Abstract
Introduction
Recurrence of atypical hemolytic uremic syndrome (aHUS) in renal allografts is common, leading to dialysis and graft failure. Pretransplant versus posttransplant initiation of eculizumab treatment in patients with aHUS has not been rigorously investigated. We hypothesized eculizumab pretransplant would reduce dialysis incidence posttransplant.
Methods
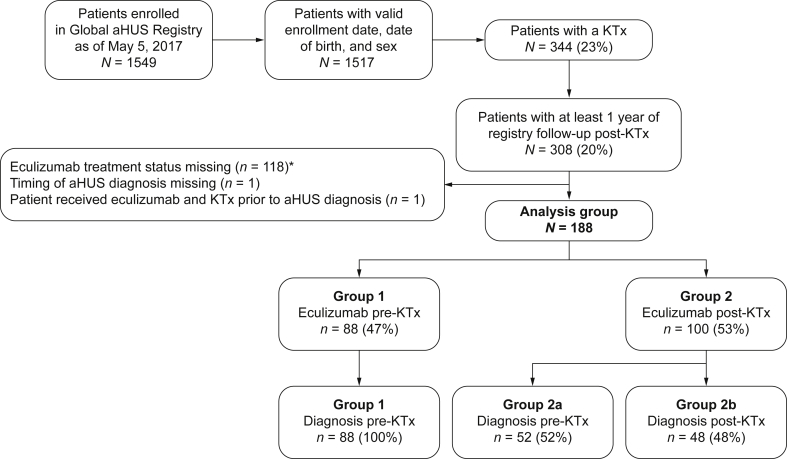
Of patients enrolled in the Global aHUS Registry (n = 1549), 344 had ≥1 kidney transplant. Of these, 188 had received eculizumab. Eighty-eight patients (47%) were diagnosed with aHUS and received eculizumab before, and during, their most recent transplant (group 1). A total of 100 patients (53%; group 2) initiated eculizumab posttransplantation. This second group was subdivided into those diagnosed with aHUS before (n = 52; group 2a) or after (n = 48; group 2b) their most recent transplant.
Results
Within 5 years of transplantation, 47 patients required dialysis; the risk of dialysis after transplantation was significantly increased in group 2b (hazard ratio [HR] 4.6; confidence interval [CI] 1.7–12.4) but not 2a (HR 2.3; CI 0.9–6.2). Graft function within 6 months of transplantation was significantly better in group 1 (median estimated glomerular filtration rate of 60.6 ml/min per 1.73 m2) compared with 31.5 and 9.6 ml/min per 1.73 m2 in groups 2a (P = 0.004) and 2b (P = 0.0001), respectively. One meningococcal infection (resolved with treatment) and 3 deaths (deemed unrelated to eculizumab) were reported.
Conclusions
Outcomes for transplant patients with aHUS treated with eculizumab were improved compared with previous reports of patients with aHUS not treated with eculizumab. Our findings suggest delayed aHUS diagnosis and therefore treatment is associated with an increased risk of dialysis posttransplantation and reduced allograft function.
Keywords: atypical hemolytic uremic syndrome, dialysis, eculizumab, kidney observational study, transplantation
Graphical abstract
See Commentary on Page 370
Atypical hemolytic uremic syndrome (aHUS) is a rare, life-threatening disease of chronic, uncontrolled complement activation leading to thrombotic microangiopathy, and often resulting in severe organ damage, including advanced chronic kidney disease. For patients with aHUS and end-stage kidney disease, transplantation was often contraindicated due to the high risk of recurrence of disease in the graft and subsequent graft loss.1, 2 Targeted complement inhibition is now available for the treatment of aHUS. Eculizumab, a humanized monoclonal antibody, binds to C5 preventing cleavage to C5a and C5b and subsequent membrane attack complex formation. Eculizumab has been shown to be effective in treating patients with kidney disease due to aHUS, normalizing hematological parameters, and restoring renal function.3, 4, 5, 6 Where available, eculizumab has profoundly changed the outcomes for patients with aHUS compared with the conventional management options such as plasma exchange. The safety and effectiveness of eculizumab treatment for posttransplant aHUS recurrence was first reported in 2009.7 The registration trials/studies for eculizumab included 26 patients who had received a total of 38 kidney transplants. Eight of the 26 patients had received a combined 12 transplants that had failed before study enrollment. Twenty-five of the 26 patients received their most recent transplant before the initiation of eculizumab. Renal and hematological endpoints improved significantly following the initiation of eculizumab, although to a smaller degree than in patients with native kidney disease, and no patients lost their graft after eculizumab initiation.8 Limited additional information is available to guide clinicians as to the timing of eculizumab therapy in relation to transplantation.8, 9, 10, 11, 12 The benefit of preoperative eculizumab therapy for patients undergoing transplantation has yet to be assessed in a patient population with a follow-up period adequate to support multivariate analysis at clinically relevant time points.
The Global aHUS Registry is the largest collection of demographic, clinical, and genetic information from patients treated with eculizumab for a diagnosis of aHUS who also received a kidney transplant in the context of routine clinical care outside of any study protocol. In this observational study, we describe 188 kidney transplant recipients with aHUS who received eculizumab and were followed postoperatively for at least 1 year to study clinically relevant endpoints.
Methods
Patient Selection
Details of the Global aHUS Registry (National Institutes of Health, www.ClinicalTrials.gov Identifier NCT01522183, managed by Alexion Pharmaceuticals, Inc., New Haven, CT) have been previously reported.13, 14 Briefly, patients of any age with a clinical diagnosis of aHUS were eligible for enrollment in this ongoing observational study subsequent to providing informed consent. Patients were enrolled from 17 countries and followed prospectively. Patients were not required to have an identified complement gene abnormality or complement factor (CF) H autoantibody, nor were they required to have previous or ongoing treatment with eculizumab. Individuals with evidence of Shiga toxin–producing Escherichia coli infection or with ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif) activity ≤10% or a subsequent diagnosis of thrombotic thrombocytopenic purpura were excluded. After enrollment, patient data were collected every 6 months. The Registry includes participants from the observational long-term follow-up clinical trial (NCT01522170) as well as other studies of the treatment of patients with aHUS with eculizumab (NCT identifiers 01770951, 00838513, 00844428, 00844545, 00844844, 01193348, 01194973).
The current analysis included Registry patients with a kidney transplant who were treated with eculizumab (Alexion Pharmaceuticals, Inc.) and followed for at least 1 year posttransplantation. Where transplantation and initiation of eculizumab occurred before enrollment, data up to enrollment were collected retrospectively. The Registry does not collect data on the type of transplantation (living vs. deceased donor), rejection episodes, details of the immunosuppression regimen, or the cause of allograft failure.
Patients were classified according to their treatment status at the time of their most recent transplant (Figure 1). In group 1, treatment was initiated before transplantation up to and including the day of surgery (postoperative day 0). All patients in group 1 had a known diagnosis of aHUS before receiving their most recent kidney transplant. Patients who initiated eculizumab but discontinued before transplantation were not included in this group. Group 2 included all patients initiating eculizumab treatment any day after postoperative day 0, either for the first time, or restarting eculizumab after transplantation having discontinued treatment before transplantation, with >14 days without treatment. Because of the number of patients in group 2 with a reported initial diagnosis of aHUS after their most recent transplant, patients were further stratified according to the timing of the last transplant in relation to time of aHUS diagnosis. Group 2a included patients who were diagnosed with aHUS before transplantation and group 2b consisted of patients who were not known to have aHUS before transplantation.
Figure 1.
Patient disposition by timing of eculizumab initiation and diagnosis. *Includes patients who discontinued eculizumab before KTx and never restarted and patients without valid dates for their eculizumab treatment. aHUS, atypical hemolytic uremic syndrome; KTx, kidney transplant.
Statistical Methods and Endpoints
Descriptive summary statistics are presented by treatment/diagnosis classification group and overall. Data are presented as frequency counts (with percentages) for categorical variables; and mean (±SD), median (range), and number of observations for continuous variables.
The primary endpoint was any dialysis, including short-term dialysis (acute; defined as a duration of up to 3 months) and graft loss (defined as chronic dialysis with a duration of more than 3 months of consecutive dialysis), as the best available proxy for allograft failure, as graft loss or biopsy findings are not specifically collected in the Registry). The dialysis incidence rate was defined as (number of new events/total patient-years)*100, where patient-years was based on time from transplantation to start date of dialysis. The 95% Poisson CIs for the incidence rate were calculated. Kaplan–Meier estimates for the time to any dialysis were also determined. For the determination of estimated glomerular filtration rate (eGFR), patients on dialysis had an imputed eGFR of 5 ml/min per 1.73 m2.
Cox proportional hazards regression was used to evaluate differences in time to any dialysis across the classification groups based on selected risk factors of interest. These covariates included age at transplantation, gender, prior transplant and prior dialysis, plasma exchange before transplantation, and eculizumab dosing. The final multivariate model was determined using the forward stepwise selection procedure, and contains covariates that were significant at the 0.10 level in the univariate analysis. The HRs and 95% CIs are presented. All analyses were conducted using SAS® version 9.2 (SAS Institute, Cary, NC).
Results
Patient Characteristics
The Global Registry enrolled 1549 patients across 220 sites between April 2012 and May 5, 2017. This analysis included 188 patients who received a kidney transplant and were treated with eculizumab at or after their most recent transplant. Table 1 summarizes patient characteristics of enrollees categorized by timing of eculizumab treatment (ongoing treatment up to or at time of transplant [group 1; n = 88] vs. treatment initiation after transplant [group 2; n = 100]). Of those patients initiating eculizumab treatment after kidney transplant, 52 patients were diagnosed with aHUS before (group 2a), and 48 were diagnosed after (group 2b), their most recent kidney transplant (Figure 1).
Table 1.
Baseline demographics
| n (%), unless stated | Group 1 |
Group 2 Eculizumab initiated after transplant |
|
|---|---|---|---|
| Eculizumab initiated at or before transplant (n = 88) | Group 2a aHUS diagnosis pretransplant (n = 52) | Group 2b aHUS diagnosis posttransplant (n = 48) | |
| Median age at most recent KTx, y (range) | 32.3 (3.0–70.2) | 33.5 (2.3–67.2) | 39.5 (2.9–75.3) |
| Age at most recent transplant, yr | |||
| <18 | 27 (31) | 14 (27) | 8 (17) |
| ≥18 | 61 (69) | 38 (73) | 40 (83) |
| Gender, female | 45 (51) | 29 (56) | 31 (65) |
| Race | |||
| Asian | 0 (0) | 1 (2) | 3 (6) |
| Black | 4 (5) | 2 (4) | 5 (10) |
| White | 83 (94) | 48 (92) | 34 (71) |
| Other | 1 (1) | 0 (0) | 6 (13) |
| Family history | 25 (28) | 11 (21) | 2 (4) |
| Total number of KTx | |||
| 1 | 64 (73) | 41 (79) | 45 (94) |
| 2 | 14 (16) | 7 (14) | 3 (6) |
| ≥3 | 10 (11) | 4 (8) | 0 (0) |
| Concomitant etiologies contributing to need for transplanta | |||
| Diabetes | 0 (0) | 1 (1) | 3 (6) |
| Hypertension | 1 (1) | 0 (0) | 4 (8) |
| Pathogenic mutation identified, n/N (%)b | |||
| CFH | 34/68 (50) | 22/37 (60) | 7/28 (25) |
| C3 | 9/42 (21) | 5/22 (23) | 1/21 (5) |
| CFI | 6/53 (11) | 0/26 (0) | 5/26 (19) |
| MCP | 9/54 (17) | 0/23 (0) | 3/24 (13) |
| CFB | 1/41 (2) | 0/18 (0) | 1/19 (5) |
| Incidence of plasma exchange before transplant | 16 (18) | 13 (25) | 3 (6) |
aHUS, atypical hemolytic uremic syndrome; CF, complement factor; KTx, kidney transplant; MCP, membrane cofactor protein.
Other etiologies reported as contributing to the need for transplant included antibody-mediated rejection, C3 glomerulopathy, glomerulonephritis, IgA nephropathy, nephrosclerosis, rejection, renal hypoplasia, and renal vasculitis.
Percentages are based on the number of patients who have the specific gene data available (n tested).
Patient age was diverse but, overall, represented an adult population. Median age at most recent transplant was 34.1 years. Forty-nine (26%) of 188 patients were younger than 18 at time of last transplant and therefore were termed pediatric patients. Most patients were female (56%). The racial background was primarily white (88%). Along with the diagnosis of aHUS, some patients had other conditions contributing to their chronic kidney disease (Table 1).
Overall, 20% (n = 38) of patients across all groups reported a family history of aHUS; however, 103 (71%) of 145 patients tested had a reported pathogenic gene mutation linked to key CFs in the alternative complement activation pathway. Gene mutations were identified in 68% of adult patients (74 of 109 tested) and in 81% of pediatric patients (29 of 36 tested). Where tested, CFH was the most frequently identified mutation (in 50%, 60%, and 25% of patients tested in groups 1, 2a, and 2b, respectively, Table 1).
There were 24 patients (28%) in group 1, 11 (21%) in group 2a, and 3 (6%) in group 2b who had received 1 or more prior transplants. In the 12 months before the most recent transplant, 32 patients had received plasma exchange, including 16 (18%) in group 1, 13 (25%) in group 2a, and 3 (6%) in group 2b (Table 1).
Most patients (73%; n = 137) were enrolled in the United States (21%, n = 40), France (19%, n = 35), Germany (16%, n = 30), Italy (9%, n = 17), and Spain (8%, n = 15). Patients meeting the inclusion criteria were also enrolled in the United Kingdom, Australia, Czech Republic, Canada, Russia, Israel, Belgium, Austria, Denmark, Sweden, Norway, and Switzerland (Figure 2). In the United States, eculizumab was most often given before transplantation when a clinical diagnosis was made before transplantation (n = 22). In contrast, in France, patients were often treated after transplantation even when the diagnosis of aHUS was made before the most recent transplant (n = 17). In France, the United States, and Germany, several patients were diagnosed after transplantation and did not initiate eculizumab therapy until after transplantation.
Figure 2.
Geographical distribution of patients analyzed in the study. The analysis population comprised patients from 17 countries, with most enrolled in Europe or North America. Shading indicates relative proportion of patients in groups 1, 2a, and 2b for each country.
Treatment Characteristics
Eculizumab was initiated at a median (range) of 3.3 (−0.07 to 36.9) years post-aHUS diagnosis. The time to initiation of eculizumab posttransplant differed between patients who were diagnosed with aHUS before transplantation (group 2a, n = 52) and those diagnosed with aHUS after transplantation (group 2b, n = 48): median time to treatment start was longer for group 2b (0.8 [range 0.008–14.9] years after transplantation) compared with group 2a (0.3 [range 0.03–14.4] years; Table 2). The dose of eculizumab was reduced in 19 patients (22%) in group 1, and 8 (15%) and 4 (8%) patients in groups 2a and 2b, respectively. As most of these patients had a series of dose modifications, reflecting an individualized treatment approach, it was not possible to calculate a clinically meaningful average dose reduction.
Table 2.
Clinical characteristics
| Median (range), unless stated | Group 1 |
Group 2 Eculizumab initiated after transplant |
|
|---|---|---|---|
| Eculizumab initiated at or before transplant (n = 88) | Group 2a aHUS diagnosis pretransplant (n = 52) | Group 2b aHUS diagnosis posttransplant (n = 48) | |
| Time from aHUS diagnosis to eculizumab initiation, yr | 4.2 (−0.04 to 36.9)a | 7.2 (0.6 to 28.4) | 0.01 (−0.1 to 14.5) |
| Time from aHUS diagnosis to most recent KTx, yr | 4.7 (0.0 to 36.9) | 5.0 (0.0 to 26.7) | −0.6 (−11.5 to −0.01) |
| Time from most recent KTx to eculizumab initiation, yr | n/a | 0.3 (0.003 to 14.4) | 0.8 (0.008 to 14.9) |
| Any discontinuation of eculizumab, n (%)b | |||
| No | 85 (97) | 50 (96) | 47 (98) |
| Yes | 3 (3) | 2 (4) | 1 (2) |
| Any dose reduction of eculizumab, n (%) | |||
| No | 69 (78) | 44 (85) | 44 (92) |
| Yes | 19 (22) | 8 (15) | 4 (8) |
| Patients with any dialysis post-KTx, n (events) | 6 (8) | 12 (20) | 29 (42) |
| Delayed graft functionc | 1 (1) | 3 (3) | 4 (4) |
| Acute dialysis | 3 (5) | 6 (13) | 16 (25) |
| Graft loss (new chronic dialysis)d | 3 (3) | 7 (7) | 17 (17) |
| Patients with any dialysis at 1-year post-KTx, n (events) | 3 (4) | 5 (7) | 11 (12) |
| Dialysis incidence rate, per 100 patient-years (95% CI) | 2.9 (1.2 to 5.6) | 7.0 (4.3 to 10.8) | 16.4 (11.8 to 22.2) |
| Graft loss incidence rate, per 100 patient-years (95% CI)d | 1.1 (0.2 to 3.1) | 2.5 (1.0 to 5.1) | 6.6 (3.9 to 10.6) |
| eGFR at 2-years posttransplant,e ml/min per 1.73 m2, median (IQR) | 70.2 (48.9 to 82.6) | 44.8 (5.0 to 67.9) | 24.2 (5.00 to 44.0) |
aHUS, atypical hemolytic uremic syndrome; CI, confidence interval; eGFR, estimated glomerular filtration rate; IQR, interquartile range; KTx, kidney transplant; n/a, not applicable.
bDiscontinuation is posttransplant only.
Clinical suspicion led to eculizumab initiation in 1 patient before diagnosis was confirmed.
Delayed graft function was defined as initiation of dialysis within 7 days of transplantation and not continued dialysis for >3 months (e.g., graft loss).
Graft loss was defined as chronic dialysis lasting >3 months.
Data collected at 2 years ± 3 months posttransplant; patients on dialysis had an imputed eGFR of 5 ml/min per 1.73 m2.
Eculizumab was discontinued in 6 patients after their most recent transplant (group 1, n = 3; group 2a, n = 2; and group 2b, n = 1). The patients in group 1 discontinued due to “investigator consideration” and “completion of posttransplant treatment” (reason was not recorded for the third patient) and were not on dialysis when discontinued. One patient from group 2a and 1 from group 2b discontinued after >3 months of dialysis (reasons were “lack of renal function improvement” and “dose withheld due to hospitalization”). The second patient in group 2a requested to discontinue after being on dialysis for 1 month, and “lack of renal function improvement” and “adverse event” were also reported. Of the 6 patients who discontinued posttransplant, all except 2 patients in group 1 restarted eculizumab treatment. Those who stopped due to >3 months of dialysis remained on dialysis when they restarted; reasons for restarting treatment did not accompany the event log for any of these patients.
Clinical and Safety Outcomes After Transplantation
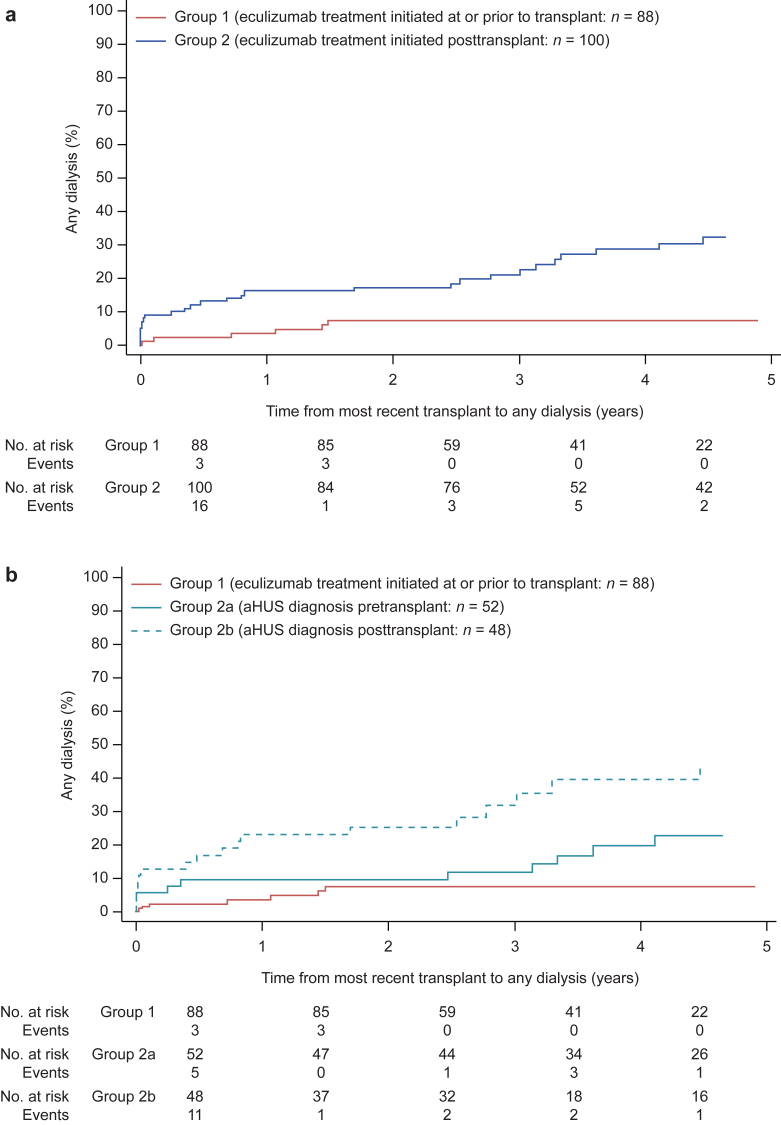
Median time from most recent transplant to first dialysis event or last follow-up was 3.0 years (range 0.003–17.3). Overall, 47 patients required dialysis at least once after transplantation (group 1, n = 6 [7%]; group 2a, n = 12 [23%]; group 2b, n = 29 [60%]) (Table 2). Eight patients (4%) experienced delayed graft function (dialysis within 7 days of transplantation), 1 in group 1, and 3 and 4 in groups 2a and 2b, respectively. An additional 17 patients received acute dialysis at time points more than 7 days after transplantation. Median duration of acute dialysis was 0.03 months (1 day) in group 1, and 1.51 and 0.40 months, in groups 2a and 2b, respectively. Graft loss occurred in 27 patients: 3 patients (3%) in group 1, 7 patients (13%) in group 2a, and 17 patients (35%) in group 2b.
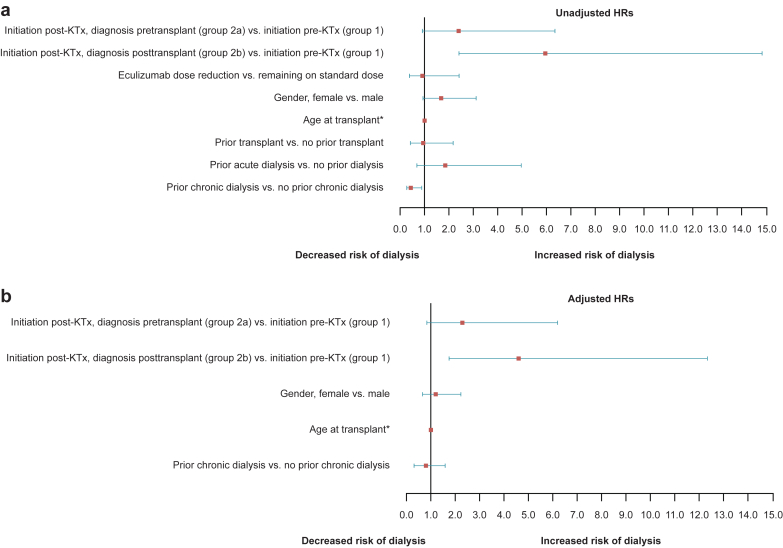
We first calculated an HR for any dialysis comparing group 1 with all patients in group 2. After accounting for multiple covariates, those patients initiating eculizumab after transplantation (group 2) were more likely to require dialysis after transplantation than those initiating eculizumab before transplantation (group 1), adjusted HR (AHR) 3.16 (1.28–7.81). Because of the increased risk of initiating dialysis after transplantation in group 2 (Figure 3a), HRs were then recalculated to separately account for a substantial number of patients with a diagnosis of aHUS after transplantation (group 2b; Figures 3b and 4). The AHR for any dialysis initiation after transplantation was significantly increased in group 2b (AHR 4.62; 95% CI 1.73–12.35) and numerically increased (nonsignificant) in group 2a (AHR 2.30; 95% CI 0.85–6.22) when compared with group 1 (control group) (Figures 3b and 4b). Furthermore, graft loss (i.e., dialysis >90 days) was increased in group 2b compared with group 1 (AHR 4.17; 95% CI 1.12–15.56). The AHRs for group 2a were numerically increased but not statistically significant (AHR 2.20; 95% CI 0.55–8.72).
Figure 3.
Cumulative proportion of patients receiving any dialysis posttransplantation. (a) Time from most recent transplant to any dialysis (cumulative proportion) for patients in group 1 and group 2. (b) Time from most recent transplant to any dialysis (cumulative proportion) for patients in group 1 and group 2 subdivided by atypical hemolytic uremic syndrome (aHUS) diagnosis date before transplantation (group 2a) and after transplantation (group 2b).
Figure 4.
Forest plot depicting hazard ratios (HRs) for time to any dialysis posttransplant. (a) Unadjusted HR (± 95% confidence interval [CI]) based on univariate analysis of each variable. (b) Adjusted HR (± 95% CI) based on multivariate analysis of all variables with P < 0.1 (eculizumab treatment status, gender, age at transplantation, prior chronic dialysis [within 12 months]). *Age was a continuous variable. KTx, kidney transplant.
Overall, early graft function (measured within 6 months posttransplant) was significantly better in group 1, with a median eGFR of 60.6 ml/min per 1.73 m2 compared with 31.5 and 9.6 ml/min per 1.73 m2 in groups 2a (P = 0.004) and 2b (P = 0.0001), respectively (Figure 5). Over time, renal function was generally maintained, and at 2 years posttransplant, eGFR values for patients in groups 2a and 2b remained significantly lower than for group 1 (Table 2). During the time patients were followed for this analysis, 1 patient (1%) in group 1 developed a meningococcal infection, which resolved with antibiotic treatment. Three deaths were reported (all in group 2a): 1 man with a glioblastoma, 1 woman with a gastrointestinal bleed, and 1 woman with a cerebral bleed. All cases were deemed unrelated to eculizumab.
Figure 5.
Median estimated glomerular filtration rate (eGFR) for patients in group 1, 2a, and 2b over time. Baseline is the first value recorded posttransplant (but within 6 months of transplantation). Values for each group are staggered at each timepoint to allow error bars to be clearly discerned and do not indicate differences in the time of measurement. Patients on dialysis had an imputed eGFR of 5 ml/min per 1.73 m2. Data are median (interquartile range [IQR]). *P < 0.01 versus group 1; †P < 0.01 versus group 2a. aHUS, atypical hemolytic uremic syndrome.
Plasma Exchange After Transplantation
Overall, 62 patients received plasma exchange after transplantation; plasma exchange was initiated in 7 patients (8%) from group 1, 27 patients (52%) from group 2a, and 28 patients (58%) from group 2b. The time to initiation of plasma exchange after transplantation is shown in Figure 6. In group 2b, plasma exchange was commonly initiated around the time of diagnosis of aHUS (Figure 6a and data not shown). In the first month posttransplant, 3, 12, and 10 patients in groups 1, 2a, and 2b initiated plasma exchange; the timing of plasma exchange for these patients is shown in Figure 6b. Initiation of eculizumab treatment commonly occurred after a delay of only several days after plasma exchange in these patients (data not shown).
Figure 6.
Plasma exchange posttransplant. Time from most recent kidney transplant to requirement for plasma exchange. (a) Cumulative proportion of patients receiving plasma exchange. Data shown up to 5 years of follow-up; 2 events occurred in group 2a and 10 events in group 2b at more than 5 years posttransplant. (b) Time to plasma exchange for patients receiving plasma exchange in the first month following transplantation. aHUS, atypical hemolytic uremic syndrome.
Conclusion
The Global aHUS Registry incorporates the efforts of 220 enrolling sites. Information is included from 1549 patients with a clinical diagnosis of aHUS, regardless of the treatment they received. Contained within the Registry is the largest cohort of patients with a diagnosis of aHUS receiving eculizumab in the setting of kidney transplantation. We analyzed the outcomes of those patients who received eculizumab in the setting of kidney transplantation (n = 188) with at least 1 year of follow-up after their most recent transplant. Overall, the population analyzed had attributes comparable to other cohorts of patients with a diagnosis of aHUS, including racial background, age, and prevalence of pathogenic gene mutations.15, 16, 17, 18 A proportion of these patients (20%) had lost 1 or more transplants previously; only the most recent transplant is considered here.
We found that a subgroup of patients initiating eculizumab after transplantation (group 2b) were more likely to require dialysis after transplantation than those initiating eculizumab before transplantation (group 1). This was discovered after a review of national practice patterns and individual patient profiles revealed a large subgroup (2b) that did not receive a diagnosis of aHUS until after transplantation. Subgroup 2b came to characterize the highest risk profile for graft failure, emphasizing the importance of the timing of diagnosis of aHUS with respect to transplantation. In our cohort, patients who were not diagnosed with aHUS until after transplantation included patients who were older, and less likely to have a family history of aHUS or have been tested for a genetic mutation. Notably, these patients had a longer time between transplantation and initiation of eculizumab than those in group 2a, supporting previous data showing that earlier eculizumab treatment is associated with improved outcomes.19 Those patients with a known pretransplant diagnosis of aHUS were treated earlier after transplantation, presumably due to an awareness of the possibility of, and more rapid recognition of, recurrent disease.
Those patients with a diagnosis before transplantation, but who did not receive eculizumab until after transplantation (group 2a), and who were treated more rapidly than group 2b, did not significantly differ in terms of dialysis initiation posttransplantation when compared with patients receiving eculizumab pretransplantation (group 1). The small number of dialysis events in any of the groups limited statistical assessment; however, the 2-year eGFR was significantly lower in both groups 2a (44.8 ml/min per 1.73 m2) and 2b (24.2 ml/min per 1.73 m2) compared with group 1 (70.2 ml/min per 1.73 m2), indicating a less favorable outcome. Patients in group 2a were also more likely than those in group 1 to be treated with plasma exchange posttransplant, before the commencement of eculizumab. Although caution should be exercised in interpreting these data, these groups were similar in demography and presence of pathogenic mutations. Our conclusions suggest that any transplant candidate with a clinical history or renal biopsy suggestive of thrombotic microangiopathy should be carefully evaluated for a possible diagnosis of aHUS and that treatment with eculizumab beginning at the time of transplantation will improve posttransplant outcome.
In this analysis, few safety concerns were reported. One meningococcal infection was identified, which resolved with antibiotics, and 3 deaths were reported, which were all deemed unrelated to eculizumab. The incidence of meningococcal infection in this cohort of kidney transplant patients with aHUS was in line with that reported in the original program of 5 clinical trials of eculizumab for the treatment of aHUS and in a prospective, observational, long-term follow-up of these studies.6, 8, 20 The case of meningococcal infection highlights the need for vaccination against Neisseria meningitidis before eculizumab initiation and/or prophylactic antibiotic therapy, as well as being alert to the signs of meningococcal disease during treatment.
Compared with historical results, the current analysis showed favorable outcomes in transplanted patients with aHUS receiving eculizumab, with graft survival (as defined by lack of posttransplant dialysis exposure) improved compared with that reported in the literature.1, 2, 21, 22 Two previous studies also reported excellent outcomes with eculizumab in patients with aHUS undergoing transplantation.8, 12 In contrast, another study suggested that eculizumab was not necessary before transplantation for patients with aHUS receiving a living organ transplant who followed a treatment protocol to reduce the risk of endothelial injury.11 Only 1 of 17 patients in that series experienced recurrent aHUS with a median follow-up of approximately 2 years. Close patient monitoring was required and it was recommended that eculizumab should be available immediately. The evidence to date indicates that eculizumab maintains long-term remission of aHUS, thus preserving graft function. The possibility of discontinuing eculizumab after sustained disease remission in patients with aHUS in their native kidneys may depend on genetic background.9 A recent study from the French aHUS registry of patients who discontinued eculizumab reported that 72% of patients with CFH variants and 50% of patients with CFI variants relapsed after a median follow-up of 22 months.23 The recently published Kidney Disease: Improving Global Outcomes guidelines and an international consensus for managing aHUS in children highlight that discontinuation is not currently recommended in transplanted patients.24, 25 Our results suggest that initiation of eculizumab therapy before transplantation reduces rates of dialysis after transplantation, and leads to improved allograft function, with the qualification that timing of aHUS diagnosis influences the posttransplant outcome, supporting our initial hypothesis.
These findings are remarkable in the context of a disease that often had devastating consequences.26, 27 Before the availability of eculizumab in 2011, death-censored graft survival at 1 year posttransplant for patients with aHUS was 76% and at 5 years, 51%, with a median graft survival of 61 months. Relapse rate ranged from 20% to approximately 80% depending on mutational background.1 Forty-three percent of relapses occurred within 1 month posttransplant and 70% within 1 year.25 These dismal outcomes were confirmed in another cohort by assessment of complement mutations in 273 patients with aHUS, which also found the highest graft failure rate in patients with identified CFH and CFI mutations (71% and 67% failure at 1 year, respectively) with similarly poor outcomes in native kidneys (end-stage kidney disease or death occurred in 77% and 60%, respectively at 3 years). Outcomes in these patients were poor regardless of age.27
In the pre-eculizumab era, plasma exchange was the only available treatment for patients with aHUS and had limited efficacy alone. Although eculizumab has been shown to be effective and offers a considerable improvement in outcomes,28, 29 the use of plasma exchange in the treatment of aHUS is still considered. Plasma exchange was received by 8% of patients in group 1 but by more than 50% of patients in groups 2a and 2b. Our results suggest that, for these patients in group 2, plasma exchange was not given prophylactically. A retrospective analysis in 2016 showed that plasma exchange was effective in 53% of nontransplanted pediatric patients treated (16 of 30), although it was possible to follow-up only 15 of 24 patients and of these, 3 died within 1.5 years.30 For a cohort of 146 patients for whom data on plasma therapy were available, Fremeaux-Bacchi et al.26 reported poor outcomes regardless of whether or not plasma therapy was administered; 44% of those on plasma therapy developed end-stage kidney disease at the first episode of aHUS. Outcomes in transplant patients treated with plasma exchange are particularly poor. In a large cohort of adult patients followed between 1995 and 2009 who had posttransplant thrombotic microangiopathy, almost half lost their graft within a year, irrespective of whether plasma exchange was used, suggesting a lack of effectiveness in this setting.1 No controlled trials comparing eculizumab and plasma exchange before transplantation have been performed and the available cost:benefit analyses are insufficient.2
There are several limitations of this analysis, all linked to the nature of an observational global registry31, 32 that draws on diverse practice information from more than 200 contributing sites. First, the Registry offers limited characterization of transplant events including living/deceased donor status, number of graft rejection episodes, transplant biopsy results, or causes of graft loss; however, with this cohort now established, future studies can target detailed characterization of these patients. Second, mutation analysis was available in as many as 145 patients, but the number of dialysis events associated with these patients was too small to support a multivariate regression model to examine the risk associated with individual mutations. This limits our current understanding as to the genetic risk profile of patients with a diagnosis of aHUS who are preparing for kidney transplantation. In part, this reflects the enrollment of patients who were diagnosed before 2011 when genetic testing was less frequently used in diagnosis. However, our findings support other reports that identify CFH as the most prevalent mutation leading to clinical manifestation of disease among patients of a similar racial/ethnic background.33, 34, 35 Third, patients with any eculizumab initiation after transplantation, regardless of transplant status or time between transplantation and initiation of dialysis or eculizumab, were included in the analysis. In some cases, eculizumab was initiated a considerable time after patients commenced dialysis; this may have reduced its impact on graft outcome. Finally, tightly restricted use of eculizumab is a recognized practice in the United States and other countries. This is consistent with the larger population (group 2) initiating eculizumab after transplantation and, presumably, at the time of an acute episode of thrombotic microangiopathy. Eculizumab may not have been available for preemptive use if the patient was not already receiving it before transplantation. The effect of national policies on clinical decision-making cannot be deciphered by this study; however, overall enrollment rate was similar among the United States, France, Germany, and Spain for the duration in which study patients were enrolled (2012–2017).
In conclusion, our findings from this observational study suggest eculizumab is beneficial for the treatment of patients with aHUS in the setting of transplantation, and carries a minimal risk of infection. More specifically, they support the hypothesis that initiation of eculizumab therapy before transplantation potentially reduces rates of dialysis after transplantation with the condition that timing of aHUS diagnosis influences the posttransplant outcome. Those patients who were diagnosed with aHUS and receiving treatment with eculizumab at the time of transplantation also enjoyed better 3-year allograft function. Overall, these data align with current guidelines suggesting that that the diagnosis of aHUS should be made as early as possible for early initiation of appropriate treatment to obtain optimal patient outcomes,24, 25 and that such recommendations also apply in the transplant setting.
Disclosure
The Global aHUS Registry is funded by Alexion Pharmaceuticals, Inc. to fulfill the US Food and Drug Administration requirement for a phase IV post-market safety assessment. NI has received honoraria from Alexion Pharmaceuticals, Inc. for advisory board membership and educational lectures. JVW has received consultancy and travel honoraria, as well as research funding from and is a member of the speaker’s bureau for Alexion Pharmaceuticals, Inc. DC has received honoraria from Alexion Pharmaceuticals, Inc. for educational lectures. All the other authors declared no competing interests.
Acknowledgments
The authors thank the patients who have enrolled in the Global aHUS Registry (NCT01522183) and their families as well as the aHUS Alliance Patient Association for communicating the importance of the subject of transplantation for patients with aHUS. The authors thank Imad Al-Dakkak and Vasilis Nikolaou (Alexion Pharmaceuticals, Inc.) for data discussions and statistical analyses. Scientific review was provided by Dr Åsa Lommelé of Alexion Pharma, GmbH, and medical writing support was provided by Matthew deSchoolmeester, PhD, of Bioscript Medical, Macclesfield, UK, funded by Alexion Pharmaceuticals, Inc.; however, the authors had final authority of the content.
Role of the Funding Source
Alexion Pharmaceuticals, Inc., provided overall study management and verified data accuracy.
Contributor Information
Andrew M. Siedlecki, Email: asiedlecki@bwh.harvard.edu.
Global aHUS Registry:
Christoph Licht, Véronique Frémeaux-Bacchi, Gema Ariceta, Gianluigi Ardissino, Fadi Fakhouri, Larry Greenbaum, Sally Johnson, Franz Schaefer, Marie Ann Scully, Leonard Woodward, Masayo Ogawa, Christoph Gasteyger, Miquel Blasco, Donata Cresseri, Galina Generolova, Nicholas Webb, Patricia Hirt-Minkowski, Natalya Lvovna Kozlovskaya, Danny Landau, Anne-Laure Lapeyraque, Chantal Loirat, Christoph Mache, Michal Malina, Leena Martola, Annick Massart, Eric Rondeau, and Lisa Sartz
Appendix
Members of the Global aHUS Registry
Christoph Licht, Véronique Frémeaux-Bacchi, Gema Ariceta, Gianluigi Ardissino, Fadi Fakhouri, Larry Greenbaum, Sally Johnson, Franz Schaefer, Marie Ann Scully, Leonard Woodward, Masayo Ogawa, and Christoph Gasteyger are Scientific Advisory Board members and Miquel Blasco (Spain), Donata Cresseri (Italy), Galina Generolova (Russia), Nicholas Webb (United Kingdom), Patricia Hirt-Minkowski (Switzerland), Natalya Lvovna Kozlovskaya (Russia), Danny Landau (Israel), Anne-Laure Lapeyraque (Canada), Chantal Loirat (France), Christoph Mache (Austria), Michal Malina (Czech Republic), Leena Martola (Finland), Annick Massart (Belgium), Eric Rondeau (France), and Lisa Sartz (Sweden) are National Coordinators members of the Global aHUS Registry.
References
- 1.Le Quintrec M., Zuber J., Moulin B. Complement genes strongly predict recurrence and graft outcome in adult renal transplant recipients with atypical hemolytic and uremic syndrome. Am J Transplant. 2013;13:663–675. doi: 10.1111/ajt.12077. [DOI] [PubMed] [Google Scholar]
- 2.Zuber J., Le Quintrec M., Krid S. Eculizumab for atypical hemolytic uremic syndrome recurrence in renal transplantation. Am J Transplant. 2012;12:3337–3354. doi: 10.1111/j.1600-6143.2012.04252.x. [DOI] [PubMed] [Google Scholar]
- 3.Legendre C.M., Licht C., Muus P. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368:2169–2181. doi: 10.1056/NEJMoa1208981. [DOI] [PubMed] [Google Scholar]
- 4.Licht C., Greenbaum L.A., Muus P. Efficacy and safety of eculizumab in atypical hemolytic uremic syndrome from 2-year extensions of phase 2 studies. Kidney Int. 2015;87:1061–1073. doi: 10.1038/ki.2014.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenbaum L.A., Fila M., Ardissino G. Eculizumab is a safe and effective treatment in pediatric patients with atypical hemolytic uremic syndrome. Kidney Int. 2016;89:701–711. doi: 10.1016/j.kint.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 6.Fakhouri F., Hourmant M., Campistol J.M. Terminal complement inhibitor eculizumab in adult patients with atypical hemolytic uremic syndrome: a single-arm, open-label trial. Am J Kidney Dis. 2016;68:84–93. doi: 10.1053/j.ajkd.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 7.Chatelet V., Fremeaux-Bacchi V., Lobbedez T. Safety and long-term efficacy of eculizumab in a renal transplant patient with recurrent atypical hemolytic-uremic syndrome. Am J Transplant. 2009;9:2644–2645. doi: 10.1111/j.1600-6143.2009.02817.x. [DOI] [PubMed] [Google Scholar]
- 8.Legendre C.M., Campistol J.M., Feldkamp T. Outcomes of patients with atypical haemolytic uraemic syndrome with native and transplanted kidneys treated with eculizumab: a pooled post hoc analysis. Transpl Int. 2017;30:1275–1283. doi: 10.1111/tri.13022. [DOI] [PubMed] [Google Scholar]
- 9.Noris M., Ruggenenti P., Remuzzi G. Kidney transplantation in patients with atypical hemolytic uremic syndrome: a therapeutic dilemma (or not)? Am J Kidney Dis. 2017;70:754–757. doi: 10.1053/j.ajkd.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Pelicano M.B., de Cordoba S.R., Diekmann F. Anti-C5 as prophylactic therapy in atypical hemolytic uremic syndrome in living-related kidney transplantation. Transplantation. 2013;96:e26–e29. doi: 10.1097/TP.0b013e31829d388d. [DOI] [PubMed] [Google Scholar]
- 11.Duineveld C., Verhave J.C., Berger S.P. Living donor kidney transplantation in atypical hemolytic uremic syndrome: a case series. Am J Kidney Dis. 2017;70:770–777. doi: 10.1053/j.ajkd.2017.06.024. [DOI] [PubMed] [Google Scholar]
- 12.Levi C., Fremeaux-Bacchi V., Zuber J. Midterm outcomes of 12 renal transplant recipients treated with eculizumab to prevent atypical hemolytic syndrome recurrence. Transplantation. 2017;101:2924–2930. doi: 10.1097/TP.0000000000001909. [DOI] [PubMed] [Google Scholar]
- 13.Licht C., Ardissino G., Ariceta G. The global aHUS registry: methodology and initial patient characteristics. BMC Nephrol. 2015;16:207. doi: 10.1186/s12882-015-0195-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schaefer F., Ardissino G., Ariceta G. Clinical and genetic predictors of atypical hemolytic uremic syndrome phenotype and outcome. Kidney Int. 2018;94:408–418. doi: 10.1016/j.kint.2018.02.029. [DOI] [PubMed] [Google Scholar]
- 15.Sperati C.J., Moliterno A.R. Thrombotic microangiopathy: focus on atypical hemolytic uremic syndrome. Hematol Oncol Clin North Am. 2015;29:541–559. doi: 10.1016/j.hoc.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Bu F., Maga T., Meyer N.C. Comprehensive genetic analysis of complement and coagulation genes in atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2014;25:55–64. doi: 10.1681/ASN.2013050453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sullivan M., Rybicki L.A., Winter A. Age-related penetrance of hereditary atypical hemolytic uremic syndrome. Ann Hum Genet. 2011;75:639–647. doi: 10.1111/j.1469-1809.2011.00671.x. [DOI] [PubMed] [Google Scholar]
- 18.Cataland S.R., Holers V.M., Geyer S. Biomarkers of terminal complement activation confirm the diagnosis of aHUS and differentiate aHUS from TTP. Blood. 2014;123:3733–3738. doi: 10.1182/blood-2013-12-547067. [DOI] [PubMed] [Google Scholar]
- 19.Vande Walle J., Delmas Y., Ardissino G. Improved renal recovery in patients with atypical hemolytic uremic syndrome following rapid initiation of eculizumab treatment. J Nephrol. 2017;30:127–134. doi: 10.1007/s40620-016-0288-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menne J., Delmas Y., Fakhouri F. Eculizumab prevents thrombotic microangiopathy in patients with atypical haemolytic uraemic syndrome in a long-term observational study. Clinical Kidney Journal. 2018 doi: 10.1093/ckj/sfy035. sfy035–sfy035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bresin E., Daina E., Noris M. Outcome of renal transplantation in patients with non-Shiga toxin-associated hemolytic uremic syndrome: prognostic significance of genetic background. Clin J Am Soc Nephrol. 2006;1:88–99. doi: 10.2215/CJN.00050505. [DOI] [PubMed] [Google Scholar]
- 22.Loirat C., Fremeaux-Bacchi V. Hemolytic uremic syndrome recurrence after renal transplantation. Pediatr Transplant. 2008;12:619–629. doi: 10.1111/j.1399-3046.2008.00910.x. [DOI] [PubMed] [Google Scholar]
- 23.Fakhouri F., Fila M., Provot F. Pathogenic variants in complement genes and risk of atypical hemolytic uremic syndrome relapse after eculizumab discontinuation. Clin J Am Soc Nephrol. 2017;12:50–59. doi: 10.2215/CJN.06440616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodship T.H., Cook H.T., Fakhouri F. Atypical hemolytic uremic syndrome and C3 glomerulopathy: conclusions from a "Kidney Disease: Improving Global Outcomes" (KDIGO) Controversies Conference. Kidney Int. 2017;91:539–551. doi: 10.1016/j.kint.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Loirat C., Fakhouri F., Ariceta G. An international consensus approach to the management of atypical hemolytic uremic syndrome in children. Pediatr Nephrol. 2016;31:15–39. doi: 10.1007/s00467-015-3076-8. [DOI] [PubMed] [Google Scholar]
- 26.Fremeaux-Bacchi V., Fakhouri F., Garnier A. Genetics and outcome of atypical hemolytic uremic syndrome: a nationwide French series comparing children and adults. Clin J Am Soc Nephrol. 2013;8:554–562. doi: 10.2215/CJN.04760512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noris M., Caprioli J., Bresin E. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol. 2010;5:1844–1859. doi: 10.2215/CJN.02210310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davin J.C., Gracchi V., Bouts A. Maintenance of kidney function following treatment with eculizumab and discontinuation of plasma exchange after a third kidney transplant for atypical hemolytic uremic syndrome associated with a CFH mutation. Am J Kidney Dis. 2010;55:708–711. doi: 10.1053/j.ajkd.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 29.Legendre C., Greenbaum L., Sheerin N. Eculizumab efficacy in aHUS patients with progressing TMA, with or without prior renal transplant. Am J Transplant. 2013;13:278–279. [Google Scholar]
- 30.Hans R., Sharma R.R., Marwaha N. Efficacy and safety of therapeutic plasma exchange by using apheresis devices in pediatric atypical hemolytic uremic syndrome patients. J Clin Apher. 2016;31:381–387. doi: 10.1002/jca.21412. [DOI] [PubMed] [Google Scholar]
- 31.Thygesen L.C., Ersboll A.K. When the entire population is the sample: strengths and limitations in register-based epidemiology. Eur J Epidemiol. 2014;29:551–558. doi: 10.1007/s10654-013-9873-0. [DOI] [PubMed] [Google Scholar]
- 32.James S., Rao S.V., Granger C.B. Registry-based randomized clinical trials—a new clinical trial paradigm. Nat Rev Cardiol. 2015;12:312–316. doi: 10.1038/nrcardio.2015.33. [DOI] [PubMed] [Google Scholar]
- 33.Zhang T., Lu J., Liang S. Comprehensive analysis of complement genes in patients with atypical hemolytic uremic syndrome. Am J Nephrol. 2016;43:160–169. doi: 10.1159/000445127. [DOI] [PubMed] [Google Scholar]
- 34.Warwicker P., Goodship T.H., Donne R.L. Genetic studies into inherited and sporadic hemolytic uremic syndrome. Kidney Int. 1998;53:836–844. doi: 10.1111/j.1523-1755.1998.00824.x. [DOI] [PubMed] [Google Scholar]
- 35.Caprioli J., Bettinaglio P., Zipfel P.F. The molecular basis of familial hemolytic uremic syndrome: mutation analysis of factor H gene reveals a hot spot in short consensus repeat 20. J Am Soc Nephrol. 2001;12:297–307. doi: 10.1681/ASN.V122297. [DOI] [PubMed] [Google Scholar]