Abstract
Background
In last years, an increasing interest emerges on the role of sub-lobar resection and lobe-specific lymph nodal dissection in the treatment of early-stage lung cancer. The aim of our study was to define the impact on cumulative incidence of recurrence (CIR) of type of surgical resection and type of nodal staging in this subset of patients. Furthermore, we evaluated the possible synergism between the different kinds of procedure.
Methods
An analysis of 969 consecutive stage I pulmonary adenocarcinoma patients, operated in six Thoracic Surgery Institutions between 2001 and 2013, was conducted. Type of surgical resection included lobectomy and sub-lobar resection; while pneumonectomy and bilobectomy were excluded from the analysis. Nodal staging procedures were classified in nodal sampling (NS), lobe-specific lymph node dissection (LS-ND) and systematic lymph node dissection (SND). Multivariable-adjusted comparisons for CIR was performed using Fine and Grey model, taking into account of death by any cause as competing event. In order to evaluate synergism between the different procedures, the test of interaction between type of surgical resection and type of nodal staging was carried out and results presented in a stratified way.
Results
Eight-hundred forty-six (87%) patients were submitted to lobectomy, while 123 (13%) to sub-lobar resection. Four-hundred fifty-five (47%) patients received SND, 98 (10%) LS-ND and 416 (43%) NS. Two-hundred forty-seven (26%) patients developed a local/distant recurrence with a 5-year CIR of 24.2%. Multivariable-adjusted comparisons showed an independent negative effect of sub-lobar resection (HR =1.52; 95% CI: 1.07–2.17), LS-ND (HR =1.74; 95% CI: 1.16–2.6) and NS (HR =1.49; 95% CI: 1.12–1.98) on CIR. Test of interaction showed a homogeneity of results among subgroups.
Conclusions
Patients affected by stage I pulmonary adenocarcinoma and submitted to lobectomy presented a significant lower recurrence rate than those submitted to sub-lobar resection. Moreover, SND presented an independent positive effect on recurrence development than other lymph node assessment strategy. Finally, lobectomy in combination with systematic lymph nodal resection showed the best results in term of CIR.
Keywords: Non-small cell lung cancer (NSCLC), lobectomy, lymphadenectomy, staging
Introduction
Non-small cell lung cancer (NSCLC) confirmed to be a major public health issue worldwide (1). In this context, 20% of whole NSCLC were diagnosticated as early stage (2), while lung adenocarcinoma accounted 70% of all NSCLC cases (3).
Currently, lobectomy with radical lymph node dissection represent the standard of care for resectable NSCLC (4,5). Nonetheless, in last years, an increasing interest emerges on the role of sub-lobar resection in the treatment of stage I lung cancer. Undeniably, the possibility to perform parenchymal sparing resection (i.e., anatomical segmentectomy and wedge resection), primarily reserved to unfit patients, to the whole cases of early stage NSCLC fascinated numerous surgical groups over the years. However, the only randomized clinical trial comparing sub-lobar resection with lobectomy for clinical stage IA NSCLC demonstrated an inferior survival and a higher local recurrence rate in the limited resection group (5). Still, interest concerning convenience of limited lung resections in early stage NSCLC remains and numerous studies could be found in recent literature, assessing their equivalence with lobectomy in term of survival and recurrence rate (6-11).
On the other hand, lymph nodal status is one of the most important prognostic factors in the management of NSCLC (12). However, optimal lymph node assessment strategy is still matter of debate amongst surgical community. Indubitably, systematic nodal dissection (13), in respect of lymph nodal sampling (NS), provides a more accurate pathological staging and allows to sterilize unknown or microscopic neoplastic lymph node spreading (14). Nevertheless, new minimally invasive biopsy techniques (15,16) and high definition imaging rise doubts on unavoidability of such aggressive nodal assessment.
Accordingly, the aim of our study was to define impact on Cumulative incidence of recurrence (CIR) of type of surgical resection and type of nodal staging. Furthermore, we evaluated the possible synergism between the different kinds of procedure.
Methods
From 2001 to 2013, patients who underwent lung surgical resection with curative intent for stage I pulmonary adenocarcinoma in six thoracic surgery institutions (Appendix A) were retrospectively reviewed.
Extended resection (i.e., combined lung and chest wall/diaphragm resections), pneumonectomy, bilobectomy or preoperative treatment regimen (e.g., chemotherapy, radiotherapy) represented the exclusion criteria from this study.
The preoperative assessment of patient encompassed chest radiographs; thoracic-, brain- and upper-abdominal computed tomography (CT) scans or whole-body 18-fluorodeoxyglucose positron emission tomography (PET), or both; fiber-optic bronchoscopy; electrocardiograms and lung function tests.
Surgical procedures were performed either through thoracotomy (muscle-sparing axillary or posterolateral) or video-assisted thoracic surgery (VATS).
Data variables and outcomes
The final data set for the analyses included the following data: age, gender, smoking habit, side of intervention, type of surgical resection, type of intraoperative lymph node assessment, pathological TNM (pTNM) stage (according to 7th edition), vascular invasion, predominant histologic pattern and histologic grade and survival data.
Type of surgical resection were divided into lobectomy and sub-lobar resection (encompassed wedge and segmental resection).
Lymph node station were classified according to IASLC 8th edition lymph node map (12). Type of intraoperative lymph node assessment was classified as follow: (I) lymph NS consisted in the removal of one or more lymph nodes, guided by preoperative or intraoperative findings; (II) lobe-specific lymph node dissection (LS-ND), encompassed the resection of only stations which are considered as the natural drain for that lobe (Appendix B) (17,18); (III) systematic nodal dissection (SND), in which, according to ESTS (13), all mediastinal tissue containing lymph nodes is dissected and removed systematically within anatomical landmarks; the hilar and the intrapulmonary lymph nodes are dissected as well, and at least three mediastinal nodes are excised as a minimum requirement.
Surgery was defined as radical (R0) when a complete tumor resection was accomplished, and incomplete in case of microscopically (R1) or macroscopically (R2) residual disease.
Histological grading was categorized into well- (G1), moderately- (G2) and poorly differentiated (G3) carcinoma according to degree of architecture and cytological atypia.
The adenocarcinoma predominant patterns were determinate according to the criteria of the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society (IASLC/ATS/ERS) (19). The following predominant patterns were clustered in the “Common Variants” group in order to simplify the analysis: Solid, Micropapillary, Acinar and Papillary.
The study was approved by the IRB of each participating center.
Statistical analysis
Categorical data are presented as number (percentage, %), continuous data as median [interquartile range (IQR)]. Missing data in evaluated predictors were multiple-imputed and combined estimates were obtained from 5 imputed data sets. Associations between type of surgery, type of intraoperative lymph node assessment and clinicopathological characteristics were investigated with the use of the χ2 test or Fisher’s exact test, when appropriate (20).
Primary outcome was CIR, calculated from the date of intervention to the date of tumor distant or local relapse. CIR were estimated using the method proposes by Gooley et al. (21), taking into account death by any cause (except of cancer related death) as competing event. Differences in CIR between groups were investigated with Fine and Grey model, taking into account of death by any cause (except of cancer related death) as competing event. Univariable and Multivariable analysis were carried out. Multivariate adjusted analysis considers age, gender, smoking habit, side of intervention, pTNM stage, vascular invasion, predominant histologic pattern and histologic grade.
Moreover, we explored a potential synergism (the so called “effect modification”) between surgical resection and intraoperative lymph node assessment in the determination of CIR. This potential synergism was evaluated from a statistical point of view by including and testing interaction terms between variables related to surgical resection and intraoperative lymph node assessment in the Fine & Gray model.
The overall survival (OS) was the secondary outcome and was defined as the time from the date of the intervention to the date of death by any cause. Survival function was estimated by Kaplan-Maier method.
All statistical analyses were performed using STATA (version 14) and R (version 3.1).
Results
According to the selection criteria, 969 patients with stage I adenocarcinoma of the lung were included in the final dataset (Figure S1). Population demographics and clinical, surgical and pathologic characteristics of the cohort were showed in Table 1.
Table 1. Patients characteristics.
| Characteristics | N non-missing | Overall (N=969) | 1st comparison | 2nd comparison | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lobectomy (N=846) | Sub-lobar resection (N=123) | P value* | SND (N=455) | LS-ND (N=98) | NS (N=416) | P value* | ||||
| Age | 969 | 0.002 | 0.009 | |||||||
| ≤70 years | 597 [62] | 537 [63] | 60 [49] | 297 [65] | 48 [49] | 252 [61] | ||||
| >70 years | 372 [38] | 309 [37] | 63 [51] | 158 [35] | 50 [51] | 164 [39] | ||||
| Gender | 969 | 0.753 | 0.426 | |||||||
| Female | 311 [32] | 270 [32] | 41 [33] | 154 [34] | 27 [28] | 130 [31] | ||||
| Male | 658 [68] | 576 [68] | 82 [67] | 301 [66] | 71 [72] | 286 [69] | ||||
| Smokers | 969 | 0.547 | 0.043 | |||||||
| No | 370 [38] | 320 [38] | 50 [41] | 180 [40] | 26 [27] | 164 [39] | ||||
| Yes | 599 [62] | 526 [62] | 73 [59] | 275 [60] | 72 [73] | 252 [61] | ||||
| pTNM stage | 969 | 0.251 | <0.001 | |||||||
| Ia | 641 [66] | 554 [65] | 87 [71] | 271 [60] | 71 [72] | 299 [72] | ||||
| Ib | 328 [34] | 292 [35] | 36 [29] | 184 [40] | 27 [28] | 117 [28] | ||||
| Vascular invasion | 815 | 0.08 | <0.001 | |||||||
| No | 643 [79] | 567 [80] | 76 [72] | 342 [78] | 59 [65] | 242 [85] | ||||
| Yes | 172 [21] | 143 [20] | 29 [28] | 98 [22] | 32 [35] | 42 [15] | ||||
| Histologic predominant pattern | 959 | 0.196 | 0.127 | |||||||
| Lepidic | 124 [13] | 102 [12] | 22 [18] | 54 [12] | 17 [17] | 53 [13] | ||||
| Common variants | 762 [79] | 671 [80] | 91 [74] | 365 [81] | 79 [81] | 318 [78] | ||||
| NOS—other variants | 73 [8] | 63 [8] | 10 [8] | 34 [8] | 2 [2] | 37 [9] | ||||
| Histologic grade | 955 | 0.131 | 0.542 | |||||||
| G1 | 202 [21] | 170 [20] | 32 [27] | 90 [20] | 17 [18] | 95 [23] | ||||
| G2 | 484 [51] | 422 [51] | 62 [52] | 238 [53] | 51 [53] | 195 [48] | ||||
| G3 | 269 [28] | 243 [29] | 26 [22] | 124 [27] | 29 [30] | 116 [29] | ||||
*, χ2 test or Fisher’s exact test. NS, lymph node sampling; LS-ND, lobe-specific lymph node dissection; SND, systematic nodal dissection.
Most of the patients are male (658, 68%) and smokers (599, 62%). Pathological stage Ia was more commonly observed (641, 66%), while the histological predominant pattern consisted of acinar adenocarcinoma (442, 46%), followed by the papillary (149, 15%), the solid (147, 15%), the lepidic (124, 12.8%) and the micropapillary (25, 3%).
Median follow-up was 63 months. A total of 686 (68%) were reported to be alive and 283 (32%) died during the follow-up period. Overall, the 5-year survival rate was 74.5% (95% CI: 71.3–77.3%) (Figure 1).
Figure 1.
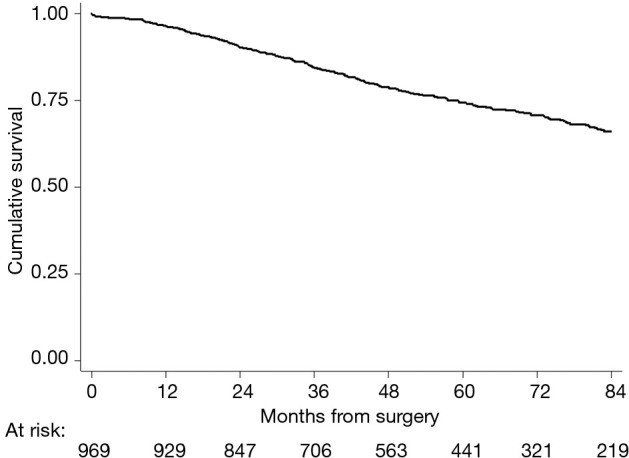
Overall survival.
Clinicopathological variables and type of surgical resection
Table 1 shows the distribution of clinicopathological variables according to the type of surgical resection: Eight-hundred forty-six (87%) patients were submitted to lobectomy, while 123 (13%) to sub-lobar resection [72 (58%) segmentectomy, 51 (42%) wedge resection].
Patients submitted to lobectomy were predominantly younger (P=0.002). No difference between type of surgical resection was observed in regard to gender, smoking habit, pTNM stage, vascular invasion, predominant histological pattern and histological grade.
Clinicopathological variables and type of lymph node assessment
Table 1 shows the distribution of clinicopathological variables according to the type of lymph nodal assessment: 455 (47%) patients received SND, 98 (10%) LS-ND and 416 (43%) NS.
Patients submitted to SND were predominantly younger (P=0.009), non-smoker (P=0.043), with higher pTNM stage (P<0.001) and less frequently presented vascular invasion (P<0.001). No difference between the types of lymph nodal assessment was observed in regard to gender, predominant histological pattern and histological grade.
CIR analysis
Nine-hundred thirty-seven patients with stage I adenocarcinoma of the lung were included in the analyses (Figure S1).
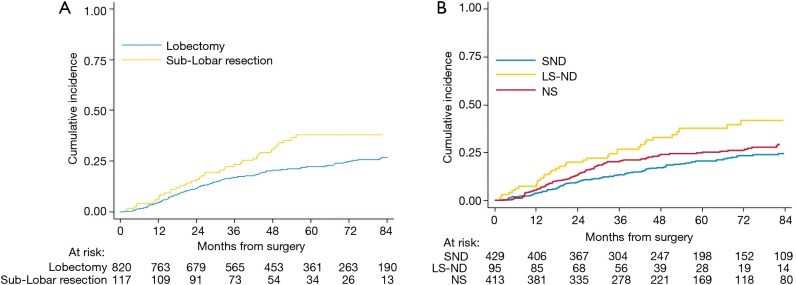
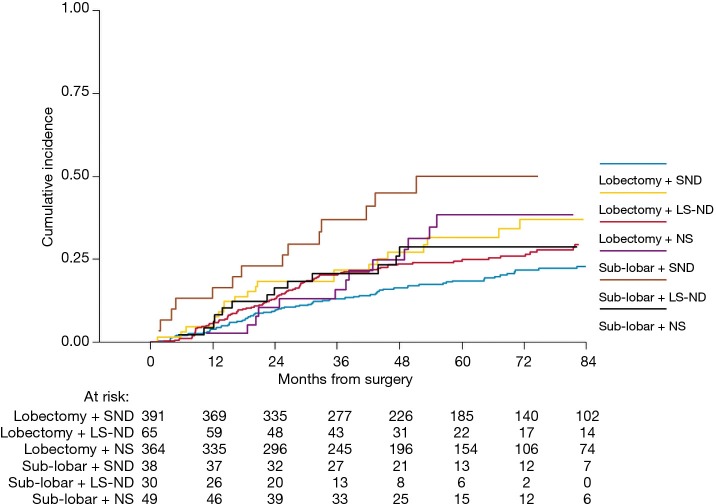
Two-hundred forty-seven (26%) patients developed a local/distant recurrence with a 5-year CIR of 24.2% (95% CI: 21.3–27.1%) (Figure 2). In univariable analysis, lobectomy (20.5% vs. sub-lobar resection 38.1%; 20.5%, vs. LS-ND 37.8% vs. NS 24.9%; P=0.014) and SND (P=0.001) were found to have a positive effect on survival (Figure 3). Combination of lobectomy plus SND showed the best recurrence rate pattern (P=0.01; Figure 4).
Figure 2.
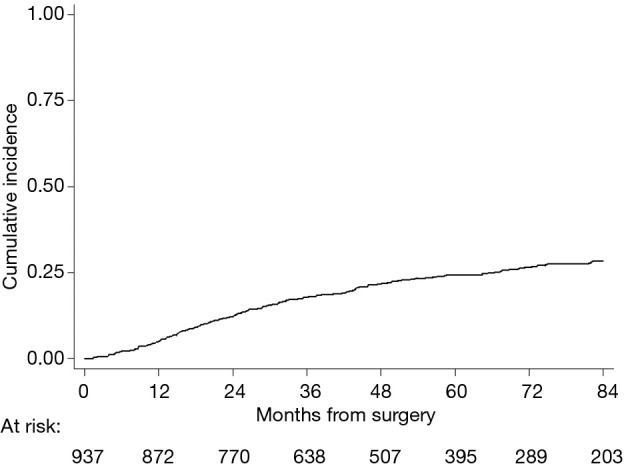
Cumulative incidence of recurrence.
Figure 3.
Cumulative incidence of recurrence. (A) According to type of surgical resection; (B) according to type of lymph node assessment. NS, lymph node sampling; LS-ND, lobe-specific lymph node dissection; SND, systematic nodal dissection.
Figure 4.
Cumulative incidence of recurrence according to treatment combination. NS, lymph node sampling; LS-ND, lobe-specific lymph node dissection; SND, systematic nodal dissection.
Multivariable-adjusted comparisons showed an independent negative effect of sub-lobar resection (HR =1.52; P=0.02; 95% CI: 1.07–2.17), LS-ND (HR =1.74; P=0.007; 95% CI: 1.16–2.6) and NS (HR =1.49; P=0.007; 95% CI: 1.12–1.98) on CIR. Test of interaction showed a homogeneity of results among subgroups (Table 2).
Table 2. Multivariable-adjusted fine and grey model for cumulative incidence of recurrence (N=969).
| Multivariable analysis* | Hazard ratio (95% CI) | P | Interaction P value |
|---|---|---|---|
| Sub-lobar resection vs. lobectomy | 0.02 | 0.268 | |
| Overall | 1.52 (1.07–2.17) | ||
| SND | 1.98 (1.14–3.42) | ||
| LS-ND | 1.87 (0.94–3.74) | ||
| NS | 1.08 (0.61–1.93) | ||
| LS-ND vs. SND | 0.007 | 0.903 | |
| Overall | 1.74 (1.16–2.6) | ||
| Lobectomy | 1.66 (1.03–2.69) | ||
| Sub-lobar resection | 1.58 (0.75–3.32) | ||
| NS vs. SND | 0.007 | 0.131 | |
| Overall | 1.49 (1.12–1.98) | ||
| Lobectomy | 1.61 (1.18–2.19) | ||
| Sub-lobar resection | 0.88 (0.43–1.82) |
*, take into account of age, gender, smoking habit, side of intervention, pTNM stage, vascular invasion, predominant histologic pattern and histologic grade. NS, lymph node sampling; LS-ND, lobe-specific lymph node dissection; SND, systematic nodal dissection.
Discussion
The central point in performing more complex surgery is to offer an oncological advantage that balances the possible additional risks due to the procedure itself. Accordingly, lobectomy and systematic nodal dissection are currently the cornerstone in the treatment of surgically resectable NSCLC. Nevertheless, in last years, several studies questioned the established standard of care, highlighted the possibility to perform parenchymal preserving resection (6-11) and less aggressive nodal assessment strategy (18,22,23) in stage I NSCLC. Indeed, the results presented by these papers are variegate and often contradictory. Moreover, the influence of the combination between type of surgical resection and of nodal assessment was rarely explored.
The results of our study conducted on a cohort of 969 stage I adenocarcinoma of the lung suggest that (I) patients submitted to lobectomy presented a significant lower recurrence rate than those submitted to sub-lobar resection, (II) systematic lymph node dissection (SND) presented an independent positive effect on recurrence development than other lymph node assessment strategy, (III) lobectomy in combination with systematic lymph nodal resection showed the best results in term of CIR.
The benefit of lobectomy over sub-lobar resection was established by the results of the study of the Lung Cancer Study Group, that represent the only multicenter randomized clinical trial on this subject published so far (5). This study, conducted by Ginsberg and Rubinstein, demonstrated an increase in overall death rate, cancer related death and locoregional recurrence rate in sub-lobar resection group. Similar results were published by various authors (6,9,11,24,25). Still, interest concerning convenience of anatomical segmentectomy and wedge resection in early stage NSCLC remains; numerous studies were conducted, assessing to find sub-group of patients that may take advantage from these procedures. As matter of fact, several groups described the equivalence in term of clinical outcomes of lobectomy and lobectomy performed by VATS (26-28). Similarly, a number of papers showed no significant difference in survival for tumours ≤2 cm in size (6-11). Interestingly, numerous authors reported a significant reduction of dissected lymph nodes in the segmentectomy group than in the lobectomy group (24,28-30).
Our results showed an independent significant advantage of lobectomy on respect of sub-lobar resection in term of recurrence rate. Moreover, this benefit remains independently from the type of lymph nodal assessment performed. Hopefully, the ongoing multicenter randomized studies currently conducted by Cancer and Leukemia Group B (CALGB 140503) and Japan Clinical Oncology Group (JCOG0802/WJOG4607L) (31-33) will solve this issue. Till then, sub-lobar resections, as alternative to lobectomy, should be proposed only in selected patients (34).
On the other hand, the IASLC staging project in the proposals for the revision of the N Descriptor in the 8th Edition of the TNM for Lung Cancer stated that “Nodal status is considered to be one of the most reliable indicators of the prognosis in patients with lung cancer and thus is indispensable in determining the optimal therapeutic options” (12). Undoubtable, systematic nodal dissection provides a higher accurate pathological staging accuracy, in reason to the higher number of lymph nodes resected (35). Moreover, systematic nodal dissection could consent the therapeutic excision of a minimal or unknown disease in mediastinal lymph nodes. However, new minimally invasive biopsy techniques (15,16) and high definition imaging rise doubts on unavoidability of such aggressive nodal assessment. Nevertheless, several studies reported an unexpected high occurrence of a node-positive occult disease in primary and secondary lung tumors (36-39) probably due to microscopic lymph nodal involvement. Contrariwise, this happened in less than 4% of cases ACOSOG Z0030 trial (23); in this study, systematic nodal dissection showed no survival benefit of over nodal sampling (NS). However, in this trial, all patients were submitted to NS and frozen section with the exclusion from the randomization for all patients with positive nodes; consequently, study conclusions could influenced be by this process. In addition, in recent years, some authors proposed a new strategy of nodal assessment, consisting in the resection of only stations which are considered as the natural drain for that lobe (17,18). Several studies compared the oncological results of this strategy with the ones of the systematic nodal dissection, mostly showing an equivalence in term of survival and recurrence rates.
Our analysis showed an independent benefit of systematic nodal dissection over both NS and lobe-specific nodal dissection. Furthermore, this benefit persists independently from the type of surgical resection performed. Accordingly, a recent metanalysis encompassed more than 1,900 patients, reported an improved OS in systematic nodal dissection group than in NS group (18).
Our study presents some limitations. First, the retrospective nature of the analysis relatively limits the strength of the results, and this should be considered by the readers when conferring clinical value to the reported evidence. Moreover, data collection from different centers represents an additional significant limitation; indeed, despite the centers do not substantially differ in the strategy of care adopted in current clinical practice, a series of confounding variables are unavoidable, and this bias potentially limiting the impact of our results. Concerning the type of surgical resection, we encompassed wedge and segmental resection as “sub-lobar resection”. On one hand this represents a statistical simplification and divers Authors have recently reported different recurrence rates between the two procedures; on the other hand, considering that lobectomy is still the gold standard even in node-negative NSCLC measuring less than 3 cm, the distinction between lobar and sub-lobar resection has a certain validity although it involves a consistent degree of approximation.
Conclusions
In the present series, lobectomy and SND confirmed to be the optimal strategy to achieve a favorable prognosis in stage I adenocarcinoma of the lung. The real value of sub-lobar resection and less aggressive nodal staging should be assessed by ongoing randomized clinical trial before being integrated in clinical practice.
Acknowledgements
None.
Appendix A.
Department of Thoracic Surgery, Azienda Ospedaliera Universitaria Città della Salute e della Scienza di Torino, Torino, Italy;
Department of Thoracic Surgery, IRCCS - Ospedale Santa Maria Nuova, Reggio Emilia, Italy;
Department of Thoracic Surgery, Amedeo Avogadro University of Eastern Piedmont, Novara, Italy;
Department of Thoracic Surgery, Azienda Ospedaliera Universitaria di Parma, Parma, Italy;
Department of Thoracic Surgery, University of Perugia Medical School, Perugia, Italy;
Department of Thoracic Surgery, San Luigi Hospital, Orbassano, Italy.
Appendix B.
Lobe-specific lymph node dissection encompassed the follow lymph node station according to IASCLC lymph node map (12):
Right upper lobar tumour: Stations 2, 4R and 7;
Left upper lobar tumour: Stations 5, 6 and 7;
Right and left middle or lower lobar tumour: Stations 7 and 9.
Figure S1.
Study flow diagram of the patient population.
Ethical Statement: The study was approved by the IRB of each participating center.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics. CA Cancer J Clin 2013;63:11-30. 10.3322/caac.21166 [DOI] [PubMed] [Google Scholar]
- 2.Available online: Seer.cancer.gov
- 3.Travis WD. Pathology of lung cancer. Clin Chest Med 2011;32:669-92. 10.1016/j.ccm.2011.08.005 [DOI] [PubMed] [Google Scholar]
- 4.NCCN Clinical practice guidelines in oncology. Non- Small Cell Lung Cancer. Version 8. 2017.
- 5.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22. 10.1016/0003-4975(95)00537-U [DOI] [PubMed] [Google Scholar]
- 6.Warren WH, Faber LP. Segmentectomy versus lobectomy in patients with stage I pulmonary carcinoma. Five-year survival and patterns of intrathoracic recurrence. J Thorac Cardiovasc Surg 1994;107:1087-93. [PubMed] [Google Scholar]
- 7.Okada M, Yoshikawa K, Hatta T, et al. Is segmentectomy with lymph node assessment an alternative to lobectomy for non-small cell lung cancer of 2 cm or smaller? Ann Thorac Surg 2001;71:956-60. 10.1016/S0003-4975(00)02223-2 [DOI] [PubMed] [Google Scholar]
- 8.Koike T, Yamato Y, Yoshiya K, et al. Intentional limited pulmonary resection for peripheral T1 N0 M0 small-sized lung cancer. J Thorac Cardiovasc Surg 2003;125:924-8. 10.1067/mtc.2003.156 [DOI] [PubMed] [Google Scholar]
- 9.El-Sherif A, Gooding WE, Santos R, et al. Outcomes of sublobar resection versus lobectomy for stage I nonsmall cell lung cancer: a 13-year analysis. Ann Thorac Surg 2006;82:408-15. 10.1016/j.athoracsur.2006.02.029 [DOI] [PubMed] [Google Scholar]
- 10.Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006;132:769-75. 10.1016/j.jtcvs.2006.02.063 [DOI] [PubMed] [Google Scholar]
- 11.Okumura M, Goto M, Ideguchi K, et al. Factors associated with outcome of segmentectomy for non-small cell lung cancer: long-term follow-up study at a single institution in Japan. Lung Cancer 2007;58:231-7. 10.1016/j.lungcan.2007.06.014 [DOI] [PubMed] [Google Scholar]
- 12.Asamura H, Chansky K, Crowley J, et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the N Descriptors in the Forthcoming 8th Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:1675-84. [DOI] [PubMed] [Google Scholar]
- 13.Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787-92. 10.1016/j.ejcts.2006.08.008 [DOI] [PubMed] [Google Scholar]
- 14.Bertoglio P, Renaud S, Guerrera F. Unless I see, I will not believe. J Thorac Dis 2017;9:2835-8. 10.21037/jtd.2017.08.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Navani N, Nankivell M, Lawrence DR, et al. Lung cancer diagnosis and staging with endobronchial ultrasoundguided transbronchial needle aspiration compared with conventional approaches: an open-label, pragmatic, randomised controlled trial. Lancet Respir Med 2015;3:282-9. 10.1016/S2213-2600(15)00029-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slavova-Azmanova NS, Lizama C, Johnson CE, et al. Impact of the introduction of EBUS on time to management decision, complications, and invasive modalities used to diagnose and stage lung cancer: a pragmatic pre-post study. BMC Cancer 2016;16:44. 10.1186/s12885-016-2081-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishiguro F, Matsuo K, Fukui T, et al. Effect of selective lymph node dissection based on patterns of lobe-specific lymph node metastases on patient outcome in patients with resectable non-small cell lung cancer: a large-scale retrospective cohort study applying a propensity score. J Thorac Cardiovasc Surg 2010;139:1001-6. 10.1016/j.jtcvs.2009.07.024 [DOI] [PubMed] [Google Scholar]
- 18.Mokhles S, Macbeth F, Treasure T, et al. Systematic lymphadenectomy versus sampling of ipsilateral mediastinal lymph-nodes during lobectomy for non-small cell lung cancer: a systematic review of randomized trials and a meta-analysis. Eur J Cardiothorac Surg 2017;51:1149-56. 10.1093/ejcts/ezw439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society: international multidisciplinary classification of lung adenocarcinoma: executive summary. Proc Am Thorac Soc 2011;8:381-5. 10.1513/pats.201107-042ST [DOI] [PubMed] [Google Scholar]
- 20.Cochran WG. The χ2 test of goodness of fit. Ann Math Stat 1952;25:315-45. 10.1214/aoms/1177729380 [DOI] [Google Scholar]
- 21.Gooley TA, Leisenring W, Crowley J, et al. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med 1999;18:695-706. [DOI] [PubMed] [Google Scholar]
- 22.Allen MS, Darling GE, Pechet TT, et al. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg 2006;81:1013-9. 10.1016/j.athoracsur.2005.06.066 [DOI] [PubMed] [Google Scholar]
- 23.Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662-70. 10.1016/j.jtcvs.2010.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landreneau RJ, Sugarbaker DJ, Mack MJ, et al. Wedge resection versus lobectomy for stage I (T1 N0 M0) non-small-cell lung cancer. J Thorac Cardiovasc Surg 1997;113:691-8. 10.1016/S0022-5223(97)70226-5 [DOI] [PubMed] [Google Scholar]
- 25.Mery CM, Pappas AN, Bueno R, et al. Similar long-term survival of elderly patients with non-small cell lung cancer treated with lobectomy or wedge resection within the Surveillance, Epidemiology, and End Results database. Chest 2005;128:237-45. 10.1378/chest.128.1.237 [DOI] [PubMed] [Google Scholar]
- 26.Sugi K, Kobayashi S, Sudou M, et al. Long-term prognosis of video-assisted limited surgery for early lung cancer. Eur J Cardiothorac Surg 2010;37:456-60. [DOI] [PubMed] [Google Scholar]
- 27.Zhong C, Fang W, Mao T, et al. Comparison of thoracoscopic segmentectomy and thoracoscopic lobectomy for small-sized stage IA lung cancer. Ann Thorac Surg 2012;94:362-7. 10.1016/j.athoracsur.2012.04.047 [DOI] [PubMed] [Google Scholar]
- 28.Yamashita S, Tokuishi K, Anami K, et al. Thoracoscopic segmentectomy for T1 classification of non-small cell lung cancer: a single center experience. Eur J Cardiothorac Surg 2012;42:83-8. 10.1093/ejcts/ezr254 [DOI] [PubMed] [Google Scholar]
- 29.Hwang Y, Kang CH, Kim HS, et al. Comparison of thoracoscopic segmentectomy and thoracoscopic lobectomy on the patients with non-small cell lung cancer: a propensity score matching study. Eur J Cardiothorac Surg 2015;48:273-8. 10.1093/ejcts/ezu422 [DOI] [PubMed] [Google Scholar]
- 30.Landreneau RJ, Normolle DP, Christie NA, et al. Recurrence and Survival Outcomes After Anatomic Segmentectomy Versus Lobectomy for Clinical Stage I Non–Small-Cell Lung Cancer: A Propensity-Matched Analysis. J Clin Oncol 2014;32:2449-55. 10.1200/JCO.2013.50.8762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blasberg JD, Pass HI, Donington JS. Sublobar resection: a movement from the Lung Cancer Study Group. J Thorac Oncol 2010;5:1583-93. 10.1097/JTO.0b013e3181e77604 [DOI] [PubMed] [Google Scholar]
- 32.Nakamura K, Saji H, Nakajima R, et al. A phase III randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607L). Jpn J Clin Oncol 2010;40:271-4. 10.1093/jjco/hyp156 [DOI] [PubMed] [Google Scholar]
- 33.Guerrera F, Renaud S, Tabbò F, et al. How to design a randomized clinical trial: tips and tricks for conduct a successful study in thoracic disease domain. J Thorac Dis 2017;9:2692-6. 10.21037/jtd.2017.06.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rami-Porta R, Tsuboi M. Sublobar resection for lung cancer. Eur Respir J 2009;33:426-35. 10.1183/09031936.00099808 [DOI] [PubMed] [Google Scholar]
- 35.Guerrera F, Errico L, Evangelista A, et al. Exploring Stage I non-small-cell lung cancer: development of a prognostic model predicting 5-year survival after surgical resection†. Eur J Cardiothorac Surg 2015;47:1037-43. 10.1093/ejcts/ezu410 [DOI] [PubMed] [Google Scholar]
- 36.Bille A, Woo KM, Ahmad U, et al. Incidence of occult pN2 disease following resection and mediastinal lymph node dissection in clinical stage I lung cancer patients. Eur J Cardiothorac Surg 2017;51:674-9. 10.1093/ejcts/ezw400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guerrera F, Renaud S, Tabbó F, et al. Epidermal growth factor receptor mutations are linked to skip N2 lymph node metastasis in resected non-small-cell lung cancer adenocarcinomas. Eur J Cardiothorac Surg 2017;51:680-8. 10.1093/ejcts/ezw362 [DOI] [PubMed] [Google Scholar]
- 38.Guerrera F, Renaud S, Schaeffer M, et al. Low Accuracy of Computed Tomography and Positron Emission Tomography to Detect Lung and Lymph Node Metastases of Colorectal Cancer. Ann Thorac Surg 2017;104:1194-9. 10.1016/j.athoracsur.2017.05.002 [DOI] [PubMed] [Google Scholar]
- 39.Decaluwé H, Petersen RH, Brunelli A, et al. Multicentric evaluation of the impact of central tumour location when comparing rates of N1 upstaging in patients undergoing video-assisted and open surgery for clinical Stage I non-small-cell lung cancer. Eur J Cardiothorac Surg 2017. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]