Background
Group C streptococcus (GCS) bacteremia can lead to a virulent infection. We describe one such case and review previous published literature.
Introduction
Streptococcus dysgalactiae subsp. equisimilis (SDSE) is a gram positive, beta-hemolytic, coccal bacterium belonging to the family Streptococcaceae and belongs to the group C and group G streptococci. Streptococcus dysgalactiae is currently divided into the subspecies Streptococcus dysgalactiae subspecies equisimilis (SDSE) and Streptococcus dysgalactiae subspecies dysgalactiae (SDSD); the former mostly associated with human disease, and the latter almost exclusively encountered in veterinary medicine. SDSE is a common colonizer of the pharynx, skin, gastrointestinal tract, and female genital tract and a part of the normal human flora. The clinical manifestations in human disease range from superficial skin infections and tonsillitis, to severe necrotizing fasciitis and bacteremia. The incidence of invasive disease has been reported to be rising.
Case report
A 72-year-old man was sent to our hospital after a mechanical fall. He was found lying on the floor at home for 3 days prior to admission. The patient has a history of Stage IVA (T2N2bM0) squamous cell carcinoma (positive HPV) of bottom of tongue and completed concurrent chemoradiotherapy with cetuximib and radiotherapy 8 months prior to admission.
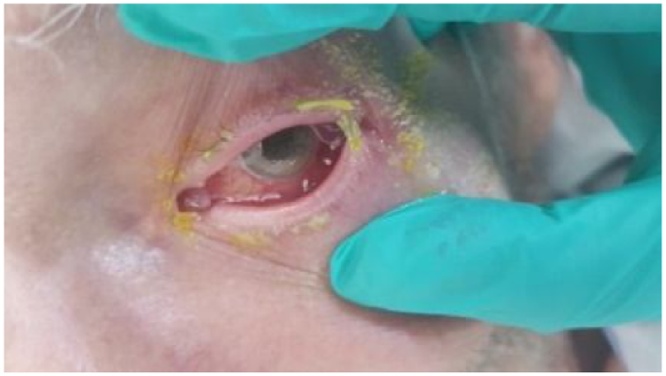
On arrival to the hospital, the patient was alert and oriented to time, place and person. He complained of weakness with pain in right wrist and left hip with epiphora and pain in left eye. His height was 182.9 cm, weight was 73 kg, and vital signs were as follows: blood pressure, 175/82 mmHg; regular heart rate, 91 beats/min; oxygen saturation, 97% on room air; respiratory rate, 18 breaths/min, and body temperature, 36.5 °C. Physical examination showed limited range of motion of both hips and minimal erythema, palpable trace effusion and tenderness to palpation over dorsal distal radius, volar wrist and carpus on right hand and wrist. His ocular examination revealed left eye matted eyelashes, mucopurulent conjunctival secretions, (Fig. 1), corneal edema, hypopyon with fibrinous membrane in pupil, no red glow seen, pupil fixed and non-reactive.
Fig. 1.
Mucopurulent conjunctival secretions.
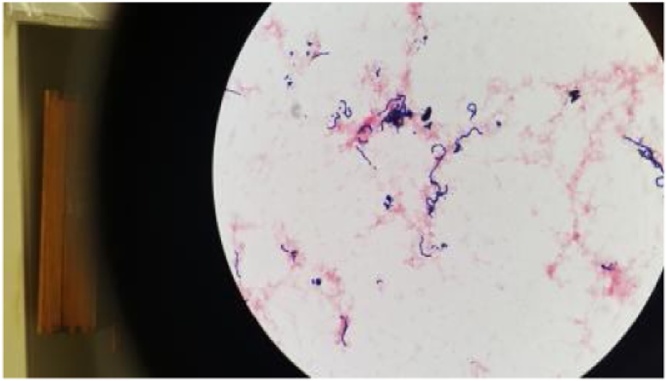
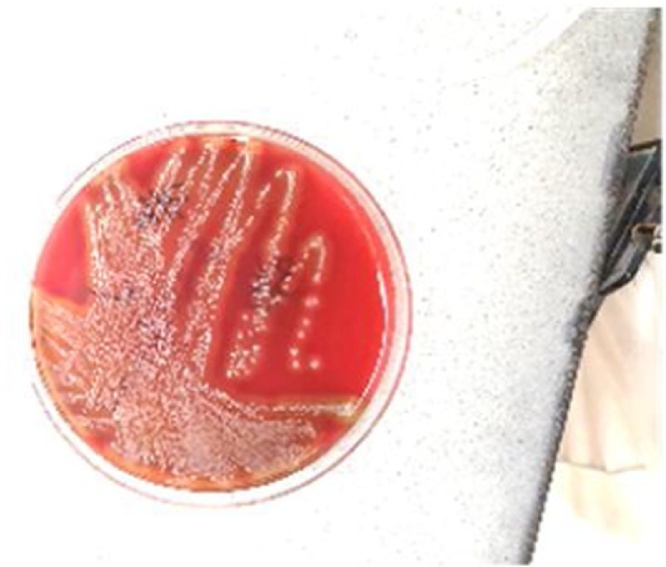
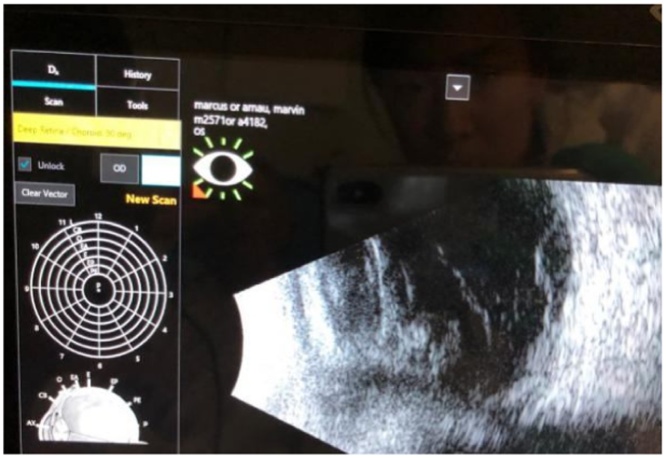
Laboratory examination showed a white blood cell count, 6.6 K/cmm, thrombocytopenia (platelet count, 47.0 K/cmm, renal impairment (blood urea nitrogen, 47.0 mg/dL; creatinine,1.4 mg/dL)and procalcitonin 5.13 ng/mL. Two sets of blood cultures were obtained and exhibited growth of Gram-positive cocci in chains (Fig. 2), which was noted to be beta-hemolytic, and Group C (Fig. 3) Streptococcus dysgalactiae subsp. equisimilis was identified by the clinical microbiology laboratory. Ophthalmology was consulted for his left eye pain, watering and decreased vision and right eye vision. B scan ultrasound of the left eye was done and showed vitreous debris (Fig. 4). The aqueous and vitreous tap of his left eye were obtained followed by intravitreal injection of vancomycin and ceftazidime. His vitreous culture yielded growth of the same Gram-positive Streptococcus dysgalactiae subsp. equisimilis. Transthoracic echocardiography (TTE) at day 3 admission showed bicuspid aortic valve with mild to moderate aortic regurgitation with normal left ventricular function, mild to moderate dilatation and normal right ventricular size and function. A TTE repeat at day 6 of admission showed aortic valve vegetation that could not exclude an abscess or anterior mitral valve leaflet vegetation. Transesophageal echocardiography (TEE) at day 9 admission showed small, mobile vegetation on the non-coronary cusp of the aortic valve and thickened aortic root (Fig. 5). Echodensity was also noted in the posterior aspect of the aortic root adjacent to the right atrium and moderate aortic valve vegetation. The mitral valve was thicken suggesting a possible microperforation of the mitral valve. There was a mobile echodensity noted on the posterior mitral valve, measuring 0.3 × 0.5 cm and mild-moderate mitral valve regurgitation.
Fig. 2.
Gram-positive cocci in chains which was noted to be beta-hemolytic.
Fig. 3.
Group C Streptococcus dysgalactiae subsp. equisimilis.
Fig. 4.
Vitreous debris from B Scan Ultrasound.
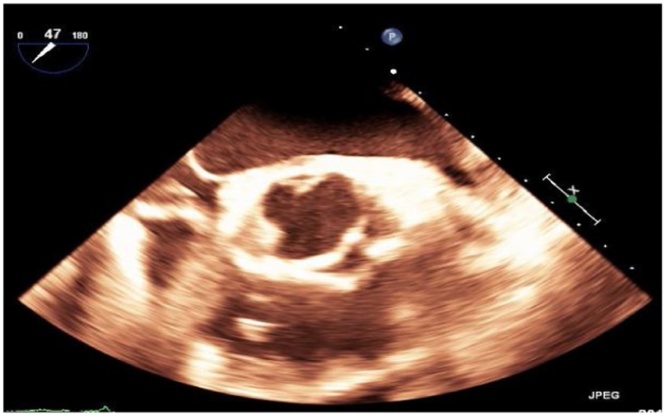
Fig. 5.
Transesophageal echocardiography (TEE) showed small, mobile vegetation on the non-coronary cusp of the aortic valve and thickened aortic root.
The patient was treated with 750 mg of intravenous vancomycin every 12 h for 2 days and followed by 4 million units of penicillin G intravenous every 4 h. Infective endocarditis (IE) using the modified Duke criteria (two major criterion) caused by SDSE. The patient left eye vision worsened to light perception and he received another intravitreal injection in left eye and was treated with topical trimethoprim/sulfamethoxazole, prednisolone acetate and atropine. He was scheduled for vitrectomy of his left eye but on the day of surgery was noted to have no light perception in left eye and as a result, vitrectomy was cancelled. The orthopedic service also examined the patient and ruled out septic arthritis. His right humerus, wrist, hand and shoulder showed no evidence of fracture or dislocation and no soft tissue swelling. Magnetic resonance imaging of the thoracic and lumbar spine with contrast showed no evidence of infection. Otolaryngologists had also examined the patient and found no infection or new lesions on his ear, nose and throat. All blood cultures that were sent throughout his treatment period were negative. The patient was eventually transferred to another facility for aortic valve replacement.
At the outside facility, the patient’s hospital course was complicated by acute heart failure secondary to worsening aortic regurgitation. The patient also developed hypoxic respiratory distress and a chest x-ray showed pulmonary edema. He was temporarily placed on BiPAP and started on dobutamine for afterload reduction and inotropic support. The following day, his bicuspid aortic valve was replaced with a prosthetic valve, and the aortic root abscess was debrided. Patient was transferred from cardiac intensive care unit to general medicine floor after 3 days for further management.
Discussion
This communication discusses a patient with Group C Streptococcuis dysgalactiae subspecies equisimilis (GCSDE) causing endogenous endophthalmitis (EE) and aortic root abscess and infective endocarditis (IE). A literature search was conducted using the keywords ‘Group C streptococcus', 'Streptococcus dysgalactiae', 'Streptococcus equisimilis',’ endogenous endophthalmitis’, and ‘infective endocarditis’.
Results of the search found a total of 104 cases of GCS heart or eye infection with 80 cases of IE and 24 with EE. Among the 80 patients with endocarditis, 73 (91%) were identified as GCSD and 10 (14%) were subspecies equisimilis (GCSDE). Of the 10 GCSDE cases with IE, 3 had peripheral septic complications including 1 meningitis and 2 gallbladder. Overall, 12 patients (15%) died, and 20% underwent valve replacement. Of the 24 reported endophtalmitis cases with GCS, 20 (83%) were blood culture positive, of which 2 were GCSDE. Overall, 44% had endocarditis with 62% having mitral valve involvement but no abscess. Twelve of EE patients had bilateral eye involvement of whom 8 (33% of total) had total loss of vision [[1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21],23].
Endogenous bacterial endophthalmitis (EBE) has been reported to constitute approximately 2–6% of all cases of endophthalmitis [22]. Blood cultures were positive in 74%–94% of patients and intraocular cultures were positive in 56% patients [22]. Fungal organisms accounted for more than 50% of all endogenous cases [22]. In 56% of patients, EBE was caused by Gram negative organisms, most commonly Klebsiella spp., E. coli, Pseudomonas aeruginosa, N. meningitidis, and Serratia marscescens. Gram positive organisms were responsible for 40% of cases. The most common Gram positive organisms were Staphylococcus aureus, group B streptococci, Streptococcus pneumoniae, Listeria monocytogenes, Nocardia asteroides, and group G streptococci [22].
A unique finding in our case was the isolation of Streptococcus dysgalactiae subsp. equisimilis from both blood and vitreous cultures., with incidental presentation and presence of myocardial abscess on TEE.
Conclusion
Peripheral septic complications are a rare but can be a serious feature of Group C Streptococcuis bacteremia. Our case describes a previously unreported presentation of Group C Streptococcuis dysgalactiae subspecies equisimilis causing endocarditis, myocardial abscess, and endophtalmitis with loss of vision.
Author statement
Paper has been revised based on 2 reviewers valuable advice.
Contributor Information
Lok Yung, Email: low9034@nyp.org.
Mahjabeen Rashid, Email: Mahjabeen.Rashid@va.gov.
Norbert Bräu, Email: Norbert.Brau@va.gov.
References
- 1.Ahmed S.I. Group C streptococcal endocarditis. Am J Med Sci. 2007;334:212–214. doi: 10.1097/MAJ.0b013e318141face. [DOI] [PubMed] [Google Scholar]
- 2.Ebneter A. A rare case of endogenous Streptococcus group C endophthalmitis associated with cellulitis. Eye. 2011;25(9):1239–1240. doi: 10.1038/eye.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yong A.S. Streptococcus dysgalactiae endocarditis presenting as acute endophthalmitis. Infect Dis Rep. 2012;4(February (1)):e16. doi: 10.4081/idr.2012.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suemori S., Sawada A., Komori S. Case of endogenous endophthalmitis caused by Streptococcus equisimilis. Clin Ophthalmol. 2010;4:917–918. doi: 10.2147/opth.s10900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gopalamurugan A.B. Bilateral endophthalmitis and ARDS complicating group G streptococcal endocarditis. Lancet. 2005;366:2062. doi: 10.1016/S0140-6736(05)67817-8. [DOI] [PubMed] [Google Scholar]
- 6.Hornblass A., To K., Coden D.J. Endogenous orbital cellulitis and endogenous endophthalmitis in subacute bacterial endocarditis. Am J Ophthalmol. 1989;108:196–197. doi: 10.1016/0002-9394(89)90021-4. [DOI] [PubMed] [Google Scholar]
- 7.Moffett D.G.J., Edward D.P. Anterior segment necrosis associated with endogenous endophthalmitis secondary to group C streptococcal septicemia. Can J Ophthalmol. 1991;26:283–287. [PubMed] [Google Scholar]
- 8.Ramaswamy G. Streptococcus equisimilis (group C) as a cause of ophthalmic infections. Am J Clin Pathol. 1983;79(March (3)):385–387. doi: 10.1093/ajcp/79.3.385. [DOI] [PubMed] [Google Scholar]
- 9.Madžar D. Endogenous endophthalmitis complicating Streptococcus equi subspecies zooepidemicus meningitis: a case report. BMC Res Notes. 2015;8(May):184. doi: 10.1186/s13104-015-1133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verweij P.E. Endophthalmitis as presenting symptom of Group G streptococcal endocarditis. Infection. 1994;22:56–57. doi: 10.1007/BF01780770. [DOI] [PubMed] [Google Scholar]
- 11.Tan J.H. Endogenous endophthalmitis due to Group G Streptococcus. Eye. 1999;13:116–117. doi: 10.1038/eye.1999.24. [DOI] [PubMed] [Google Scholar]
- 12.Ziakas N.G. Endogenous group G Streptococcus endophthalmitis following a dental procedure. Eur J Ophthal. 2004;14:59–60. doi: 10.1177/112067210401400110. [DOI] [PubMed] [Google Scholar]
- 13.Deighton C. Beta haemolytic streptococci and musculoskeletal sepsis in adults. Ann Rheum Dis. 1993;52(June (6)):483–487. doi: 10.1136/ard.52.6.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steckelberg R.C., Wang A.T., Wilson W. Infective endophthalmitis due to Group G Streptococcal infection in a patient with metastatic ovarian carcinoma. BMJ Case Rep. 2011;2011(August) doi: 10.1136/bcr.07.2011.4526. pii: bcr0720114526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bloom C., Thomson E.C., Main J. An elderly lady with sudden blindness and a sore foot. J Infect. 2006;52(February (2)):e53–e54. doi: 10.1016/j.jinf.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Nagelberg H.P., Petashnick D.E., To K.W., Woodcome H.A., Jr. Group B streptococcal endophthalmitis. Am J Ophthalmol. 1994;117(Apr (4)):498–500. doi: 10.1016/s0002-9394(14)70010-8. [DOI] [PubMed] [Google Scholar]
- 17.Farber B.P., Weinbaum D.L., Dummer J.S. Metastatic bacterial endophthalmitis. Arch Intern Med. 1985;145(January (1)):62–64. [PubMed] [Google Scholar]
- 18.Blair D.C., Martin D.B. Beta hemolytic streptococcal endocarditis: predominance of non-group A organisms. Am J Med Sci. 1978;276(November–December (3)):269–277. doi: 10.1097/00000441-197811000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Puliafito C.A., Baker A.S., Haaf J. Infectious endophthalmitis: review of 36 cases. Ophthalmology. 1982;89:921–929. [PubMed] [Google Scholar]
- 20.Berkey P.B., Rolston K.V. Group G streptococcal as a cause of bacterial endophthalmitis. Arch Ophthalmol. 1988;106:171–172. doi: 10.1001/archopht.1988.01060130181014. [DOI] [PubMed] [Google Scholar]
- 21.Mirani P. Group G streptococcal endophthalmitis: case report and revie of the literature. Infect Dis Clin Pract. 2005;13:24–26. [Google Scholar]
- 22.Jackson T.L., Eykyn S.J., Graham E.M., Stanford M.R. Endogenous bacterial endophthalmitis: a 17-year prospective series and review of 267 reported cases. Surv Ophthalmol. 2003;48(July–August (4)):403–423. doi: 10.1016/s0039-6257(03)00054-7. [DOI] [PubMed] [Google Scholar]
- 23.Connell P.P., O’Neill E.C., Fabinyi D. Endogenous endophthalmitis: 10-year experience at a tertiary referral centre. Eye. 2011;25(1):66–72. doi: 10.1038/eye.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]