Abstract
Introduction
Pregnancy-related acute kidney injury is the most common cause of renal cortical necrosis (RCN). Atypical hemolytic uremic syndrome (aHUS) as a cause of RCN in pregnant/postpartum is underevaluated. In the current article, we describe a series of cases of pregnancy-related RCN.
Methods
All cases with acute kidney injury (AKI) in the setting of pregnancy and postpartum state were included. Diagnosis of RCN was made by contrast-enhanced computerized tomography (nonenhancing renal cortex, enhancing medulla, and no excretion of contrast medium) or on a renal biopsy. aHUS was diagnosed in the presence of microangiopathic hemolytic anemia (thrombocytopenia, elevated lactate dehydrogenase with schistocytes on peripheral smear examination, or low haptoglobin).
Results
A total of 21 (17.5%) patients presented with RCN during pregnancy, all in the postpartum state. Twenty patients (95.2%) showed microangiopathic hemolytic anemia consistent with HUS and 1 (4.8%) patient had biopsy-proven thrombotic microangiopathy. Low complement 3 or activation of an alternate complement pathway was seen in 9 of 15 patients in which it was done. At the end of 6 months, only 2 (9.5%) patients had partial recovery of renal functions, 5 (23.8%) patients died, and 14 remained (66.7%) on hemodialysis.
Conclusion
The clinical and laboratory features are highly suggestive of aHUS in more than three-fourths of cases with postpartum RCN. Investigations are needed to look for genetic abnormalities in the complement pathway.
Keywords: AKI, HUS, pregnancy, renal cortical necrosis
RCN is the most catastrophic form of AKI.1 Thought to be as a result of severe renal ischemia secondary to hemorrhage or microvascular thrombosis, RCN is encountered in the developing world as a complication of pregnancy.1 The exact pathogenesis of RCN in the obstetric setting has remained unsolved.2, 3, 4 Hemolysis and microangiopathy have been reported, but were ascribed to disseminated intravascular coagulation, perhaps secondary to sepsis or amniotic fluid embolism. aHUS, the commonest cause of RCN in the nonobstetric setting,1 has recently been identified in developed countries as a distinct cause of AKI in the late pregnancy or postpartum period.5, 6 In recent years, we have increasingly identified clinical findings consistent with aHUS in patients presenting with obstetric AKI. In this present report, we describe the contemporary clinico-epidemiological profile and outcome of obstetric RCN from a developing country and highlight the possible contribution of aHUS to this condition.
Material and Methods
The current report is a part of the prospective study evaluating economic consequences of patients with obstetric AKI referred to the Postgraduate Institute of Medical Education and Research, Chandigarh, between August 2015 and November 2017. Patients with known chronic kidney disease and organ transplant recipients were excluded. The Institute Ethics Committee approved the study protocol and all patients provided informed consent.
All patients were managed as per standard of care, including dialysis and other supportive therapy. Investigations included complete blood count, peripheral smear examination, serum lactate dehydrogenase, serum haptoglobin, liver function tests, and renal function tests. We performed complement 3 and alternate pathway functional assay in 15 patients.
aHUS was diagnosed in the presence of microangiopathic hemolytic anemia (thrombocytopenia, elevated lactate dehydrogenase with schistocytes on peripheral smear examination, or low haptoglobin).7 Preeclampsia was diagnosed on the basis of standard clinical criteria. A diagnosis of definite sepsis was made when the patient had fever, leukocytosis, and growth of microorganisms on blood culture, whereas probable sepsis was diagnosed when the patient had fever, leukocytosis, and elevated procalcitonin but without microbiological proof.
Patients who failed to show improvement in urine output after 2 weeks underwent contrast-enhanced computerized tomography. RCN was diagnosed on the basis of characteristic imaging findings (nonenhancing renal cortex, enhancing medulla, and no excretion of contrast medium),8 or kidney biopsy when contrast-enhanced computerized tomography was inconclusive. All patients were followed for at least 6 months.
Results
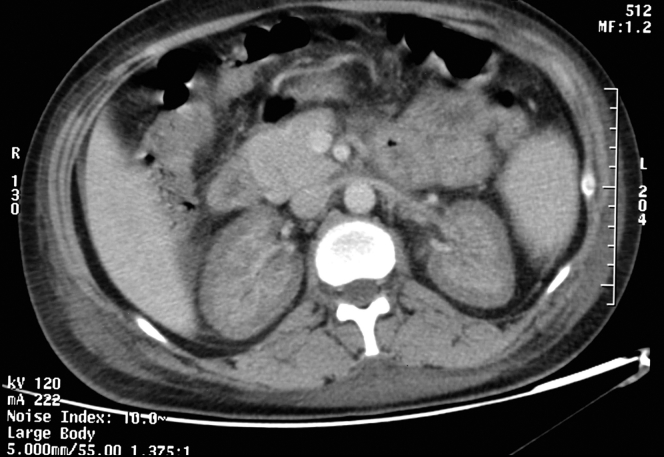
Of 120 patients with obstetric AKI referred to our hospital during the study period, 21 (17.5%) were diagnosed with RCN. The diagnosis was confirmed by demonstration of classical findings on contrast-enhanced computerized tomography (Figure 1) in 20 cases, and in 1 on kidney biopsy. Table 1 depicts the demographic and clinical details of all patients. In all cases, clinical manifestations appeared in the intra- or postpartum period. The mean age of the patients was 26.38 ± 4.33 (range 20–38) years. Fifteen patients (71.42%) had received regular antenatal care. Seven (33.3%) patients had preeclampsia. A total of 9 (42.8%) cases had postpartum hemorrhage preceding AKI. All cases received initial management in other health facilities and were referred to our hospital after a mean interval of 4.3 days following delivery. Three cases had received dialysis before referral. Three (14.2%) patients showed bacteremia in cultures drawn within 48 hours of arrival, and another 9 (42.8%) were treated for presumed sepsis during the hospital stay.
Figure 1.
Both the kidneys show thin rim of enhancement in the subcapsular area with lack of enhancement in the cortical area, consistent with renal cortical necrosis.
Table 1.
Clinical, laboratory parameters, and outcome of all the patients
| Patient no. | Age, yr | Gestational age, wk | Preeclampsia | History of blood loss | Sepsis | Hemoglobin, g/dl | Platelet count (× 109/l) | LDH (U/l) | Schistocytes on peripheral smear | Plasma haptoglobin, mg/dl | Serum creatinine at presentation, mg/dl | Total bilirubin, mg/dl | AST, U/l | ALT, U/l | C3, g/l | APFA | RCN diagnosed by | Outcome at 6 mo |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 01 | 29 | 38 | No | Yes | No | 4.9 | 8 | 3687 | Yes | 4.07 | 3.6 | 1 | 103 | 143 | 104.56 | 51.76 | CECT | Died |
| 02 | 23 | 38 | No | No | Definite | 8.6 | 30 | 3054 | NA | 7.3 | 4.9 | 5.8 | 1640 | 560 | 78.24 | -0.11 | CECT | On dialysis |
| 03 | 25 | 38 | No | No | Probable | 9.5 | 16 | 1177 | Yes | 20.89 | 7.0 | 5.5 | 71 | 21 | 84.24 | 64.64 | CECT | Dieda |
| 04 | 23 | 30 | Yes | Yes | No | 4.5 | 58 | 2045 | Yes | 8.02 | 5.1 | 0.5 | 106 | 120 | 58.32 | 27.68 | CECT | On dialysis |
| 05 | 27 | 38 | Yes | No | No | 8.1 | 94 | 5046 | Yes | 7.52 | 1.8 | 1.78 | 1031 | 322 | 163.84 | 8.90 | CECT | On dialysis |
| 06 | 26 | 38 | No | Yes | No | 7.1 | 101 | 2912 | Yes | 5.47 | 1.9 | 0.7 | 50 | 28 | 105.6 | 68.73 | CECT | Serum creatinine 3.2 mg/dl |
| 07 | 29 | 38 | No | No | Probable | 9.3 | 42 | 3219 | NA | 5.85 | 1.5 | 7.2 | 396 | 242 | 94.16 | 26.10 | CECT | On dialysis |
| 08 | 29 | 38 | Yes | Yes | No | 8.7 | 77 | 3752 | Yes | 12.36 | 4.7 | 0.9 | 51 | 95 | 37.12 | 64.12 | CECT | On dialysis |
| 09 | 38 | 35 | Yes | Yes | No | 6.9 | 48 | 4031 | Yes | 17.47 | 2.6 | 0.5 | 37 | 35 | 41.76 | 45.51 | CECT | On dialysis |
| 10 | 30 | 38 | No | No | No | 8.4 | 295 | 1044 | No | 40.43 | 6.0 | 1.74 | 1335 | 1457 | 106.0 | 29.34 | Biopsy | On dialysis |
| 11 | 20 | 38 | No | No | Probable | 9.2 | 40 | 4071 | Yes | 20.39 | 3.7 | 1.6 | 205 | 180 | 54.24 | 8.53 | CECT | On dialysis |
| 12 | 22 | 38 | No | Yes | Probable | 7.4 | 91 | 3816 | Yes | 37.76 | 3.1 | 0.6 | 24 | 5 | 81.28 | 35.15 | CECT | On dialysis |
| 13 | 24 | 38 | Yes | No | Definite | 8.5 | 12 | 1026 | Yes | 9.69 | 2.7 | 5.3 | 409 | 98 | 179 | 75.35 | CECT | Died |
| 14 | 32 | 38 | No | No | Probable | 8.2 | 21 | 4420 | Yes | 8.14 | 2.6 | 3.9 | 93 | 92 | 32.15 | 13.23 | CECT | Serum creatinine 3 mg/dl |
| 15 | 29 | 38 | Yes | Yes | No | 6.4 | 23 | 2046 | No | 4.17 | 1.3 | 1 | 108 | 67 | 18.23 | 3.058 | CECT | On dialysis |
| 16 | 24 | 38 | No | Yes | Probable | 5.2 | 35 | 8390 | Yes | 6.97 | 5.0 | 0.86 | 240 | 120 | ND | ND | CECT | Died |
| 17 | 21 | 36 | No | Yes | Probable | 6.5 | 22 | 1282 | Yes | 36.26 | 10 | 5.9 | 102 | 46 | ND | ND | CECT | On dialysis |
| 18 | 32 | 37 | No | No | Probable | 7.9 | 88 | 1778 | Yes | 17 | 6.0 | 6.3 | 6020 | 1763 | ND | ND | CECT | On dialysis |
| 19 | 26 | 38 | No | No | Definite | 8.7 | 25 | 1617 | NA | 9 | 8.0 | 0.83 | 1636 | 244 | ND | ND | CECT | Died |
| 20 | 23 | 37 | No | No | No | 9.2 | 19 | 3850 | Yes | 15.7 | 2.8 | 1.3 | 420 | 245 | ND | ND | CECT | Died |
| 21 | 24 | 34 | Yes | No | Probable | 6.0 | 47 | 2347 | Yes | 21 | 6.0 | 1 | 23 | 44 | ND | ND | CECT | On dialysis |
ALT, alanine aminotransferase; APFA, alternative pathway functional assay; AST, aspartate aminotransferase; C3, complement 3; CECT, contrast-enhanced computerized tomography; LDH, lactate dehydrogenase; NA, not available; ND, not done.
Died at 5 months due to infection on dialysis.
As shown in Table 1, 20 (95.2%) patients exhibited classical microangiopathic hemolytic anemia consistent with HUS. In 1 patient, the kidney biopsy showed thrombotic microangiopathy. A total of 9 of 15 cases showed low complement 3 and/or alternate pathway functional assay, suggesting activation of the alternative pathway of the complement system. Nine (42.8%) patients had evidence of HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome.9 None of the patients were treated with plasma exchange either because of financial reasons (n =15) or because disease was considered irreversible and hence treatment was deemed to be futile (n = 6). Two (9.5%) patients had spontaneous recovery of urine output with improvement in glomerular filtration rate and were able to come off dialysis. Five (23.8%) patients died in hospital, all due to health care–associated infections. The remaining 14 remained dialysis-dependent at discharge.
Discussion
Through this report, we raise the possibility of postpartum aHUS as an important contributor to RCN, one of the most devastating complications of pregnancy in the developing world.
In recent years, postpartum aHUS secondary to abnormalities in complement pathway has been described in reports from the developed world.5, 6 However, this entity is not well-recognized in the developing world, where obstetric AKI is far more common.10, 11 In the study by Sahay et al.12 39% of RCN cases were due to obstetric AKI, but HUS was reported only in 7.3%.
We believe aHUS is underrecognized in this setting in developing countries. The timing of referral and availability of investigations could have a bearing on the detection of diagnostic findings: the classical findings of microangiopathic hemolytic anemia could have disappeared in the case of late presentation and workup. Frimat et al.3 reported 18 instances of RCN in patients with pregnancy-related AKI due to postpartum hemorrhage; all the patients had thrombocytopenia, and more than one-half of them had evidence of hemolysis. A high prevalence of RCN has indeed been shown in primary HUS.13
In the past, RCN was attributed to massive hemorrhage or sepsis, without full understanding of the mechanism. We identified bleeding, preeclampsia, and possible sepsis in 43%, 33%, and 57% cases, respectively. Sixty percent of the patients in the present report had evidence of activated alternative pathway of the complement cascade (hypocomplementemia and/or low alternate pathway functional assay), which strongly supports the possibility of aHUS.
What could have led to the development of aHUS and RCN? We speculate that these patients might have inherited defects of the complement pathway that are unmasked in the peripartum period, especially in the presence of complications, which acted as triggers for complement activation. Confirmation of this hypothesis requires studies to evaluate variations in genes regulating the complement pathway. Genetic abnormalities have been shown in 41% to 86% of the previously reported cases of postpartum aHUS.5, 6, 14
Forty-two percent of the patients had HELLP syndrome. Presence of HELLP is not inconsistent with coexistent aHUS; the features overlap considerably and may not be mutually exclusive. Recent reports have hinted at dysregulation of the alternative complement pathway in patients with preeclampsia/HELLP syndrome.15, 16, 17
All patients in the current study were referred from rural hospitals, and 40% of patients had not received adequate antenatal care despite the existence of government programs to improve access to medical care for pregnant women. We were unable to ascertain the quality of immediate postpartum care these patients received. In contrast, none of the patients who were followed up in the antenatal clinic of our hospital developed AKI, suggesting that inappropriate management in the early postpartum period might have triggered the cascade of events that culminate in aHUS and RCN.
The message from these cases carries important diagnostic and therapeutic implications. The constellation of findings in these cases strongly suggest a diagnosis of aHUS. In the western countries, identification of hematological abnormalities suggestive of microangiopathic hemolytic anemia in the appropriate clinical context, such as postpartum AKI, triggers the diagnosis of aHUS and institution of therapy with plasma exchange or complement inhibitors, most commonly eculizumab. Presence of advanced AKI practically rules out thrombotic thrombocytopenic purpura, the other possible cause of thrombotic microangiopathy. Case series have shown dramatically improved prognosis with the complement inhibition.18 However, eculizumab is not available in large parts of the developing world, including India, and even if it was available, it would be extremely expensive and hence unaffordable for these patients.
To conclude, aHUS should be considered strongly in the setting of oliguric postobstetric AKI. Early diagnosis could allow timely intervention with plasmapheresis and thereby prevent the development of catastrophic RCN. Studies are needed to identify abnormalities in complement pathway genes.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This study was supported by a grant from the International Society of Nephrology (grant number 05-037) to RR and the Department of Biotechnology, Ministry of Science and Technology, Government of India, to VJ.
References
- 1.Chugh K.S., Jha V., Sakhuja V., Joshi K. Acute renal cortical necrosis—a study of 113 patients. Ren Fail. 1994;16:37–47. doi: 10.3109/08860229409044846. [DOI] [PubMed] [Google Scholar]
- 2.Prakash J., Vohra R., Wani I.A. Decreasing incidence of renal cortical necrosis in patients with acute renal failure in developing countries: a single-centre experience of 22 years from Eastern India. Nephrol Dial Transplant. 2007;22:1213–1217. doi: 10.1093/ndt/gfl761. [DOI] [PubMed] [Google Scholar]
- 3.Frimat M., Decambron M., Lebas C. Renal cortical necrosis in postpartum hemorrhage: a case series. Am J Kidney Dis. 2016;68:50–57. doi: 10.1053/j.ajkd.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 4.Prakash J., Pant P., Singh A.K. Renal cortical necrosis is a disappearing entity in obstetric acute kidney injury in developing countries: our three decades of experience from India. Ren Fail. 2015;37:1185–1189. doi: 10.3109/0886022X.2015.1062340. [DOI] [PubMed] [Google Scholar]
- 5.Huerta A., Arjona E., Portoles J. A retrospective study of pregnancy-associated atypical hemolytic uremic syndrome. Kidney Int. 2018;93:450–459. doi: 10.1016/j.kint.2017.06.022. [DOI] [PubMed] [Google Scholar]
- 6.Fakhouri F., Roumenina L., Provot F. Pregnancy-associated hemolytic uremic syndrome revisited in the era of complement gene mutations. J Am Soc Nephrol. 2010;21:859–867. doi: 10.1681/ASN.2009070706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sinha A., Gulati A., Saini S. Prompt plasma exchanges and immunosuppressive treatment improves the outcomes of anti-factor H autoantibody-associated hemolytic uremic syndrome in children. Kidney Int. 2014;85:1151–1160. doi: 10.1038/ki.2013.373. [DOI] [PubMed] [Google Scholar]
- 8.Goergen T.G., Lindstrom R.R., Tan H., Lilley J.J. CT appearance of acute renal cortical necrosis. Am J Roentgenol. 1981;137:176–177. doi: 10.2214/ajr.137.1.176. [DOI] [PubMed] [Google Scholar]
- 9.Sibai B.M., Ramadan M.K., Usta I. Maternal morbidity and mortality in 442 pregnancies with hemolysis, elevated liver enzymes, and low platelets (HELLP syndrome) Am J Obstet Gynecol. 1993;169:1000–1006. doi: 10.1016/0002-9378(93)90043-i. [DOI] [PubMed] [Google Scholar]
- 10.Krishna A., Singh R., Prasad N. Maternal, fetal and renal outcomes of pregnancy-associated acute kidney injury requiring dialysis. Indian J Nephrol. 2015;25:77–81. doi: 10.4103/0971-4065.136890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sivakumar V., Sivaramakrishna G., Sainaresh V.V. Pregnancy-related acute renal failure: a ten-year experience. Saudi J Kidney Dis Transpl. 2011;22:352–353. [PubMed] [Google Scholar]
- 12.Sahay M., Swarnalata, Swain M., Padua M. Renal cortical necrosis in tropics. Saudi J Kidney Dis Transplant. 2013;24:725. doi: 10.4103/1319-2442.113864. [DOI] [PubMed] [Google Scholar]
- 13.Tinaztepe K., Akkök N., Tinaztepe B. Hemolytic-uremic syndrome (HUS): a clinicopathological study of 15 cases. Turk J Pediatr. 1993;35:23–36. [PubMed] [Google Scholar]
- 14.Bruel A., Kavanagh D., Noris M. Hemolytic uremic syndrome in pregnancy and postpartum. Clin J Am Soc Nephrol. 2017;12:1237–1247. doi: 10.2215/CJN.00280117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alrahmani L., Willrich M.A.V. The complement alternative pathway and preeclampsia. Curr Hypertens Rep. 2018;20:40. doi: 10.1007/s11906-018-0836-4. [DOI] [PubMed] [Google Scholar]
- 16.Lynch A.M., Salmon J.E. Dysregulated complement activation as a common pathway of injury in preeclampsia and other pregnancy complications. Placenta. 2010;31:561–567. doi: 10.1016/j.placenta.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaught A.J., Gavriilaki E., Hueppchen N. Direct evidence of complement activation in HELLP syndrome: a link to atypical hemolytic uremic syndrome. Exp Hematol. 2016;44:390–398. doi: 10.1016/j.exphem.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cavero T., Rabasco C., López A. Eculizumab in secondary atypical haemolytic uraemic syndrome. Nephrol Dial Transplant. 2017;32:466–474. doi: 10.1093/ndt/gfw453. [DOI] [PMC free article] [PubMed] [Google Scholar]