34 (4.1) | 36 (1.5) | <0.001 |
| Ischemic heart disease | 30 (3.6) | 50 (2.1) | 0.020 |
| Hypertension | 321 (38.6) | 593 (24.8) | <0.001 |
| Osteoporosis | 143 (17.2) | 184 (7.7) | <0.001 |
| Depression | 43 (5.2) | 106 (4.4) | 0.443 |
| Lung cancer | 2 (0.2) | 5 (0.2) | 1.000 |
| DM | 136 (16.3) | 212 (8.9) | <0.001 |
| Bronchiectasis | 6 (0.7) | 14 (0.6) | 0.798 |
| Pulmonary tuberculosis | 59 (7.1) | 156 (6.5) | 0.629 |
| COPD | 161 (19.4) | 223 (9.3) | <0.001 |
*, data are presented as mean ± SD. Other variables are presented as a number (percent). §, smoking history was categorized as “current smoker”, “ex-smoker”, or “never smoker”. Current-smoker was defined as a person who had smoked more than 100 cigarettes in their lifetime, and ex-smoker was defined as one who had not smoked in the last 28 days. #, comorbid diseases were ascertained from answers to the question: “Were you diagnosed with a comorbid disease by a doctor?” For example, stroke was ascertained by the question: “Were you diagnosed with stroke by a doctor?” BMI, body mass index; COPD, chronic obstructive pulmonary disease; FEV1, a forced expiratory volume in one second; FVC, a forced vital capacity; DM, diabetes mellitus; PFT, pulmonary function test.
Characteristics of participants with COPD
The clinical characteristics of participants with COPD are shown in Table 2. The mean age of the 384 participants with COPD was 65 years; two-thirds of the participants with COPD were men and were ever-smokers. The mean FEV1 values were 2.2 L and 77% of the predicted value. Participants with COPD were more likely to have experienced cardiovascular diseases (e.g., hypertension or ischemic heart disease) or pulmonary diseases (e.g., lung cancer, bronchiectasis, or pulmonary tuberculosis) than those without COPD.
Table 2. Characteristics of participants with COPD in our cohort (n=3,223).
| Variable | COPD | P | |
|---|---|---|---|
| Yes (n=384) | No (n=2,839) | ||
| Age (years)* | 65±10 | 56±11 | <0.001 |
| Male, n (%) | 280 (72.9) | 1,133 (39.9) | <0.001 |
| Smoking history§, n (%) | <0.001 | ||
| Never smokers | 117 (30.5) | 1,743 (61.4) | |
| Ex-smokers | 143 (37.2) | 560 (19.7) | |
| Current-smokers | 124 (32.3) | 520 (18.3) | |
| Missing | 0 (0.0) | 16 (0.6) | |
| Smoking amount (pack-year)* | 26±26 | 11±20 | <0.001 |
| BMI (kg/m2)* | 23±3 | 24±3 | <0.001 |
| PFT* | |||
| FEV1 (L) | 2.2±0.7 | 2.7±0.7 | <0.001 |
| FEV1 (% predicted value) | 77±17 | 94±12 | <0.001 |
| FVC (L) | 3.5±0.9 | 3.4±0.9 | 0.046 |
| FVC (% predicted value) | 89±15 | 92±12 | <0.001 |
| Education status, n (%) | <0.001 | ||
| Elementary school | 192 (50.0) | 1,019 (35.9) | |
| Middle school | 68 (17.7) | 469 (16.5) | |
| High school | 74 (19.3) | 803 (28.3) | |
| University | 49 (12.8) | 538 (19.0) | |
| Missing | 1 (0.3) | 10 (0.4) | |
| Comorbidity#, n (%) | |||
| Stroke | 13 (3.4) | 57 (2.0) | 0.092 |
| Ischemic heart disease | 15 (3.9) | 65 (2.3) | 0.077 |
| Hypertension | 142 (37.0) | 772 (27.2) | <0.001 |
| Osteoporosis | 32 (8.3) | 295 (10.4) | 0.210 |
| Depression | 12 (3.1) | 137 (4.8) | 0.154 |
| Lung cancer | 3 (0.8) | 4 (0.1) | 0.041 |
| DM | 53 (13.8) | 295 (10.4) | 0.054 |
| Bronchiectasis | 11 (2.9) | 9 (0.3) | <0.001 |
| Pulmonary tuberculosis | 50 (13.0) | 165 (5.8) | <0.001 |
*, data are presented as mean ± SD. Other variables are presented as a number (percent). §, smoking history was categorized as “current smoker”, “ex-smoker”, or “never smoker”. Current-smoker was defined as a person who had smoked more than 100 cigarettes in their lifetime, and ex-smoker was defined as one who had not smoked in the last 28 days. #, comorbid diseases were ascertained from answers to the question: “Were you diagnosed with a comorbid disease by a doctor?” For example, stroke was ascertained by the question: “Were you diagnosed with stroke by a doctor?” BMI, body mass index; COPD, chronic obstructive pulmonary disease; FEV1, a forced expiratory volume in one second; FVC, a forced vital capacity; DM, diabetes mellitus; PFT, pulmonary function test.
Multiple logistic regression analysis to identify risk factors for COPD development
To investigate the association between living below the poverty line and the prevalence of COPD, we performed multivariate logistic regression analysis using backward elimination. The prevalence of COPD was significantly related to being older (OR =1.1; 95% CI: 1.06–1.09; P<0.001), being male (OR =3.6; 95% CI: 2.64–4.89; P<0.001), smoking more (OR =1.0; 95% CI: 1.00–1.01; P=0.004), having a lower BMI (OR =0.9; 95% CI: 0.89–0.97; P<0.001), having a history of pulmonary tuberculosis (OR =1.8; 95% CI: 1.23–2.64; P=0.002), and living below the poverty line (OR =1.4; 95% CI: 1.08–1.82; P=0.012) (Table 3).
Table 3. Risk factors for COPD in all participants (n=3,223).
| Risk factors | Odds ratio (95% CI) | P |
|---|---|---|
| Univariate analysis | ||
| Age (years) | 1.1 (1.07–1.09) | <0.001 |
| Male | 4.1 (3.20–5.14) | <0.001 |
| Smoking amount (pack-year) | 1.1 (1.02–1.03) | <0.001 |
| BMI | 0.9 (0.86–0.93) | <0.001 |
| Education status | <0.001 | |
| University | Reference | |
| High school | 1.0 (0.69–1.48) | |
| Middle school | 1.6 (1.08–2.35) | |
| Elementary school | 2.1 (1.49–2.88) | |
| History of pulmonary tuberculosis | 2.4 (1.73–3.39) | <0.001 |
| Living below the poverty line | 2.3 (1.87–2.91) | <0.001 |
| Multivariate analysis | ||
| Age (years) | 1.1 (1.06–1.09) | <0.001 |
| Male | 3.6 (2.64–4.89) | <0.001 |
| Smoking amount (pack-year) | 1.0 (1.00–1.01) | 0.004 |
| BMI | 0.9 (0.89–0.97) | <0.001 |
| History of pulmonary tuberculosis | 1.8 (1.23–2.64) | 0.002 |
| Living below the poverty line | 1.4 (1.08–1.82) | 0.012 |
Multivariate logistic regression analysis using backward elimination was performed to evaluate the association between living below the poverty line and the prevalence of COPD, after adjusting for age, sex, smoking amount, BMI, education status, and the history of pulmonary tuberculosis. COPD, chronic obstructive pulmonary disease; BMI, body mass index.
Association between living below the poverty line and COPD in participants older than 65 years
The baseline characteristics of 888 participants older than 65 years are shown in Table 4. Of those aged over 65 years, 460 (51.8%) met the definition of living below the relative poverty line. Those living below the poverty line were older than those living above the poverty line. Smoking pattern and percent predicted value of the PFT did not differ significantly between participants living above and below the relative poverty line. Those individuals living below the poverty line had lower educational levels than those living above the poverty line. Of the 888 participants older than 65 years, 122 of the 460 (26.5%) living below the poverty line, and 88 of the 428 (20.6%) living above the poverty line had COPD (P=0.040).
Table 4. Baseline characteristics in participants older than 65 years (n=888).
| Variable | Living below poverty line (n=460) | Living above poverty line (n=428) | P |
|---|---|---|---|
| Age (years)* | 72±5 | 70±5 | <0.001 |
| Male, n (%) | 175 (38.0) | 197 (46.0) | 0.017 |
| Smoking history§, n (%) | 0.261 | ||
| Never smokers | 283 (61.5) | 246 (57.5) | |
| Ex-smokers | 118 (25.7) | 130 (30.4) | |
| Current-smokers | 58 (12.6) | 51 (11.9) | |
| Missing | 1 (0.2) | 1 (0.2) | |
| Smoking amount (pack-year)* | 15±25 | 16±26 | 0.357 |
| BMI (kg/m2)* | 24±3 | 24±3 | 0.181 |
| PFT* | |||
| FEV1 (L) | 2.0±0.5 | 2.2±0.6 | <0.001 |
| FEV1 (% predicted value) | 92±18 | 92±17 | 0.927 |
| FVC (L) | 2.8±0.7 | 3.0±0.8 | 0.001 |
| FVC (% predicted value) | 88±14 | 87±15 | 0.361 |
| Educational level, n (%) | <0.001 | ||
| Elementary school | 368 (80.0) | 272 (63.6) | |
| Middle school | 50 (10.9) | 47 (11.0) | |
| High school | 28 (6.1) | 59 (13.8) | |
| University | 13 (2.8) | 49 (11.4) | |
| Missing | 1 (0.2) | 1 (0.2) | |
| Level of stress, n (%) | 1.000 | ||
| Little | 144 (31.3) | 121 (28.3) | |
| Some | 196 (42.6) | 215 (50.2) | |
| Much | 96 (20.9) | 71 (16.6) | |
| Very much | 23 (5.0) | 21 (4.9) | |
| Missing | 1 (0.2) | 0 (0.0) | |
| Comorbidity#, n (%) | |||
| Stroke | 23 (5.0) | 15 (3.5) | 0.321 |
| Ischemic heart disease | 20 (4.3) | 21 (4.9) | 0.750 |
| Hypertension | 221 (48.0) | 210 (49.1) | 0.788 |
| Osteoporosis | 109 (23.7) | 82 (19.2) | 0.103 |
| Depression | 18 (3.9) | 27 (6.3) | 0.126 |
| Lung cancer | 1 (0.2) | 0 (0.0) | 1.000 |
| DM | 81 (17.6) | 76 (17.8) | 1.000 |
| Bronchiectasis | 3 (0.7) | 5 (1.2) | 0.492 |
| Pulmonary tuberculosis | 33 (7.2) | 39 (9.1) | 0.326 |
| COPD | 122 (26.5) | 88 (20.6) | 0.040 |
*, data are presented as mean ± SD. Other variables are presented as a number (percent). §, smoking history was categorized as “current smoker”, “ex-smoker”, or “never smoker”. Current-smoker was defined as a person who had smoked more than 100 cigarettes in their lifetime, and ex-smoker was defined as one who had not smoked in the last 28 days. #, comorbid diseases were ascertained from answers to the question: “Were you diagnosed with a comorbid disease by a doctor?” For example, stroke was ascertained by the question: “Were you diagnosed with stroke by a doctor?” BMI, body mass index; COPD, chronic obstructive pulmonary disease; FEV1, a forced expiratory volume in one second; FVC, a forced vital capacity; DM, diabetes mellitus; PFT, pulmonary function test.
In participants aged more than 65 years, the prevalence of COPD was significantly related to being male (OR =3.6; 95% CI: 2.36–5.55; P<0.001), smoking more (OR =1.0; 95% CI: 1.00–1.02; P=0.027), having a lower BMI (OR =0.9; 95% CI: 0.86–0.97; P=0.002), and living below the poverty line (OR =1.5; 95% CI: 1.06–2.13; P=0.021) (Table 5).
Table 5. Risk factors for COPD in participants older than 65 years (n=888).
| Risk factors | Odds ratio (95% CI) | P |
|---|---|---|
| Univariate analysis | ||
| Age (years) | 1.1 (1.02–1.09) | 0.003 |
| Male | 4.8 (3.45–6.78) | <0.001 |
| Smoking amount (pack-year) | 1.0 (1.02–1.03) | <0.001 |
| BMI | 0.9 (0.83–0.92) | <0.001 |
| Education status | 0.097 | |
| University | Reference | |
| High school | 1.5 (0.84–2.68) | |
| Middle school | 1.4 (0.84–2.32) | |
| Elementary school | 1.6 (1.03–2.63) | |
| History of pulmonary tuberculosis | 2.1 (1.25–3.44) | 0.005 |
| Living below the poverty line | 1.4 (1.02–1.91) | 0.037 |
| Multivariate analysis | ||
| Male | 3.6 (2.36–5.55) | <0.001 |
| Smoking amount (pack-year) | 1.0 (1.00–1.02) | 0.027 |
| BMI | 0.9 (0.86–0.97) | 0.002 |
| Living below the poverty line | 1.5 (1.06–2.13) | 0.021 |
Multivariate logistic regression analysis using backward elimination was performed to evaluate the association between living below the poverty line and the prevalence of COPD, after adjusting for age, sex, smoking amount, BMI, and the history of pulmonary tuberculosis. BMI, body mass index; COPD, chronic obstructive pulmonary disease.
Discussion
This cross-sectional, nationwide, population-based study was designed to identify whether living below the relative poverty line was related to the prevalence of COPD. We found that living below the relative poverty line was significantly related to the prevalence of COPD. In our study, participants living below the poverty line had a 1.4-time higher risk of COPD development compared with those living above the poverty line (OR =1.4; 95% CI: 1.08–1.82; P=0.012). Considering that COPD patients living in poverty may not be diagnosed and treated effectively because of the cost involved, national financial support of these people would be important for decreasing the prevalence of COPD and preventing disease progression.
Although poverty is a known risk factor for developing COPD, the definitions of poverty differ between studies; some have used educational level, socioeconomic status, or income (12-17,20,22,28-32). Poverty is an abstract and multifaceted concept with a complex definition and reflects contemporary socioeconomic and cultural conditions. Therefore, we can approach to various and different concept about poverty. However, these conceptual approaches are not necessarily useful for formulating a national economic policy. For this reason, many governments establish a poverty line, which reflects the socioeconomic and cultural conditions, when establishing their policy of national economic support. The concept of the poverty line is important in the real world. Although previous studies have shown clearly that poverty is related to the prevalence of COPD, the data from these studies may not reflect in the real world because of the absence of the concept of poverty line. For this reason, we evaluated the relationship between poverty and the prevalence of COPD using the poverty line. The poverty line is defined in two ways-as the absolute poverty line or relative poverty line. The absolute poverty line is not an optimal indicator of national economic support in the middle- or high-income countries because few live below the absolute poverty line. In these countries, most governments use the relative poverty line to identify poor participants who may receive economic support (6-9). In our study, participants living below the poverty line had a 1.4-time higher risk of COPD development compare with those living above the poverty line. Although our result is similar to that of previous studies, our study provides specific evidence to show why governments should care for people living below the relative poverty line. Our results suggest that government may be able to reduce the prevalence of COPD in people living below the poverty line by improving their environment. In addition, COPD patients living in poverty are at increased risk of disease progression, acute exacerbations, and death because they are less able to access hospital services because of the cost. Therefore, governments should attempt to identify COPD patients living below the poverty line and provide financial support to allow these people to seek appropriate medical care. These activities may help to reduce the prevalence of COPD and prevent disease progression. Eventually, these activities may help to decrease the socioeconomic burden of COPD.
We also analyzed the relationship between relative poverty and the prevalence of COPD in elderly people. In our study, elderly people living below the poverty line were 1.5-time more likely to be at risk of COPD development than those living above the poverty line (OR =1.5; P=0.021; Table 5). This result is important from the public health perspective. The global population is aging rapidly (33,34). An increase in the population of the elderly will increase demand for primary and long-term health care, which may greatly increase the socioeconomic burden (33,34). In addition, elderly people typically have low economic status after retirement (34). Because of the cost, impoverished older people with COPD have less access to hospital services than their younger counterparts, which also increases their risk of disease progression, acute exacerbation, and death (35-37). For these reasons, early detection and treatment of COPD in the elderly population living below the poverty line are critically important public health issues.
We cannot provide an explicit mechanism to explain the relationship between COPD and poverty because this study was cross-sectional. Previous reports have suggested that prenatal exposure, more frequent lower respiratory tract infections during childhood, housing conditions, exposure to air pollution or environmental tobacco smoke, and other lifestyle factors may contribute to the higher prevalence of COPD among those living in poverty (10). In our study, smoking history and smoking amount did not differ significantly between those living below and above the poverty line. Therefore, the association between poverty and COPD may be affected by factors other than smoking. Further studies using a prospective cohort study design are needed to identify the mechanisms underlying the associations between COPD and poverty.
This study had some limitations. First, we included some patients with asthma because the diagnosis of COPD was based on pre-bronchodilator PFT results. However, many epidemiological studies have used only pre-bronchodilator PFT data (38,39). This was because post-bronchodilator PFTs are extremely difficult to administer in nationwide epidemiological studies because of concerns about safety. Second, it is possible that we underestimated the number of participants with low incomes because we used private data to determine monthly income. Third, we did not include all known risk factors of COPD in the multivariate analysis, such as genetic factor, occupational exposure, biomass fuels, because of the retrospective study design. Fourth, because it was a cross-sectional study, our study did not identify factors that may influence the association between living below the poverty line and the prevalence of COPD. Finally, the percentage of participants with acceptable and reproducible spirometry results was low. However, technical and personal errors were minimized by the use of criteria for both the acceptability and reproducibility of spirometry results (23).
Conclusions
In conclusion, those living below the relative poverty line have an increased prevalence of COPD, especially older people with COPD. From the perspective of COPD disease control, policy makers should consider providing national economic support for the early detection and management of COPD in people living below the relative poverty line.
Acknowledgements
None.
Ethical Statement: The study was approved by the Institutional Review Board of the Korea Centers for Disease Control and Prevention (approval number: 2009-01CON-03-2C) and informed consent was obtained from all participants prior to initial data collection.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.McCally M, Haines A, Fein O, et al. Poverty and ill health: physicians can, and should, make a difference. Ann Intern Med 1998;129:726-33. 10.7326/0003-4819-129-9-199811010-00009 [DOI] [PubMed] [Google Scholar]
- 2.Laterveer L, Niessen LW, Yazbeck AS. Pro-poor health policies in poverty reduction strategies. Health Policy Plan 2003;18:138-45. 10.1093/heapol/czg018 [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Global report for research on infectious diseases of poverty. Geneva, Switzerland: WHO, 2012. [Google Scholar]
- 4.Mackenbach JP, Stirbu I, Roskam AJ, et al. Socioeconomic inequalities in health in 22 European countries. N Engl J Med 2008;358:2468-81. 10.1056/NEJMsa0707519 [DOI] [PubMed] [Google Scholar]
- 5.Sweileh WM, Al-Jabi SW, Sawalha AF, et al. Bibliometric analysis of medicine-related publications on poverty (2005-2015). Springerplus 2016;5:1888. 10.1186/s40064-016-3593-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization (2016). Poverty and health. Available online: http://www.who.int/topics/poverty/en/
- 7.Marmot M. Social determinants of health inequalities. Lancet 2005;365:1099-104. 10.1016/S0140-6736(05)74234-3 [DOI] [PubMed] [Google Scholar]
- 8.Fritzell J, Rehnberg J, Bacchus Hertzman J, et al. Absolute or relative? A comparative analysis of the relationship between poverty and mortality. Int J Public Health 2015;60:101-10. 10.1007/s00038-014-0614-2 [DOI] [PubMed] [Google Scholar]
- 9.Goedhart T, Halberstadt V, van Praag BMS, et al. The poverty line: Concept and measurement. J Hum Resour 1977;12:503-20. 10.2307/145372 [DOI] [Google Scholar]
- 10.Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013;187:347-65. 10.1164/rccm.201204-0596PP [DOI] [PubMed] [Google Scholar]
- 11.Blasi F, Cesana G, Conti S, et al. The clinical and economic impact of exacerbations of chronic obstructive pulmonary disease: a cohort of hospitalized patients. PLoS One 2014;9:e101228. 10.1371/journal.pone.0101228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grigsby M, Siddharthan T, Chowdhury MA, et al. Socioeconomic status and COPD among low- and middle-income countries. Int J Chron Obstruct Pulmon Dis 2016;11:2497-507. 10.2147/COPD.S111145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho KH, Nam CM, Lee EJ, et al. Effects of individual and neighborhood socioeconomic status on the risk of all-cause mortality in chronic obstructive pulmonary disease: A nationwide population-based cohort study, 2002-2013. Respir Med 2016;114:9-17. 10.1016/j.rmed.2016.03.003 [DOI] [PubMed] [Google Scholar]
- 14.Sommer I, Griebler U, Mahlknecht P, et al. Socioeconomic inequalities in non-communicable diseases and their risk factors: an overview of systematic reviews. BMC Public Health 2015;15:914. 10.1186/s12889-015-2227-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gershon AS, Dolmage TE, Stephenson A, et al. Chronic obstructive pulmonary disease and socioeconomic status: a systematic review. COPD 2012;9:216-26. 10.3109/15412555.2011.648030 [DOI] [PubMed] [Google Scholar]
- 16.Burney P, Jithoo A, Kato B, et al. Chronic obstructive pulmonary disease mortality and prevalence: the associations with smoking and poverty--a BOLD analysis. Thorax 2014;69:465-73. 10.1136/thoraxjnl-2013-204460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lange P, Marott JL, Vestbo J, et al. Socioeconomic status and prognosis of COPD in Denmark. COPD 2014;11:431-7. 10.3109/15412555.2013.869580 [DOI] [PubMed] [Google Scholar]
- 18.Sahni S, Talwar A, Khanijo S, et al. Socioeconomic status and its relationship to chronic respiratory disease. Adv Respir Med 2017;85:97-108. 10.5603/ARM.2017.0016 [DOI] [PubMed] [Google Scholar]
- 19.Burney PG, Patel J, Newson R, et al. Global and regional trends in COPD mortality, 1990-2010. Eur Respir J 2015;45:1239-47. 10.1183/09031936.00142414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tøttenborg SS, Lange P, Johnsen SP, et al. Socioeconomic inequalities in adherence to inhaled maintenance medications and clinical prognosis of COPD. Respir Med 2016;119:160-7. 10.1016/j.rmed.2016.09.007 [DOI] [PubMed] [Google Scholar]
- 21.Hwang JA, Kim YS, Leem AY, et al. Clinical Implications of Sarcopenia on Decreased Bone Density in Men With COPD. Chest 2017;151:1018-27. 10.1016/j.chest.2016.12.006 [DOI] [PubMed] [Google Scholar]
- 22.Kim DS, Kim YS, Jung KS, et al. Prevalence of chronic obstructive pulmonary disease in Korea: a population-based spirometry survey. Am J Respir Crit Care Med 2005;172:842-7. 10.1164/rccm.200502-259OC [DOI] [PubMed] [Google Scholar]
- 23.Yoo KH, Kim YS, Sheen SS, et al. Prevalence of chronic obstructive pulmonary disease in Korea: the fourth Korean National Health and Nutrition Examination Survey, 2008. Respirology 2011;16:659-65. 10.1111/j.1440-1843.2011.01951.x [DOI] [PubMed] [Google Scholar]
- 24.Hwang YI, Park YB, Yoo KH. Recent Trends in the Prevalence of Chronic Obstructive Pulmonary Disease in Korea. Tuberc Respir Dis (Seoul) 2017;80:226-9. 10.4046/trd.2017.80.3.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korean Statistical Information Service. Daejeon: KOSIS. Available online: http://kosis.kr
- 26.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38. 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 27.National Collaborating Centre for Chronic Conditions National clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary care. Thorax 2004;59 Suppl 1:1-232. [PMC free article] [PubMed] [Google Scholar]
- 28.Prescott E, Vestbo J. Socioeconomic status and chronic obstructive pulmonary disease. Thorax 1999;54:737-41. 10.1136/thx.54.8.737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yin P, Zhang M, Li Y, et al. Prevalence of COPD and its association with socioeconomic status in China: findings from China Chronic Disease Risk Factor Surveillance 2007. BMC Public Health 2011;11:586. 10.1186/1471-2458-11-586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prescott E, Lange P, Vestbo J. Socioeconomic status, lung function and admission to hospital for COPD: results from the Copenhagen City Heart Study. Eur Respir J 1999;13:1109-14. 10.1034/j.1399-3003.1999.13e28.x [DOI] [PubMed] [Google Scholar]
- 31.Chen Y, Breithaupt K, Muhajarine N. Occurrence of chronic obstructive pulmonary disease among Canadians and sex-related risk factors. J Clin Epidemiol 2000;53:755-61. 10.1016/S0895-4356(99)00211-5 [DOI] [PubMed] [Google Scholar]
- 32.Kanervisto M, Vasankari T, Laitinen T, et al. Low socioeconomic status is associated with chronic obstructive airway diseases. Respir Med 2011;105:1140-6. 10.1016/j.rmed.2011.03.008 [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization. World report on ageing and health. Geneva, Switzerland: WHO, 2015. [Google Scholar]
- 34.Crystal S, Shea DG, Reyes AM. Cumulative Advantage, Cumulative Disadvantage, and Evolving Patterns of Late-Life Inequality. Gerontologist 2017;57:910-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atkinson Smith M. Use of the Vulnerable Populations Conceptual Model to Assess the Impoverished Elderly. Am J Nurse Pract 2011;15:42-6. [Google Scholar]
- 36.Fotokian Z, Mohammadi Shahboulaghi F, Fallahi-Khoshknab M, et al. The empowerment of elderly patients with chronic obstructive pulmonary disease: Managing life with the disease. PLoS One 2017;12:e0174028. 10.1371/journal.pone.0174028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fried TR, Fragoso CAV, Rabow MW. Caring for the older person with chronic obstructive pulmonary disease. JAMA 2012;308:1254-63. 10.1001/jama.2012.12422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mannino DM, Thorn D, Swensen A, et al. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J 2008;32:962-9. 10.1183/09031936.00012408 [DOI] [PubMed] [Google Scholar]
- 39.Lange P, Celli B, Agusti A, et al. Lung-Function Trajectories Leading to Chronic Obstructive Pulmonary Disease. N Engl J Med 2015;373:111-22. 10.1056/NEJMoa1411532 [DOI] [PubMed] [Google Scholar]