Abstract
Background
Thymic epithelial tumors are rare thoracic tumors for which pathological diagnosis is challenging due to the definition of multiple subtypes, tumor heterogeneity, and variations in interobserver reproducibility. In this study, we aimed at analyzing the quality of pathological reporting in line with the consistency between initial diagnosis and final diagnosis after expert review through a collaboration between the largest thoracic oncology center in Estonia, and one expert center in France.
Methods
Hospital electronic database and pathology databases from the Tallinn North Estonia Medical Centre were searched for thymic and mediastinal tumors from 2010 to 2017. Pathology specimens were referred to the Pathology Department of the Lyon University hospital. Overall, 55 tissue specimens from 49 patients were included.
Results
From pathology reports, tumor size, diagnosis, and invasion had been mentioned in ≥80% of cases, while resection status and staging were assessed in only 48% and 17% of cases, respectively. The initial diagnosis was consistent with that of the review in 60% of cases. Diagnostic concordance for thymoma subtypes was low (Cohen’s kappa 0.34, 95% CI: 0.16–0.52). Overall, a major change in the management of 8 (16%) patients had to be made after pathological review: 3 patients had a normal thymus according to the reference centre, while thymoma B1 or B2 had been diagnosed locally; 5 additional patients had a final diagnosis of non-thymic tumor.
Conclusions
Implementing structured pathology reports may help to decrease discrepancies in the diagnosis of thymic epithelial tumors. The development of expert networks is an opportunity to improve diagnosis and patient care, particularly in regard to rare cancers.
Keywords: Thymic epithelial tumors, pathology, diagnostic discrepancy
Introduction
According to the rare cancer network RARECAREnet, the European crude incidence rate of epithelial tumors of thymus was 1.7 per million per year for the period 2000–2007, resulting in 1,000 new cases annually (1). Thymic tumors account for less than 2% of all malignancies, but represent the most frequent tumors of the anterior mediastinum (2). The major level of complexity when making the diagnosis of thymic epithelial tumors is heterogeneity, reflected also within the World Health Organization (WHO) classification (3); whereby thymic epithelial tumors are classified into thymomas, thymic carcinomas, thymic neuroendocrine tumors and combined thymic carcinomas. Thymomas are further classified into type A, type AB, type B1, type B2 and type B3 thymoma, micronodular thymoma with lymphoid stroma and metaplastic thymoma. Rare thymoma subtypes include microscopic thymoma, sclerosing thymoma and lipofibroadenoma (4). In regard to differential diagnosis, other mediastinal malignancies should be excluded, such as lymphomas and germ-cell tumors, as well as thymic mesenchymal tumors and benign thymic hyperplasia. Besides the complexity within the WHO classification, the tumor, node, metastasis (TNM) staging system has been lacking for thymic malignancies, as nodal involvement is relatively uncommon and local spread of the primary tumor has been considered relevant (5,6).
Physicians treating patients with rare cancers often face diagnostic and therapeutic challenges (7-9). Due to their low frequency and limited hands-on experience, but also as a result of poor definition of histopathological features, wide interobserver variations exist even among pathologists in highly specialized centers (10-13). While the interobserver reproducibility of the WHO classification of thymic epithelial tumors has been questioned over time, the recent International Thymic Malignancies Interest Group (ITMIG) consensus statement proposes major and minor morphological, as well as immunohistochemical criteria to better individualize each thymic epithelial tumors entity; these criteria were defined based on a series of 188 prototypic and difficult-to-classify cases (4,14). In the routine practice setting, due to lack of centralization and rarity of the tumor type, collaborative networking is needed to improve diagnostic accuracy, which can lead to major change in the clinical management of patients in 7-30% of cases (8,15).
Meanwhile, in the setting of collaborative networks, such as the European Reference networks (16), tools to ensure accurate communication among centers are being developed. In 2016, the International Collaboration on Cancer Reporting (ICCR) introduced the recommended dataset for reporting of thymic epithelial tumors (17). This dataset contains the required and recommended data elements for structured pathology reporting. So far, such structured reporting for thymic tumors has not been widely implemented. Thymic malignancies in Estonia may thus serve as a model to describe the challenges small countries and centers face when treating rare cancers.
In this study, we aimed at analyzing the quality of pathological reporting in line with the consistency between initial diagnosis and final diagnosis after expert review through a collaboration between the largest thoracic oncology center in Estonia, and one expert center in France.
Methods
Study population
All inpatient and outpatient visits in the North Estonia Medical Centre from 2010 to 2017 where the main clinical diagnosis was benign or malignant thoracic or mediastinal tumor with the ICD-10 (International Classification of Diseases 10th Revision) code D15, D38, C37 or C38 were selected for initial review. Cases where follow-up visits included confirmed tumors such as throat cancer, lung cancer, germ cell tumor, lymphoma or non-neoplastic condition were excluded. Medical records for the remaining patients were extensively reviewed. All patients with a final clinical diagnosis of thymic tumor, regardless of whether the patient had an initial or follow-up visit during the study period, were included. Patients with at least one pathology specimen were included, whereas patients diagnosed only radiologically were excluded. In addition, the pathology database of the North Estonia Medical Centre was searched for specimens from mediastinum or thymus from 2010 to 2017.
Baseline demographic and clinical characteristics of the study population were retrieved from hospital medical records. For thymic malignancies, Masaoka stage, if not present in pathology report, was updated retrospectively according to radiological and clinical information. Clinical follow-up and current disease status were obtained from the nationwide Electronic Health Record with the cut-off date 1st June 2017. Date of death, if applicable, was retrieved from the Estonian Causes of Death Registry with the cut-off date 1st June 2017.
Histopathological review
Quality of local pathology reports was reviewed retrospectively according to the ICCR dataset. Surgical specimen reports were assessed by presence or absence of key data elements: tumor or specimen size, extent of direct invasion, resection margin status, final histologic diagnosis according to the WHO classification of tumors, lymph node involvement according to ITMIG lymph node map (18) and pathological stage according to modified Masaoka staging system for thymomas (19), and UICC TNM in case of carcinomas, which were standard at the time of initial diagnosis (20).
For the pathological review, paraffin blocks with formalin or AFA fixated tissue and haematoxylin-eosin-saffron slides were referred to the Department of Pathology of the Lyon University Hospital. ITMIG consensus major and minor morphological and immunohistochemical criteria were used to make the diagnosis of cases, together with the 2015 WHO reference book (3). The major histological criteria must be either present or absent and presence of minor criteria is more varied. In addition, the histologic subtypes are compared by clearly stated criteria and illustrated with morphological patterns and selected immunohistochemical stains (15). If not performed at the time of diagnosis, any additional immunohistochemical stains were performed for each subtype accordingly. To overcome difficulties in assessing keratin expression related to the presence of dendritic cells extensions, P63 stains were used in type B1 and B2 thymomas. CD5 and CD117/KIT expression was assessed on epithelial cells for type B3 thymomas and thymic carcinomas. Immunostains for GLUT-1 were performed in type A and B3 thymomas, and in thymic carcinomas.
Statistics
Descriptive statistics were used to characterize patients at study entry. Follow-up duration was measured from the date of first biopsy. Disease-free survival was defined as the time between radical surgery or end of curative-intent treatment to first recurrence of the tumor. The presence or absence of six key data elements of resected specimens and eight immunohistochemical stains for all thymic malignancies was recorded. Concordance rate as percentage of diagnoses consistent between referral and expert pathologic review was calculated. Cohen’s kappa was calculated for main diagnostic categories composed according to their clinical implications—thymoma regardless of subtype, thymic carcinoma, normal thymus, and other neoplasms.
Results
Patient characteristics
Medical records from 660 patients encompassing 1,249 medical visits were initially retrieved from the hospital electronic database based on the ICD-10 codes for benign or malignant neoplasms of thorax, thymus or mediastinum. Final dataset included 49 unique patients with the mean age at diagnosis being 59 (range, 22–88) years; 27 patients (55%) were female (Table 1). In regard to the treatment of the thymic lesion, 35 (71%) patients had radical surgery, 6 (12%) received palliative treatment while 8 (16%) refused or had contraindications for surgery. The surgical techniques used were transsternal resection in 14 patients, thoracotomy in 10 patients, and thoracoscopy in 11 patients; 8 patients had extensive resection of the lung, and 3 of the superior vena cava. Fifteen percent (6 of 41) of patients presented with autoimmune disease, myasthenia or myalgia at the time of diagnosis, and 27% (11 of 41) had second malignancies before or after diagnoses of thymic tumor.
Table 1. Demographic and baseline clinical characteristics of patients with thymic tumors and other mediastinal tumors included for central review.
| Characteristic | N=49 [%] |
|---|---|
| Gender | |
| F | 27 [55] |
| M | 22 [45] |
| Age at diagnosis (median, range) | 61 [22–88] |
| Biopsy | 29 [59] |
| Radical surgery | 35 [71] |
| Tumor type | |
| Thymic | 41 [84] |
| Other | 8 [16] |
| Masaoka stage1,2 | |
| I | 11 [27] |
| II | 12 [29] |
| III | 10 [24] |
| IV | 8 [20] |
| Autoimmune disease1 | 6 [15] |
| Radiotherapy1 | 6 [15] |
| Chemotherapy1 | 9 [22] |
| Median follow up in years from initial diagnosis (range) | 4 [0–16] |
| Follow up status | |
| Dead | 12 [25] |
| Disease free | 32 [65] |
| Stable disease | 5 [10] |
1, only patients with thymic tumors; 2, updated retrospectively based on medical records if not present in pathology report.
Initial histopathological diagnoses
Overall, 78% of patients had a surgical specimen available, this includes patients with radical surgery and surgical biopsies, 59% of patients had a core needle biopsy specimen available (Table 1). In regard to the quality of pathological reporting, reports were written by 9 different pathologists, of whom one has special interests in mediastinal tumors. Clinical and radiological information was accessible to the pathologist at the time of diagnosis in all cases. The reports were written as free text, which allowed variability in the use of different data elements. The quality of pathology reports was assessed, with tumor size, diagnosis, and invasion mentioned in ≥80% of cases, while resection status and staging were assessed in 14 (48%) and 5 (17%) of cases, respectively (Table 2). There was no specific immunohistochemical panel in use and the choice and amount of stains was different between pathologists. The most frequently used were epithelial cell markers p63, CKAE1/AE3 and CK MNF116. Other markers were less frequently and inconsistently used. The amount of different stains varied from 0 to 20 and included various stains for differential diagnosis of metastatic tumors in case of biopsies.
Table 2. Quality of local pathology reports of thymic malignancies according to the recommendations from the International Collaboration on Cancer Reporting.
| Characteristic | N [%] |
|---|---|
| Data elements marked in surgical specimen reports (N=29) | |
| Final histologic diagnosis | 29 [100] |
| Specimen or tumor size | 26 [90] |
| Extent of direct invasion | 25 [86] |
| Resection margin status | 14 [48] |
| Lymph node involvement according to ITMIG lymph node map1 | 3 [33] |
| Pathological staging (TNM 7th version or Masaoka-Koga) | 5 [17] |
| IHC stains performed on biopsy or surgical specimen (N=41) | |
| Any IHC stains | 40 [98] |
| p63, CK MNF116 or CK AE1/AE3 | 38 [93] |
| Ki67 | 25 [61] |
| CD5 | 21 [51] |
| TdT | 21 [51] |
| CD3 | 19 [46] |
| CD20 | 19 [46] |
| CD117 | 16 [39] |
| GLUT12 | 0 [0] |
1, only on 9 surgical specimens with resected lymph nodes; 2, GLUT1 was recommended by reference centre, but not used in Estonia. CK, cytokeratins; IHC stain, immunohistochemical stain.
Histopathological review at the expert center
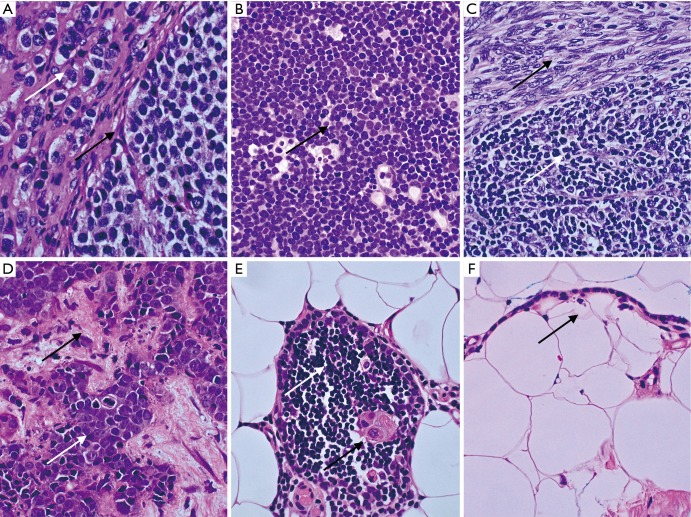
Results of histopathological review are presented in Table 3. Initial diagnosis was consistent with that of the review in 60% of cases. Minor discrepancies regarding thymoma subtype were observed in 20% of cases, whereas concordance rate among thymoma subtypes was 51% (Table 3). Overall, a major change in the management of 8 (16%) patients had to be made after pathological review: 3 patients had a normal thymus according to the reference center, whereas thymoma B1 or B2 had been diagnosed locally, including one patient with severe myasthenia gravis; 5 patients had implications for treatment due to major differences in pathohistological diagnoses: one carcinoid tumor on expert review, whereas sarcoma had been diagnosed locally; one T cell lymphoma vs. thymoma B2; three cases of thymoma vs. squamous cell carcinoma (Figure 1).
Table 3. Concordance rate for pathohistological diagnoses between local and reference centre.
| Diagnoses | Reference centre (N) | Local centre (N) | Concordance rate (%)1 |
|---|---|---|---|
| Thymoma type A | 3 | 3 | 512 |
| Thymoma type AB | 18 | 14 | |
| Thymoma type B1 | 3 | ||
| Thymoma type B2 | 7 | 6 | |
| Thymoma type B3 | 4 | ||
| Thymic carcinoma | 6 | 8 | 75 |
| Normal thymus | 7 | 4 | 57 |
| Micronodular thymoma with lymphoid stroma | 3 | 2 | 25 |
| Carcinoid | 2 | 1 | 50 |
1, proportion of samples that are concordant for the total number of pairs with respective diagnoses; 2, percent of observed agreements for all thymoma subtypes combined.
Figure 1.
Representative cases with diagnostic difficulties and with major implications on treatment from the North Estonian Medical Centre (Olympus BX53 equipped with Olympus DP27 camera and Olympus cellSens software). The tissue sections were stained by haematoxylin and eosin (HE) staining (magnification, ×600). (A) Thymic carcinoid tumor. Nuclei with salt-and-pepper chromatin (white arrow) and nested growth pattern of uniform cells (black arrow). Stains for chromogranin A and synaptophysin were positive; (B) T lymphoblastic lymphoma. Destructive growth pattern of monotonous LCA positive lymphoblasts with high mitotic activity (black arrow); (C) AB thymoma. Biphasic tumor with spindle cell epithelial component (black arrow) resembling type A and lymphocyte-rich component resembling type B1 (white arrow); (D) metastatic thymic carcinoma in liver biopsy. Carcinoma cells (white arrow) with high grade atypia, necrosis and central mitosis in the background of desmoplastic stroma (black arrow); (E) thymic tissue with normal lymphocytes (white arrow) and Hassall body (black arrow) surrounded by mediastinal adipose tissue; (F) unilocular cyst lined with thymic cuboid epithelium (black arrow) in mediastinal adipose tissue. LCA, leukocyte common antigen.
Concordance rates between the two centers were calculated for diagnostic categories composed according to their clinical implications. Cohen’s kappa was good when calculated for four diagnostic categories—thymoma regardless of subtype, thymic carcinoma, normal thymus, or other neoplasms [0.79 (95% CI: 0.64–0.94; n=55)]. However, the probability of agreement was as low as 50% (Cohen’s kappa 0.34, 95% CI: 0.16–0.52), when calculated for thymoma subtypes (n=36 in total, n=32 reference center, n=34 initial diagnosis). Overall agreement between histopathologists in classifying thymic epithelial tumors was moderate (Cohen’s kappa 0.52, 95% CI: 0.346–0.698; n=44 in total, n=40 reference center, n=42 initial diagnosis). The most difficult was actually classifying B2 thymomas.
Discussion
Taken together, our results demonstrated (I) the moderate quality of pathology reports at the local center, presenting major components recommended based on the WHO classification, despite absence of structured reporting; (II) the initial diagnosis made at the local center was consistent with that of the review in only 60% of cases, what was actually mostly related to thymoma subtyping; and ultimately; (III) a rate of major discrepancies leading to change in the management of patients in 16% of cases.
Due to the retrospective nature of this study, it was not possible to analyse why certain required data elements were often missing in local pathology reports. This could partially be attributed to the free text allowing personal preferences in reporting. Local reports were written by 9 different pathologists, none specialized in thoracic oncology. Due to the lack of a common immunohistochemical analysis panel and structured reporting at local laboratory, we were not able to analyse why certain diagnoses had been made. Generally, no discussion on differential diagnoses was given. As no official TNM classification system has been in use for thymic malignancies, this could partially explain why pathological staging was described in only 17% of reports. However, the extent of invasion according to the commonly used Masaoka-Koga classification was presented in 86% of cases.
The latest WHO classification for thymic tumors was published in 2015 (3). In comparison to the previous version, the most significant modification was the definition of major and minor criteria to make the diagnosis of histologic subtypes of thymomas. In this study, our second aim was to assess the reproducibility of diagnosing tumors originating from the anterior mediastinum. Diagnostic concordance for thymoma subtypes between reference and local centers was low (Cohen’s kappa 0.34, 95% CI: 0.16–0.52). Classifying thymomas into subtypes correlates with prognosis, but actually has a limited impact on treatment plans based on the recent clinical practice guidelines from the ESMO (21). The most important issue in regard to clinical management is to differentiate thymomas and thymic carcinomas from non-tumoral thymus and other tumors, the former being more prone to perioperative chemotherapy. In this aspect the concordance in our study was actually good with a kappa of 0.79 (95% CI: 0.64–0.94). In our study, there was only one expert reviewer per case. Hence, we were not able to account for interobserver variability in the reference center. The cases in the study were from 2010–2017. The expert reviewer in France used the 2015 WHO and the 2014 ITMIG consensus. This is potentially a confounding factor, however, the paper highlights how different is the baseline workup of specimens in a real-life setting and how it impacts the diagnosis. Most of the discrepancies were actually not related to a classification change, but to the way how pathologists’ approached certain tumor type.
Generally, our data are in agreement with those of Sakakura et al. in Japan, who observed that the concordance rate was the lowest for thymoma AB subtype (30%), whereas in our study it was the lowest for thymoma B3 subtype (0%) (22). Simplification of the subgroup classification significantly improved interobserver agreement in two retrospective multicenter studies among a panel of pathologists in the UK, the Netherlands and Germany (23,24). In contrast, new versions of histopathological classifications aim at improving prognostic and predictive value of diagnoses, but this may also lead to lower reproducibility due to increasing complexity. Diagnostic difficulties mainly arise from borderline cases, tumors with atypia, high mitotic activity and necrosis, and tumors with more than one histological pattern (15,25). Currently, the WHO major and minor diagnostic criteria for thymic epithelial tumors are listed as descriptive text, instead a more precise comparative table format could be helpful. In addition, molecular subtyping of thymic malignancies could further refine the diagnostic accuracy in the future. For example in the case of gliomas, a diagnostic algorithm based on molecular profiling was proposed in 2015, after the histopathological review indicated that a consensus in diagnoses for certain subtypes of tumors was less than 15% (10). The updated 2016 WHO classification of central nervous system tumors now integrates genetic markers into diagnoses (26). More recently, it was demonstrated that the loss of CDKN2A(p16) by FISH could facilitate detection of the sarcomatoid component in ambiguous cases of mesothelioma (27). Hence, categorization of thymic epithelial tumors by more reproducible molecular tests could improve subjective morphology-based diagnoses and overcome issues with complex diagnoses and is a subject for further research.
Synoptic (structured) reporting in pathology is the use of templates to create a standardized report on the data that is critical for clinical decision making. Synoptic reporting is based on standard classification as well as prognostic factors and is equally useful for common and rare cancers. In a recent systematic review, implementation of synoptic reporting resulted in an increased completeness and quality of pathology reports, particularly in surgical elements such as resection margins, type of local invasion, and mean number of lymph nodes removed (28). Synoptic reporting should be implemented for rare tumors, particularly in small centers, to improve overall quality. Initially, introduction of new format is more time-consuming as it requires changes in hospital electronic systems and pathologists’ personal preferences. We hereby suggest here data elements from the ICCR dataset for reporting thymic epithelial tumors. The use of a specific recommended panel of immunohistochemical stains could further improve diagnostic accuracy, in the setting of low number of cases seen in rare tumors – for example in Estonia, thymic tumors are diagnosed less than 7 times every year.
In regard to quality in pathology, actions should be taken to specialise people, for example at the time of this study none of the pathologists were focusing specifically on thoracic pathology. It has been shown that reproducibility decreases significantly with the number of pathologists involved, particularly in rare cancers and among general pathologists (23,29). When a rare diagnoses is primarily based on morphology, i.e., pathologists’ experience, it is not possible to reach a correct diagnoses even with access to all relevant equipment, and therefore, the possibility to consult externally is of major importance.
No similar studies have been conducted in Estonia, Eastern Europe or Nordic countries, the observed findings may reflect regional differences. Other similar studies have commonly compared the diagnoses among expert thoracic pathologists in large cancer centres. Estonia with a population of 1.3 million has two centers specialized for thoracic oncology. Approximately 20% of patients with rare cancers in the EU are treated in small countries with a population of 10 million or less, comprising of 18 member states. This is an original work as it highlights the challenges across Europe that may be improved with networks formally established and recognized. The recent recognition of the European Reference Network EURACAN leads to exchange between expert centers and local centers, both for diagnosis in the routine practice setting, and for research projects (16).
The European Commission recently approved the European Reference Networks for—referred to as the G8 domain—handles a network of 20+ healthcare providers; the objectives of EURACAN include the updating and the assessment of current guidelines, the development of educational programs, dissemination and communication with patients’ groups, and the establishment of research projects, from the diagnostic workup of the disease to the therapeutic strategies. Meanwhile, regional network, more dedicated to clinical management of patients, are being established. In France, RYTHMIC (Réseau tumeurs THYMiques et Cancer; www.rythmic.org) is a nationwide network for thymic malignancies, which was appointed in 2012 by the French National Cancer Institute, as part of its rare cancer program. Since then, the management of all patients diagnosed with thymic tumors has been discussed at a national multidisciplinary tumor board, which is organized twice a month basis using a web-based conferencing system. Decision-making is based on consensual recommendations that were originally established based on available evidence and are updated and approved each year by all members of the network. A prospective database of all patients is hosted by the French Thoracic Cancer Intergroup. Overall, more than 2,000 patients have been enrolled in 5 years, leading to a significant expertise in the diagnosis and management of these tumors. Similar thymoma-dedicated networks are now being implemented in Spain and Italy (the TYME collaborative group).
Conclusions
In conclusion, our study further supports the value of synoptic reporting, particularly in case of rare cancers where clinical experience is limited, and this should be implemented in local centers to optimize the diagnosis process. The development of expert networks is an opportunity to improve diagnosis and patient care, particularly of rare cancers.
Acknowledgements
Funding: To conduct this research and to prepare the manuscript KO, AAR and KL have received research grant from the North Estonia Medical Centre, Tallinn, Estonia; NG and LC have received support from the European Reference Network EURACAN HP-ERN-SGA EURACAN - 769 029.
Ethical Statement: The study was approved by regional ethics committee of Tallinn Medical Ethics Committee (No. 1377).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Gatta G, Capocaccia R, Botta L, et al. Burden and centralised treatment in Europe of rare tumours: Results of RARECAREnet-a population-based study. Lancet Oncol 2017;18:1022-39. 10.1016/S1470-2045(17)30445-X [DOI] [PubMed] [Google Scholar]
- 2.Scorsetti M, Leo F, Trama A, et al. Thymoma and thymic carcinomas. Crit Rev Oncol Hematol 2016;99:332-50. 10.1016/j.critrevonc.2016.01.012 [DOI] [PubMed] [Google Scholar]
- 3.WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. Fourth edition. Available online: http://apps.who.int/bookorders/anglais/detart1.jsp?codlan=1&codcol=70&codcch=4007
- 4.den Bakker MA, Roden AC, Marx A, Marino M. Histologic Classification of Thymoma: A Practical Guide for Routine Cases. J Thorac Oncol 2014;9:S125-S130. 10.1097/JTO.0000000000000297 [DOI] [PubMed] [Google Scholar]
- 5.Filosso PL, Ruffini E, Lausi PO, et al. Historical perspectives: The evolution of the thymic epithelial tumors staging system. Lung Cancer 2014;83:126-32. 10.1016/j.lungcan.2013.09.013 [DOI] [PubMed] [Google Scholar]
- 6.Detterbeck FC, Stratton KM, Giroux DM, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: Proposal for an Evidence-Based Stage Classification System for the Forthcoming (8th) Edition of the TNM Classification of Malignant Tumors. J Thorac Oncol 2014;9:S65-S72. 10.1097/JTO.0000000000000290 [DOI] [PubMed] [Google Scholar]
- 7.Boyd N, Dancey JE, Gilks CB, Huntsman DG. Rare cancers: A sea of opportunity. Lancet Oncol 2016;17:e52-e61. 10.1016/S1470-2045(15)00386-1 [DOI] [PubMed] [Google Scholar]
- 8.Blay JY, Coindre JM, Ducimetière F, Ray-Coquard I. The value of research collaborations and consortia in rare cancers. Lancet Oncol 2016;17:e62-e69. 10.1016/S1470-2045(15)00388-5 [DOI] [PubMed] [Google Scholar]
- 9.Billingham L, Malottki K, Steven N. Research methods to change clinical practice for patients with rare cancers. Lancet Oncol 2016;17:e70-e80. 10.1016/S1470-2045(15)00396-4 [DOI] [PubMed] [Google Scholar]
- 10.Kros JM, Huizer K, Hernández-Laín A, et al. Evidence-based diagnostic algorithm for glioma: Analysis of the results of pathology panel review and molecular parameters of EORTC 26951 and 26882 trials. J Clin Oncol 2015;33:1943-50. 10.1200/JCO.2014.59.0166 [DOI] [PubMed] [Google Scholar]
- 11.Marx A, Chan JK, Coindre JM, et al. The 2015 World Health Organization Classification of Tumors of the Thymus: Continuity and Changes. J Thorac Oncol 2015;10:1383-95. 10.1097/JTO.0000000000000654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roden AC, Yi ES, Jenkins SM, et al. Reproducibility of 3 Histologic Classifications and 3 Staging Systems for Thymic Epithelial Neoplasms and Its Effect on Prognosis. Am J Surg Pathol 2015;39:427-41. 10.1097/PAS.0000000000000391 [DOI] [PubMed] [Google Scholar]
- 13.Laurent C, Baron M, Amara N, et al. Impact of expert pathologic review of lymphoma diagnosis: Study of patients from the French Lymphopath network. J Clin Oncol 2017;35:2008-17. 10.1200/JCO.2016.71.2083 [DOI] [PubMed] [Google Scholar]
- 14.Meurgey A, Girard N, Merveilleux du Vignaux C, et al. Assessment of the ITMIG Statement on the WHO Histological Classification and of the Eighth TNM Staging of Thymic Epithelial Tumors of a Series of 188 Thymic Epithelial Tumors. J Thorac Oncol 2017;12:1571-81. 10.1016/j.jtho.2017.06.072 [DOI] [PubMed] [Google Scholar]
- 15.Marx A, Ströbel P, Badve SS, et al. ITMIG consensus statement on the use of the WHO histological classification of thymoma and thymic carcinoma: refined definitions, histological criteria, and reporting. J Thorac Oncol 2014;9:596-611. 10.1097/JTO.0000000000000154 [DOI] [PubMed] [Google Scholar]
- 16.Imbimbo M, Maury JM, Garassino M, et al. Mesothelioma and thymic tumors: Treatment challenges in (outside) a network setting. Eur J Surg Oncol 2019;45:75-80. 10.1016/j.ejso.2018.01.078 [DOI] [PubMed] [Google Scholar]
- 17.Nicholson AG, Detterbeck F, Marx A, et al. Dataset for reporting of thymic epithelial tumours: recommendations from the International Collaboration on Cancer Reporting (ICCR). Histopathology 2017;70:522-38. 10.1111/his.13099 [DOI] [PubMed] [Google Scholar]
- 18.Bhora FY, Chen DJ, Detterbeck FC, et al. The ITMIG/IASLC Thymic Epithelial Tumors Staging Project: A Proposed Lymph Node Map for Thymic Epithelial Tumors in the Forthcoming 8th Edition of the TNM Classification of Malignant Tumors ITMIG DEFINITIONS AND POLICIES METHODS Process for Development of Recommendations. J Thorac Oncol 2014;9:S88-S96. [DOI] [PubMed] [Google Scholar]
- 19.Masaoka A. Staging system of thymoma. J Thorac Oncol 2010;5:S304-12. 10.1097/JTO.0b013e3181f20c05 [DOI] [PubMed] [Google Scholar]
- 20.International Union against Cancer (UICC) TNM Classification of Malignant Tumours 7th edition. Available online: http://meteor.aihw.gov.au/content/index.phtml/itemId/403583
- 21.Girard N, Ruffini E, Marx A, Faivre-Finn C, Peters S, ESMO Guidelines Committee Thymic epithelial tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26:v40-v55. 10.1093/annonc/mdv277 [DOI] [PubMed] [Google Scholar]
- 22.Sakakura N, Tateyama H, Nakamura S, et al. Diagnostic reproducibility of thymic epithelial tumors using the World Health Organization classification: note for thoracic clinicians. Gen Thorac Cardiovasc Surg 2013;61:89-95. 10.1007/s11748-012-0187-z [DOI] [PubMed] [Google Scholar]
- 23.Verghese ET, Den Bakker MA, Campbell A, et al. Interobserver variation in the classification of thymic tumours - A multicentre study using the WHO classification system. Histopathology 2008;53:218-23. 10.1111/j.1365-2559.2008.03088.x [DOI] [PubMed] [Google Scholar]
- 24.Rieker RJ, Hoegel J, Morresi-Hauf A, et al. Histologic classification of thymic epithelial tumors: comparison of established classification schemes. Int J cancer 2002;98:900-6. 10.1002/ijc.10255 [DOI] [PubMed] [Google Scholar]
- 25.Wu J, Fang W, Chen G. The enlightenments from ITMIG Consensus on WHO histological classification of thymoma and thymic carcinoma: Refined definitions, histological criteria, and reporting. J Thorac Dis 2016;8:738-43. 10.21037/jtd.2016.01.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.WHO Classification of Tumours of the Central Nervous System, Revised. Fourth Edition. Available online: http://apps.who.int/bookorders/WHP/detart1.jsp?sesslan=1&codlan=1&codcol=70&codcch=24001
- 27.Galateau Salle F, Le Stang N, Nicholson AG, et al. New Insights on Diagnostic Reproducibility of Biphasic Mesotheliomas: A Multi-Institutional Evaluation by the International Mesothelioma Panel From the MESOPATH Reference Center. J Thorac Oncol 2018;13:1189-203. 10.1016/j.jtho.2018.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sluijter CE, van Lonkhuijzen LR, van Slooten HJ, et al. The effects of implementing synoptic pathology reporting in cancer diagnosis: a systematic review. Virchows Arch 2016;468:639-49. 10.1007/s00428-016-1935-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zucali PA, Di Tommaso L, Petrini I, et al. Reproducibility of the WHO classification of thymomas: Practical implications. Lung Cancer 2013;79:236-41. 10.1016/j.lungcan.2012.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]