Abstract
Introduction
There were concerns raised regarding a high prevalence of chronic kidney disease (CKD) in Uddanam, a fertile subtropical low-altitude territory in the southern Indian state of Andhra Pradesh. The present study was undertaken to ascertain the prevalence of CKD, disease characteristics, and risk factor profile in this area.
Methods
We selected 2210 subjects (age >18 years) using multistage sampling. After obtaining demographic and anthropometric data, urinary protein-creatinine ratio, serum creatinine, and blood glucose were measured in all the subjects. Glomerular filtration rate was estimated (eGFR) using the Modification of Diet in Renal Disease equation.
Results
Mean age of the subjects was 43.2 ± 14.2 years (range: 18–98), 44.3% were men and 55.7% were women. Mean eGFR of subjects was 94.3 ± 33.4. Low eGFR (<60 ml/min per 1.73 m2) was seen in 307 (13.98%) patients with a mean eGFR of 34.8 ± 16.6. The prevalence of subjects having low eGFR and with proteinuria (CKD) was 18.23%. Major risk factors, such as diabetes, long-standing hypertension, and significant proteinuria, were absent in 73% of patients with CKD, implying that a significant proportion of the population is afflicted with the entity “CKD of unknown etiology (CKDu).”
Conclusion
The prevalence of CKD and CKDu in Uddanam is much higher than other earlier studies in either rural or urban communities in India. We suggest that there is a dire need to review health policies and allocate resources for prevention and treatment of CKD in the Uddanam region.
Keywords: chronic kidney disease, proteinuria, Uddanam
See Commentary on Page 367
CKD and end-stage renal disease have emerged as important public health problems and causes of morbidity and mortality all over the world. In developing countries, the prevalence of CKD is rapidly increasing and surpassing the prevalence of CKD in developed countries.1 In addition, a new form of severe CKD affecting adults in their fourth and fifth decades, not due to traditional risk factors like diabetes and long-standing hypertension, has been reported from Sri Lanka, several central American countries, and Egypt during the past 2 decades.2, 3 This has been named CKD of unknown etiology (CKDu), and it is fatal due to late recognition and rapid disease progression.4 It is believed that the prevalence of CKD and CKDu is on the rise in India. Earlier studies in limited geographical areas in India have shown that the prevalence of CKD was less than 1%,5 whereas in a recent study in rural parts of Karnataka, India, a growing prevalence of CKD of 6.8% was reported.6 It also has been estimated that age-adjusted incidence of end-stage renal disease in India is 229 per million population7; however, there are no national reports establishing the prevalence of CKD across the country. In the first report of Indian CKD registry that published data from 52,273 adults, the most common cause of CKD was diabetes (31%).8 An interesting observation in this study was that a significant proportion (16%) had CKDu, and the patients were middle-aged, poor, and presented with advanced CKD.
The Uddanam region is a fertile, subtropical, low-altitude territory, which is well-known for coconut and cashew farms in the Srikakulam district of the southern Indian state of Andhra Pradesh. Several concerns were raised regarding the high prevalence of CKD due to environmental causes in the Uddanam region, resulting in a high death rate. Local surveys conducted pointed toward some possible causes that could contribute to the disease, but no systematic epidemiological studies were conducted and no published data are available.9 In these circumstances, we have undertaken a systematic population study with the following objectives: to establish prevalence and the characteristics of CKD in the population of Uddanam and examine whether CKD in Uddanam can be explained by traditional risk factors like diabetes and long-standing hypertension, or if this is CKDu. A study on the prevalence of CKD, identification of CKDu, and the associated risk factors will help in providing a basis for health care policy and planning preventive measures.
Methods
Study Design and Sample Size
A pilot study was conducted in the Uddanam area on 170 subjects, and the prevalence (p) of CKD was found to be 15.9%. Based on this prevalence, the sample size for a cross-sectional study was calculated with the following formula10:
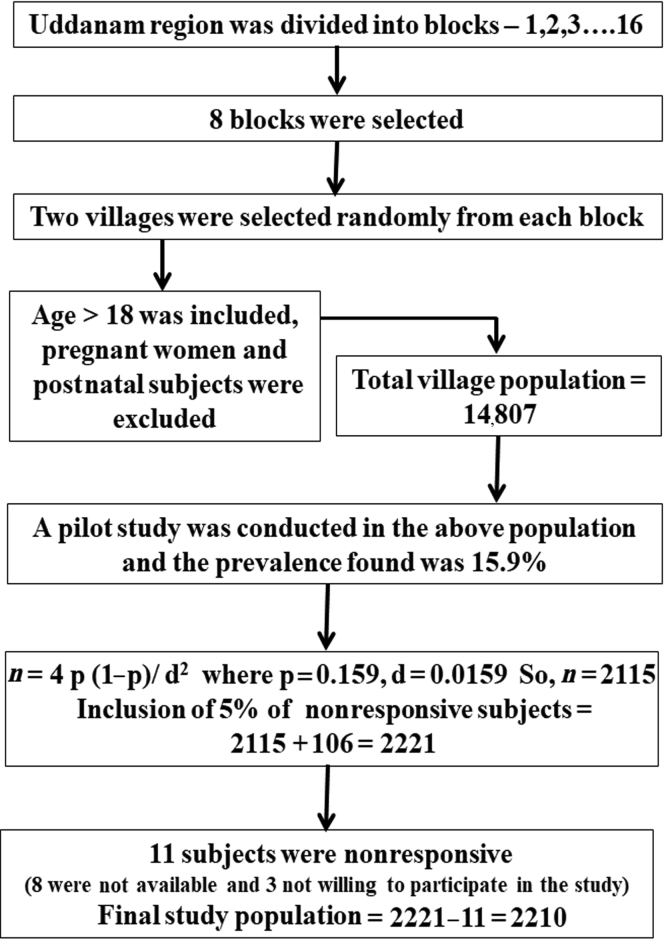
A cross-sectional study was conducted in Uddanam. The calculated sample size was 2115. It was assumed that 5% of subjects would be nonresponsive and hence the sample size would be 2221. These subjects were selected using a multistage sampling method (Figure 1). The Uddanam region was divided into 16 blocks. Of 16, 8 blocks were selected using a systematic random sampling method. Two villages were selected from each block, using a simple random sampling method. Subjects who were 18 years or older were included. Pregnant and postnatal women were excluded. The total population of 16 villages was found to be 14,807. Fifteen percent of the rural population equaling to 2221 was selected randomly for the study; however, 11 were nonresponders (8 were not available and 3 were not willing to participate in the study). Hence, data were collected from 2210 subjects. Informed consent was obtained from all the subjects (N = 2210). The study was approved by the Institutional Ethics Committee of King George Hospital, Andhra Medical College, Visakhapatnam.
Figure 1.
Schematic representation of sampling method of investigation: multistage sampling method was applied to estimate the study population in Uddanam region.
Characteristics of the Subject Population and Sample Collection
The field work was carried out by the staff of Andhra Medical College, Visakhapatnam. All subjects were administered a structured questionnaire. Subjects were questioned about the presence or absence of symptoms suggestive of renal disease. Past medical history of diabetes mellitus, hypertension, ischemic heart disease, and stroke was elicited. Questions pertaining to tobacco smoking and alcohol consumption were asked. Blood pressure measurements were taken for the entire group using standard instruments, which were calibrated daily. Random midstream urine samples were taken from all individuals. Blood tests were done for all participants, after taking their informed consent. A total of 980 blood samples from men and 1230 blood samples from women were collected; 5 ml of blood was drawn in the fasting state for creatinine and glucose estimation.
Definitions and Evaluation Criteria
Blood samples were collected for creatinine and glucose. All samples were stored at 2 to 8 °C, using a cold pack placed in thermos containers. Urine samples were collected, labeled, and details of collection were recorded. Cold chain, the system of storing and transporting blood and urine samples at recommended temperatures from the place of collection to the point of analysis, was maintained and monitored. The blood and urine samples were sent to the core laboratory in Visakhapatnam and analyzed on the same day.
Hypertension
Hypertension was defined as blood pressure more than 140/90 mm Hg or if the patient was on medication for hypertension or had a positive self-reported history of hypertension. Hypertension of more than 5 years’ duration was taken as long-standing hypertension.
Diabetes
Diabetes was defined as fasting blood glucose >126 mg/dl or random blood glucose ≥200 mg/dl or on any medication for diabetes.
Chronic Kidney Disease
CKD stages were defined by National Kidney Foundation, USA, under Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines.11, 12 CKD is defined as the presence of either kidney damage or glomerular filtration rate (GFR) <60 ml/min per 1.73 m2. Protein-creatinine ratio >0.2 and serum creatinine >1.2 were taken as the indicators of kidney damage, and GFR was estimated using predictive equations.12, 13, 14
Statistical Analysis
Data analysis was carried out using SPSS V22 statistical software (IBM Corp., Chicago, IL). For descriptive statistics, categorical variables were reported as a proportion, whereas continuous variables were reported as means and SDs, when distributions were considered approximately normal. The outcome variable was eGFR. Exposure variables considered were age, gender, education, occupation (farmer/nonfarmer), exposure to agrochemicals, tobacco smoking, alcohol intake, diabetes, hypertension, long-standing hypertension, and proteinuria. The unadjusted relationships between the exposure variables and the presence or absence of CKD were examined in univariate logistic regression analyses. Simple logistic regression analysis was also applied taking CKDu as the dependent variable and age, gender, education, occupation (farmer/nonfarmer), pesticide contact, tobacco smoking, and alcohol consumption as independent variables. Multivariate logistic regression analysis was performed to evaluate the simultaneous effects of various exposure variables, with adjustment for the potential confounding effects of other factors mentioned previously. All the potential risk factors in the initial logistic regression model were included. Covariates included in the initial model were age, education, occupation (farmer/nonfarmer), direct exposure to agrochemicals (handling, spraying), tobacco smoking, alcohol consumption, diabetes, hypertension, and long-standing hypertension. In the multiple logistic regression model, the variables included were those that were significant at the 20% (P < 0.20) level in the initial model to accommodate more explanatory variables and to reduce type II error. The Backward Likelihood Ratio method was applied at the 10% level of significance to fit the model, and adjusted odds ratios were found. Confidence intervals from these analyses were based on SEs. In all the analyses, a P < 0.05 was considered significant.
Results
Characteristics of the Study Population
All the 2210 subjects were older than 18 and the mean age was 43.2 ± 14.2; 63% of the subjects were younger than 47 years. Most (65.4%) were agricultural farmers. Women constituted 55.7% of the population studied. There were 42.3% who did not have any education, whereas 57.7% were educated up to the primary level (Table 1). There were 57.6% who were of normal weight. Hypertension was seen in 369 (16.7%) subjects and duration of hypertension was less than 5 years in 227 (61.5%), whereas 142 (38.5%) were hypertensive for more than 5 years (Table 2, Table 3, Table 4, Table 5). Of 369 hypertensive subjects, 314 (85.1%) were known hypertensive and 55 (14.9%) were discovered to be hypertensive during the present evaluation. Diabetes was found in 159 (7.2%) subjects (Table 2, Table 3).
Table 1.
Demographic characteristics of the study population (N = 2210)
| Characteristics | Number | Percentage |
|---|---|---|
| Age, yr | ||
| 18–27 | 347 | 15.7 |
| 28–37 | 501 | 22.7 |
| 38–47 | 544 | 24.6 |
| 48–57 | 395 | 17.8 |
| 58–67 | 315 | 14.2 |
| >68 | 108 | 4.9 |
| Total | 2210 | |
| Sex distribution | ||
| Men | 980 | 44.3 |
| Women | 1230 | 55.7 |
| Education | ||
| Yes | 1276 | 57.7 |
| No | 934 | 42.3 |
| Occupation | ||
| Farmer | 1446 | 65.4 |
| Nonfarmer | 764 | 34.6 |
| Exposed to pesticides | ||
| Yes | 326 | 14.7 |
| No | 1884 | 85.3 |
| Tobacco smoking | ||
| Yes | 381 | 17.2 |
| No | 1829 | 82.8 |
| Alcohol consumption | ||
| Yes | 466 | 21.1 |
| No | 1744 | 78.9 |
Table 2.
Distribution of hypertension, long-standing hypertension, and diabetes in the total subject population (N = 2210)
| Total study population, N = 2210 |
Long-standing hypertension (≥5 yr) |
Diabetes |
||||||
|---|---|---|---|---|---|---|---|---|
| Women | Men | Total | Women | Men | Total | Women | Men | Total |
| 1230 | 980 | 2210 | 73 | 69 | 142 | 78 | 81 | 159 |
| Hypertension (<5 yr) |
No diabetes |
No diabetes + hypertension (<5 yr) |
||||||
|---|---|---|---|---|---|---|---|---|
| Women | Men | Total | Women | Men | Total | Women | Men | Total |
| 129 | 98 | 227 | 1152 | 899 | 2051 | 1093 | 846 | 1939 |
Table 3.
Distribution of hypertension, long-standing hypertension, and diabetes in total subject population (N = 2210)
| Subject population N = 2210 |
Long-standing hypertension (≥5 yr) |
Diabetes |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| PCR | Women | Men | Total | Women | Men | Total | Women | Men | Total |
| ≤0.2 | 50.8 | 40.1 | 91.0 | 33.6 | 40.9 | 74.5 | 41.5 | 41.5 | 83.0 |
| >0.2 | 4.8 | 4.2 | 9.0 | 18.2 | 7.3 | 25.5 | 7.5 | 9.4 | 17.0 |
|
N = 2210 |
Hypertension (<5 yr) |
No diabetes |
No diabetes + hypertension (<5 y) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| PCR | Women | Men | Total | Women | Men | Total | Women | Men | Total |
| ≤0.2 | 34.2 | 30.0 | 64.2 | 51.5 | 40.0 | 91.6 | 52.2 | 40.1 | 92.3 |
| >0.2 | 18.0 | 17.8 | 35.8 | 4.6 | 3.8 | 8.4 | 4.0 | 3.8 | 7.7 |
Values given in the table are percentages (%). Proteinuria is PCR >0.2; long-standing hypertension is >5 years of diagnosis.
PCR, protein-creatinine ratio.
Table 4.
Distribution of hypertension, long-standing hypertension, and diabetes in CKD population (n = 403)
| CKD population n = 403 |
Long-standing hypertension (≥5 yr) |
Diabetes |
||||||
|---|---|---|---|---|---|---|---|---|
| Women | Men | Total | Women | Men | Total | Women | Men | Total |
| 216 | 187 | 403 | 28 | 27 | 55 | 31 | 28 | 59 |
| Hypertension (<5 yr) |
No diabetes |
No diabetes + hypertension (<5 yr) |
||||||
|---|---|---|---|---|---|---|---|---|
| Women | Men | Total | Women | Men | Total | Women | Men | Total |
| 47 | 31 | 78 | 185 | 159 | 344 | 164 | 141 | 305 |
CKD, chronic kidney disease.
Table 5.
Distribution of hypertension, long-standing hypertension, and diabetes in CKD population (n = 403)
| CKD = 403 |
Long-standing hypertension (≥5 yr) |
Diabetes |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| PCR | Women | Men | Total | Women | Men | Total | Women | Men | Total |
| ≤0.2 | 27.0 | 23.3 | 50.4 | 25.5 | 19.6 | 45.1 | 32.2 | 22.0 | 54.2 |
| >0.2 | 26.6 | 23.1 | 49.6 | 39.2 | 15.7 | 54.9 | 20.3 | 25.4 | 45.8 |
|
n = 403 |
Hypertension (<5 yr) |
No diabetes |
No diabetes + hypertension (<5 yr) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| PCR | Women | Men | Total | Women | Men | Total | Women | Men | Total |
| ≤0.2 | 27.4 | 23.9 | 51.3 | 26.2 | 23.5 | 49.7 | 26.8 | 24.2 | 51.0 |
| >0.2 | 24.5 | 24.2 | 48.7 | 27.6 | 22.7 | 50.3 | 25.2 | 23.9 | 49.0 |
Values given in the table are percentages (%). Proteinuria is PCR >0.2; long-standing hypertension is >5 years of diagnosis.
CKD, chronic kidney disease; PCR, protein-creatinine ratio.
The mean serum creatinine was 1.2 ± 1.0 mg/dl. The range of serum creatinine was between 0.06 and 29.0 mg/dl. Serum creatinine more than 1.2 mg/dl was observed in 286 (12.94%) subjects. Of them, 169 (59%) were men and 117 (41%) were women. eGFR was estimated using Modification of Diet in Renal Disease-K/DOQI equation 4. Mean eGFR was found to be 94.3 ± 33.4. Low eGFR (<60 ml/min per 1.73 m2) was seen in 307 (13.9%). Urine examination revealed significant proteinuria (protein-creatinine ratio [PCR] >0.2) in 200 (9.04%); of these, 96 (48%) had normal eGFR and 104 (52%) had low eGFR. The population stratification according to the National Kidney Foundation-K/DOQI criteria is shown in Table 6. When proteinuria and/or decreased eGFR were taken as markers, CKD was found in 403 subjects (18.2%). Of these, only 88 (3.9%) knew that they had kidney disease. The distribution of patients with CKD for hypertension, diabetes, and long-standing hypertension is given in Table 4, Table 5.
Table 6.
Association of demographic, habitual, and health characteristics with CKD versus non-CKD group
| Variable | Category | CKD |
Crude OR (95% CI) | P | |||
|---|---|---|---|---|---|---|---|
| Positive |
Negative |
||||||
| n | % | n | % | ||||
| Age, yr | >67 | 52 | 12.9 | 56 | 3.1 | 11 (6.4–18.9) | <0.001 |
| 58–67 | 105 | 26.1 | 210 | 11.6 | 5.93 (3.7–9.3) | <0.001 | |
| 48–57 | 97 | 24.1 | 298 | 16.5 | 3.86 (2.4–6.1) | <0.001 | |
| 38–47 | 83 | 20.6 | 461 | 25.5 | 2.13 (1.3–3.4) | <0.001 | |
| 28–37 | 39 | 9.7 | 462 | 25.6 | 1 (0.6–1.6) | 0.001 | |
| 18–27 | 27 | 6.7 | 320 | 17.7 | Ref | ||
| Gender | Women | 216 | 53.6 | 1014 | 56.1 | 0.9 (0.7–1.1) | 0.360 |
| Men | 187 | 46.4 | 793 | 43.9 | |||
| Education | Educated | 195 | 48.4 | 1080 | 59.8 | 0.63 (0.5–0.8) | <0.001 |
| Uneducated | 208 | 51.6 | 727 | 40.2 | |||
| Occupation | Farmer | 290 | 72.0 | 1151 | 63.7 | 1.46 (1.1–1.8) | 0.002 |
| Nonfarmer | 113 | 28.0 | 656 | 36.3 | |||
| Farmers - Pesticide contact | Exposed to pesticides | 69 | 17.1 | 257 | 14.2 | 1.25 (0.9–1.7) | 0.140 |
| Not exposed to pesticides | 334 | 82.9 | 1550 | 85.8 | |||
| Tobacco smoking | Yes | 82 | 20.3 | 299 | 16.5 | 1.28 (0.9–1.7) | 0.080 |
| No | 321 | 79.7 | 1508 | 83.5 | |||
| Alcohol consumption | Yes | 105 | 26.1 | 361 | 19.9 | 1.4 (1.1–1.8) | 0.009 |
| No | 298 | 73.9 | 1446 | 80.1 | |||
| Diabetes | Yes | 59 | 14.1 | 100 | 5.6 | 2.75 (1.9–3.9) | <0.001 |
| No | 344 | 85.9 | 1707 | 94.4 | |||
| Hypertension | Yes | 133 | 33.0 | 236 | 13.1 | 3.28 (2.5–4.2) | <0.001 |
| No | 270 | 67.0 | 1571 | 86.9 | |||
| Long-standing hypertension | Yes | 55 | 13.6 | 87 | 4.8 | 3.12 (2.2–4.4) | <0.001 |
| No | 348 | 86.4 | 1720 | 95.2 | |||
| Proteinuria | Yes | 200 | 49.6 | 0 | 0.0 | — | — |
| No | 203 | 50.4 | 1807 | 100 | |||
CI, confidence interval; CKD, chronic kidney disease; OR, odds ratio.
Characteristics of CKD
In the present study, 403 subjects had CKD. Of these, 216 (53.6%) were women and 187 (46.4%) were men (Table 4). Mean age of patients with CKD was 51.8 ± 13.7, whereas the mean age of the non-CKD population was 41.4 ± 13.7, which is statistically significant (P < 0.001). The demographic, lifestyle, and clinical characteristics of patients with CKD are shown in Table 6. Seventy percent of the patients with CKD were between 38 and 67 years old. Most patients with CKD (71.9%) were agricultural farmers and 51.6% did not have any education. There were 58.6% who had normal body weight. A total of 261 (64%) patients had serum creatinine greater than 1.2 mg/dl; of these, 144 (55%) were men and 117 (45%) were women. Hypertension was found in 133 (33%). The duration of hypertension was less than 5 years in 78 (58.7%), whereas 55 (41.3%) had been hypertensive for more than 5 years (long-standing hypertension). The prevalence of diabetes and hypertension in subjects with CKD was found to be 14.6% and 33.0%, and it was 5.5% and 13.0% in the non-CKD group (Table 6). CKD due to long-standing hypertension, as evidenced by left ventricular hypertrophy on electrocardiogram, was found in 43 (11.7%) of 369 hypertensive patients. A total of 203 (50.4%) subjects among patients with CKD had PCR ≤0.2 (Table 5). The mean serum creatinine was 2.2 ± 1.8 mg/dl, with range of 0.8 to 29.0. Mean eGFR was found to be 40.3 ± 14.8. As per National Kidney Foundation-K/DOQI stagewise classification of CKD, 13.2%, 10.7%, 29.8%, 17.4%, 18.6%, and 10.4% of patients were found in stages I, II, IIIA, IIIB, IV, and V, respectively (Table 7). Genderwise and stagewise distribution of patients with CKD is given in Table 8. Proteinuria alone (CKD stages I and II) without decreased GFR was seen in 96 (23.8%). Three (0.7%) patients had nephrotic range (PCR >3) proteinuria.
Table 7.
Agewise and stagewise population distribution of patients with CKD
| eGFR values (stages of CKD) | |||||||
|---|---|---|---|---|---|---|---|
| Age, yr | Total | <15 (Stage V) | 15–29 (Stage IV) | 30–43 (Stage IIIB) | 44–59 (Stage IIIA) | 60–89 (Stage II) | ≥90 (Stage I) |
| 18–27 | 27 | 0 | 2 | 3 | 5 | 3 | 14 |
| 28–37 | 39 | 5 | 2 | 5 | 10 | 3 | 14 |
| 38–47 | 83 | 9 | 15 | 16 | 21 | 9 | 13 |
| 48–57 | 97 | 9 | 19 | 17 | 34 | 11 | 7 |
| 58–67 | 105 | 13 | 22 | 21 | 32 | 13 | 4 |
| >68 | 52 | 6 | 15 | 8 | 18 | 4 | 1 |
| Total (%) | 403 | 42 (10.4) | 75 (18.6) | 70 (17.4) | 120 (29.8) | 43 (10.7) | 53 (13.2) |
CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate.
Table 8.
Genderwise and stagewise population distribution of patients with CKD
| Stagewise distribution of subjects with CKD | |||
|---|---|---|---|
| eGFR and CKD stage | Total | Men | Women |
| <15 (Stage V) | 42 | 16 | 26 |
| 15–29 (Stage IV) | 75 | 36 | 39 |
| 30–43 (stage IIIB) | 70 | 32 | 38 |
| 44–59 (Stage IIIA) | 120 | 56 | 64 |
| 60–89 (Stage II) | 43 | 26 | 17 |
| ≥90 (Stage I) | 53 | 21 | 32 |
| Total (%) | 403 | 187 (46.4) | 216 (53.6) |
CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate.
On simple logistic regression analysis, there was significant relationship of CKD with age, education status, farming, alcohol consumption, diabetes, hypertension, and long-standing hypertension (Table 6); however, there was no statistically significant relationship of CKD with gender distribution, pesticide contact, and tobacco smoking.
Multiple Logistic Regression Analysis
Multiple logistic regression analysis of the study population for CKD showed significance for risk factors such as age, tobacco smoking, alcohol, education, diabetes, and long-standing hypertension (Table 9). Gender difference in the study population was not included in the multiple regression analysis because it did not have any association with CKD in univariate analysis even at a 20% significance level. Patients with chronic or long-standing hypertension were 2.1 times (95% confidence interval 0.7–2.8) more likely to get CKD than those without chronic hypertension. Similarly, patients with diabetes were 1.65 times (95% confidence interval 0.7–4.8) more likely to get CKD compared with patients without diabetes. The adjusted odds ratio indicated that demographic characteristics, such as age, farming, and alcohol consumption, and clinical parameters, such as diabetes and long-standing hypertension, were found to be the risk factors for CKD in the Uddanam area.
Table 9.
Simultaneous effect of multiple variables with CKD using Multiple Logistic Regression Analysis
| Variable | Adjusted OR (95% CI) | P |
|---|---|---|
| Age, yr | ||
| >67 | 8.88 (5.0–15.7) | <0.001 |
| 58–67 | 5.22 (3.2–8.4) | <0.001 |
| 48–57 | 3.52 (2.2–5.6) | <0.001 |
| 38–47 | 1.87 (1.2–2.9) | 0.009 |
| 28–37 | 0.96 (0.5–1.6) | 0.872 |
| Education, yes | 0.79 (0.6–1.0) | 0.060 |
| Occupation farmer | 1.39 (1.1–1.8) | 0.013 |
| Tobacco smoking | 0.63 (0.4–0.8) | 0.004 |
| Alcohol consumption | 1.32 (0.9–1.7) | 0.057 |
| Diabetes | 1.65 (1.1–2.4) | 0.009 |
| Long-standing hypertension | 2.08 (1.4–3.0) | <0.001 |
CI, confidence interval; CKD, chronic kidney disease; OR, odds ratio.
Characteristics of CKDu
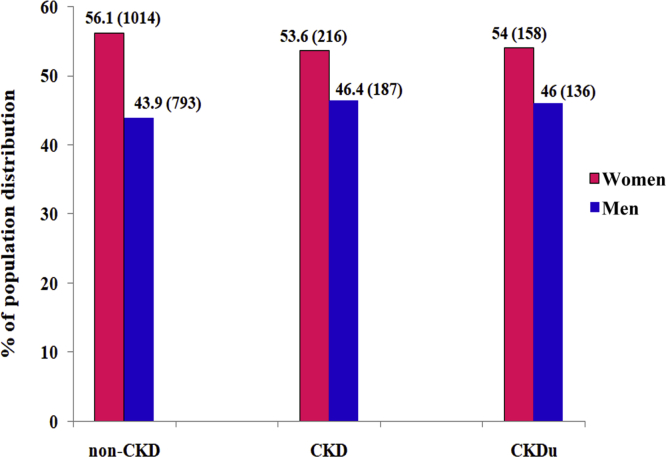
Patients with CKD who did not have diabetes or long-standing hypertension and whose PCR was ≤1 were considered to have CKDu.15 A total of 294 (73%) of patients with CKD in the Uddanam region had CKDu. Their mean age was 50.9 ± 12.3 (Table 10). There were 136 (46%) men and 158 (54%) women in the CKDu population (Table 10). The mean eGFR was found to be 40.2 ± 14.8 (Table 10). The mean systolic blood pressure was 128.0 ± 25.7 and diastolic blood pressure was 84.3 ± 15.8 mm Hg (Table 10). The mean blood pressure in both CKD and CKDu was normal, although a quarter of the patients were in advanced CKD stages IV and V (Table 10). Stagewise distribution of CKDu and distribution in men and women are shown in Table 11, Table 12. As per National Kidney Foundation-K/DOQI stagewise classification of CKD, 16.0%, 9.5%, 32.7%, 17.0%, 18.7%, and 6.1% of patients with CKDu were found in stages I, II, IIIA, IIIB, IV, and V, respectively (Table 11). Patients with CKDu did not have any symptoms even when they were in advanced stages IV and V of kidney failure. Sixty-nine percent of the CKDu population was between 38 and 67 years old. Most of the patients with CKDu were agricultural workers (73.1%), without even primary education (53.7%), nonsmokers (81.6%), and nonalcoholics (75.5%) (Table 13). On univariate analysis, it was observed that, except for age, no other parameter was statistically significant (Table 13). Hence, multiple logistic regression analysis was not performed. The percentage of population distribution of both men and women was linear in non-CKD, CKD, and CKDu groups (Figure 2).
Table 10.
Clinical and biochemical characteristics of the subject population (study population, CKD, non-CKD, and CKDu) in the Uddanam region
| Total population = 2210 |
CKD = 403 |
non-CKD = 1807 |
CKDu = 294 |
|
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Age | 43.1 ± 14.2 | 51.8 ± 13.7 | 41.4 ± 13.6 | 50.9 ± 12.3 |
| Weight | 55 ± 12.4 | 54.3 ± 11.5 | 55.2 ± 12.5 | 53.3 ± 11.1 |
| Serum creatinine | 1.3 ± 1.0 | 3.00 ± 2.7 | 0.8 ± 0.2 | 2.2 ± 1.8 |
| Serum urea | 29.6 ± 25.6 | 75.2 ± 52.8 | 21.6 ± 6.7 | 40.3 ± 35.4 |
| Body mass index | 22.6 ± 4.6 | 22.3 ± 4.6 | 22.6 ± 4.7 | 21.8 ± 4.9 |
| eGFR | 94.3 ± 33.4 | 40.3 ± 14.7 | 103.2 ± 25.2 | 40.3 ± 14.8 |
| Systolic BP | 127.3 ± 23.5 | 137.2 ± 28.3 | 125.8 ± 22.3 | 127.5 ± 25.7 |
| Diastolic BP | 82.2 ± 13.9 | 85.8 ± 16.8 | 81.6 ± 13.4 | 84.3 ± 15.8 |
| Proteinuria (PCR) | 0.2 ± 0.1 | 1.5 ± 0.9 | 0.4 ± 0.1 | 0.3 ± 0.1 |
BP, blood pressure; CKD, chronic kidney disease; CKDu, chronic kidney disease of unknown etiology; eGFR, estimated glomerular filtration rate; PCR, protein-creatinine ratio.
Table 11.
Agewise and stagewise population distribution of patients with CKDu
| eGFR values (stages of CKDu) | |||||||
|---|---|---|---|---|---|---|---|
| Age, yr | Total | <15 (Stage V) | 15–29 (Stage IV) | 30–43 (Stage IIIB) | 44–59 (Stage IIIA) | 60–89 (Stage II) | ≥90 (Stage I) |
| 18–27 | 26 | 0 | 2 | 3 | 4 | 3 | 14 |
| 28–37 | 35 | 3 | 1 | 5 | 10 | 3 | 13 |
| 38–47 | 65 | 5 | 12 | 12 | 18 | 7 | 11 |
| 48–57 | 71 | 4 | 15 | 11 | 30 | 6 | 5 |
| 58–67 | 68 | 4 | 18 | 13 | 23 | 7 | 3 |
| >68 | 29 | 2 | 7 | 6 | 11 | 2 | 1 |
| Total (%) | 294 | 18 (6.1) | 55 (18.7) | 50 (17.0) | 96 (32.7) | 28 (9.5) | 47 (16.0) |
CKDu, chronic kidney disease of unknown etiology; eGFR, estimated glomerular filtration rate.
Table 12.
Genderwise and stagewise population distribution of patients with CKDu
| Stagewise distribution of subjects with CKDu | |||
|---|---|---|---|
| eGFR and CKD stage | Total | Men | Women |
| <15 (Stage V) | 18 | 6 | 12 |
| 15–29 (Stage IV) | 55 | 23 | 32 |
| 30–43 (stage IIIB) | 50 | 25 | 25 |
| 44–59 (Stage IIIA) | 96 | 45 | 51 |
| 60–89 (Stage II) | 28 | 18 | 10 |
| ≥90 (Stage I) | 47 | 19 | 28 |
| Total | 294 | 136 (46.3) | 158 (53.7) |
CKDu, chronic kidney disease of unknown etiology; eGFR, estimated glomerular filtration rate.
Table 13.
Association of demographic and habitual characteristics with CKDu
| Variable | Category | CKDu |
Non-CKDu |
Crude OR (95% CI) | P | ||
|---|---|---|---|---|---|---|---|
| Count | % | Count | % | ||||
| Age, yr | >67 | 29 | 9.9 | 23 | 21.1 | 0.048 (0.006–0.385) | 0.004 |
| 58–67 | 68 | 23.1 | 37 | 33.9 | 0.071 (0.009–0.542) | 0.011 | |
| 48–57 | 71 | 24.1 | 27 | 24.8 | 0.1 (0.013–0.772) | 0.027 | |
| 38–47 | 65 | 22.1 | 17 | 15.6 | 0.149 (0.019–1.18) | 0.071 | |
| 28–37 | 35 | 11.9 | 4 | 3.7 | 0.337 (0.035–3.191) | 0.343 | |
| 18–27 | 26 | 8.8 | 1 | 0.9 | Ref | – | |
| Gender | Women | 158 | 53.7 | 60 | 55.0 | 1.083 (0.697–1.684) | 0.723 |
| Men | 136 | 46.3 | 49 | 45.0 | |||
| Education | Educated | 136 | 46.3 | 59 | 54.1 | 0.729 (0.469–1.134) | 0.161 |
| Uneducated | 158 | 53.7 | 50 | 45.9 | |||
| Occupation | Farmer | 215 | 73.1 | 75 | 68.8 | 1.234 (0.763–1.994) | 0.391 |
| Nonfarmer | 79 | 26.9 | 34 | 31.2 | |||
| Farmers - pesticides contact | Exposed to pesticides | 62 | 21.1 | 26 | 23.9 | 0.853 (0.506–1.438) | 0.551 |
| Not exposed to pesticides | 232 | 78.9 | 83 | 76.1 | |||
| Tobacco smoking | Yes | 54 | 18.4 | 28 | 25.7 | 0.651 (0.386–1.096) | 0.106 |
| No | 240 | 81.6 | 81 | 74.3 | |||
| Alcohol consumption | Yes | 72 | 24.5 | 33 | 30.3 | 0.747 (0.459–1.216) | 0.241 |
| No | 222 | 75.5 | 76 | 69.7 | |||
CI, confidence interval; CKDu, chronic kidney disease of unknown etiology; OR, odds ratio.
Figure 2.
Genderwise population distribution of patients without chronic kidney disease (CKD) and patients with CKD or CKD of unknown etiology (CKDu). Numerical values given in parentheses represent number of subjects.
Discussion
Over the past 2 decades, there were widespread concerns among the general public and medical fraternity regarding the high prevalence of CKD in the Uddanam region of Andhra Pradesh. Hence, we undertook this study to determine the prevalence of CKD and associated risk factors among the rural population of Uddanam. In our study, the prevalence of low eGFR was 13.9% and prevalence of CKD was 18.23%. This uncanny high prevalence of CKD in a specific segment of the population is highly significant and is evidence pointing to an epidemic of CKD in this region. Although published data indicate an increasing prevalence of CKD in India, community-based studies are few and most of them were done in urban centers. CKD prevalence of 0.79% was reported based on serum creatinine estimation in the south zone population of New Delhi,5 whereas the prevalence of decreased Modification of Diet in Renal Disease-GFR was 4.2% in the north Indian population.16 In another study undertaken in the rural population of south India, it was found that the prevalence of decreased Modification of Diet in Renal Disease-GFR was 4.35% and prevalence of CKD was 6.3%.6 In population-based studies, the prevalence of decreased GFR was 4.7% to 8.1% in Europe17, 18 and 4.5% to 7.7% in the United States.19, 20 The prevalence of CKD in Uddanam is at least 3 to 4 times higher than the prevalence reported in any of the previously mentioned studies. Several global epidemics of CKD have been reported, some with known etiology and in others the etiology remains unclear. In Sri Lanka, El Salvador, and Nicaragua, CKD is reported as a major public health problem causing significant mortality, and these regions are considered to be the hot-spots of CKD. The prevalence of CKD was between 13% and 18% in these 3 regions.21, 22 Interestingly, the prevalence of CKD in Uddanam is the same as reported from any of the hot-spots in other geographical foci.
The prevalence of diabetes was found to be 7.2% in our study population, which is comparable to the previous Indian studies in rural populations where the prevalence was reported as 6% to 7%. The prevalence of diabetic kidney disease in our study was 14.6%, which is in stark contrast to the data from the Indian CKD registry in which diabetic nephropathy was the preeminent cause of CKD in 31% of patients.8 We used similar criteria for identifying diabetic nephropathy. It is possible that some of these patients had nondiabetic kidney disease and more strict criteria for diagnosis of diabetic nephropathy could have further reduced the prevalence of diabetic kidney disease. Only 11.9% of our patients had high proteinuria (PCR >1), indicating that most patients with diabetes had CKD because of tubulo-interstitial disease rather than diabetic nephropathy.
Hypertension was seen in 16.7% of the total subject population; 85.1% of subjects gave a history of hypertension, indicating a large percentage of subjects were aware of their hypertensive status. In our study among all the hypertensive patients, 38.4% had long-standing hypertension, whereas among patients of chronic kidney disease, only 13.6% had long-standing hypertension. These results reveal that in a high proportion of patients with CKD, the 2 traditional etiological factors were strikingly absent.
Several Indian studies have used dipstick-positive proteinuria as the criteria for chronic kidney disease. The prevalence of proteinuria varied from 0.5% to 4.4% in these studies.5, 16, 22 However, we have estimated PCR as a marker of proteinuria and report PCR >0.2 in 9.04% of the entire study population. This has led us to identify more patients in CKD stages I and II. Among patients with CKD, 49.6% had PCR >0.2. Of these, only a minority (8.4%) of subjects had high-range proteinuria (PCR >1). We feel that PCR is an easy and effective way of identifying CKD in population studies.
In our study, pesticide contact and hypertension did not fit in the multiple logistic regression model while evaluating the simultaneous effects of exposure variables. The reason that hypertension did not fit might be because of the covariance effect of long-standing hypertension. In studies of Meso-American nephropathy and Sri Lankan CKD, the prevalence of CKD varied conspicuously with age and occupation, such as agricultural works, sugarcane cutting, and mining, which are considered as heat stress occupations.21 Similarly we found the influence of occupation on the prevalence of CKD in Uddanam. CKD prevalence also varied with altitude and climatic conditions.23 As the Uddanam region is uniformly at 50 feet above sea level, there was no effect of the altitude on prevalence.
The prevalence of CKD and CKDu was relatively high in Uddanam. In our study, 294 (73%) patients with CKD had PCR <1 and did not have diabetes or long-standing hypertension. This type of CKD of unexplained etiology is strikingly common in the Uddanam region and to the best of our knowledge, has not been reported from any other parts of India. A similar prevalence of more than 50% CKDu among patients with CKD was reported in cross-sectional studies from Nicaragua and Sri Lanka.2, 15
Univariate regression analysis of our data did not show significant association of gender disparity, education, farming, contact with pesticides, or habits such as tobacco smoking and alcohol consumption with CKDu in Uddanam. Our findings strongly indicate that both men and women are equally exposed to unknown factors that make them prone to CKD. The slow progress of disease, minimal urinary protein, and absence of chronic hypertension or diabetes strongly suggest that tubulo-interstitial nephritis is the pathology of CKD.
Although this cross-sectional study cannot address the causality, we have made an attempt to explore risk factors that characterize CKDu. We performed water and soil analysis for heavy metals (Zn, Mn, Cd, Si, Fe, Al, and As) and organo-metallic pesticides. In this preliminary study, the levels of silica in 110 water samples collected from various sources in Uddanam were found to be high. Further studies are necessary to find the association of silica with CKD.
In summary, this study provides evidence regarding CKDu as a true public health threat in Uddanam. The absence of a strong association between CKD and conventional risk factors reiterates the importance to search for new etiological factors. Considering the significant impact of CKDu in this region, in-depth studies are required to find etiological factors and implement preventive measures.
Strengths and Limitations of the Study
The strength of the study lies in its sampling methodology. We used multistage sampling, which covered a large area of investigation. We conducted house-to-house surveys with random sampling without bias for gender, occupation, literacy, or socioeconomic status. Questionnaires were administered and data were collected by medical graduates. This has ensured good compliance from the subjects and accuracy of the data. Biochemical tests were carried out in a single accredited reference laboratory. Proteinuria was quantified by PCR instead of the dip-stick method, which identified more cases in CKD stages I and II and eliminated false-positive cases.
There are a few limitations in the study. It was based on a single measurement of serum creatinine for each individual and estimation of GFR by using formulae rather than direct measurement. However, because this is a large population-based study in a specific geographic area, high prevalence of low eGFR in a single spot determination is significant. Kidney biopsy studies of early CKD stages I and II would probably have helped to identify etiological factors of CKD. In the second phase of our study, we have included kidney biopsy for histopathological examination and estimation of heavy metals in the biopsy specimen for establishing the type of kidney disease and possible etiological factors. Other diagnostic tests, such as ultrasonography of the kidney, would have identified other kidney pathologies. We could not perform ultrasonography in a population-based study in view of existing legal sanctions in the country.
Conclusion
This is the first report on prevalence and risk factors of CKDu in India. In this study, we report 18.23% prevalence of CKD in a rural population in the Uddanam region of India. This is 4 to 18 times higher than the prevalence reported in any population-based study carried out in India. This study provides new evidence of high prevalence of CKD of epidemic proportions, which has emerged as a large public health threat involving consequent financial burden. This form of CKD appears to be unrelated to traditional risk factors, such as diabetes and chronic hypertension. Seventy-three percent of patients with CKD in Uddanam were identified as CKDu. This disease is similar to CKDu reported from Sri Lanka and rural Nicaragua; however, unlike CKDu in Sri Lanka or rural Nicaragua, we did not find any association with age, sex, education, employment in agricultural fields, or habits such as tobacco smoking and alcohol consumption. Therefore, we propose that there might be a new etiological factor or multiple factors responsible for Uddanam kidney disease. There is a compelling need for a cohort study for unravelling the etiology. Urinary biomarker and renal biopsy studies in early stages of CKD population may give an insight into the hitherto unknown etiology. In addition, it is important to make note of the existing disease burden, identify the population with early CKD, prevent progression of the disease, and establish treatment facilities for patients with advanced kidney disease so that morbidity and mortality can be contained.
Disclosure
All the authors declared no competing interests.
Acknowledgments
We sincerely thank students and faculty of the Department of Community Medicine, Andhra Medical College, Visakhapatnam, for their support and cooperation in collection of data.
References
- 1.Mills K.T., Xu Y., Zhang W. A systematic analysis of world-wide population-based data on the global burden of chronic kidney disease in 2010. Kidney Int. 2015;88:950–957. doi: 10.1038/ki.2015.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Athuraliya N.T., Abeysekera T.D.J., Amerasinghe P.H. Uncertain etiologies of proteinuric-chronic kidney disease in rural Sri Lanka. Kidney Int. 2011;80:1212–1221. doi: 10.1038/ki.2011.258. [DOI] [PubMed] [Google Scholar]
- 3.Correa-Rotter R., Wesseling C., Johnson R.J. CKD of unknown origin in Central America: the case for a Mesoamerican nephropathy. Am J Kidney Dis. 2014;63:506–520. doi: 10.1053/j.ajkd.2013.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jha V., Garcia-Garcia G., Iseki K. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;20:260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal S.K., Dash S.C., Irshad M. Prevalence of chronic renal failure in adults in Delhi, India. Nephrol Dial Transplant. 2005;20:1638–1642. doi: 10.1093/ndt/gfh855. [DOI] [PubMed] [Google Scholar]
- 6.Anupama Y.J., Uma G. Prevalence of chronic kidney disease among adults in a rural community in South India: results from the kidney disease screening (KIDS) project. Indian J Nephrol. 2014;24:214–221. doi: 10.4103/0971-4065.132990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Modi G.K., Jha V. The incidence of end-stage renal disease in India: a population-based study. Kidney Int. 2006;70:2131–2133. doi: 10.1038/sj.ki.5001958. [DOI] [PubMed] [Google Scholar]
- 8.Rajapurkar M.M., John G.T., Kirpalani A.L. What do we know about chronic kidney disease in India: first report of the Indian CKD registry. BMC Nephrol. 2012;13:1–8. doi: 10.1186/1471-2369-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gadde P., Sanikommu S., Manumanthu R. Uddanam nephropathy in India: a challenge for epidemiologists. Bull World Health Organ. 2017;95:848–849. doi: 10.2471/BLT.17.196758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pourhoseingholi M.A., Vahedi M., Rahimzadeh M. Sample size calculation in medical studies. Gastroenterol Hepatol Bed Bench. 2013;6:14–17. [PMC free article] [PubMed] [Google Scholar]
- 11.Jaffe M. Ueber den Niederschlag welchen Pikrinsäure in normalen Harn erzeugt und über eine neue reaction des Kreatinins. Z Physiol Chem. 1886;10:391–400. [in German] [Google Scholar]
- 12.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Kidney Disease Outcome Quality Initiative. Am J Kidney Dis. 2002;39:S1–S246. [PubMed] [Google Scholar]
- 13.Hallan S.I., Orth S.R. The KDOQI 2002 classification of chronic kidney disease: for whom the bell tolls. Nephrol Dial Transplant. 2010;25:2832–2836. doi: 10.1093/ndt/gfq370. [DOI] [PubMed] [Google Scholar]
- 14.Ginsberg J.M., Chang B.S., Matarese R.A. Use of single voided urine samples to estimate quantitative proteinuria. N Engl J Med. 1983;309:1543–1546. doi: 10.1056/NEJM198312223092503. [DOI] [PubMed] [Google Scholar]
- 15.Orantes C.M., Herrera R., Almaguer M. Chronic kidney disease and associated risk factors in the Bajo Lempa region of El Salvador: Nefrolempa study, 2009. MEDICC Rev. 2011;13:14–22. doi: 10.37757/MR2011V13.N4.5. [DOI] [PubMed] [Google Scholar]
- 16.Singh N.P., Ingle G.K., Saini V.K. Prevalence of low glomerular filtration rate, proteinuria and associated risk factors in North India using Cockcroft-Gault and Modification of Diet in Renal Disease equation: an observational, cross-sectional study. BMC Nephrol. 2009;10:1–13. doi: 10.1186/1471-2369-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Q.L., Rothenbacher D. Prevalence of chronic kidney disease in population-based studies: systematic review. BMC Public Health. 2008;8:117. doi: 10.1186/1471-2458-8-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruck K., Stel V.S., Gambaro G. CKD prevalence varies across the European general population. J Am Soc Nephrol. 2016;27:2135–2147. doi: 10.1681/ASN.2015050542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coresh J., Selvin E., Stevens L.A. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 20.Hill N.R., Fatoba S.T., Oke J.L. Global prevalence of chronic kidney disease—a systematic review and meta-analysis. PLoS One. 2016;11:18. doi: 10.1371/journal.pone.0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Donnell J.K., Tobey M., Weiner D.E. Prevalence of and risk factors for chronic kidney disease in rural Nicaragua. Nephrol Dial Transplant. 2010;1:1–8. doi: 10.1093/ndt/gfq385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed I., John G.T., Kirubakaran M.G. Prevalence of proteinuria in rural adult population in Tamil Nadu. Indian J Med Res. 2006;124:185–188. [PubMed] [Google Scholar]
- 23.Torres C., Aragón A., González M. Decreased kidney function of unknown cause in Nicaragua: a community-based survey. Am J Kidney Dis. 2010;55:485–496. doi: 10.1053/j.ajkd.2009.12.012. [DOI] [PubMed] [Google Scholar]