Abstract
Background
Anti-PD/PD-L1-targeted immunotherapy is associated with remarkably high rates of durable clinical responses in patients across a range of tumor types, although their high incidence of skin, gastrointestinal, and endocrine side effects with their use. The risk of pneumonitis associated with checkpoint inhibition therapy is not well described.
Methods
A systematic review of the literature was conducted on randomized clinical trials (RCTs) comparing anti-PD/PD-L1 mono-immunotherapy (IMM) to chemotherapy (CTH) protocols in cancer patients. The primary endpoint was the pneumonitis rate in IMM compared to CTH. Secondary endpoints were (I) high-grade pneumonitis rate in IMM compared to CTH and (II) tumor response rate, progression-free survival (PFS), and overall survival (OS) between IMM and CTH. Random model and leave-one-out-analysis were performed.
Results
Thirteen RCTs studying 7,246 patients were included; 3,704 (51.12%) patients in the IMM arm and 3,542 (48.88%) patients in the chemotherapy arm. Seven non-small cell lung cancer (NSCLC) RCTs were included with 4,164 patients; 2,101 in the IMM arm and 2,063 patients in the CTH arm. Three RCTs were on melanoma patients (n=1,390). Nine RCTs compared mono-immunotherapy to CTH [docetaxel in 5 studies (38.5%), platinum-based in 2 studies (15.4%), dacarbazine in 1 study (7.7%) and everolimus in 1 study]. Both high-grade and all-grade pneumonitis were higher among patients in the IMM arm when compared to the CTH arm (OR =4.39, 95% CI: 1.65–11.69, P=0.003 and OR =2.46, 95% CI: 1.29–4.6, P=0.007). Tumor response rate was significantly better in the immunotherapy arm (OR =2.31, 95% CI: 1.62–3.29, P<0.001). PFS and OS were longer in patients who received IMM compared to patients in the CTH arm (HR =0.75, 95% CI: 0.65–0.85, P<0.001, and HR =0.71, 95% CI: 0.66–0.77, P<0.001).
Conclusions
The incidence of high-grade and all-grade pneumonitis is higher in anti-PD-1 therapy but not in anti-PD-L1 therapy when compared to traditional CTH regimens for NSCLC and melanoma. High-grade adverse events were otherwise more common in the CTH arm. Tumor response rate, PFS, and OS are all substantially improved with IMM over CTH. These results can be used to guide therapy selection and set expectations for treatment effect in these patients.
Keywords: Immunotherapy, pneumonitis, pembrolizumab, survival, response
Introduction
Checkpoint inhibition therapy, specifically antibodies targeting programmed cell death protein 1 (PD-1) and PD-L1 on lymphocytes and tumor cells, plays an important role in downregulating immunologic tumor escape mechanisms (1). Although these therapies have shown remarkable success in treatment of various malignancies including NSCLC and melanoma (2,3), anti-PD-1 and anti-PD-L1 have unique toxic effects which are referred to as immune-related adverse events (IRAEs) (4). Although multiple organ system involvement has been reported, pneumonitis in particular has emerged as a relatively uncommon but serious and potentially life-threatening IRAE resulting in pneumonitis-related deaths in Phase I trials (4).
Pneumonitis is defined as inflammation of the lung parenchyma, and has been described in <10% of patients receiving anti-PD-1/PD-L1 therapy either alone or in combination, and appears to occur more commonly in patients with lung cancer (5,6). In this study, we aimed to analyze all grades of pneumonitis in anti-PD-1/anti-PD-L1 treated patients in comparison to standardized chemotherapy (CTH) protocols.
Methods
Search strategy and study selection
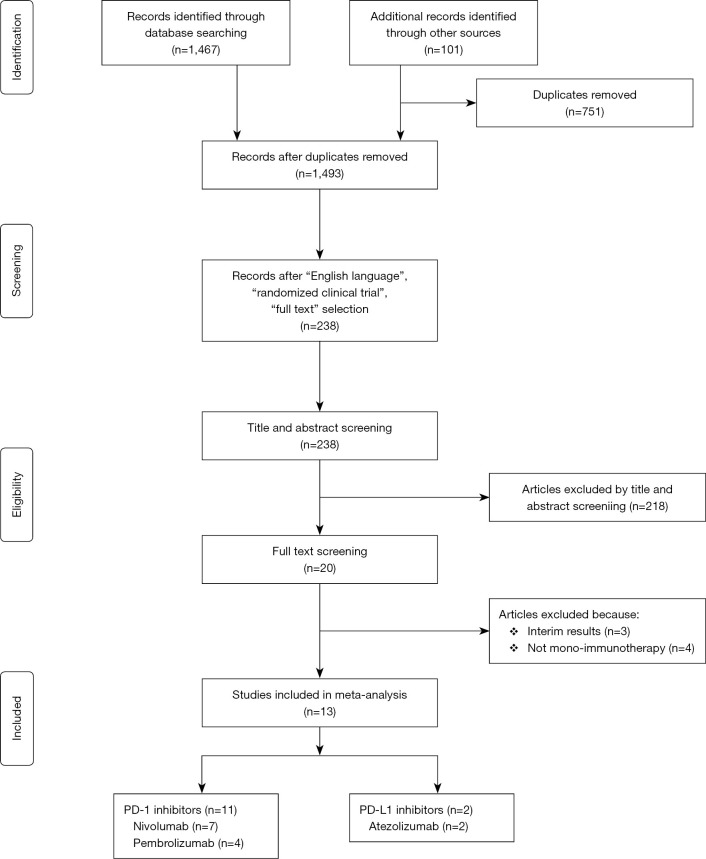
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (7) (Figure S1). In March 2018, the PubMed, MEDLINE and EMBASE databases were searched for publications containing anti-PD-1 and anti-PD-L1 immunotherapy (IMM) by the words “Nivolumab” OR “Pembrolizumab” OR “Atezolizumab” OR “Durvalumab” OR “Avelumab” OR “BMS936559” OR “Pidilizumab” that were obtained from a previously published review (8). All studies comparing mono-immunotherapy versus other single/multiple treatments that reported all grades of pneumonitis were included. The bibliography of all studies and related meta-analyses were searched to identify further articles that could potentially be recruited, i.e., backward snowballing.
Figure S1.
PRISMA of our meta-analysis. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Inclusion criteria were phase II or III comparative randomized clinical trials (RCTs) that had two arms (in the form of IMM vs. CTH/targeted therapy). These studies reported pulmonary complications including pneumonitis, pneumonia, interstitial lung disease, pleural effusion, and aspiration pneumonia. Exclusion criteria were ongoing trials; non-comparative RCT, phase I RCT, RCT with monotherapy/single arm or dose-escalation trials, RCT with three or more comparative arms, two-armed studies but in the form of IMM vs. IMM or IMM vs. Placebo, less than 50 patients, no pulmonary complication reported, non-English articles, and no full-text available.
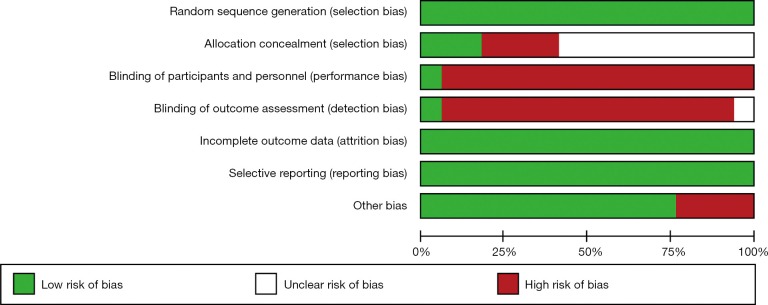
Two authors (Massimo Baudo and Mohamed Rahouma) independently reviewed the electronic reports identified by the searches. In case of discrepancies, they were resolved by the 3rd author’s (Mario Gaudino) opinion and consensus meeting. The quality of included studies was assessed using The Cochrane Collaboration’s tool for assessing risk of bias in RCTs (9) (Figure S2).
Figure S2.
Risk of bias in RCTs assessed by using the Cochrane Collaboration’s tool. RCTs, randomized clinical trials.
Study outcomes
The primary endpoint was the pneumonitis rate in IMM compared to CTH. Secondary endpoints were (I) high-grade pneumonitis rate in IMM compared to CTH and (II) tumor response rate, progression-free survival (PFS), and overall survival (OS) between IMM and CTH. Subgroup analyses were conducted for the occurrence of pneumonitis based on the cancer type [NSCLC, melanoma and others (including head & neck, renal cell carcinoma and urothelial carcinoma)] and immunotherapy treatment type (anti-PD1 or anti-PD-L1). High grade adverse events were defined as grade 3 (severe complications), grade 4 (life threatening complications), and grade 5 (death) as reported by National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAEv.4) (10).
Data extraction and statistical analysis
Microsoft Office Excel 2010 program (Microsoft, Redmond, Washington) was used for data extraction. Data were expressed in the same way they were expressed in the included studies (i.e., frequency and percentage for categorical variables and mean ± standard deviation or median and range (or interquartile range) for continuous variables).
Data on study design, study period, country, study center, trial phase (II or III), cancer type, comparison arms, doses of drug administered, inclusion/exclusion criteria, treatments arms, sample size, PD-L1 tumor cell expression percent groups, pathology and post-immunotherapy surgery, were retrieved. The following patient characteristics were registered: age, sex, smoking, Eastern Cooperative Oncology Group Performance Status (ECOG-PS), stage, response rates, PFS, OS, pneumonitis (see earlier; all grade/high grade), response (complete and partial responders, using Response Evaluation Criteria in Solid Tumor (RECIST) criteria, were considered as responders), and all-cause mortality.
All-cause mortalities were derived from the natural logarithm of the provided hazard ratio (HR); the standard error (SE) was derived from the 95% confidence interval (95% CI) or log rank P value (11). Odds ratios (ORs) with 95% CI for pneumonitis events were calculated by means of the DerSimonian-Laird [inverse variance (IV)] method (12). Relative risk [risk ratio (RR)] with 95% CI was similarly calculated for events with incidence higher than 10% to avoid exaggeration of the risk (13). Random-effect model was used for statistical outcome pooling, computing risk estimates with CI.
Funnel plots were used for assessment of publication bias by graphical inspection. Hypothesis testing for equivalence was set at the two-tailed 0.05 level. Hypothesis testing for statistical homogeneity was set at the two-tailed 0.10 level and was based on the Cochran Q test, with I2 values of 0–25%, 26–50%, and 51–100% representing low, moderate, and high heterogeneity, respectively (14).
Sensitivity analysis using “leave one out analysis” and meta-regression were performed and results were reported as regression coefficient (i.e., Beta). Variables included in meta-regression were age, gender, performance status (PS), smokers and radiotherapy.
This meta-analysis was performed using meta and metafor packages in R (version 3.3.3 R Project for Statistical Computing). Review Manager Version 5.3 (The Cochrane Collaboration, The Nordic Cochrane Centre and Copenhagen, Denmark) was used to perform the risk of bias assessment.
Results
Eligible studies and characteristics of studies
An outline of the systematic review process is shown in Figure S1. For clinical outcomes, 1,568 studies were identified. After removal of duplicates, 1,493 studies were screened. Twenty full text articles were assessed for eligibility. Thirteen RCTs met our inclusion criteria with 7,246 patients included [3,704 (51.12%) in the IMM arm and 3,542 (48.88%) patients in the other arm]. Seven NSCLC RCTs were included with 4,164 patients (2,101 in the IMM arm and 2,063 patients in the other arm). Three RCTs were on melanoma patients (n=1,390). The RCTs compared mono-immunotherapy to CTH (Docetaxel in 5 of them, Platinum-based in 2 studies, Dacarbazine in 1 study and Everolimus in 1 study), while the remaining studies reported different investigator’s choice of chemotherapy (Tables 1,2). The pooled mean follow-up was 10.9 and 8.9 months in the IMM and CTH arms, respectively. All-grade pneumonitis occurred in 3.13% of the IMM arm compared to 2.06% in the CTH arm, while high-grade pneumonitis occurred in 1.32% of the IMM arm compared to 0.45% in the CTH arm. Pneumonitis-related mortality occurred in 0.17% among the IMM compared to 0% among the CTH group. Among chemotherapy regimens, everolimus had the highest percentage of patients developing all-grade pneumonitis (14.61%), followed by docetaxel (0.52%), then platinum-based CTH (0.24%) and dacarbazine (0%). in contrast to the IMM. Similarly, everolimus had the highest rate of high-grade pneumonitis (2.77%), followed by platinum-based CTH and docetaxel (0.24% vs. 0.17%), then dacarbazine (0%), in contrast to the IMM (Table S1).
Table 1. Criteria of the included studies.
| Author | Year | Study period | Country | Center | Study type | Trial phase | Cancer type | Comparison arms (IMM vs. CTH) | Doses of drug administration¶ |
|---|---|---|---|---|---|---|---|---|---|
| Fehrenbacher (15) | 2016 | 2013–2014 | International | Multicenter | RCT | Phase II | NSCLC | Atezolizumab vs. Docetaxel | Atezolizumab 1,200 mg vs. Docetaxel 75 mg/m2 |
| Brahmer (2) | 2015 | 2012–2013 | International | Multicenter | RCT | Phase III | Squamous cell NSCLC | Nivolumab vs. Docetaxel | Nivolumab 3 mg/kg vs. Docetaxel 75 mg/m2 |
| Borghaei (16) | 2015 | 2012–2013 | International | Multicenter | RCT | Phase III | NSCLC | Nivolumab vs. Docetaxel | Nivolumab 3 mg/kg vs. Docetaxel 75 mg/m2 |
| Motzer (17) | 2016 | 2012–2014 | International | Multicenter | RCT | Phase III | Renal-cell carcinoma with a clear-cell component | Nivolumab vs. Everolimus | Nivolumab 3 mg/kg vs. Everolimus 10 mg |
| Reck (18) | 2016 | 2014–2015 | International | Multicenter | RCT | Phase III | PD-L1-positive NSCLC | Pembrolizumab vs. Platinum based CHT | Pembrolizumab 200 mg vs. the investigator’s choice of one of 5 platinum-based CTH regimens for 4 to 6 cycles: carboplatin + pemetrexed, cisplatin + pemetrexed, carboplatin + gemcitabine, cisplatin + gemcitabine, or carboplatin + paclitaxel |
| Robert (3) | 2014 | 2013–2014 | International | Multicenter | RCT | Phase III | Melanoma | Nivolumab vs. Dacarbazine | Nivolumab 3 mg/kg vs. Dacarbazine 1,000 mg/m2 |
| Weber (19) | 2015 | 2012–2014 | International | Multicenter | RCT | Phase III | Melanoma | Nivolumab vs. ICC (either dacarbazine or carboplatin + paclitaxel) | Nivolumab 3 mg/kg vs. ICC (either dacarbazine 1,000 mg/m2 or carboplatin area under the curve 6 + paclitaxel 175 mg/m2 |
| Ferris (20) | 2016 | 2014–2015 | International | Multicenter | RCT | Phase III | Head and neck squamous cell carcinoma | Nivolumab vs. Standard, single-agent systemic therapy (methotrexate, docetaxel or cetuximab) | Nivolumab 3 mg/kg every 2 weeks. Standard therapy either methotrexate 40 to 60 mg/m2 or docetaxel 30 to 40 mg/m2 or cetuximab 250 mg/m2 after a loading dose of 400 mg/m2 |
| Bellmunt (21) | 2017 | 2014–2015 | International | Multicenter | RCT | Phase III | Urothelial Carcinoma | Pembrolizumab vs. investigator's choice of chemotherapy between paclitaxel, docetaxel or vinflunine | Pembrolizumab 200 mg vs. paclitaxel (at a dose of 175 mg/m2), docetaxel (at a dose of 75 mg/m2), or vinflunine (at a dose of 320 mg/m2) |
| Hamid (22) | 2017 | 2012–2013 | International | Multicenter | RCT | Phase II | Advanced melanoma | Pembrolizumab vs. investigator's choice of chemotherapy between carboplatin, carboplatin + paclitaxel, dacarbazine, paclitaxel alone or oral temozolomide | Pembrolizumab 2 mg/kg |
| Rittmeyer (23) | 2017 | 2014 | International | Multicenter | RCT | Phase III | Squamous and NSCLC | Atezolizumab vs. docetaxel | Atezolizumab 1,200 mg or docetaxel 75 mg/m2 |
| Herbst (24) | 2016 | 2013–2015 | International | Multicenter | RCT | Phase II/III | Lung cancers | Pembrolizumab vs. Docetaxel | Pembrolizumab 2 mg/kg vs. docetaxel 75 mg/m2 |
| Carbone (25) | 2017 | 2014–2015 | International | Multicenter | RCT | Phase III | NSCLC | Nivolumab vs. Platinum-based CTH | Nivolumab IV at a dose of 3 mg per kilogram of body weight vs. platinum-based chemotherapy |
¶, name of chemotherapy and number of studies details—Docetaxel: 5, Platinum-based: 2, Everolimus: 1, Dacarbazine: 1, Either Dacarbazine or Carboplatin+ Paclitaxel: 1, Either Methotrexate or Docetaxel: 1, Either Paclitaxel, Docetaxel or Vinflunine: 1, Either Carboplatin plus Paclitaxel, Dacarbazine, Paclitaxel alone or oral Temozolomide: 1. CTH, chemotherapy; IMM, immunotherapy; NSCLC, non-small cell lung cancer; RCT, randomized clinical trial.
Table 2. Criteria of the included studies.
| Author | Inclusion criteria | Exclusion criteria | PDL tumor cell expression % groups | Pathology: IMM vs. CTH | Surgery |
|---|---|---|---|---|---|
| Fehrenbacher (15) | Progression post-platinum chemotherapy ≥18 yrs, ECOG 0 or 1, measurable disease by RECIST v1.1, adequate end-organ function, provided tumor specimens for central PD-L1 testing on FFPE sections before enrolment | CNS metastases, history of pneumonitis, autoimmune or chronic viral disease, or previous treatment with docetaxel, CD137 agonists, immune check point inhibitors | 0: 96 (67%); 1: 19 (13%); 2: 14 (10%); 3: 15 (10%) |
Non-squamous 66% each; squamous 34% each |
NR |
| Brahmer (2) | Stage IIIB or IV squamous-cell NSCLC with recurrence after one prior platinum-containing regimen, ≥18 yrs, ECOG PS of 0 or 1, a pretreatment tumor-tissue specimen for biomarker analyses, treated, stable brain metastases. Prior maintenance therapy, including an EGFR-TKI, was allowed | Autoimmune disease, symptomatic interstitial lung disease, systemic immunosuppression, prior therapy with T-cell co-stimulation or checkpoint-targeted agents, or prior docetaxel therapy, received >1 prior systemic therapy for metastatic disease | <1%: 54 (40%); ≥1%: 63 (47%); <5%: 75 (56%); ≥5%: 42 (31%); <10%: 81 (60%); ≥10%: 36 (27%); not evaluable: 18 (13%) |
Squamous-cell NSCLC 100% | Prior surgery: 69 51% vs. 56% |
| Borghaei (16) | Stage IIIB or IV or recurrent non-squamous NSCLC after radiation or surgical resection and had recurrence or progression with 1 prior platinum-based doublet CTH, ≥18 years old, ECOG-PS of 0 or 1, adequate hematologic, hepatic, and renal function and stable CNS metastases | Autoimmune disease, symptomatic interstitial lung disease, systemic immunosuppression, prior treatment with immune-stimulatory antitumor agents including checkpoint-targeted agents, and prior use of docetaxel | <1%: 108 (47%); ≥1%: 123 (53%); <5%: 136 (59%); ≥5%: 95 (41%); <10%: 145 (63%); ≥10%: 86 (37%); not quantifiable: 61 (21%) |
Adenocarcinoma: 92% vs. 94%; large cell carcinoma: 2% vs. 2%; bronchoalveolar Ca: 2% vs. 0%; other 4% vs. 3% |
Prior surgery: 69% vs. 75% |
| Motzer (17) | Adult, advanced or metastatic renal-cell carcinoma with a clear-cell component and measurable disease according to RECIST version 1.1, received 1–2 antiangiogenic therapy. No >3 previous regimens of systemic therapy and disease progression during or after the last treatment and within 6 months before study enrollment. All patients had a Karnofsky performance status of at least 70 | CNS metastasis, previous mTOR inhibitor or glucocorticoids treatment | ≥1%: 94 (25%); <1%: 276 (75%); ≥5%: 44 (12%); <5%: 326 (88%); non quantifiable: 40 (10%) |
Advanced or metastatic renal-cell carcinoma with a clear-cell component (100%) | Previous nephrectomy: 364 (89%) vs. 359 (87%) |
| Reck (18) | ≥18 years old, stage IV NSCLC, no previous systemic therapy for metastatic disease, ECOG-PS of 0 or 1, one measurable lesion (RECIST version 1.1), life expectancy ≥3 months, a PD-L1 tumor proportion score of ≥50%. | Systemic glucocorticoids, other immunosuppressive treatment, brain metastases, active autoimmune disease, active interstitial lung disease, or a history of pneumonitis for which they had received glucocorticoids | PD-L1 tumor proportion score ≥50% | Squamous: 18.8% vs.17.9%; non-squamous: 81.2% vs. 82.1% |
NR |
| Robert (3) | Confirmed, unresectable, previously untreated stage III or IV melanoma without a BRAF mutation, ≥18 years old, ECOG-PS of 0 or 1, and the availability of tumor tissue for PD-L1 analysis | Brain metastases, uveal melanoma or serious autoimmune disease | Positive (at least 5%): 74 (35.2%); negative or indeterminate: 64.8%) |
Metastatic melanoma without BRAF mutation 100% | Subsequent surgery: 8.6% vs. 12% |
| Weber (19) | ≥18 years old, histologically confirmed, unresectable stage IIIC or IV metastatic melanoma, ECOG PS of 0 or 1, BRAF wild-type tumors with progression after anti-CTLA-4 treatment, BRAFV600 mutation-positive tumor mutation with progression on anti-CTLA-4 treatment and a BRAF inhibitor, WBCs ≥2,000×109/L; neutrophil count ≥1,500×109/L; platelet count of 100×109/L; hemoglobin ≥90 g/L, and adequate end-organ function, | Active brain metastases, previous treatment with immune check point inhibitors, grade 4 toxic effects, or infliximab usage to manage adverse events from previous ipilimumab treatment; a primary ocular melanoma, grade 4 laboratory test abnormality, active, or suspected autoimmune disease, active brain or leptomeningeal metastasis, grade 2 or worse eye pain or reduction of visual activity related to previous anti-CTLA-4 treatment, and previous malignancies | Pretreatment PD-L1-positive (≥5% staining); 49% | Advanced melanoma 100% | NR |
| Ferris (20) | ≥18 years old, recurrent squamous-cell carcinoma of the head and neck (including metastatic disease) of the oral cavity, pharynx, or larynx that was not amenable to curative treatment; tumor progression or recurrence within 6 months after the last dose of platinum-containing chemotherapy; an ECOG performance-status score of 0 or 1; adequate bone marrow, hepatic, and renal function; and measurable disease according to RECIST version 1.1 | Active brain metastases, autoimmune disease, or systemic immunosuppression; known human immunodeficiency virus or hepatitis B or C virus infection; and previous therapy targeting T-cell costimulating or immune checkpoint pathways | ≥1%: 88 (36.7%); ≥5%: 54 (22.5%); ≥10%: 43 (17.9%); <1%: 73 (30.4%); <5%: 107 (44.6%); <10%: 118 (49.2%) |
Squamous cell carcinoma (100%) | NR |
| Bellmunt (21) | ≥18 years old, urothelial carcinoma of the renal pelvis, ureter, bladder, or urethra that showed predominantly transitional-cell, had progression after platinum-based chemotherapy for advanced disease or recurrence within 12 months after the receipt of platinum-based adjuvant or neoadjuvant therapy for localized muscle-invasive disease, had received ≤2 lines of systemic chemotherapy for advanced disease previously, had at least one measurable lesion according to RECIST version 1.1 and had performance status score of 0, 1, or 2 | Prior anti-PD-1, anti-PD-L1, or anti-CTLA-4 therapy | Tumor PD-L1 combined positive score ≥10%: 74 (28.5%) | Pure transitional-cell features in histologic testing: 186/270 (68.9%) vs. 197/270 (73.0%) | NR |
| Hamid (22) | ≥18 years old and unresectable stage III or stage IV melanoma not amenable to local therapy; confirmed disease progression within 24 weeks of last ipilimumab dose; previous BRAF or MEK inhibitor therapy or both (if BRAFV600 mutant-positive); resolution or improvement of ipilimumab-related adverse events to grade 0–1 and prednisone dose 10 mg/day or less for at least 2 weeks before the first dose of study drug and ECOG performance status 0 or 1 | Active brain metastases or carcinomatous meningitis, active autoimmune disease, active infection requiring systemic therapy, known history of HIV infection, active hepatitis B virus or hepatitis C virus infection, a history of grade 4 ipilimumab-related adverse events or grade 3 ipilimumab-related adverse events lasting longer than 12 weeks, or previous treatment with any other anti-PD-1 or anti-PD-L1 therapy | PD-L1 status— IMM (2 mg/kg) vs. IMM (10 mg/kg) vs. CTH; Positive: 99 (55.0%) vs. 97 (53.6%) vs. 98 (54.7%); Negative: 48 (26.7%) vs. 46 (25.4%) vs. 40 (22.3%); Unknown: 33 (18.3%) vs. 38 (21.0%) vs. 41 (22.9%) |
Advanced melanoma (100%) | NR |
| Rittmeyer (23) | Squamous or non-squamous non-small-cell lung cancer, 18 years or older, measurable disease per RECIST; version 1.1, and ECOG of 0 or 1. Patients had received 1–2 previous cytotoxic chemotherapy regimens for stage IIIB or IV NSCLC | Autoimmune disease and prior treatments with docetaxel, CD137 agonists, anti-CTLA4, or therapies targeting the PD-L1 and PD-1 pathway | PD-L1 subgroups— TC3 or IC3: 72 (17%) vs. 65 (15%); TC2/3 or IC2/3: 129 (30%) vs. 136 (32%); TC1/2/3 or IC1/2/3: 241 (57%) vs. 222 (52%); TC0 and IC0: 180 (42%) vs. 199 (47%) |
Non-squamous: 313 (74%) vs. 315 (74%); squamous: 112 (26%) vs. 110 (26%) | NR |
| Herbst (24) | ≥18 years, with progression as per RECIST; version 1.1 after ≥2 cycles of platinum-doublet chemotherapy, as well as an appropriate TKI for those with an EGFR-sensitizing mutation or ALK gene rearrangement; measurable disease as per RECIST; ECOG of 0 or 1; provision of a tumour sample; and PD-L1 expression on at least 1% of tumour cells | Previous treatment with PD-1 checkpoint inhibitors or docetaxel, known active brain metastases or carcinomatous meningitis, active autoimmune disease requiring systemic steroids, and interstitial lung disease or history of pneumonitis requiring systemic steroids | >50% 139 (40%); 1–49% 205 (60%) |
Squamous 76 (22%) vs. 66 (19%); non-squamous 240 (70%) vs. 240 (70%); other 9 (3%) vs. 10 (3%); unknown 19 (6%) vs. 27 (8%) |
NR |
| Carbone (25) | Squamous-cell or non-squamous stage IV or recurrent NSCLC, ECOG performance-status score of 0 or 1, and measurable disease according to RECIST version 1.1, had received no previous systemic anti- cancer therapy as primary therapy for advanced or metastatic disease, not be taking glucocorticoids. Previous palliative radiotherapy and previous adjuvant or neoadjuvant chemotherapy. Patients with CNS metastases were eligible if they had been adequately treated and had been asymptomatic for at least 2 weeks before randomization | Autoimmune disease or known EGFR mutations or ALK translocations that were sensitive to available targeted therapy | PD-L1 expression level— ≥5%: 208 (77%) vs. 210 (78%); ≥50%: 88 (32%) vs. 126 (47%) |
Squamous cell carcinoma: 66 (24%) vs. 64 (24%); non-squamous cell carcinoma: 205 (76%) vs. 206 (76%) |
NR |
CNS, central nervous system; FFPE, formalin-fixed paraffin-embedded; LDH, lactic acid dehydrogenase; PS, performance status, RECIST, Response Evaluation Criteria in Solid Tumors; ULN, upper limit of the normal range; WBC, white blood cells.
Table S1. Literature search strategies in Embase 1974 to 2018 March 10, Ovid MEDLINE(R), Epub Ahead of Print, In-Process & Other Non-Indexed Citations and Daily 1946 to March 10, 2018 and PubMed¶.
| Searches | Results |
|---|---|
| anti-pdl1.mp. [mp=ti, ab, hw, tn, ot, dm, mf, dv, kw, fx, dq, nm, kf, px, rx, an, ui, sy] | 425 |
| anti-pd1.mp. [mp=ti, ab, hw, tn, ot, dm, mf, dv, kw, fx, dq, nm, kf, px, rx, an, ui, sy] | 2,226 |
| nivolumab.mp. [mp=ti, ab, hw, tn, ot, dm, mf, dv, kw, fx, dq, nm, kf, px, rx, an, ui, sy] | 12,629 |
| pembrolizumab.mp. [mp=ti, ab, hw, tn, ot, dm, mf, dv, kw, fx, dq, nm, kf, px, rx, an, ui, sy] | 9,956 |
| Atezolizumab.mp. [mp=ti, ab, hw, tn, ot, dm, mf, dv, kw, fx, dq, nm, kf, px, rx, an, ui, sy] | 2,883 |
| Durvalumab.mp. [mp=ti, ab, hw, tn, ot, dm, mf, dv, kw, fx, dq, nm, kf, px, rx, an, ui, sy] | 1,952 |
| Avelumab.mp. [mp=ti, ab, hw, tn, ot, dm, mf, dv, kw, fx, dq, nm, kf, px, rx, an, ui, sy] | 1,235 |
| Pidilizumab.mp. [mp=ti, ab, hw, tn, ot, dm, mf, dv, kw, fx, dq, nm, kf, px, rx, an, ui, sy] | 415 |
| BMS936559.mp. [mp=ti, ab, hw, tn, ot, dm, mf, dv, kw, fx, dq, nm, kf, px, rx, an, ui, sy] | 4 |
| 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 | 20,350 |
| limit 10 to English language | 19,825 |
| limit 11 to full text | 1,137 |
| limit 12 to humans | 1,024 |
¶, PubMed code that we used for the literature search: “anti-pdl1”[All Fields] OR “anti-pd1”[All Fields] OR “nivolumab”[All Fields] OR “pembrolizumab”[All Fields] OR “Atezolizumab”[All Fields] OR “Durvalumab”[All Fields] OR “Avelumab”[All Fields] OR “Pidilizumab”[All Fields] OR “BMS936559”[All Fields]. Then, from these results we excluded progressively according to language and type of article as described in our PRISMA chart.
Meta-analysis of the outcomes
Pneumonitis in immunotherapy
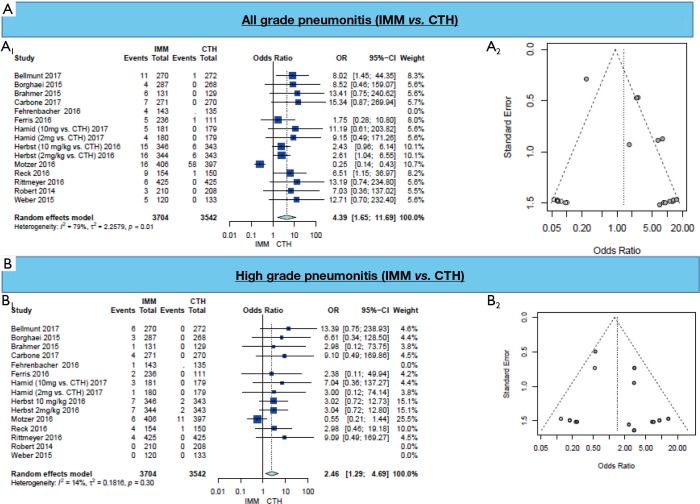
A higher rate of all-grade pneumonitis was found in all immunotherapy arms (OR =4.39, 95% CI: 1.65–11.69, P=0.003) (Figure 1A, Table 3). On subgroup analysis, the occurrence of all-grade pneumonitis was highest among patients with NSCLC (OR =3.54, 95% CI: 2.02–6.22) and melanoma (OR =9.82, 95% CI: 2.27–42.42), but not in head & neck, renal cell carcinoma and urothelial carcinoma patients (OR =1.62, 95% CI: 0.12–21.11). Subgroup analysis of immunotherapy type (anti-PD-1, nivolumab or pembrolizumab or anti-PD-L1, atezolizumab) showed that only anti-PD-1 treatment is associated with higher all-grade pneumonitis (OR =4.11, 95% CI: 1.50–11.22) (Table 3).
Figure 1.
Results of meta-analysis of pneumonitis grades. (A) All grades pneumonitis (A1; Forest plot, A2; funnel plot) and (B) high grades pneumonitis (B1; Forest plot, B2; funnel plot).
Table 3. Summary of outcomes among our work.
| Outcome or subgroup | Studies | Participants | Effect estimate (95% CI) | Heterogeneity | Overall effect | Higher in |
|---|---|---|---|---|---|---|
| All-grade pneumonitis | 14¶ | 7,246 | OR =4.39 (1.65, 11.69) | I2=79%, P<0.001 | Z=2.96, P=0.003 | IMM |
| Lung cancer | 7 | 4,164 | OR =3.54 (2.02, 6.22) | I2=0% | – | IMM |
| Melanoma | 4 | 1,692 | OR =9.82 (2.27, 42.42) | I2=0% | – | IMM |
| Others | 3 | 1,390 | OR =1.62 (0.12, 21.11) | I2=87.3% | – | None |
| Anti-PD-1Ϯ | 13¶ | 6,118 | OR =4.11 (1.50, 11.22) | I2=79.7% | – | IMM |
| Anti-PD-L1Ϯ | 1 | 850 | OR =13.19 (0.74, 234.80) | – | – | None |
| High-grade pneumonitis* | 12 | 7,246 | OR =2.46 (1.29, 4.69) | I2=14.4%, P=0.3037 | Z=2.72, P=0.007 | IMM |
| Lung cancer | 7 | 4,164 | OR =3.70 (1.72, 7.96) | I2=0% | – | IMM |
| Melanoma | 2 | 1,692 | OR =4.75 (0.54, 41.97) | I2=0% | – | None |
| Others | 3 | 1,390 | OR =1.78 (0.24, 12.98) | I2=57.9% | – | None |
| Anti-PD-1Ϯ | 11 | 6,118 | OR =2.32 (1.19, 4.51) | I2=15.4% | – | IMM |
| Anti-PD-L1Ϯ | 1 | 850 | OR =9.09 (0.49, 169.27) | – | – | None |
| High-grade treatment related morbidities | 15 | 7,160 | OR =0.31 (0.24, 0.40) | I2=80.7%, P<0.001 | Z=−8.88, P<0.001 | CTH |
| High-grade treatment related morbidities | 15 | 7,160 | RR =0.46 (0.37, 0.56) | I2=87.5%, P<0.001 | Z=−7.22, P<0.001 | CTH |
| Response rate (IMM vs. CTH) | 15¶ | 7,160 | OR =2.31 (1.62, 3.29) | I2=84.4%, P<0.001 | Z=4.64, P<0.001 | IMM |
| Response rate (IMM vs. CTH) | 15¶ | 7,160 | RR =2.00 (1.49, 2.67) | I2=84.1%, P<0.001 | Z=4.63, P<0.001 | IMM |
| Overall survival | 14 | – | HR =0.71 (0.66, 0.77) | I2=25.1%, P=0.184 | Z=−8.39, P<0.001 | IMM |
| Progression free survival | 15 | – | HR =0.75 (0.65, 0.85) | I2=82.3%, P<0.001 | Z=−4.26, P<0.001 | IMM |
Ϯ, anti-PD-1 was the immunotherapy reported in the majority of the included articles, while anti-PD-L1 was reported in 2 articles; 1 of them (Fehrenbacher 2016) (15) reported pneumonitis in immunotherapy (anti-PD-L1) arm only; ¶, some studies were included twice as they reported two different immunotherapy doses. CTH, chemotherapy; IMM, immunotherapy; HR, hazard ratio; OR, odds ratio.
Similarly, the occurrence of high-grade pneumonitis in the IMM arm was found among all included studies (OR =2.46, 95% CI: 1.29–4.69, P=0.007) (Figure 1B, Table 3). On subgroup analysis, high-grade pneumonitis was higher among NSCLC patients (OR =3.70, 95% CI: 1.72–7.96), while there was no difference among melanoma patients [OR =4.75 (0.54, 41.97)] or other cohorts (OR =1.78, 95% CI: 0.24–12.98). Subgroup analysis of immunotherapy type showed that anti-PD-1 treatment is associated with higher pneumonitis (OR =2.32, 95% CI: 1.19–4.51); this effect was not seen in anti-PD-L1 (Table 3).
High-grade morbidity
Grade 3–5 adverse events occurred more frequently in the CTH than the IMM arm (RR =0.46, 95% CI: 0.37–0.56, P<0.001) (Table 3).
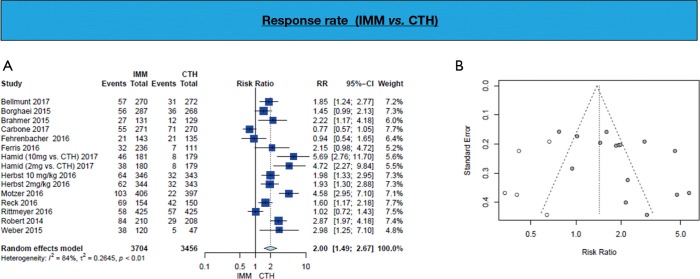
Response in immunotherapy
Response rate is in favor of immunotherapy arm (RR =2.00, 95% CI: 1.49–2.67; P<0.001) (Figure 2, Table 3).
Figure 2.
Response rate (A; Forest plot, B; funnel plot) shows higher response in IMM group 2.26 times those in CTH group. IMM, immunotherapy; CTH, chemotherapy.
Survival in immunotherapy
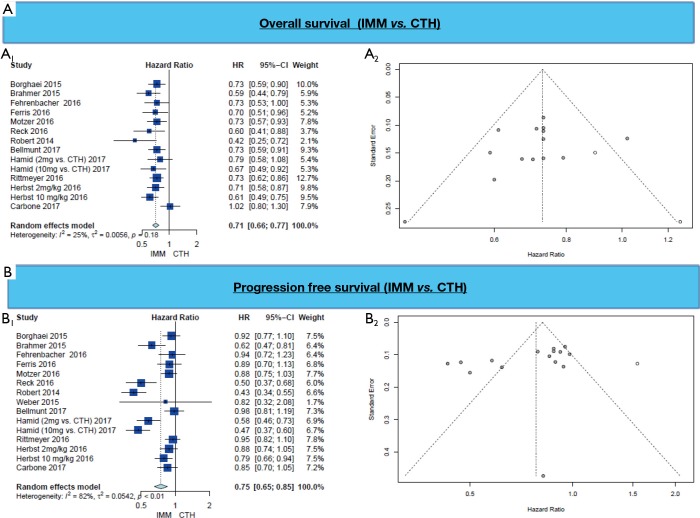
PFS and OS are longer in patients who received IMM in comparison to patient in the chemotherapy arm (HR =0.75, 95% CI: 0.65–0.85, P<0.001 and HR =0.71, 95% CI: 0.66–0.77, P<0.001 respectively) (Figure 3, Table 3).
Figure 3.
(A) Overall survival (A1; Forest plot, A2; funnel plot) and (B) Progression free survival (B1; Forest plot, B2; funnel plot).
Sensitivity analysis
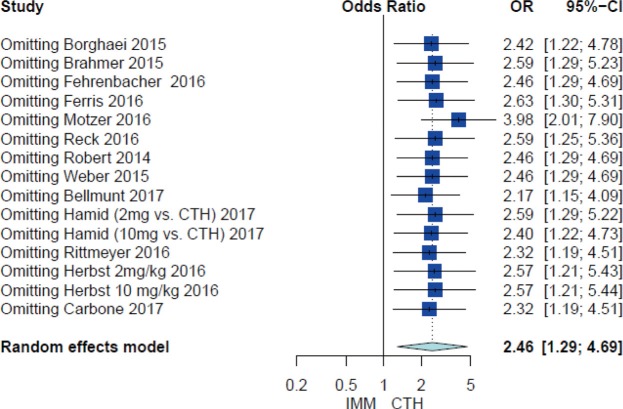
Leave one out analysis of high grade pneumonitis studies was conducted and revealed robustness of the result (Figure S3).
Figure S3.
Leave one out analysis of high grade pneumonitis studies.
Meta-regression
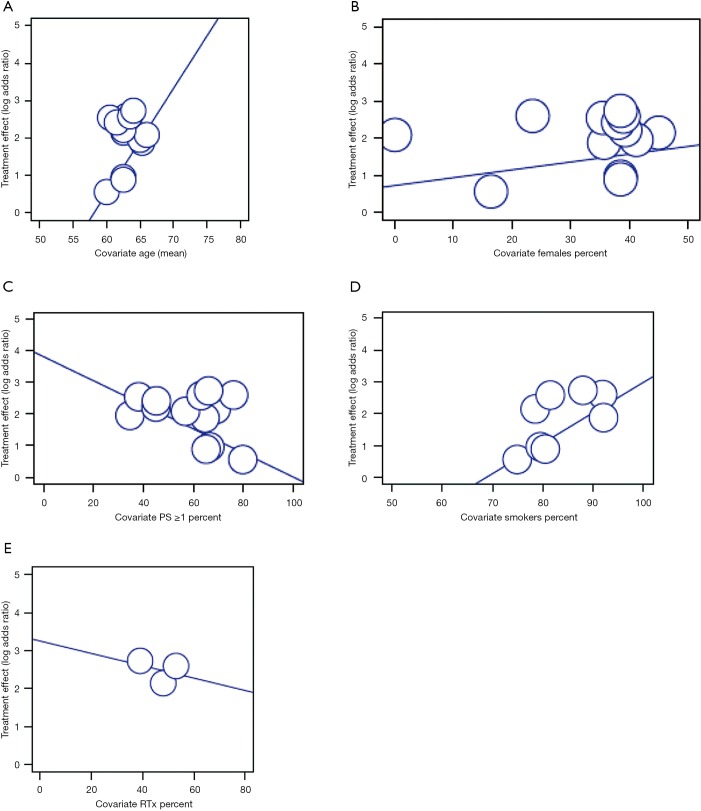
Six levels of meta-regressions were done assessing the effect of different variables (age, gender, performance status, smoking and radiation) on all-grade and high-grade pneumonitis first in all studies, then among NSCLC related studies, and among anti-PD-1 studies alone. This showed an obvious trend of higher pneumonitis among smokers (Beta=0.10, P value =0.104) and elderly patients (Beta=0.41, P=0.072). The same trend was noted regarding smoking in NSCLC studies (Beta=0.10, P=0.146) (Table S2 and Figure S4).
Table S2. Incidence of all and high grades pneumonitis among different chemotherapeutic agent compared to immunotherapy in the corresponding studies.
| Pneumonitis | No. of studies | IMM (%) | CTH (%) | CTH agent |
|---|---|---|---|---|
| All grade pneumonitis | Five (Borghaei 2015, Brahmer 2015, Fehrenbacher 2016, Herbst 2016, Rittmeyer 2016) | 3.04 | 0.52 | Docetaxel |
| Two (Carbone 2017, Reck 2016) | 3.76 | 0.24 | Platinum-based CTH | |
| Rittmeyer 2016 | 1.43 | 0.00 | Dacarbazine | |
| Motzer 2016 | 3.94 | 14.61 | Everolimus | |
| High grade pneumonitis | Five (Borghaei 2015, Brahmer 2015, Fehrenbacher 2016, Herbst 2016, Rittmeyer 2016) | 1.37 | 0.17 | Docetaxel |
| Two (Carbone 2017, Reck 2016) | 1.88 | 0.24 | Platinum-based CTH | |
| Rittmeyer 2016 | 0 | 0 | Dacarbazine | |
| Motzer 2016 | 1.48 | 2.77 | Everolimus |
The remaining studies mentioned different investigator’s choice of chemotherapy. CTH, chemotherapy; IMM, immunotherapy.
Figure S4.
Meta-regression of high grades pneumonitis.
Discussion
In the present meta-analysis, the risk of all-grade pneumonitis was higher among all immunotherapy arms when compared to standard chemotherapy regimens in NSCLC, melanoma, and other tumor types, similar to previous studies (26,27). Similarly, the incidence of high-grade pneumonitis secondary to anti-PD-1 treatment was higher in IMM compared to CTH as reported previously (28). The risk of pneumonitis was highest in smokers, elderly patients, and patients with NSCLC when compared to other tumor types. Interestingly, this effect was not seen in anti-PD-L1 treatment. High grade morbidity overall was higher in the traditional chemotherapy arm than IMM, suggesting that while pneumonitis is a potential limitation to IMM, it may still overall have a superior safety profile to CTH. Similarly, tumor response, PFS, and OS were all favored the immunotherapy arm.
This is the first meta-analysis to report the incidence of pneumonitis in IMM in relation to different chemotherapeutic agents and to assess the effect of age, gender, smoking, performance status and radiotherapy on incidence of pneumonitis with immunotherapy through meta-regression. In 2016, Nishino and his colleagues (27) compared the incidence of PD-1 inhibitors related pneumonitis among different tumor types (NSCLC, melanoma and renal cell carcinoma) and therapeutic regimens including nivolumab or pembrolizumab. Among their study, the majority of the included articles were Phase I RCTs (n=12 articles), whereas our study also evaluated efficacy as only phase II and III studies were included.
Prior meta-analyses on this topic report an all-grade pneumonitis incidence between 2.28–3.69%, high-grade pneumonitis between 1.65–2.87%, and an incidence of all-grade pneumonitis up to 4.27% in the NSCLC subgroup (Table S3) (26,28-30). Anti-PD-1 was associated with higher incidence of all grade pneumonitis between 3.62–3.3.90%, while anti-PD-L1 was associated with either insignificant incidence or protective effect against pneumonitis (Table S4) (26,28-30). Our findings were similar, and in the current analysis, all-grade pneumonitis was most common with everolimus with a rate of over 1 in 7. To our knowledge, no pneumonitis cases were reported with dacarbazine (DTIC) (Figure S5).
Table S3. Published meta-analyses on immunotherapy-induced pneumonitis.
| First author, year | Studies | Patients | Cancer types | Results: all studies | Results: only NSCLC | Results: among anti-PD-1 |
|---|---|---|---|---|---|---|
| Abdel-Rahman, 2016 (29) | 11 | 6,671 | All | AGP: OR =3.96 (95% CI, 2.02–7.79); HGP: OR =2.87 (95% CI, 0.90–9.20) | AGP: OR =3.25 (95% CI, 1.51–7.00) |
NR |
| Costa, 2017 (30) | 9 | 5,353 | All | AGP: RR =2.28 (95% CI, 0.76–6.88) | NR | NR |
| Khunger, 2017 (28) | 19 | 5,038 | NSCLC | NR | NR | AGP (anti-PD-1) =3.62 (95% CI, 2.36–4.87); HGP (anti-PD-1) =1.15 (95% CI, 0.57–1.73); AGP (anti-PD-L1) =1.37 (95% CI, 0.80–1.94); HGP (anti-PD-L1) =0.41 (95% CI, 0.00–0.85) |
| Wu, 2017 (26) | 16 | 6,360 | All | AGP =3.29% (95% CI, 2.72–3.98); HGP =1.65% (95% CI, 1.25–2.16) | AGP =4.27% (95% CI, 3.26–5.58); HGP =2.04% (95% CI, 1.37–3.03) |
AGP (anti-PD-1 vs. CTH): OR =3.90 (95% CI, 1.93–7.85); HGP (anti-PD-1 vs. CTH): OR =3.55 (95% CI, 1.29–9.76) |
NSCLC, non-small cell lung cancer; AGP, all-grade pneumonitis; HGP, high-grade pneumonitis; NR, not reported.
Table S4. Meta-regression results of different variables on all and high grades.
| Pneumonitis | Beta ± SE, P value | ||
|---|---|---|---|
| All studies | Only NSCLC | Among anti-PD-1 | |
| All grade pneumonitis | |||
| Age | 0.2798±0.2411, P=0.2459 | 0.4290±0.3231, P=0.1842 | 0.2663±0.2433, P=0.2738 |
| Female% | 0.0210±0.0348, P=0.5452 | −0.0663±0.0883, P=0.4526 | 0.0180±0.0354, P=0.6110 |
| PS ≥1 | −0.0380±0.0260, P=0.1440 | 0.0866±0.1266, P=0.4943 | −0.0378±0.0260, P=0.1459 |
| Smokers | 0.0954±0.0588, P=0.1043¶ | 0.0946±0.0651, P=0.1461¶ | 0.0952±0.0588, P=0.1051¶ |
| RTH | −0.0162±0.1463, P=0.9117 | −0.0162±0.1463, P=0.9117 | -0.0162±0.1463, P=0.9117 |
| High grade pneumonitis | |||
| Age | 0.4080±0.2269, P=0.0721¶ | 0.0415±0.3712, P=0.9110 | 0.3856±0.2286, P=0.0916¶ |
| Female% | 0.0254±0.0319, P=0.4265 | 0.0351±0.0970, P=0.7177 | 0.0194±0.0329, P=0.5565 |
| PS ≥1 | −0.0239±0.0448, P=0.5937 | −0.0148±0.1421, P=0.9173 | −0.0229±0.0448, P=0.6087 |
| Smokers | 0.0051±0.0701, P=0.9416 | −0.0023±0.0753, P=0.9762 | 0.0080±0.0702, P=0.9095 |
| RTH | −0.0727±0.1545, P=0.6381 | −0.0727±0.1545, P=0.6381 | −0.0727±0.1545, P=0.6381 |
*, positive beta reflects an increase in pneumonitis with increase in the variable, while negative beta reflects a decrease in pneumonitis with increase in the variable; ¶, those in italic show trends toward statistical significance. NSCLC, non-small cell lung cancer; PS, performance status; RTH, radiotherapy; SE, slandered error.
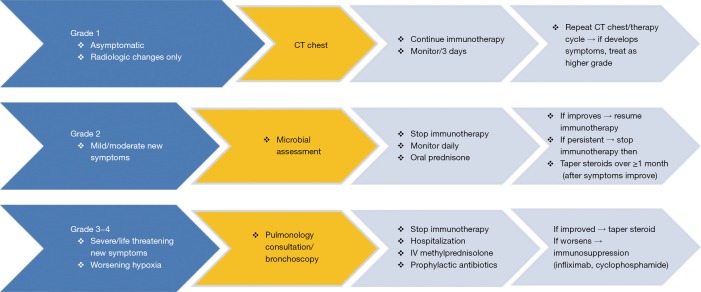
Figure S5.
Management of pneumonitis associated with anti-PD-1/PD-L1 (Those in yellow should be done for any pneumonitis grade).
Wu and colleagues (26) conducted a meta-analysis to evaluate the incidence of pneumonitis in Phase II, III and IV RCT with participants receiving PD-1 inhibitors. They reached the same conclusion that PD-1 inhibitors were associated with an increased risk of pneumonitis in a dose-independent manner, compared with routine chemotherapeutic agents with different frequency and severity in various tumor types. Similar to our results, Khunger et al. reported in his recently published single arm (immunotherapy arm) meta-analysis that there was a higher incidence of pneumonitis with anti-PD-1 compared to anti-PD-L1 (28). Finally, we found that immunotherapy had lower high-grades treatment related morbidities compared to chemotherapy similar to prior results by Costa et al. (30).
The occurrence of pneumonitis seen in this meta-analysis is relatively rare, anti-PD-1 specific, and most pronounced in smokers, the elderly, and patients with primary lung cancer. This is likely due to underlying lung impairment in these patients (26). Interestingly, we did not find a similar effect from prior radiation in lung cancer studies in the meta-regression analysis, but this may be attributed in part to the small number of studies that reported radiation (n=3) (2,16,25). Although the risk of pneumonitis must be considered prior to initiating checkpoint inhibition therapy, our results confirm that in all other metrics, immunotherapy has a superior profile to traditional CTH in NSCLC and melanoma. Most clinical trials exclude patients with prior radiation, radiation pneumonitis, interstitial lung disease, autoimmune disease, clinically significant lung disease, hypoxia and decreased performance status, so we are not able to assess the full risk of pneumonitis in these at-risk groups.
This study is limited by limited clinical time that immunotherapy has been available; therefore follow-up time is short and the number of clinical trials is not large enough to fully evaluate the safety of PD-1 inhibitors and their side effects. Heterogeneity in individual studies was addressed partially through subgroup analysis, although this remains a limitation in particular due to the relatively small sample size. Finally, our analysis was conducted at the study level rather than individual patient data level, meaning the potential variables at the patient level were not involved in the analysis (31).
Conclusions
The incidence of high-grade and all-grade pneumonitis is higher in anti-PD-1 therapy but not in anti-PD-L1 therapy when compared to traditional CTH regimens for NSCLC and melanoma. High-grade adverse events were otherwise more common in the CTH arm. Tumor response rate, PFS, and OS are all substantially improved with IMM over CTH. These results can be used to guide therapy selection and set expectations for treatment effect in these patients.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677-704. 10.1146/annurev.immunol.26.021607.090331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373:123-35. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320-30. 10.1056/NEJMoa1412082 [DOI] [PubMed] [Google Scholar]
- 4.Barbee MS, Ogunniyi A, Horvat TZ, et al. Current status and future directions of the immune checkpoint inhibitors ipilimumab, pembrolizumab, and nivolumab in oncology. Ann Pharmacother 2015;49:907-37. 10.1177/1060028015586218 [DOI] [PubMed] [Google Scholar]
- 5.Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol 2015;33:2004-12. 10.1200/JCO.2014.58.3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rizvi NA, Brahmer JR, Ou SH, et al. Safety and Clinical Activity of MEDI4736, an Anti-Programmed Cell Death-Ligand 1 (PD-L1) Antibody, in Patients with Non-Small Cell Lung Cancer (NSCLC). American Society of Clinical Oncology; 2015. Available online: http://ascopubs.org/doi/abs/10.1200/jco.2015.33.15_suppl.8032, accessed April 22, 2017.
- 7.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6:e1000100. 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bersanelli M, Buti S. From targeting the tumor to targeting the immune system: Transversal challenges in oncology with the inhibition of the PD-1/PD-L1 axis. World J Clin Oncol 2017;8:37. 10.5306/wjco.v8.i1.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Protocol Development | CTEP. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm, accessed April 5, 2017.
- 11.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815-34. [DOI] [PubMed] [Google Scholar]
- 12.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 13.Viera AJ. Odds ratios and risk ratios: what’s the difference and why does it matter? South Med J 2008;101:730-4. 10.1097/SMJ.0b013e31817a7ee4 [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. 10.1016/S0140-6736(16)00587-0 [DOI] [PubMed] [Google Scholar]
- 16.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015;373:1627-39. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015;373:1803-13. 10.1056/NEJMoa1510665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016;375:1823-33. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 19.Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2015;16:375-84. 10.1016/S1470-2045(15)70076-8 [DOI] [PubMed] [Google Scholar]
- 20.Ferris RL, Blumenschein G, Jr, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 2016;375:1856-67. 10.1056/NEJMoa1602252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bellmunt J, De Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 2017;376:1015-26. 10.1056/NEJMoa1613683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamid O, Puzanov I, Dummer R, et al. Final analysis of a randomised trial comparing pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory advanced melanoma. Eur J Cancer 2017;86:37-45. 10.1016/j.ejca.2017.07.022 [DOI] [PubMed] [Google Scholar]
- 23.Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. 10.1016/S0140-6736(16)32517-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 25.Carbone DP, Reck M, Paz-Ares L, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med 2017;376:2415-26. 10.1056/NEJMoa1613493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu J, Hong D, Zhang X, et al. PD-1 inhibitors increase the incidence and risk of pneumonitis in cancer patients in a dose-independent manner: a meta-analysis. Scientific Reports 2017;7:44173. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5341153/, accessed April 22, 2017. 10.1038/srep44173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishino M, Giobbie-Hurder A, Hatabu H, et al. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncol 2016;2:1607-16. 10.1001/jamaoncol.2016.2453 [DOI] [PubMed] [Google Scholar]
- 28.Khunger M, Rakshit S, Pasupuleti V, et al. Incidence of pneumonitis with use of programmed death 1 and programmed death-ligand 1 inhibitors in non-small cell lung cancer: a systematic review and meta-analysis of trials. Chest 2017;152:271-81. 10.1016/j.chest.2017.04.177 [DOI] [PubMed] [Google Scholar]
- 29.Abdel-Rahman O, Fouad M. Risk of pneumonitis in cancer patients treated with immune checkpoint inhibitors: a meta-analysis. Ther Adv Respir Dis 2016;10:183-93. 10.1177/1753465816636557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Costa R, Carneiro BA, Agulnik M, et al. Toxicity profile of approved anti-PD-1 monoclonal antibodies in solid tumors: a systematic review and meta-analysis of randomized clinical trials. Oncotarget 2017;8:8910. 10.18632/oncotarget.13315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol 2015;26:2375-91. [DOI] [PMC free article] [PubMed] [Google Scholar]