Abstract
Introduction
Poor sleep associates with adverse chronic kidney disease (CKD) outcomes yet the biological mechanisms underlying this relation remain unclear. One proposed mechanism is via allostatic load, a cumulative biologic measure of stress.
Methods
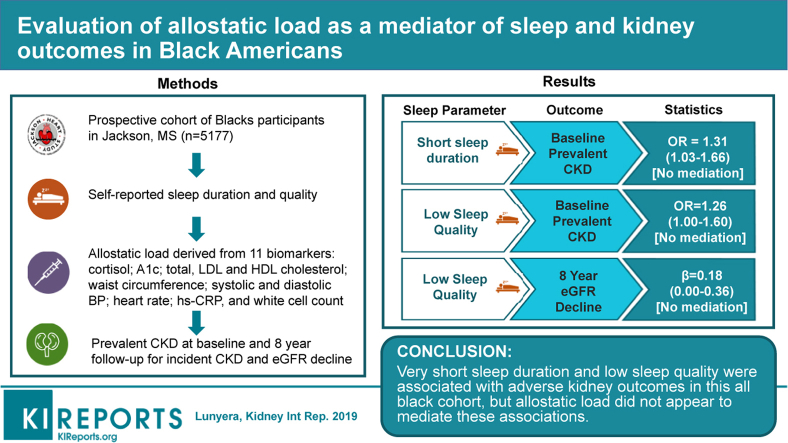
Using data from 5177 Jackson Heart Study participants with sleep measures available, we examined the association of self-reported sleep duration: very short, short, recommended, and long (≤5, 6, 7–8, or ≥9 hours per 24 hours, respectively) and sleep quality (high, moderate, low) with prevalent baseline CKD, and estimated glomerular filtration rate (eGFR) decline and incident CKD at follow-up. CKD was defined as eGFR <60 ml/min per 1.73 m2 or urine albumin-to-creatinine ratio ≥30 mg/g. Models were adjusted for demographics, comorbidities, and kidney function. We further evaluated allostatic load (quantified at baseline using 11 biomarkers from neuroendocrine, metabolic, autonomic, and immune domains) as a mediator of these relations using a process analysis approach.
Results
Participants with very short sleep duration (vs. 7–8 hours) had greater odds of prevalent CKD (odds ratio [OR] 1.31, 95% confidence interval [CI] 1.03–1.66). Very short, short, or long sleep duration (vs. 7–8 hours) was not associated with kidney outcomes over a median follow-up of 8 years. Low sleep quality (vs. high) associated with greater odds of prevalent CKD (OR 1.26, 95% CI 1.00–1.60) and 0.18 ml/min per 1.73 m2 (95% CI 0.00–0.36) faster eGFR decline per year. Allostatic load did not mediate the associations of sleep duration or sleep quality with kidney outcomes.
Conclusions
Very short sleep duration and low sleep quality were associated with adverse kidney outcomes in this all-black cohort, but allostatic load did not appear to mediate these associations.
Keywords: African Americans, kidney diseases, sleep, sleep deprivation
Graphical abstract
Emerging data substantiate associations of poor sleep patterns, such as short sleep duration, with adverse CKD outcomes.1, 2, 3, 4 Black Americans disproportionately experience poor sleep patterns5 and are at increased risk for CKD and end-stage renal disease compared with nonblack individuals.6 However, despite these observed associations, studies have not evaluated the relation between poor sleep and adverse CKD outcomes specifically in black individuals.
The mechanisms underlying the relation between sleep and kidney outcomes also remain unclear. One suggested mechanism is via chronic derangements in physiological systems by which sleep exerts its reparative effects on the body.5 For instance, short sleep duration increases sympathetic activity and vascular tone,7 and sleep deprivation impairs release of hormonal regulators of metabolic and vascular function, such as cortisol, aldosterone, and melatonin from the hypothalamic-pituitary-adrenal system.8, 9, 10, 11 A composite measure of dysregulation in these systems is termed allostatic load, a multidimensional biologic construct subsuming biomarkers across the physiologic domains of neuroendocrine, autonomic, immune, and metabolic function. As a biomarker that captures preclinical adverse health consequences of stress exposure in the social environment, allostatic load has been linked with cardiovascular disease and diabetes,12, 13 and is posited to adversely affect kidney outcomes.14, 15
Therefore, we examined the potential mediating effect of allostatic load on the association between poor sleep patterns (sleep duration and sleep quality) and CKD outcomes in black Americans by using data from the Jackson Heart Study (JHS).
Methods
Study Population
The JHS is a prospective cohort study comprising 5306 black Americans, 21 to 94 years of age at enrollment, from the tricounty (Hinds, Madison, and Rankin) area of the Jackson, MS, metropolitan area. JHS enrolled participants from 2000 to 2004 using probability-based sampling and random sampling with the Jackson driver’s license registry and a commercially available list as the sampling frame, as previously detailed.16, 17 First-degree relatives of JHS participants and prior participants from the Atherosclerosis Risk in Communities were also recruited to participate in the study.
Participants who provided written informed consent completed the JHS baseline examination (Exam 1: 2000–2004), and 2 subsequent follow-up examinations (Exam 2: 2005–2008; and Exam 3: 2009–2013). Institutional review boards at the University of Mississippi Medical Center, Jackson State University, and Tougaloo College approved the JHS protocol. For this analysis, we excluded participants missing sleep assessments and key covariates.
Sleep Duration and Quality Assessment
The JHS assessed sleep using a modified version of the Berlin Sleep Questionnaire.18 As part of this assessment, participants self-reported their habitual sleep duration at baseline in response to the question, “During the past month, excluding naps, how many hours of actual sleep did you get at night (or day, if you work at night) on average?” We categorized sleep duration into very short (≤5 hours), short (6 hours), recommended (7 to 8 hours), and long (≥9 hours) per 24 hours, based on prior studies that have shown 7 to 8 hours to be beneficial for health.1, 19
Participants rated their habitual sleep quality in response to the question, “During the past month, how would you rate your sleep quality overall?” Possible responses included excellent, very good, good, fair, or poor. In our primary analysis, we categorized sleep quality into 3 categories: high (included “excellent” and “very good”), moderate (included “good”), and low (included “fair” and “poor”). In a sensitivity analysis, we dichotomized sleep quality into high (included “excellent”, “very good”, and “good”) and low (included “fair” and “poor”).
Allostatic Load Assessment
We assessed allostatic load using 11 biomarkers representing 4 physiologic domains: neuroendocrine, metabolic, autonomic, and immune function. Cortisol captured neuroendocrine function; metabolic function encompassed hemoglobin A1c, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and waist circumference. Autonomic function featured systolic blood pressure, diastolic blood pressure, and resting heart rate; immune function comprised C-reactive protein and white blood cell count. We converted values of the 11 biomarkers to z-scores and averaged the z-scores within the respective domains. The z-score for high-density lipoprotein cholesterol was multiplied by −1 to account for its protective effect on adverse health outcomes, and the z-score for waist circumference was computed separately for men and women, and then recombined into 1 score to account for sex differences. Allostatic load comprised the average z-score across the 4 domains; higher scores indicate greater cumulative physiologic derangement.
Demographics and Comorbidities
We included the following sociodemographic covariates in our analysis: age, sex, educational attainment (≤ vs. >high school diploma), household income (≤1.5 vs. >1.5 times the poverty level), employment status (working for pay [full- or part-time] vs. not working for pay), alcohol consumption (within the past year vs. not), smoking status (current or former vs. never), physical activity, nutritional status, and depression. We defined physical activity and nutritional status based on the American Heart Association’s Life Simple 7 guideline.20 Categories for physical activity included poor (0 minutes of moderate and vigorous physical activity); intermediate (>0 to <150 minutes of moderate physical activity, >0 to <75 minutes of vigorous physical activity, or >0 to <150 minutes of combined moderate and vigorous physical activity); and ideal (≥150 minutes of moderate physical activity, ≥75 minutes of vigorous physical activity, or ≥150 minutes of combined moderate and vigorous physical activity). Nutritional status, defined based on 5 recommended dietary components (fruits and vegetables ≥4.5 cups per day; fish >3.5 ounces twice per week; sodium <1500 mg per day; sugary beverages <450 kcal per week; and whole grains ≥3 servings per day), comprised poor diet (0–1 components) versus intermediate or ideal diet (2–5 components). Participants self-reported depressive symptoms based on the 20-item Center for Epidemiological Studies-Depression questionnaire. We defined depression as a composite score of ≥16 on the Center for Epidemiological Studies-Depression questionnaire.
CKD Outcome Assessment
Our primary CKD outcomes included prevalent CKD at baseline, eGFR decline, and incident CKD. We defined prevalent CKD as either eGFR <60 ml/min per 1.73 m2 assessed using the CKD-EPI equation21 or albuminuria, defined as spot urine (or 24-hour if spot was missing) albumin-to-creatinine ratio ≥30 mg/g. eGFR decline was defined as the annual rate of kidney function decline during follow-up (Exam 1 to Exam 3), and it was determined using the equation: 365.25 × (eGFR at Exam 1 – eGFR at Exam 3) / (number of days between Exam 1 and Exam 3). Among those without CKD at Exam 1, we defined incident CKD as either eGFR <60 ml/min per 1.73 m2 at Exam 3 along with ≥25% decline in eGFR during follow-up or albuminuria at Exam 3. Isotope dilution mass spectrometry–calibrated serum creatinine was used for all GFR estimations.22
Statistical Analysis
We described demographic and clinical characteristics of participants by categories of sleep duration using mean ± SD or median [interquartile range] for continuous variables and frequency (percent) for categorical covariates. We reported the distribution of allostatic load and baseline kidney function across categories of sleep duration using mean ± SD.
We used a process analysis approach,23 which is a special case of structural equation modeling, to evaluate allostatic load as a mediator of sleep and CKD outcomes. Unlike the commonly used Causal Steps Approach to mediation analysis that relies on certain stepwise criteria, but without a formal hypothesis test or quantification of the mediated effect,24 the process analysis approach uses hypothesis tests to evaluate presence of (and to quantify) mediation. Briefly, we used generalized linear regression models with an identity (for continuous outcomes) or logit (for binary outcomes) link to estimate parameters that assessed presence of mediation based on 2 separate pathways, quantified by the indirect and direct associations (Supplementary Figure S1). The indirect association (henceforth referred to as mediated association) evaluated the association of sleep with CKD outcomes via allostatic load, and the direct association evaluated the association of sleep with CKD outcomes independent of the effect of allostatic load on CKD. We used the quantile bootstrap approach, with 5000 replications, to test the significance of the mediated association; this approach had the advantage of relaxing the normality assumption regarding the sampling distribution of the mediated associations. We inferred evidence of mediation if the mediated association of at least 1 sleep category was non-zero relative to the reference sleep category.22 All regression models were adjusted for age, sex, educational attainment, household income, employment status, alcohol consumption, and body mass index. We also adjusted the eGFR decline model for baseline eGFR and albumin-to-creatinine ratio. We assessed and confirmed model quality for fit, predictive accuracy, and model assumptions.
We conducted several sensitivity analyses. Because the main mediation models mentioned previously did not include diabetes and hypertension to avoid overadjustment because allostatic load comprises HBA1c (diabetes) and blood pressure (hypertension), we stratified the main models by hypertension and diabetes to account for their potential confounding effects. We also investigated the association of sleep with kidney outcomes using different categorizations (6 hours per 24 hours as the reference category for sleep duration and a dichotomized sleep quality variable) to determine if the results of the primary analysis were sensitive to categorization. Given that depression confounds the effect of sleep on adverse health outcomes, we additionally adjusted for depression in the main mediation analysis among a subset of JHS participants for whom JHS assessed depression. Finally, we also examined the mediated associations of allostatic load on the relation between sleep duration and CKD outcomes separately for each domain of allostatic load.
All hypothesis tests were 2-sided at the 0.05 level without adjustment for multiple comparisons, or imputation for missing data. We used SAS 9.4 (SAS Institute, Cary, NC) and R 3.3.0 (R Core Team, Vienna, Austria) for all analyses.
Results
Sociodemographic and Behavioral Characteristics of Participants
Of the 5306 JHS participants, 5177 (98%) had complete data for sleep duration. These participants were predominantly women (63%), mean age was 55 years (SD 13), and most had greater than high school diploma (62%) or worked for pay (63%). Most (n = 2085; 40%) slept the recommended 7 to 8 hours; 1286 (25%), 1521 (29%), and 285 (6%) reported very short (≤5 hours), short (6 hours), and long (≥9 hours) sleep duration, respectively. Participants with short sleep duration were generally similar to those with 7 to 8 hours. However, those with very short sleep (vs. 7–8 hours) were more likely to be men (39% vs. 35%); cigarette smoker (35% vs. 30%); and to have poor physical activity (54% vs. 47%) or low sleep quality (70% vs. 16%). Those with long sleep (vs. 7–8) were more likely to have lower-middle income status (52% vs. 33%), poor physical activity (54% vs. 47%), and to be a cigarette smoker (39% vs. 30%), but less likely to have more than a high school diploma (48% vs. 62%) or to work for pay (40% vs. 62%) (Table 1).
Table 1.
Baseline characteristics of participants stratified by categories of sleep duration
| Baseline characteristics | Overall N = 5177 | Sleep duration |
|||
|---|---|---|---|---|---|
| Very short (≤5 hours) n = 1286 | Short (6 hours) n = 1521 | 7–8 hours n = 2085 | Long (≥9 hours) n = 285 | ||
| Demographics | |||||
| Age, mean ± SD, yr | 55.3 ± 12.8 | 54.3 ± 12.5 | 54.5 ± 12.4 | 55.8 ± 13.0 | 59.5 ± 13.9 |
| Male, n (%) | 1893 (36.6) | 498 (38.7) | 576 (37.9) | 729 (35.0) | 90 (31.6) |
| Lower-middle income,an (%) | 1742 (33.6) | 457 (35.5) | 458 (30.1) | 679 (32.6) | 148 (51.9) |
| Education: >high school diploma, n (%) | 3211 (62.0) | 776 (60.3) | 1012 (66.5) | 1286 (61.7) | 137 (48.1) |
| Working for pay, n (%) | 3250 (62.8) | 803 (62.4) | 1049 (69.0) | 1283 (61.5) | 115 (40.4) |
| Behavioral factors and comorbidity | |||||
| BMI, mean ± SD, kg/m2 | 31.8 ± 7.3 | 32.4 ± 7.5 | 31.7 ± 7.3 | 31.5 ± 7.2 | 31.1 ± 6.9 |
| Alcohol use, n (%) | 2379 (46.0) | 615 (47.8) | 724 (47.6) | 946 (45.4) | 94 (33.0) |
| Current or former smoker, n (%) | 1664 (32.1) | 451 (35.1) | 467 (30.7) | 634 (30.4) | 112 (39.3) |
| AHA diet score, n (%) | |||||
| Poor | 2544 (49.1) | 641 (49.8) | 753 (49.5) | 1021 (49.0) | 129 (45.3) |
| Intermediate or ideal | 2004 (38.7) | 462 (35.9) | 603 (39.6) | 821 (39.4) | 118 (41.4) |
| AHA physical activity score, n (%) | |||||
| Poor | 2537 (49.0) | 690 (53.7) | 712 (46.8) | 981 (47.1) | 154 (54.0) |
| Intermediate | 1643 (31.7) | 384 (29.9) | 486 (32.0) | 689 (33.0) | 84 (29.5) |
| Ideal | 997 (19.3) | 212 (16.5) | 323 (21.2) | 415 (19.9) | 47 (16.5) |
| Depression score, mean ± SD | 10.8±8.1 | 13.4±9.2 | 10.2±7.5 | 9.6±7.3 | 11.5±8.6 |
| Sleep quality, n (%) | |||||
| High | 1608 (31.1) | 138 (10.7) | 400 (26.3) | 912 (43.7) | 158 (55.4) |
| Moderate | 1782 (34.4) | 251 (19.5) | 611 (40.2) | 835 (40.0) | 85 (29.8) |
| Low | 1786 (34.5) | 897 (69.8) | 510 (33.5) | 337 (16.2) | 42 (14.7) |
AHA, American Heart Association; BMI, body mass index.
Family income ≤1.5 times the poverty level.
Distribution of Allostatic Load and CKD Outcomes
Among 5177 (98%) JHS participants with the required data for computing allostatic load, the composite allostatic load z-score ranged from −1.1 to 6.7 and its mean was 0 (SD 0.44). Participants with very short sleep and those with 7 to 8 hours had comparable mean allostatic load z-score (0.01 [SD 0.43] vs. 0.01 [SD 0.44]). However, compared with the recommended 7 to 8 hours, those with short sleep had lower mean allostatic load z-score: 0.01 (SD 0.44) vs. −0.02 (SD 0.45), and those with long sleep had higher mean allostatic load z-score: 0.07 (SD 0.43) versus 0.01 (SD 0.44) (Table 2).
Table 2.
Distribution of allostatic load and CKD outcomes stratified by categories of sleep duration
| Characteristica | No. included (% of JHS) | Overall N = 5177 | Sleep duration |
|||
|---|---|---|---|---|---|---|
| Very short ≤5 hours n = 1286 | Short 6 hours n = 1521 | 7–8 hours n = 2085 | Long ≥9 hours n = 285 | |||
| Allostatic load | ||||||
| z-scores | ||||||
| All domains | 5177 (97.7) | 0 ± 0.44 | 0.01 ± 0.43 | -0.02 ± 0.45 | 0.01 ± 0.44 | 0.07 ± 0.43 |
| Neuroendocrine | 5074 (98.0) | 0 ± 1.00 | -0.01 ± 1.05 | -0.06 ± 0.95 | 0.01 ± 0.99 | 0.23 ± 1.09 |
| Metabolic | 5177 (100) | 0 ± 0.59 | 0.02 ± 0.60 | -0.02 ± 0.57 | 0.01 ± 0.59 | -0.04 ± 0.62 |
| Autonomic | 5177 (100) | 0 ± 0.69 | 0.02 ± 0.70 | -0.01 ± 0.70 | -0.01 ± 0.67 | 0.03 ± 0.73 |
| Immune | 5105 (98.6) | 0 ± 0.81 | -0.01 ± 0.67 | -0.01 ± 0.93 | 0.02 ± 0.83 | 0.05 ± 0.63 |
| Biological measures | ||||||
| Cortisol, mmol/l | 5074 (95.7) | 9.9 ± 4.1 | 9.8 ± 4.3 | 9.6 ± 3.9 | 9.9 ± 4.1 | 10.8 ± 4.5 |
| HBA1c, % | 4980 (93.9) | 6.0 ± 1.3 | 6.0 ± 1.3 | 5.9 ± 1.3 | 6.0 ± 1.3 | 6.1 ± 1.4 |
| LDL cholesterol, mg/dl | 5027 (94.8) | 125.9 ± 36.5 | 125.9 ±37.2 | 125.2 ±35.8 | 127.0 ±36.3 | 122.0 ±38.2 |
| HDL cholesterol, mg/dl | 5072 (95.7) | 51.6 ± 14.6 | 51.8 ± 15.2 | 51.3 ± 14.4 | 51.4 ± 14.3 | 53.3 ± 15.3 |
| Total cholesterol, mg/dl | 5072 (95.7) | 198.7 ± 39.9 | 199.0 ± 40.6 | 197.3 ± 38.4 | 199.6 ± 40.4 | 197.2 ± 41.1 |
| Waist circumference, cm | 5175 (97.6) | 100.7 ± 16.2 | 102.2 ± 16.8 | 100.2 ± 16.0 | 100.1 ± 15.9 | 100.0 ± 15.6 |
| Systolic BP, mm Hg | 5169 (97.5) | 127.5 ± 16.8 | 127.3 ± 16.8 | 127.1 ± 16.5 | 127.5 ± 16.8 | 129.3 ± 19.0 |
| Diastolic BP, mm Hg | 5169 (97.5) | 75.8 ± 8.8 | 76.0 ± 9.0 | 75.8 ± 8.9 | 75.6 ± 8.4 | 75.1 ± 9.4 |
| Resting heart rate, beats per minute | 5165 (97.4) | 64.5 ± 10.6 | 64.8 ± 10.8 | 64.4 ± 10.7 | 64.2 ±10.3 | 65.1 ± 11.2 |
| CRP, median [IQR], mg/dl | 5078 (95.8) | 0.26 [0.11, 0.56] | 0.27 [0.11, 0.58] | 0.25 [0.10, 0.55] | 0.26 [0.11, 0.57] | 0.30 [0.14, 0.61] |
| WBC, 1000 cells/cmm | 4586 (86.5) | 5.6 ± 2.1 | 5.6 ± 1.9 | 5.5 ± 1.7 | 5.7 ± 2.5 | 5.8 ± 1.7 |
| CKD outcomes | ||||||
| Baseline eGFR, ml/min per 1.73 m2 | 5109 (96.4) | 94.2 ± 21.9 | 94.8 ± 22.4 | 95.2 ± 21.2 | 94.0 ± 21.5 | 87.9 ± 25.4 |
| Baseline ACR, median [IQR], mg/g | 3259 (61.5) | 6 [4, 12] | 6 [4, 13] | 6 [4, 11] | 6 [4, 13] | 7 [5, 18] |
| Events | ||||||
| Prevalent CKD at baseline, n (%) | 3373 (63.6) | 656 (12.7) | 176 (13.7) | 168 (11.0) | 253 (12.1) | 59 (20.7) |
| Annual eGFR decline | 3658 (69.0) | 1.27 ± 1.97 | 1.23 ± 2.01 | 1.16 ± 1.84 | 1.36 ± 2.04 | 1.35 ± 1.88 |
| Incident CKD at follow-up, n (%) | 2028 (38.3) | 250 (12.3) | 76 (3.7) | 57 (2.8) | 107 (5.3) | 10 (0.5) |
ACR, albumin-to-creatinine ratio; BP, blood pressure; CKD, chronic kidney disease; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; IQR, interquartile range; JHS, Jackson Heart Study; LDL, low-density lipoprotein; WBC, white blood cells.
Values are mean ± SD unless stated otherwise.
Mean eGFR at baseline among 5109 (96%) JHS participants was 94 ml/min per 1.73 m2 (SD 22). Mean eGFR was comparable among participants with very short, short, and the recommended 7 to 8 hours of sleep: eGFR 95 (SD 22) versus 95 (SD 21) versus 94 (SD 22) ml/min per 1.73 m2, respectively; however, it was lower in those with long sleep: eGFR 88 (SD 25) ml/min per 1.73 m2. Baseline albumin-to-creatinine ratio was assessed in 3259 (62%) JHS participants and it was comparable across all sleep duration categories (Table 2).
Evaluating Allostatic Load as a Mediator of Sleep and CKD Outcomes
Sleep and Prevalent CKD
Among 3373 (64%) participants with CKD measures available at baseline, 650 (13%) had prevalent CKD. Participants with very short sleep (vs. 7–8 hours) had greater odds of prevalent CKD at baseline: OR for direct effect 1.31, 95% CI 1.03 to 1.66. Allostatic load did not mediate this association: OR for mediated association: 0.99, 95% CI 0.94 to 1.04. Short or long sleep duration (vs. 7–8 hours) was not associated with odds of prevalent CKD. Low (vs. high) sleep quality associated with 26% increased odds of prevalent CKD at baseline: OR for direct association 1.26, 95% CI 1.00 to 1.60. There was no mediated association via allostatic load: OR 1.02, 95% CI 0.98 to 1.07 (Table 3).
Table 3.
Direct and mediated (via allostatic load) effects of sleep pattern on CKD outcomes in the Jackson Heart Study
| Sleep measures | Prevalent CKD |
Incident CKD |
eGFR decline |
|||
|---|---|---|---|---|---|---|
| Direct effect OR (95% CI) | Mediated effect OR (95% CI) | Direct effect OR (95% CI) | Mediated effect OR (95% CI) | Direct effect β (95% CI) | Mediated effect β (95% CI) | |
| No. of events/N | 656/3373 | 656/3373 | 250/2028 | 250/2028 | 2392 | 2392 |
| Sleep duration | ||||||
| Very short | 1.31 (1.03 to 1.66) | 0.99 (0.94 to 1.04) | 1.35 (0.96 to 1.89) | 0.98 (0.94 to 1.01) | −0.10 (−0.29 to 0.09) | −0.01 (−0.02 to 0.00) |
| Short | 0.96 (0.76 to 1.22) | 0.98 (0.94 to 1.03) | 0.76 (0.53 to 1.09) | 0.99 (0.95 to 1.02) | −0.20 (−0.38 to −0.03) | 0.00 (−0.02 to 0.01) |
| 7–8 hours | Ref | Ref | Ref | Ref | Ref | Ref |
| Long | 1.27 (0.88 to 1.83) | 1.05 (0.97 to 1.14) | 0.60 (0.30 to 1.23) | 1.02 (0.94 to 1.09) | −0.23 (−0.57 to 0.11) | 0.00 (−0.02 to 0.03) |
| Sleep quality | ||||||
| High | Ref | Ref | Ref | Ref | Ref | Ref |
| Moderate | 1.09 (0.86 to 1.38) | 1.01 (0.97 to 1.06) | 0.87 (0.61 to 1.23) | 1.01 (0.97 to 1.04) | 0.13 (−0.05 to 0.31) | 0.00 (−0.01 to 0.01) |
| Low | 1.26 (1.00 to 1.60) | 1.02 (0.98 to 1.07) | 1.25 (0.89 to 1.77) | 1.00 (0.96 to 1.04) | 0.18 (0.00 to 0.36) | 0.00 (−0.01 to 0.01) |
CI, confidence interval, CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; OR, odds ratio.
All models adjusted for age, sex, educational attainment, household income, employment status, alcohol consumption, and body mass index; the eGFR decline model additionally adjusted for baseline albumin-to-creatinine ratio and eGFR.
Sleep and Incident CKD
After a median follow-up of 8 years, 250 (12%) of those whose CKD status could be determined (n = 2028; 38%) developed incident CKD. Very short, short, or long sleep duration (vs. 7–8 hours) was not significantly associated with incident CKD. Similarly, low sleep quality (vs. high) was not significantly associated with incident CKD (OR 1.25, 95% CI 0.89–1.77). Allostatic load did not mediate the associations of sleep duration or sleep quality with incident CKD (Table 3).
Sleep and eGFR Decline
The mean eGFR decline among 3658 (69%) JHS participants over follow-up was 1.3 ml/min per 1.73 m2 per year (SD 2.0). Very short or long sleep duration (vs. 7–8 hours) was not associated with eGFR decline. Short sleep duration (6 hours vs. 7–8 hours) associated with a 0.20 ml/min per 1.73 m2 (95% CI 0.38–0.03) slower eGFR decline per year over follow-up. There was no mediated association via allostatic load: 0.00 ml/min per 1.73 m2 per year (95% CI −0.02 to 0.01). Low sleep quality (vs. high) associated with 0.18 ml/min per 1.73 m2 (95% CI 0.00–0.36) faster eGFR decline per year; this association was not mediated via allostatic load: 0.00 ml/min per 1.73 m2 per year, 95% CI −0.01 to 0.01 (Table 3).
Sensitivity Analyses
In sensitivity analyses, associations between very short (≤5 hours) sleep duration and adverse kidney outcomes were more evident when short (6 hours) sleep duration was the reference sleep category (Supplementary Table S1). For instance, very short sleep (vs. 6 hours) associated with increased odds of incident CKD: OR 1.77, 95% CI 1.20 to 2.60. Allostatic load did not mediate this association: OR 0.99, 95% CI 0.96 to 1.03. Associations between sleep and kidney outcomes were robust with additional adjustment for depression (Supplementary Table S2). Results of mediation pathways via each of the 4 domains of allostatic load were generally consistent with that of the global allostatic load score (results not shown).
In analyses stratified by hypertension (Table 4) and diabetes (Table 5), the associations between sleep and kidney outcomes were evident only among participants with diabetes: short sleep duration (vs. 7–8 hours) associated with 0.68 ml/min per 1.73 m2 (95% CI 0.07–1.30) slower eGFR decline per year among those with diabetes.
Table 4.
Direct and mediated (via allostatic load) effects of sleep pattern on CKD outcomes; stratified by baseline hypertension status
| Sleep measures stratified by hypertension status | Prevalent CKD |
Incident CKD |
eGFR decline |
|||
|---|---|---|---|---|---|---|
| Direct effect OR (95% CI) | Mediated effect OR (95% CI) | Direct effect OR (95% CI) | Mediated effect OR (95% CI) | Direct effect β (95% CI) | Mediated effect β (95% CI) | |
| Hypertension | ||||||
| No. of events/N | 320/1303 | 320/1303 | 141/754 | 141/754 | 939 | 939 |
| Sleep duration | ||||||
| Very short | 1.31 (0.94 to 1.85) | 0.98 (0.91 to 1.05) | 1.31 (0.80 to 2.14) | 0.99 (0.93 to 1.04) | 0.00 (−0.33 to 0.32) | −0.01 (−0.04 to 0.01) |
| Short | 1.00 (0.72 to 1.40) | 0.97 (0.90 to 1.02) | 0.88 (0.54 to 1.42) | 1.00 (0.94 to 1.05) | −0.32 (−0.62 to −0.02) | 0.00 (−0.03 to 0.01) |
| 7–8 hours | Ref | Ref | Ref | Ref | Ref | Ref |
| Long | 0.91 (0.53 to 1.57) | 0.99 (0.88 to 1.11) | 0.70 (0.29 to 1.71) | 0.99 (0.90 to 1.10) | −0.14 (−0.69 to 0.41) | 0.01 (−0.02 to 0.05) |
| No hypertension | ||||||
| No. of events/N | 77/1067 | 77/1067 | 51/754 | 51/754 | 834 | 834 |
| Sleep duration | ||||||
| Very short | 1.64 (0.88 to 3.04) | 0.96 (0.89 to 1.01) | 1.53 (0.75 to 3.12) | 0.95 (0.86 to 1.01) | −0.04 (−0.33 to 0.25) | −0.01 (−0.04 to 0.01) |
| Short | 1.32 (0.72 to 2.41) | 0.99 (0.92 to 1.04) | 0.87 (0.40 to 1.89) | 0.98 (0.91 to 1.07) | −0.03 (−0.30 to 0.25) | 0.00 (−0.03 to 0.01) |
| 7–8 hours | Ref | Ref | Ref | Ref | Ref | Ref |
| Long | 1.72 (0.64 to 4.60) | 1.05 (0.94 to 1.21) | 0.32 (0.04 to 2.64) | 1.09 (0.93 to 1.37) | −0.42 (−0.95 to 0.12) | 0.01 (−0.03 to 0.06) |
CI, confidence interval, CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; OR, odds ratio.
All models adjusted for age, sex, educational attainment, household income, employment status, alcohol consumption, and body mass index; the eGFR decline model additionally adjusted for baseline albumin-to-creatinine ratio and eGFR.
Table 5.
Direct and mediated (via allostatic load) effects of sleep pattern on CKD outcomes; stratified by baseline diabetes status
| Sleep measures stratified by diabetes status | Prevalent CKD |
Incident CKD |
eGFR decline |
|||
|---|---|---|---|---|---|---|
| Direct effect OR (95% CI) | Mediated effect OR (95% CI) | Direct effect OR (95% CI) | Mediated effect OR (95% CI) | Direct effect β (95% CI) | Mediated effect β (95% CI) | |
| Diabetes | ||||||
| No. of events/N | 166/485 | 166/485 | 69/242 | 69/242 | 329 | 329 |
| Sleep duration | ||||||
| Very short | 1.66 (0.99 to 2.77) | 0.91 (0.80 to 1.02) | 1.56 (0.71 to 3.40) | 0.91 (0.70 to 1.11) | 0.01 (−0.65 to 0.66) | −0.07 (−0.22 to 0.03) |
| Short | 1.00 (0.60 to 1.69) | 0.91 (0.79 to 1.00) | 1.27 (0.59 to 2.71) | 0.90 (0.70 to 1.09) | −0.68 (−1.30 to −0.07) | −0.08 (−0.22 to 0.01) |
| 7–8 hours | Ref | Ref | Ref | Ref | Ref | Ref |
| Long | 0.66 (0.31 to 1.42) | 1.03 (0.88 to 1.22) | 0.92 (0.28 to 3.00) | 1.05 (0.80 to 1.43) | −0.34 (−1.31 to 0.63) | 0.04 (−0.10 to 0.23) |
| No diabetes | ||||||
| No. of events/N | 227/1881 | 227/1881 | 123/1266 | 123/1266 | 1443 | 1443 |
| Sleep duration | ||||||
| Very short | 1.23 (0.84 to 1.78) | 1.00 (0.95 to 1.05) | 1.20 (0.75 to 1.93) | 0.99 (0.97 to 1.01) | −0.03 (−0.25 to 0.19) | 0.00 (−0.01 to 0.02) |
| Short | 1.09 (0.76 to 1.56) | 1.00 (0.95 to 1.04) | 0.71 (0.43 to 1.16) | 1.00 (0.98 to 1.02) | −0.09 (−0.30 to 0.12) | 0.00 (−0.01 to 0.01) |
| 7–8 hours | Ref | Ref | Ref | Ref | Ref | Ref |
| Long | 1.38 (0.75 to 2.54) | 0.99 (0.90 to 1.09) | 0.35 (0.10 to 1.21) | 1.00 (0.96 to 1.05) | −0.27 (−0.68 to 0.14) | 0.00 (−0.03 to 0.02) |
CI, confidence interval, CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; OR, odds ratio.
All models adjusted for age, sex, educational attainment, household income, employment status, alcohol consumption, and body mass index; the eGFR decline model additionally adjusted for baseline albumin-to-creatinine ratio and eGFR.
Discussion
In a large prospective cohort of black American individuals, very short sleep duration (≤5 hours) and low sleep quality were independently associated with greater prevalence of CKD at baseline. Low sleep quality also associated with faster eGFR decline per year over long-term follow-up. Short sleep duration (6 hours), compared with the recommended 7 to 8 hours, associated with slower annual eGFR decline over follow-up. Allostatic load did not mediate the associations between sleep duration or sleep quality with kidney outcomes. These results suggest a role of poor sleep as a risk factor for development of CKD in black individuals, but its effect on kidney outcomes does not appear to be mediated via allostatic load. Furthermore, sleep duration of 6 hours appears to confer the most favorable kidney outcomes in this all-black cohort.
To our knowledge, this is the first study to evaluate allostatic load as a mediator of the association between sleep and kidney health in a large cohort of black Americans. Unfavorable sleep patterns are especially common among black Americans25, 26 and may contribute to health disparities in this group,1 yet the mechanisms by which poor sleep affects kidney health have not been elucidated to-date. Prior work has established the relation of suboptimal sleep patterns in the general population with development of chronic diseases, such as diabetes,27 hypertension,28 and cardiovascular disease.29 Notably, these relations persist when evaluating objectively measured or subjectively reported sleep duration or quality. Further, the potential damage caused by suboptimal sleep is evident when examining its relation with kidney health; poor sleep patterns have been linked to rapid kidney function decline,1 incident albuminuria,2, 3 and progression of CKD.4
However, although poor sleep consistently associates with adverse health in epidemiologic studies, its causal role as it pertains to development of CKD and known CKD risk factors, such as hypertension and diabetes, remains unclear.30 For instance, considering that poor sleep is often a manifestation of illness, including CKD, the association of suboptimal sleep characteristics (e.g., short sleep duration and poor sleep quality) with unfavorable kidney health in observational studies, particularly cross-sectional studies, may reflect a reverse causal phenomenon.31 Therefore, identifying preclinical biologic pathways by which poor sleep potentially affects development and progression of CKD may elucidate whether the association of poor sleep with CKD outcomes is causal or due to a spurious relationship between sleep and kidney health.30
Allostatic load has been proposed as a potential mediator of the association between sleep and kidney outcomes, as its role as a key biologic mediator of the cumulative effects of social determinants on health becomes increasingly evident.14 Derangements in domains of allostatic load, such as immune dysregulation,32 neuroendocrine dysfunction,33 and metabolic dysfunction,34 have been previously linked with suboptimal sleep patterns in the general population. However, these relations have not been consistently found in black populations.35, 36 Our findings also suggest that allostatic load may not be an important mediator of associations between sleep and poor kidney outcomes in black individuals. This suggests that the detrimental effects of suboptimal sleep on health via these biologic mediators might be blunted by factors that are unique to the black population, including behavioral adaptive mechanisms like coping,37, 38, 39 which is hypothesized to modify the adverse health impact of external stressors in black individuals. Therefore, future work examining the relation between sleep and allostatic load should also evaluate the potential role of such behavioral adaptive strategies such as cognitive reappraisal and emotion expression on this relation.40
In addition to allostatic load, other physiologic mediators of the association between sleep patterns and kidney outcomes warrant investigation in future work. For example, in experimental settings, sleep deprivation has been shown to disrupt homeostasis in components of the renin-angiotensin-aldosterone system (e.g., nocturnal release of aldosterone and excretion of electrolytes).41, 42 Furthermore, observational studies associate sleep duration with markers of endothelial dysfunction.43 Thus, given that derangements in the renin-angiotensin-aldosterone system and endothelial dysfunction44 are well-established mechanisms underlying kidney damage, studies should examine their potential role in the observed association between sleep and CKD to determine the nature of this relation.
We noted some limitations. First, the sleep measures that we used in our analysis were self-reported and assessed once over a 24-hour period; prior work suggests that although subjective reports of usual sleep correlate moderately with objectively measured sleep, individuals tend to overestimate their sleep duration.45 Thus, further work using objectively measured sleep should explore the validity and reliability of our findings, particularly regarding the amount of sleep that confers the most favorable kidney outcomes. Furthermore, although we defined allostatic load based on the most commonly used definition, future work should examine if our findings are robust to other proposed definitions of allostatic load.46, 47 In addition, given the observational design of the JHS, we were not able to account for residual confounding; randomized studies will be needed to provide conclusive evidence regarding the role of allostatic load on the sleep-CKD relation. Because the JHS was limited to 1 geographic region, our findings may not be generalizable to other populations in the United States. Last, considering that the JHS measured both sleep and allostatic load at baseline, the lack of a temporal relation between exposure and mediator limits causal inference regarding the hypothesized mediation via allostatic load of the effects of sleep on kidney health. Despite these limitations, our study had important strengths. First, the JHS measured biomarkers required for computing allostatic load in a large cohort of community-dwelling black individuals. Second, most participants in the JHS were followed for a median of 8 years to allow for development of longitudinal kidney outcomes. The availability of prospective data also allowed for mediation analytic approaches.
In conclusion, in a prospective cohort of black Americans, very short sleep duration and low sleep quality were associated with greater prevalence of CKD at baseline and low sleep quality associated with faster kidney function decline over a median follow-up of 8 years, but allostatic load did not appear to mediate these associations. Future work should investigate the role of other biologic mediators in the relation between sleep and CKD outcomes in black individuals.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The authors thank the staffs and participants of the Jackson Heart Study.
The Jackson Heart Study is supported and conducted in collaboration with Jackson State University (HHSN268201300049C and HHSN268201300050C), Tougaloo College (HHSN268201300048C), and the University of Mississippi Medical Center (HHSN268201300046C and HHSN268201300047C) contracts from the National Heart, Lung, and Blood Institute and the National Institute for Minority Health and Health Disparities. This work, in part, was funded by the Intramural Program at the National Institutes of Health, National Institute of Environmental Health Sciences (Z1AES103325-01). The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute, the National Institutes of Health, or the US Department of Health and Human Services.
Footnotes
Figure S1. Model pathways for mediation analysis of the effect of sleep pattern on CKD outcomes via allostatic load.
Table S1. Direct and mediated (via allostatic load) effects of sleep pattern on CKD outcomes with sleep duration of 6 hours per 24 hours as the reference category.
Table S2. Direct and mediated (via allostatic load) effects of sleep pattern on CKD outcomes in a subset of Jackson Heart Study participants who had data for depression.
Supplementary material is linked to the online version of the paper at www.kireports.org/.
Supplementary Material
Model pathways for mediation analysis of the effect of sleep pattern on CKD outcomes via allostatic load.
Direct and mediated (via allostatic load) effects of sleep pattern on CKD outcomes with sleep duration of 6 hours per 24 hours as the reference category.
Direct and mediated (via allostatic load) effects of sleep pattern on CKD outcomes in a subset of Jackson Heart Study participants who had data for depression.
References
- 1.McMullan C.J., Curhan G.C., Forman J.P. Association of short sleep duration and rapid decline in renal function. Kidney Int. 2016;89:1324–1330. doi: 10.1016/j.kint.2015.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheungpasitporn W., Thongprayoon C., Gonzalez-Suarez M.L. The effects of short sleep duration on proteinuria and chronic kidney disease: a systematic review and meta-analysis. Nephrol Dial Transplant. 2017;32:991–996. doi: 10.1093/ndt/gfw072. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto R., Nagasawa Y., Iwatani H. Self-reported sleep duration and prediction of proteinuria: a retrospective cohort study. Am J Kidney Dis. 2012;59:343–355. doi: 10.1053/j.ajkd.2011.08.032. [DOI] [PubMed] [Google Scholar]
- 4.Ricardo A.C., Knutson K., Chen J. The Association of sleep duration and quality with CKD progression. J Am Soc Nephrol. 2017;28:3708–3715. doi: 10.1681/ASN.2016121288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson C.L., Redline S., Emmons K.M. Sleep as a potential fundamental contributor to disparities in cardiovascular health. Annu Rev Public Health. 2015;36:417–440. doi: 10.1146/annurev-publhealth-031914-122838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsu C.Y., Lin F., Vittinghoff E., Shlipak M.G. Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. J Am Soc Nephrol. 2003;14:2902–2907. doi: 10.1097/01.asn.0000091586.46532.b4. [DOI] [PubMed] [Google Scholar]
- 7.Castro-Diehl C., Diez Roux A.V., Redline S. Sleep duration and quality in relation to autonomic nervous system measures: The Multi-Ethnic Study of Atherosclerosis (MESA) Sleep. 2016;39:1927–1940. doi: 10.5665/sleep.6218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmid S.M., Hallschmid M., Schultes B. The metabolic burden of sleep loss. Lancet Diabetes Endocrinol. 2015;3:52–62. doi: 10.1016/S2213-8587(14)70012-9. [DOI] [PubMed] [Google Scholar]
- 9.Spiegel K., Leproult R., Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 10.Buxton O.M., Cain S.W., O’ Connor S.P. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med. 2012;4:129ra43. doi: 10.1126/scitranslmed.3003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMullan C.J., Schernhammer E.S., Rimm E.B. Melatonin secretion and the incidence of type 2 diabetes. JAMA. 2013;309:1388–1396. doi: 10.1001/jama.2013.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sabbah W., Watt R.G., Sheiham A., Tsakos G. Effects of allostatic load on the social gradient in ischaemic heart disease and periodontal disease: evidence from the Third National Health and Nutrition Examination Survey. J Epidemiol Community Health. 2008;62:415–420. doi: 10.1136/jech.2007.064188. [DOI] [PubMed] [Google Scholar]
- 13.Duru O.K., Harawa N.T., Kermah D., Norris K.C. Allostatic load burden and racial disparities in mortality. J Natl Med Assoc. 2012;104:89–95. doi: 10.1016/s0027-9684(15)30120-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruce M.A., Griffith D.M., Thorpe R.J., Jr. Stress and the kidney. Adv Chronic Kidney Dis. 2015;22:46–53. doi: 10.1053/j.ackd.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norton J.M., Moxey-Mims M.M., Eggers P.W. Social determinants of racial disparities in CKD. J Am Soc Nephrol. 2016;27:2576–2595. doi: 10.1681/ASN.2016010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor H.A., Jr. The Jackson Heart Study of the future. Ethn Dis. 2012;22(3 suppl 1):S1-49–S1-54. [PubMed] [Google Scholar]
- 17.Fuqua S.R., Wyatt S.B., Andrew M.E. Recruiting African-American research participation in the Jackson Heart Study: methods, response rates, and sample description. Ethn Dis. 2005;15(4 suppl 6):S6-18–S6-29. [PubMed] [Google Scholar]
- 18.Fülöp T., Hickson D.A., Wyatt S.B. Sleep-disordered breathing symptoms among African-Americans in the Jackson Heart Study. Sleep Med. 2012;13:1039–1049. doi: 10.1016/j.sleep.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson D.A., Lisabeth L., Lewis T.T. The contribution of psychosocial stressors to sleep among African Americans in the Jackson Heart Study. Sleep. 2016;39:1411–1419. doi: 10.5665/sleep.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sacco R.L. The new American Heart Association 2020 goal: achieving ideal cardiovascular health. J Cardiovasc Med (Hagerstown) 2011;12:255–257. doi: 10.2459/JCM.0b013e328343e986. [DOI] [PubMed] [Google Scholar]
- 21.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang W., Young B.A., Fülöp T. Effects of serum creatinine calibration on estimated renal function in African Americans: the Jackson Heart Study. Am J Med Sci. 2015;349:379–384. doi: 10.1097/MAJ.0000000000000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayes A.F., Preacher K.J. Statistical mediation analysis with a multicategorical independent variable. Br J Math Stat Psychol. 2014;67:451–470. doi: 10.1111/bmsp.12028. [DOI] [PubMed] [Google Scholar]
- 24.Baron R.M., Kenny D.A. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 25.Jackson C.L., Redline S., Kawachi I. Racial disparities in short sleep duration by occupation and industry. Am J Epidemiol. 2013;178:1442–1451. doi: 10.1093/aje/kwt159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen X., Wang R., Zee P. Racial/ethnic differences in sleep disturbances: The Multi-Ethnic Study of Atherosclerosis (MESA) Sleep. 2015;38:877–888. doi: 10.5665/sleep.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shan Z., Ma H., Xie M. Sleep duration and risk of type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 2015;38:529–537. doi: 10.2337/dc14-2073. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y., Mei H., Jiang Y.R. Relationship between duration of sleep and hypertension in adults: a meta-analysis. J Clin Sleep Med. 2015;11:1047–1056. doi: 10.5664/jcsm.5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang D., Li W., Cui X. Sleep duration and risk of coronary heart disease: a systematic review and meta-analysis of prospective cohort studies. Int J Cardiol. 2016;219:231–239. doi: 10.1016/j.ijcard.2016.06.027. [DOI] [PubMed] [Google Scholar]
- 30.Okun M.L. Biological consequences of disturbed sleep: important mediators of health? Jpn Psychol Res. 2011;53:163–176. doi: 10.1111/j.1468-5884.2011.00463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maung S.C., El Sara A., Chapman C. Sleep disorders and chronic kidney disease. World J Nephrol. 2016;5:224–232. doi: 10.5527/wjn.v5.i3.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grandner M.A., Buxton O.M., Jackson N. Extreme sleep durations and increased C-reactive protein: effects of sex and ethnoracial group. Sleep. 2013;36:769–779E. doi: 10.5665/sleep.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tajiri E., Yoshimura E., Hatamoto Y. Effect of sleep curtailment on dietary behavior and physical activity: a randomized crossover trial. Physiol Behav. 2018;184:60–67. doi: 10.1016/j.physbeh.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Rangaraj V.R., Knutson K.L. Association between sleep deficiency and cardiometabolic disease: implications for health disparities. Sleep Med. 2016;18:19–35. doi: 10.1016/j.sleep.2015.02.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wiener R.C., Zhang R., Shankar A. Elevated serum C-reactive protein and markers of sleep disordered breathing. Int J Vasc Med. 2012;2012:914593. doi: 10.1155/2012/914593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanagasabai T., Chaput J.P. Sleep duration and the associated cardiometabolic risk scores in adults. Sleep Health. 2017;3:195–203. doi: 10.1016/j.sleh.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 37.Fernandez C.A., Loucks E.B., Arheart K.L. Evaluating the effects of coping style on allostatic load, by sex: The Jackson Heart Study, 2000–2004. Prev Chronic Dis. 2015;12:E165. doi: 10.5888/pcd12.150166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okhomina V.I., Glover L., Taylor H., Sims M. Dimensions of and responses to perceived discrimination and subclinical disease among African-Americans in the Jackson Heart Study. J Racial Ethn Health Disparities. 2018;5:1084–1092. doi: 10.1007/s40615-017-0457-7. [DOI] [PubMed] [Google Scholar]
- 39.Lunyera J., Davenport C.A., Bhavsar N.A. Nondepressive psychosocial factors and CKD outcomes in Black Americans. Clin J Am Soc Nephrol. 2018;13:213–222. doi: 10.2215/CJN.06430617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson S.J., Jaremka L.M., Fagundes C.P. Shortened sleep fuels inflammatory responses to marital conflict: emotion regulation matters. Psychoneuroendocrinology. 2017;79:74–83. doi: 10.1016/j.psyneuen.2017.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Charloux A., Gronfier C., Chapotot F. Sleep deprivation blunts the night time increase in aldosterone release in humans. J Sleep Res. 2001;10:27–33. doi: 10.1046/j.1365-2869.2001.00235.x. [DOI] [PubMed] [Google Scholar]
- 42.Kamperis K., Hagstroem S., Radvanska E. Excess diuresis and natriuresis during acute sleep deprivation in healthy adults. Am J Physiol Renal Physiol. 2010;299:F404–F411. doi: 10.1152/ajprenal.00126.2010. [DOI] [PubMed] [Google Scholar]
- 43.Hall M.H., Mulukutla S., Kline C.E. Objective sleep duration is prospectively associated with endothelial health. Sleep. 2017;40(1) doi: 10.1093/sleep/zsw003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rossi G.P., Seccia T.M., Barton M. Endothelial factors in the pathogenesis and treatment of chronic kidney disease Part I: general mechanisms: a joint consensus statement from the European Society of Hypertension Working Group on Endothelin and Endothelial Factors and The Japanese Society of Hypertension. J Hypertens. 2018;36:451–461. doi: 10.1097/HJH.0000000000001599. [DOI] [PubMed] [Google Scholar]
- 45.Lauderdale D.S., Knutson K.L., Yan L.L. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;19:838–845. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duong M.T., Bingham B.A., Aldana P.C. Variation in the calculation of allostatic load score: 21 examples from NHANES. J Racial Ethn Health Disparities. 2017;4:455–461. doi: 10.1007/s40615-016-0246-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnson S.C., Cavallaro F.L., Leon D.A. A systematic review of allostatic load in relation to socioeconomic position: poor fidelity and major inconsistencies in biomarkers employed. Soc Sci Med. 2017;192:66–73. doi: 10.1016/j.socscimed.2017.09.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Model pathways for mediation analysis of the effect of sleep pattern on CKD outcomes via allostatic load.
Direct and mediated (via allostatic load) effects of sleep pattern on CKD outcomes with sleep duration of 6 hours per 24 hours as the reference category.
Direct and mediated (via allostatic load) effects of sleep pattern on CKD outcomes in a subset of Jackson Heart Study participants who had data for depression.