Introduction
Widespread use of combined antiretroviral therapy (cART) has reduced morbidity and mortality of HIV-infected patients, increasing life expectancy and quality of life.1 Despite this success, kidney disease remains a problem in this population with cART associated with both acute kidney injury (AKI) and chronic kidney disease (CKD)2,S1
The incidence of AKI in HIV outpatients ranges from 2.7 to 6.9 per 100 person-years, whereas hospital-related AKI is between 6% and 18%.2 AKI is commonly multifactorial, with volume depletion, sepsis, and drug nephrotoxicity the most frequent etiologies.2, 3 Drug-induced nephrotoxicity accounts for up to 30% of AKI episodes in critically ill patients.S2 There is increasing evidence that cART can lead to a wide variety of nephrotoxic effects and mild to severe AKI.4 Many factors contribute to drug-induced AKI in patients with HIV2; the most frequent mechanisms of cART nephrotoxicity are summarized in Table 1.2
Table 1.
Causes of AKI in HIV-positive patients and manifestations of antiretroviral toxicity2
| Acute kidney injury |
|---|
| Common HIV nonspecific causes |
| Opportunistic infectionsa |
| Kidney hypoperfusion and ischemia |
| Acute interstitial nephritis |
| Rhabdomyolysis |
| Urinary tract obstruction: blood clots, fungus balls, or crystalluriab |
| HIV-specific glomerulopathiesa |
| Drugsb |
| Antiretroviral nephrotoxicityc | |||
|---|---|---|---|
| Antiretroviral group | Kidney damage mechanism | Kidney manifestations | |
| NRTI | Abacavir Didanosine Lamivudine Stavudine Zidovudine |
Inhibition of mitochondrial DNA polymerase; oxidative phosphorylation and endogenous nucleotide kinases | AKI, AIN (case report) Fanconi or Fanconi-like syndrome Type B lactic acidosis Nephrogenic diabetes insipidus (case reports) |
| NtRTI | Tenofovir | Direct proximal tubular epithelial cell toxicity Intracellular accumulation Mitochondrial depletion |
Fanconi syndrome Nephrogenic diabetes insipidus AKI Osteomalacia |
| NNRTI | Efavirenz Nevirapine |
Unknown Hypersensitivity |
Minimal change disease (case report) Urolithiasis (case report) AKI (case reports) |
| PIs | Indinavir Atazanavir Nelfinavir Amprenavir Saquinavir Lopinavir Ritonavir Darunavir |
Intratubular drug precipitation due to poor solubility (mainly for indinavir, atazanavir) | AKI and CKD Acute and chronic interstitial nephritis Nephrolithiasis, asymptomatic crystalluria, crystalline nephropathy Papillary necrosis |
| IIs | Raltegravir | Skeletal muscle toxicity | Rhabdomyolysis and AKI (case reports) |
AIN, acute interstitial nephritis; AKI, acute kidney injury; CKD, chronic kidney disease; IIs, integrase inhibitors; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; NtRTI, nucleotide reverse transcriptase; PIs, protease inhibitors.
Less common since cART introduction.
More common since cART introduction.
Including antiretroviral drugs listed in Antiretroviral Nephrotoxicity.
Herein we report a rare case of severe AKI with Fanconi-like syndrome associated with cART nephrotoxicity. Kidney biopsy revealed 2 major forms of kidney injury: crystalline nephropathy from darunavir (DRV) and severe proximal tubulopathy due to tenofovir disoproxil fumarate (TDF).
Case Presentation
A 61-year-old white man with HIV infection was admitted to the emergency department with severe AKI and anemia. The patient had a past history of avascular necrosis of the left femoral head 3 years prior, when he refused surgical intervention initiating self-medication with nonsteroidal anti-inflammatory drugs; 1 year later, hypertension and CKD were diagnosed (estimated glomerular filtration rate 56 ml/min per 1.73 m2). HIV infection was diagnosed 9 months before admission and 2 months later cART was initiated (he was treatment-naïve) comprising ritonavir-boosted DRV, TDF, and emtricitabine. At that time the baseline kidney function corresponded to 1.63 mg/dl of serum creatinine with estimated glomerular filtration rate 45 ml/min per 1.73 m2 with no urinary abnormalities. One month before admission, the patient ingested nonsteroidal anti-inflammatory drugs for hip pain. Subsequently, he presented with several days of malaise, anorexia, and fatigue 2 days before admission. Remarkable findings on physical examination included hypotension, dehydration, marked pallor, and cachexia.
Laboratory analysis revealed hemoglobin 4.8 g/dl, ferritin 1400 ng/ml, reticulocytes 5%, serum creatinine 16.3 mg/dl, blood urea nitrogen 161 mg/dl, normal serum electrolyte, and anion gap metabolic acidosis (HCO3 13.3, delta HCO3 10.9; anion gap 25.7, delta anion gap 13.7 mEq/l, and delta delta 1.26 ratio) with normoglycemia and serum phosphate 6.2 mg/dl. Urinalysis revealed pH 5.5, glucose 1000 mg/dl, and non-nephrotic proteinuria with urinary protein-creatinine ratio (0.837 g/g) higher than albumin-creatinine ratio (0.120 g/g) implying tubular proteinuria; and urinary hemoglobin (+3). CD4 was 178 cells/mm3 and viral load <20 copies RNA/ml.
The patient received 4 units of packed red blood cells and i.v. saline solution. Metabolic acidosis was corrected with i.v. bicarbonate and the cART regimen was modified, withdrawing TDF and beginning lamivudine.
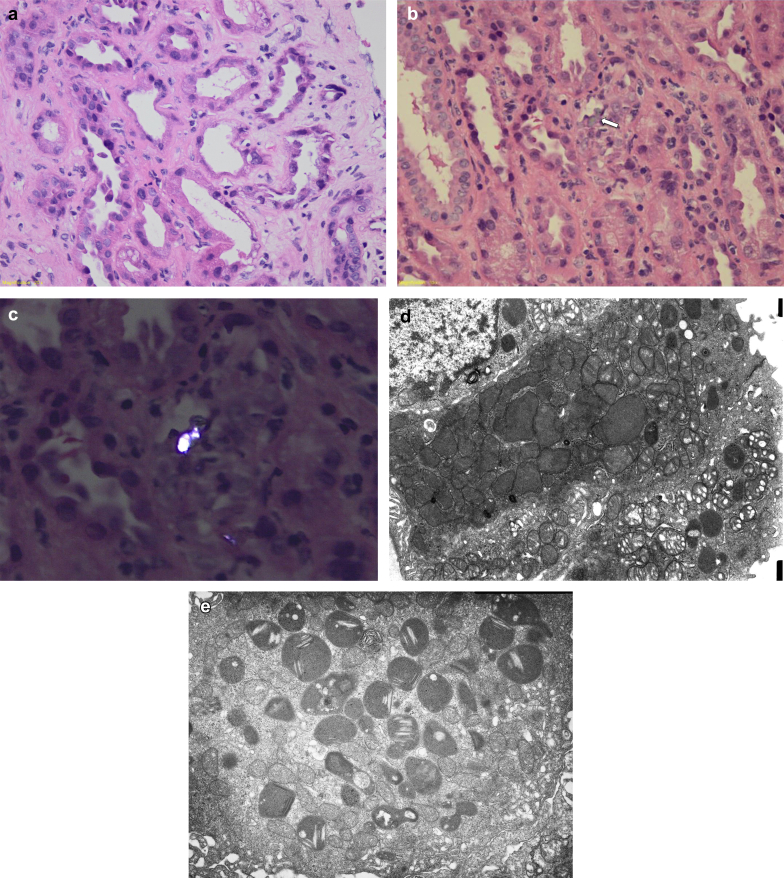
Because of the lack of kidney recovery, biopsy was performed 26 days after admission. Light microscopy showed normal glomeruli, with significant tubular injury characterized by lumen dilatation, loss of brush border, and degenerative and reactive features of tubular cells and detachment of tubular epithelium. Some vacuoles and empty geometric-like spaces were also observed in the cytoplasm of tubular cells. There was stromal edema and sparse inflammatory infiltration mostly characterized by lymphocytes (Figure 1a, hematoxylin-eosin original magnification ×10). These lesions affected primarily the proximal tubules. Granular casts were present in distal tubular lumens and there was a focal tubulitis with an inflammatory infiltrate in the interstitium, consisting of lymphocytes, plasma cells, and rare eosinophils, but also some crystals were found inside the cytoplasm of tubular cells (Figure 1b, hematoxylin-eosin original magnification ×10), which were birefringent and multirefractive on polarized light (Figure 1c, polarized hematoxylin-eosin original magnification ×20). Electron microscopy confirmed significant proximal tubular injury characterized by tubular cells with degenerative features and loss of microvilli. Of note, the cytoplasm contained plenty of dysmorphic mitochondria, with variations of size and with a paucity of cristae, some were small with swollen cristae (Figure 1d, electron microscopy original magnification ×8000). Other ultrastructural findings included detached epithelial cells, with brush border effacement and tubular epithelial mitochondria with irregular shapes and degenerative changes with sparse abnormal cristae (Figure 1e, electron microscopy original magnification ×5000). Tubular cell lysosomes showed exuberant needle-shaped and thin lamellar crystals approximately 5 nm in thickness (Figure 1e, electron microscopy original magnification ×10,000).
Figure 1.
Kidney biopsy. Light microscopy: (a) (hematoxylin-eosin [HE], original magnification ×10), proximal tubules with lumen dilatation, loss of brush border, and degenerative and reactive aspects of tubular cells and detachment of tubular epithelium. Some vacuoles and empty geometric-like spaces are also observed in the cytoplasm of tubular cells. There is stromal edema and sparse inflammatory infiltration, mostly characterized by rare lymphocytes. (b) In the center of the image, there is a crystal (arrow) in the cytoplasm of a tubular cell with lymphocytes permeating through the epithelium, which has degenerative aspect (HE, original magnification ×10). (c) The darunavir crystal is birefringent and multirefractive on polarized light (polarized HE, original magnification ×20). Electron microscopy (EM): (d) Tubular epithelial cells with degenerative aspects and loss of microvilli and cytoplasm containing mitochondria with irregular shapes, some of them dysmorphic, with variation of size and sparse abnormal cristae and some others smaller with swollen cristae (original magnification ×5000). (e) Tubular cell lysosomes with abundant needle-shaped and thin lamellar crystals, approximately 5 nm in thickness (EM, original magnification ×10,000).
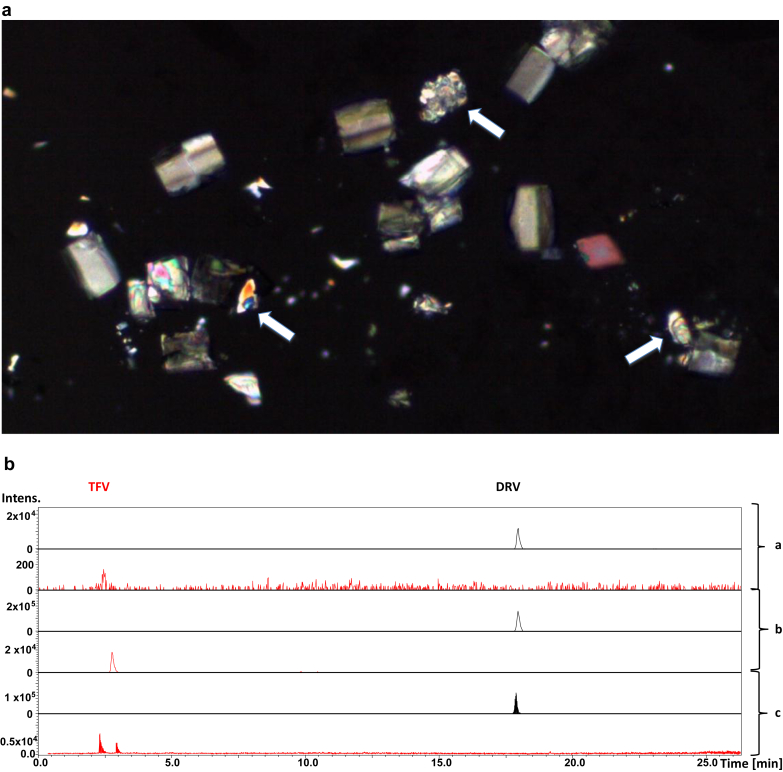
The urinary sediment examination was repeated without centrifugation and a large number of different crystals were detected by polarized microscopy (Figure 2a). In addition to calcium oxalate and uric acid crystals, other irregular crystals were observed that were most likely DRV. Liquid chromatography with high-resolution mass spectrometry method was used for simultaneous identification and quantification of DRV and tenofovir in the urine sample. Although tenofovir and DRV were identified in the free liquid urine (Figure 2b, b section), only DRV was found in the crystal (Figure 2b, a section). Identification was confirmed on comparison with standard solutions of tenofovir and DRV (Figure 2b, c section). More information is found in Supplementary Table S1.
Figure 2.
Urinary crystals. (a) Several crystals are noted under polarized light, including calcium oxalate, uric acid, and darunavir crystals (white arrows). (b) Liquid chromatography with high-resolution mass spectrometry. Extracted ion chromatograms for darunavir (DRV) in black and tenofovir (TFV) in red. Urinary crystals contained only DRV in the section marked a, free liquid urine contained both DRV and TFV in b, and standard solutions of DRV and TFV showing their respective retention times in c. The retention times of DRV and/or TFV in the free liquid urine and urine crystal were identical to the ones of the standard solutions.
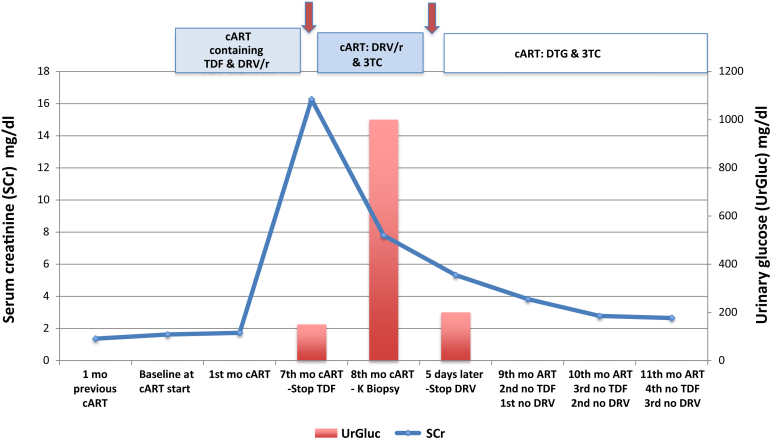
Based on these findings, DRV was subsequently withdrawn (36 days of admission) and serum creatinine rapidly decreased to 5.32 mg/dl and reached 2.65 mg/dl after 5 months. Urine abnormalities also resolved. The clinical evolution is shown in Figure 3. Of note, there was no past history of urinary complaints or lithiasis.
Figure 3.
Clinical evolution. Changes in serum creatinine level (solid line) and urinary glucose (red columns) since HIV diagnosis and initiation of combined antiretroviral therapy. Conversion factor for serum creatinine in mg/dl to mol/l 88.4. ART, antiretroviral therapy; cARVT, combined antiretroviral therapy; DRV/r, darunavir-boosted ritonavir; DTG, dolutegravir; mo, months of evolution; SCr, serum creatinine; TDF, tenofovir; UrGluc, urinary glucose; 3TC, lamivudine.
Discussion
Herein we describe a rare case of severe AKI in a patient with underlying 3a CKD and recent cART initiation who developed nephrotoxicity from biopsy-proven DRV-induced crystalline nephropathy and severe proximal tubulopathy with mitochondrial injury from TDF. It is likely that these 2 forms of kidney injury could have been further exacerbated by exposure to nonsteroidal anti-inflammatory drugs and underlying CKD.
We first discuss TDF-related kidney injury, which was manifested as proximal tubulopathy and AKI with mitochondrial damage noted on kidney biopsy. Based on ART guidelines, combination therapy with TDF and emtricitabine has been the first-choice nucleoside reverse transcriptase inhibitor.S3 However, this combination is not recommended when creatinine clearance is below 30 to 50 ml/min, as both drugs are mainly eliminated via the kidneys. In the case reported there was no dose adjustment at ART initiation and therefore the potential nephrotoxic effect was increased. TDF requires dose adjustment to avoid tubular toxicity.5 TDF is excreted unchanged in urine and it is cleared by a combination of glomerular filtration and proximal tubular secretion.S4 Active transport occurs through basolateral human organic ion transporter-1 followed by secretion into the tubular lumen through the apical transporter multidrug resistance–associated protein 4.6 Dysfunction of efflux transporters can lead to drug accumulation into the proximal tubular cellsS5 resulting in high intracellular concentrations that can deplete mitochondrial DNA and injure mitochondria.7 Inhibition of basolateral uptake of TDF may protect against tubular injury by reducing intracellular concentrations.8 Drug-drug interactions increase the risk of TDF-related kidney toxicity by increasing renal exposure to TDF. Pharmaco-enhancers such as ritonavir increase plasma concentrations of TDF and increase nephrotoxicity. Nonsteroidal anti-inflammatory drug intake by our patient was likely important in decreasing glomerular filtration rate and further increasing TDF accumulation with development of severe AKI.
As noted in our case, TDF toxicity resulted in severe proximal tubular injury characterized by glycosuria and tubular proteinuria. Following TDF withdrawal, kidney function began to recover with improving proximal tubular function, but AKI only partially recovered. TDF-induced AKI cases demonstrate toxic acute tubular necrosis, with distinctive proximal tubular eosinophilic inclusions representing giant mitochondria visible by light microscopy and mitochondrial enlargement, depletion, and dysmorphic changes by electron microscopy.7 These same ultrastructural findings were observed in our case and noted in other published cases.S6 TDF also causes chronic tubulointerstitial nephritis and CKD, which may account for the lack of complete reversibility observed in our patient.S7
The second kidney lesion noted in our patient was unexpected, and discovered after review of the kidney biopsy specimen. The protease inhibitor DRV was confirmed as the cause of crystalline-induced AKI. Intratubular crystals and crystals within tubular cell cytoplasm were observed on light microscopy and within lysosomes on electron microscopy. Reexamination of the urine sediment revealed crystals on polarization that were proven to be DRV on analysis using liquid chromatography high-resolution spectrometry. This provided evidence that supported DRV as the causative crystals in the tubular lumens and tubular cell lysosomes. Thus, like the protease inhibitors indinavir and atazanavir, DRV also can induce crystalline nephropathy.
Protease inhibitors are known to cause kidney injury, which can manifest as crystalluria, leukocyturia, nephritis, nephrolithiasis, nephropathy, and urolithiasis.S8 The protease inhibitors are poorly soluble in urine and tend to precipitate in the urine at physiologic pH, in the setting of volume depletion, underlying kidney disease, and other factors; however, reports of nephrotoxicity associated with DRV are quite rare. Ritonavir-boosted DRV has been associated with increased urinary DRV levels with DRV 13 times higher in the urine than the plasma.9 Of these, 4 of 51 patients treated with ritonavir-boosted DRV had urinary crystals containing DRV (7.8%), but there were no differences in urine and plasma levels of DRV, treatment duration, or urine pH.9 The incidence of nephrolithiasis is lower with ritonavir-boosted DRV than ritonavir-boosted atazanavir, which resulted in the recommendation of its use in patients at risk for CKD.S9
In conclusion, we report a case of AKI with proximal tubulopathy due to the dual toxicity of TDF and DRV in a patient with underlying CKD. Acute tubular injury and proximal tubulopathy were primarily due to TDF-induced mitochondrial injury. Further tubular injury from crystalline-related kidney injury was due to DRV as manifested by intratubular and cellular DRV crystals. As such, this is the first report of crystalline nephropathy caused by DRV. Health care providers should be aware of this potential complication when evaluating patients receiving this drug who develop AKI. Teaching points are shown in Table 2.
Table 2.
Teaching points
| - ART can lead to a number of nephrotoxic effects including AKI, tubulopathies, and CKD |
| - Reduced GFR and drug-drug interactions increase the risk of TDF-related kidney toxicity by increasing renal exposure to tenofovir |
| - Nonsteroidal anti-inflammatory drugs decrease GFR and further increase ART accumulation and risk for AKI |
| - TDF toxicity can result in severe proximal tubular injury, AKI, and CKD |
| - DRV is another protease inhibitor that is poorly soluble in urine and can cause crystalline-induced AKI |
| - Drug discontinuation is mandated to reverse nephrotoxicity |
| - Urinary sediment analysis should be included in monitoring drugs associated with crystal formation |
| - Kidney biopsy is mandatory for diagnosis of ART-induced nephrotoxicity and severe AKI |
| - High-resolution spectrometry is a useful tool for identification of drug-induced urinary crystals |
AKI, acute kidney injury; ART, antiretroviral therapy; CKD, chronic kidney disease; DRV, darunavir; GFR, glomerular filtration rate; TDF, tenofovir disoproxil fumarate.
Disclosure
All the authors declared no competing interests.
Acknowledgments
JM and AMMA have support from Fundação para a Ciência e a Tecnologia (FCT), Portugal, through research grants UID/QUI/00100/2013 and IF/01091/2013/CP1163/CT0001, and Portugal 2020 (RNEM-LISBOA-01-0145-FEDER-402-022125) to Instituto Superior Técnico. Sofia Pereira from CEDOC, Universidade Nova de Lisboa, Patricia Pacheco from Infectious Disease Department and Luis Inchaustegui from Nephrology Department of Hospital Fernando Fonseca, and Conceiçäo Oliveira from Instituto Superior Técnico, Universidade de Lisboa, are gratefully acknowledged.
Footnotes
Table S1. Chromatographic and mass spectrometry data registered for darunavir and/or tenofovir in the crystal and urine samples.
Supplementary References.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Chromatographic and mass spectrometry data registered for darunavir and/or tenofovir in the crystal and urine samples.
References
- 1.May M.T., Gompels M., Delpech V. Impact on life expectancy of HIV-1 positive individuals of CD4+ cell count and viral load response to antiretroviral therapy. AIDS. 2014;28:1193–2002. doi: 10.1097/QAD.0000000000000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campos P., Ortiz A., Soto K. HIV and kidney diseases: 35 years of history and consequences. Clin Kidney J. 2016;9:772–781. doi: 10.1093/ckj/sfw104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perazella M.A. Acute renal failure in HIV-infected patients: a brief review of common causes. Am J Med Sci. 2000;319:385–391. doi: 10.1097/00000441-200006000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Kalyesubula R., Perazella M.A. Nephrotoxicity of HAART. AIDS Res Treat. 2011;2011:562790. doi: 10.1155/2011/562790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ezinga M., Wetzels J.F.M., Bosch M.E.W. Long-term treatment with tenofovir: prevalence of kidney tubular dysfunction and its association with tenofovir plasma concentration. Antivir Ther. 2014;19:765–771. doi: 10.3851/IMP2761. [DOI] [PubMed] [Google Scholar]
- 6.Imaoka T., Kusuhara H., Adachi M. Functional involvement of multidrug resistance-associated protein 4 (MRP4/ABCC4) in the renal elimination of the antiviral drugs adefovir and tenofovir. Mol Pharmacol. 2006;71:619–627. doi: 10.1124/mol.106.028233. [DOI] [PubMed] [Google Scholar]
- 7.Herlitz L.C., Mohan S., Stokes M.B. Tenofovir nephrotoxicity: acute tubular necrosis with distinctive clinical, pathological, and mitochondrial abnormalities. Kidney Int. 2010;78:1171–1177. doi: 10.1038/ki.2010.318. [DOI] [PubMed] [Google Scholar]
- 8.Izzedine H., Thibault V., Valantin M. Tenofovir/probenecid combination in HIV/HBV-coinfected patients: how to escape Fanconi syndrome recurrence? AIDS. 2010;24:1078–1079. doi: 10.1097/QAD.0b013e3283313f54. [DOI] [PubMed] [Google Scholar]
- 9.de Lastours V., De Silva E.F.R., Daudon M. High levels of atazanavir and darunavir in urine and crystalluria in asymptomatic patients. J Antimicrob Chemother. 2013;68:1850–1856. doi: 10.1093/jac/dkt125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Chromatographic and mass spectrometry data registered for darunavir and/or tenofovir in the crystal and urine samples.