Summary
We have previously developed a cocktail of nine small molecules to convert human fetal astrocytes into neurons, but a nine-molecule recipe is difficult for clinical applications. Here, we identify a chemical formula with only three to four small molecules for astrocyte-to-neuron conversion. We demonstrate that modulation of three to four signaling pathways among Notch, glycogen synthase kinase 3, transforming growth factor β, and bone morphogenetic protein pathways is sufficient to change an astrocyte into a neuron. The chemically converted human neurons can survive >7 months in culture, fire repetitive action potentials, and display robust synaptic burst activities. Interestingly, cortical astrocyte-converted neurons are mostly glutamatergic, while midbrain astrocyte-converted neurons can yield some GABAergic neurons in addition to glutamatergic neurons. When administered in vivo through intracranial or intraperitoneal injection, the four-drug combination can significantly increase adult hippocampal neurogenesis. Together, human fetal astrocytes can be chemically converted into functional neurons using three to four small molecules, bringing us one step forward for developing future drug therapy.
Keywords: astrocyte-to-neuron conversion, small molecule, chemical conversion, reprogramming, adult neurogenesis, glutamatergic neuron
Highlights
-
•
Chemical reprogramming of human astrocytes into neurons with three to four small molecules
-
•
Notch/GSK-3/TGF-β/BMP pathways are critical for astrocyte-to-neuron conversion
-
•
Human fetal astrocytes are chemically converted into glutamatergic neurons
-
•
In vivo administration of four core drugs increases hippocampal adult neurogenesis
In this article, Chen and colleagues identified a chemical formula using only three to four small molecules to reprogram human fetal astrocytes into functional neurons, paving the way for a potential drug therapy for neuroregeneration and brain repair.
Introduction
Neuronal loss is the leading cause of symptoms in patients with neural injury or neurodegenerative disorders. Following nerve injury, glial cells including astrocytes, NG2 cells, and microglia will proliferate and become reactive glial cells to form glial scarring in order to protect neighboring tissues from further damage (Burda and Sofroniew, 2014). However, the continuous presence of glial scars also inhibits neuronal growth and synaptic transmission in the injured area (Filous and Silver, 2016, Yiu and He, 2006). Thus, reactive glial cells are a double-edged sword that can have both neuroprotective and neuroinhibitory functions during the progression of injury or diseases. Glial scarring has been widely reported after traumatic brain injury, stroke, and spinal cord injury, but efforts to remove the neuroinhibitory effect of glial scarring has only resulted in limited success (Filous and Silver, 2016, Pekny and Nilsson, 2005).
We have recently demonstrated that reactive astrocytes and NG2 cells can be directly reprogrammed into functional neurons inside mouse brains with the expression of a single neural transcription factor, NEUROD1 (Guo et al., 2014). Other transcription factors, such as neurogenin 2 (NGN2), ASCL1, and SOX2, have also been reported to reprogram glial cells into neurons both in vitro and in vivo (Berninger et al., 2007, Grande et al., 2013, Heinrich et al., 2010, Liu et al., 2015, Niu et al., 2013, Su et al., 2014, Torper et al., 2015). Direct conversion from glial cells into neurons inside the brain or spinal cord without cell transplantation can avoid the problems of tumor formation, aberrant differentiation, and immunorejection that are often associated with stem cell transplantation (Li and Chen, 2016). The majority of glia-to-neuron conversion research has been carried out using virus-mediated ectopic expression of transcription factors, which requires production of viruses and sophisticated intracranial or intra-spinal injection procedures. However, small-molecule-mediated chemical reprogramming has been developed to allow cell trans-differentiation without viruses (Cao et al., 2016, Cheng et al., 2014, Hu et al., 2015, Li et al., 2015, Zhang et al., 2015, Zhang et al., 2016a, Zhao et al., 2015). Our lab recently developed a chemical protocol to reprogram human astrocytes (HAs) into functional neurons using a cocktail of nine small molecules (Zhang et al., 2015). These nine molecules need to be sequentially administered to reprogram HAs into neurons, making its clinical translation quite difficult due to the large number of small molecules used and the complicated timing of drug application.
In this study, we identify a chemical protocol composed of only three to four small molecules (DAPT, CHIR99021, SB431542, and LDN193189) that can more efficiently reprogram HAs into neurons. By substituting each of these four drugs (core drugs) with functional analogs, we demonstrate that simultaneous modulation of four signaling pathways including Notch, glycogen synthase kinase 3β (GSK-3β), transforming growth factor β (TGF-β), and bone morphogenetic protein (BMP) pathways, is sufficient to reprogram HAs into neurons. Even modulating three out of the four signaling pathways can convert HAs into neurons. Our chemically converted human neurons are highly functional and can survive >7 mo in cell culture. Moreover, when applied in vivo, core drugs significantly increase the adult neurogenesis in the mouse hippocampus. Therefore, we have identified a simple chemical formula for astrocyte-to-neuron conversion, bringing us one step closer toward developing future drug therapy for brain repair.
Result
Identification of Four Core Drugs for Astrocyte-to-Neuron Conversion
We have recently identified a combination of nine small molecules, including SB431542, LDN193189, TTNPB, Thiazovivin, CHIR99021, DAPT, valproic acid (VPA), smoothened agonist (SAG), and purmorphamine (Purmo), to reprogram human fetal astrocytes into functional neurons (Zhang et al., 2015). However, for therapeutic purpose, a nine-molecule cocktail added in a sequential manner is rather difficult to translate into clinical applications. Here, to search for a more practical formula for chemical reprogramming, we purchased human fetal astrocytes (HA1800) from ScienCell (San Diego, CA) as described previously (Zhang et al., 2015). Because human fetal astrocytes might contain neuroprogenitors, we have subcultured the astrocytes for at least ten generations in the presence of 10% fetal bovine serum (FBS) to minimize progenitors (Zhang et al., 2015). After more than ten passages, the majority of our astrocytes were immunopositive for astrocytic markers such as glial fibrillary acidic protein(GFAP), S100β, and glutamine synthetase (Figures 1A, 1B, and S1A), but rarely for neuroprogenitor markers SOX2 (Figures S1B and S1C) and NESTIN (Figures S2E and S2F). Long-term culture of our HAs in neural differentiation medium for 1 month also rarely yielded any neurons (Figures S1D and S1E). At the transcriptome level (Figure S1F), in comparison with published datasets on HAs (Zhang et al., 2016b) or human neural stem cells (hNSCs) (Modrek et al., 2017), our HA samples (red box, GSE123397) were closely related to the fetal astrocytes from human brain, but clearly different from the hNSCs (green box). Figure S1G shows the heatmap of gene expression of typical astrocyte and NSC markers. Our HA samples showed low expression of STMN1, DLX1, and NES but high expression of FN1, COL1A1, VIM, and MYC, which was opposite to that of hNSCs. Together, the significant difference between our HA samples and NSCs suggests that after ten passages in 10% FBS, neuroprogenitors are minimized in our HA cultures.
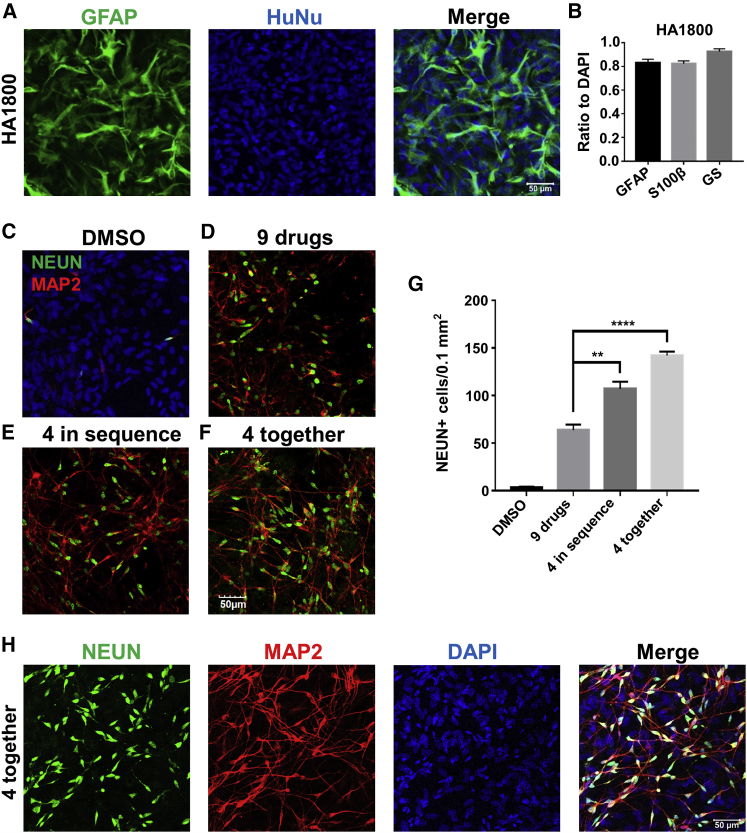
Figure 1.
Identification of Four Core Drugs to Chemically Reprogram HA into Neurons
(A) HA (1800, ScienCell) were characterized by immunostaining of astrocyte marker GFAP (green) and human nuclei (HuNu).
(B) Quantified data showing the majority of cells immunopositive for astrocyte markers (GFAP+, 84.7% ± 2.7%; S100β+, 82.3% ± 2.3%; glutamine synthetase [GS]+, 92.5% ± 2.5%). N = 3 batches.
(C) HA treated with 0.2% DMSO had few neurons when assessed with neuronal markers NEUN (green) and MAP2 (red).
(D) Sequential application of a nine-drug cocktail for 8 days resulted in a significant number of neurons as described recently (Zhang et al., 2015).
(E and F) The four core drugs (DAPT, CHIR99021, SB431542, and LDN193189) were administered either in sequence (E) or together (F) for 6 days, and both yielded a significant number of neurons.
(G) Quantified data (NEUN) showing higher reprogramming efficiency when four drugs (DCSL) were added in sequence or together as a mixture. ∗∗p < 0.01, ∗∗∗∗p < 0.0001, one-way ANOVA followed with Dunnett's multiple comparison test. Data are represented in means ± SEM. The immunostaining was performed at 14 days after the beginning of drug treatment. N = 3 batches.
(H) Representative images of neurons converted by four drugs together at day 14. Neurons were labeled by NEUN (green) and MAP2 (red), while DAPI (blue) showed all cell nuclei. Scale bars, 50 μm.
After characterizing HA properties, we repeated and confirmed successful chemical conversion of HAs into neurons using the nine-molecule cocktail (Zhang et al., 2015) (Figures 1C and 1D). We then investigated how to reduce the number of drugs for chemical reprogramming by studying the effect of each individual drug among the nine-molecule cocktail. Interestingly, four molecules including SB431542 (S), LDN193189 (L), CHIR99021 (C), and DAPT (D) appeared to be most critical in maintaining high reprogramming efficiency (Zhang et al., 2015). Therefore, we tested these four drugs (SLCD) together to determine their reprogramming capability. Unexpectedly, when we applied SB431542 (5 μM) and LDN193189 (0.25 μM) for 2 days, and then replaced with CHIR99021 (1.5 μM) and DAPT (5 μM) for 4 days, it yielded more neurons than the nine-drug formula (Figures 1E and 1G). When we applied the four drugs (SLCD) altogether for 6 days, it yielded even more neurons (Figures 1F–1H). Quantitatively, the neuronal yield (converted neurons/astrocyte number before drug treatment) reached 71% when the four drugs (SLCD) were applied together (Figures S1H and S1I). Therefore, the four drugs together (SLCD) are referred as “core drugs” hereafter, and the core drug-converted neurons are referred to as induced neurons or “iNs” to be consistent with other studies (Yang et al., 2011). It is worth mentioning that the chemically converted iNs and the remaining non-converted astrocytes formed a neuron-astrocyte co-culture condition, with the iNs migrating onto the surface of astrocytes, and the astrocytes served as a feeder layer to promote neuronal survival and maturation (Figure S1J) (Tang et al., 2013).
We then performed a lineage-tracing experiment using GFAP:GFP retroviruses to label HAs with GFP before drug treatment (Figure S2A). The GFP-labeled astrocytes became NeuN+ (RBFOX3) neurons after core drug treatment (Figure S2A, top row), but remained GFAP+ astrocytes in the DMSO control group (Figure S2A, bottom row). To observe the astrocyte-to-neuron conversion process, we performed long-term time-lapse imaging for eight consecutive days on a group of GFP-labeled HAs during and after core drug treatment (Video S1). As shown in the video, the initial flat HAs infected with GFP lentiviruses gradually changed their morphology into neuron-like cells with extended long neurites after core drug treatment (see Video S1 in Supplemental Information). Following time-lapse imaging, we further performed post-fix immunostaining and confirmed that the GFP-labeled neuron-like cells after core drug treatment were indeed immunopositive for NeuN (Figure S2B). Importantly, we did not observe significant cell proliferation during the 8 days of time-lapse imaging (Video S1), consistent with the Ki67 immunostaining results showing an inhibition of cell proliferation by core drug treatment (Figures S2C and S2D, ∼130 Ki67+ cells/field before drug treatment and ∼10 Ki67+ cells/field after drug treatment). Moreover, NESTIN staining rarely showed neuroprogenitor cells induced by core drugs (Figures S2E and S2F). Together, through lineage tracing, time-lapse imaging, and Ki67 and NESTIN staining, we show that HAs can be directly converted into neurons by four molecules.
Can HAs spontaneously turn into neurons during our experimental process? To test this idea, we performed DCX and TUJ1 (TUBB3) staining but did not observe a significant number of newborn neurons in the DMSO control group from day 2 to day 7 (Figures S3A and S3B, quantified in Figures S3C–S3F). In the core drug-treated group, DCX and TUJ1 signals did not change much at day 2 and day 4, but dramatically increased at day 7 (Figures S3A–S3F), suggesting that it takes ∼1 week for HAs to be chemically converted into neurons. At day 14 of the DMSO control group, we did see a few neurons, which were eliminated if the N2 supplement was removed from the culture medium (Figures S3G–S3J), suggesting that the N2 supplement may have a very weak neural induction role in cell cultures.
We further tested whether our core drugs could reprogram different sources of HAs into neurons. After purchasing a different line of HAs from Lonza (cc-2565), we found that our core drugs (4-day treatment of 2.5 μM SB431542, 0.125 μM LDN193189, 0.75 μM CHIR99021, and 2.5 μM DAPT) also induced a large number of iNs (Figures S3K–S3N, immunostained at day 10). Therefore, we conclude that HAs from different sources can be chemically converted into neurons.
Transcriptional Regulation and MECP2-REST Signaling Change during Chemical Reprogramming
What is the molecular mechanism underlying the core drug reprogramming? Our real-time PCR experiments showed that the basic-helix-loop-helix neural transcription factors, including both NEUROD1 and NGN2, were significantly upregulated at day 1 and day 4 by core drug treatment (Figures 2A and 2B), whereas the astrocytic GFAP expression was downregulated (Figure S4A). Interestingly, each individual drug among the four core drugs upregulated NGN2 level (Figure 2A), and the NEUROD1 level was upregulated by LDN193189, SB431542, and DAPT (Figure 2B). VPA, an HDAC inhibitor that alters histone acetylation and gene transcription, was found to induce a significant increase of both NEUROD1 and NGN2 expression (Figures 2A and 2B). However, when VPA was added together with the four core drugs, it unexpectedly decreased the reprogramming efficiency (Figures S4B and S4C). We then further tested core drugs in combination with other individual drugs including ROCK inhibitor Tzv, retinoic acid receptor agonist TTNPB, sonic hedgehog activator SAG, and Purmo. Addition of Tzv to the core drugs showed no effect (Figure S4D), while addition of TTNPB decreased the reprogramming efficiency (Figure S4E and quantified in S4G). Addition of SAG and Purmo significantly increased astrocytic proliferation, resulting in overgrown astrocytes and decrease of neurons (data not shown). These results suggest that alteration of extra signaling pathways in addition to the four pathways modulated by core drugs might result in reduced conversion efficiency.
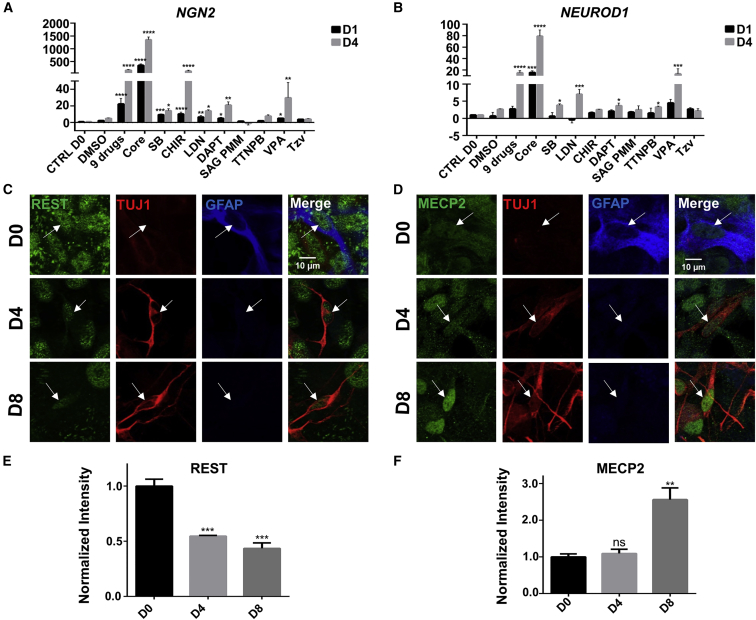
Figure 2.
Transcriptional Regulation during Chemical Reprogramming
(A and B) Real-time qPCR analyses revealed transcriptional activation of NGN2 (A) and NEUROD1 (B) by core drug treatment. Note than NGN2 was activated earlier than NEUROD1, and that the core drug group showed higher levels of NGN2 and NEUROD1 than the nine-drug group. Among individual drugs, SB431542, CHIR99021, LDN193189, DAPT, and VPA increased NGN2 to a significant level, whereas SB431542, LDN193189, DAPT, TTNPB, and VPA significantly increased the expression of NEUROD1. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001, one-way ANOVA after log2 transformation, Dunnett's multiple comparison test. Data are represented in means ± SEM. N = 3 batches.
(C and D) Immunostaining showed a gradual decrease of REST (green) during chemical reprogramming of astrocytes (GFAP, blue) into neurons (TUJ1, red) (C). Immunostaining of MECP2 (green) showed a gradual increase during chemical reprogramming (D). Scale bars, 10 μm.
(E and F) Quantified data showing the opposite change of REST and MECP2 during chemical reprogramming. ∗∗p < 0.01, ∗∗∗p < 0.001, one-way ANOVA followed by Dunnett's multiple comparison test. Data are represented in means ± SEM. N = 3 batches.
Besides transcriptional regulation of NGN2 and NEUROD1, we further discovered a significant change of REST (RE1-silencing transcription factor) and MECP2 (methyl CpG binding protein 2) signals during core drug-mediated chemical reprogramming (Figures 2C–2F). REST is a transcriptional repressor that can silence neuronal genes in stem cells and non-neuronal cells (Ballas et al., 2005). During neuronal differentiation, REST is downregulated in neurons. We found a significant decrease of REST expression during chemical reprogramming process, particularly in Tuj1+ neurons (Figure 2C, quantified in Figure 2E), indicating the suppression of REST by core drugs during chemical conversion. In contrast, the expression level of MECP2, a nuclear protein that can regulate the expression of many genes including suppressing REST (Skene et al., 2010), increased significantly after core drug treatment (Figure 2D, quantified in Figure 2F). MECP2 has been reported to be highly expressed in neurons, much more than that in astrocytes (Kishi and Macklis, 2004, Skene et al., 2010). Consistently, we found that the increase of MECP2 signal was coinciding with an increase of Tuj1 and a decrease of GFAP signal after core drug treatment. Together, the changes of REST and MECP2 expression level indicate a cell fate change from astrocytes to neurons induced by core drug treatment.
Neuronal Identity after Core Drug Reprogramming
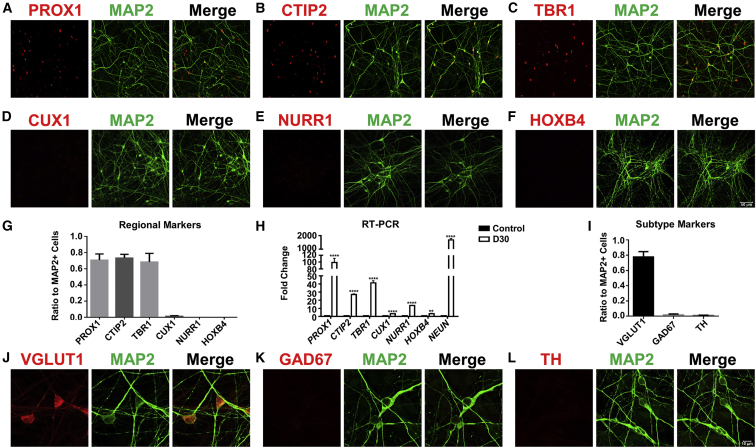
We next characterized the neuronal identity after chemical reprogramming by performing immunostaining on a series of neuronal markers. The core drug-converted human neurons (3 months after drug treatment) were mainly immunopositive for PROX1, CTIP2 (BCL11B), and TBR1 (Figures 3A–3C), but negative for other neuronal markers such as CUX1, NURR1 (NR4A2), and HOXB4 (Figures 3D–3G; quantified in Figure 3H). These results were further confirmed with real-time PCR analysis (Figure 3I). When examining neurotransmitter subtypes, we found that the majority of neurons converted from human cortical astrocytes were immunopositive for VGlut1 (SLC17A7) (78%), a glutamatergic neuron marker, but very few were GABAergic (GAD67 [GAD1], 2%) or dopaminergic neurons (TH, 1%) (Figures 3J–3M). However, when testing our core drug combination in midbrain HAs (ScienCell, 1850), we found a large proportion of converted iNs co-expressing GABA and GAD67 (Figure S5A–S5F), suggesting that different lineages of astrocytes originated from different brain regions may be chemically converted into different subtypes of iNs. Therefore, human cortical astrocytes can be converted into cortical glutamatergic neurons, and different lineages of astrocytes may have their own intrinsic factors to influence the final cell fate after conversion.
Figure 3.
Characterization of Human Neuron Subtypes after Core Drug-Mediated Reprogramming
(A–F) Representative images illustrating the immunostaining of hippocampal neuron marker PROX1 (A, red), forebrain deep layer marker CTIP2 (B, red), cortical neuron marker TBR1 (C, red), cortical superficial layer marker CUX1 (D, red), midbrain marker NURR1 (E, red), hindbrain marker HOXB4 (F, red), and neuronal dendritic marker MAP2 (A–F, green). Scale bar, 50 μm.
(G) MAP2-positive neurons were also immunopositive for PROX1 (71% ± 8%), CTIP2 (73% ± 4%), and TBR1 (68% ± 11%), but not other markers. N ≥ 3 batches.
(H) Real-time PCR experiments were performed at 1 month after core drug treatment to assess different neuronal gene expression level. All data were normalized to human astrocyte control. ∗∗p < 0.01, ∗∗∗∗p < 0.0001, unpaired t test after log2 transformation. Data are represented in means ± SEM. N = 3 batches.
(I–L) Immunostaining (I) with different neuronal subtype markers revealed that the human astrocyte-converted iNs (MAP2, green) after core drug treatment were mostly glutamatergic (VGlut1, red) (J), and rarely GABAergic (GAD67, red) (K) or dopaminergic (TH, red) (L).
Data are represented in mean ± SEM. N = 3 batches. Scale bar, 10 μm.
Functional Characterization of Core Drug-Converted iNs
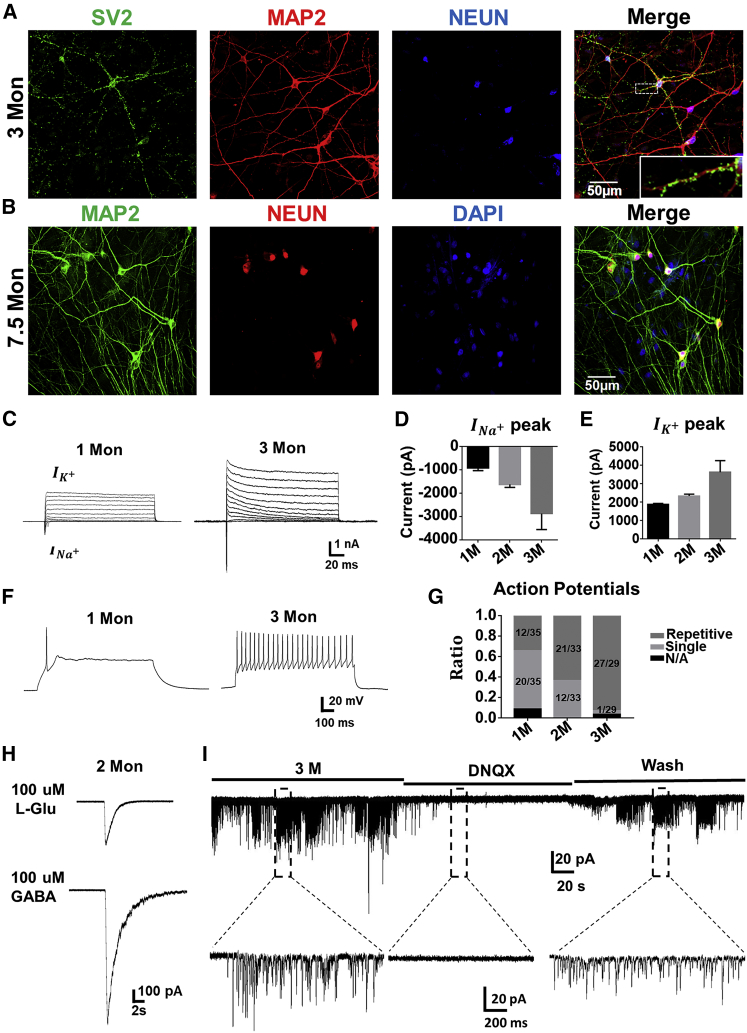
We further investigated whether the core drug-converted iNs were functionally connected to each other. Different from mouse neuron cultures, we found that the core drug-converted human neurons could survive for 3–7 months in our cell culture condition and form robust synaptic connections (Figures 4A and 4B). Some core drug-converted human neurons could survive as long as 7.5 months (Figure 4B), providing an extended time window for future studies on drug screening or disease modeling. Functionally, patch-clamp recordings revealed that our chemically converted iNs showed large sodium and potassium currents (Figure 4C) as well as repetitive action potentials (Figure 4F). As expected, converted iNs showed a clear developmental time course of functional maturation when analyzed from 1- to 3-month cultures after chemical conversion. Specifically, sodium currents increased from 1 nA at 1-month culture to 3 nA at 3-month culture, while potassium currents increased from ∼2 nA at 1-month culture to ∼3.5 nA at 3-month culture (Figures 4D and 4E). The number of cells firing repetitive action potentials increased from 12/35 at 1-month culture to 27/29 at 3-month culture (Figure 4G). The membrane capacitance also increased (Figure S5G, indicating cell growth), together with a decrease of membrane resistance (Figure S5H, an indication of channel and receptor expression) from 1- to 3-month cultures. We also detected glutamate- and GABA-induced receptor currents in 2-month-old converted iNs (Figure 4H), which was consistent with the immunostaining of glutamate receptors (GluR1 and GluR2 subunits) (Figures S5J and S5K) and GABAA receptors (γ2 and α5 subunits) (Figures S5L and S5M). Moreover, we recorded robust spontaneous synaptic activities, some in burst activity pattern, in 3-month-old cultures after chemical conversion (Figure 4I), suggesting the formation of a highly integrated neural network among the converted iNs. Notably, most of the synaptic events were blocked by glutamate receptor antagonist DNQX (Figure 4I), consistent with their glutamatergic identity shown in Figure 3. Therefore, the four core drugs (SLCD) can chemically reprogram HAs into highly functional neurons.
Figure 4.
Functional Characterization of the Core Drug-Converted Human Neurons
(A and B) Human astrocyte-converted iNs (MAP2, red; NEUN, blue) induced by core drug treatment showed robust synaptic puncta (SV2, green) after 3 months of culture. N = 3 batches (A). Neurons reprogrammed from HA could survive as long as 7.5 months. N = 3 batches (B). Scale bars, 50 μm.
(C–E) Representative traces of sodium and potassium currents recorded from converted iNs (C), with quantitative analyses showing the developmental increase of peak INa+ (D) and IK+ (E).
(F and G) Whole-cell recordings revealed an increase of action potential firing from 1- to 3-month-old cultures after core drug-mediated chemical conversion (F), with quantitative result showing the ratio of converted iNs having repetitive action potential firing at different time points (G).
(H) Representative traces showing glutamate-induced (100 μM) or GABA-induced (100 μM) currents in 2-month-old converted iNs.
(I) Representative trace showing robust synaptic burst activities, which were blocked by glutamate receptor antagonist DNQX, confirming glutamatergic property.
Data are represented as means ± SEM. N ≥ 29.
Signaling Pathways Critical for Chemical Reprogramming
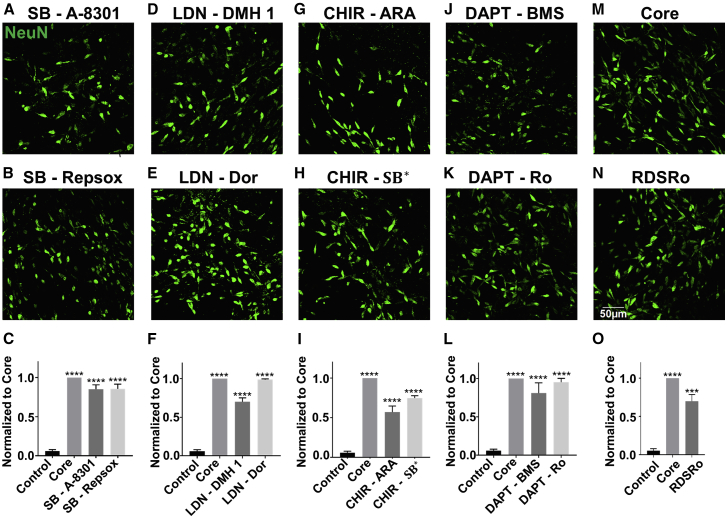
After identifying the four core drugs (SLCD), we further tested whether these particular four drugs were unique for chemical reprogramming, or rather they could be replaced by other functional analogs modulating similar signaling pathways. For example, SB431542 is a TGF-β inhibitor and inhibits a subfamily of activin receptor-like kinase (ALK) receptors including ALK4, ALK5, and ALK7 (Inman et al., 2002). We therefore substituted SB431542 with its functional analogs Repsox (1 μM) or A-8301 (0.25 μM), which also inhibit TGF-β pathway. Interestingly, we found that HAs were also efficiently reprogramed into neurons by substituting SB431542 with Repsox or A-8301 among the four-drug combination (Figures 5A–5C). Thus, inhibiting the TGF-β signaling pathway in general, rather than any particular inhibitor, is critical for chemical reprogramming. Similarly, replacing LDN193189, an inhibitor of BMP through inhibiting another ALK subfamily (ALK2 and ALK3), with other two BMP inhibitors Dorsomorphin (1 μM) or DMH 1 (1.5 μM), also resulted in a significant neuronal reprogramming (Figures 5D–5F). CHIR99021 is a selective inhibitor of GSK-3 and indirectly activates the WNT signaling pathway. CHIR99021 has been used in several studies to generate neurons from ESCs or iPSCs (Fasano et al., 2010, Kriks et al., 2011), and WNT has been shown to regulate postnatal and adult neurogenesis (Lie et al., 2005, Zhang et al., 2011). Replacement of CHIR99021 by two other GSK-3 inhibitors, SB216763 (1 μM; different from TGF-β inhibitor SB431542) or AR-A014418 (6 μM), resulted in a large number of neurons but less than the effect of CHIR99021 itself (Figures 5G–5I). Lastly, DAPT inhibits Notch signaling pathway by targeting γ-secretase and is involved in the maintenance of the proliferative and undifferentiated state of embryonic stem cells during development (Urban and Guillemot, 2014). Replacing DAPT with other two Notch inhibitors, BMS906024 (2 μM) or RO4929097 (0.5 μM), resulted in comparable reprogramming efficiency (Figures 5J–5L), suggesting an important role of Notch signaling in neuronal conversion. After testing each individual signaling pathway with at least three different inhibitors, we replaced all of the original four core drugs (SLCD) with their functional analogs, Repsox, Dorsomorphin, SB216763, and RO4929097 (RDSRo). The four functional analogs (RDSRo) together also generated a large number of converted iNs, with the conversion efficiency reaching ∼70% of the original core drug group (Figures 5M–5O). Similar to the core drug group (SLCD), the RDSRo group also induced mainly glutamatergic neurons but not GABAergic or dopaminergic neurons (Figures S6A–S6C) and formed local synaptic connections (Figure S6D). Consistently, real-time PCR analysis revealed that both core drug group or their various substitution groups all downregulated glial genes such as Glt1 (SLC1A2) (Figures S6E and S6F). Furthermore, real-time PCR experiments identified significant changes of gene targets that are correlated with the four signaling pathways, including NEUROGENIN 1 for the GSK-3β pathway (Hirabayashi et al., 2004), UNC13A for the Notch pathway (Li et al., 2012), ID1 for the BMP pathway (Morikawa et al., 2011), and COL1A1 for the TGF-β pathway (Verrecchia et al., 2001) (Figure S6G). Altogether, these results suggest that modulation of four signaling pathways including TGF-β, BMP, GSK-3, and Notch in HAs is sufficient for reprogramming into functional neurons.
Figure 5.
Drug Replacement Revealed Key Signaling Pathways Involved in Chemical Reprogramming
(A–C) Among core drugs, replacing SB431542 with its functional analog A-8301 (A) or Repsox (B) yielded similar numbers of reprogrammed neurons (87% ± 4% for A-8301 and 89% ± 6% for Repsox replacement group) (C). Immunostaining of NEUN was performed 14 days after the start of drug treatment.
(D–F) Replacing LDN193189 with its functional analog DMH 1 (D) or Dorsomorphin (E) resulted in similar conversion efficiency (70% ± 5% for DMH 1 and 97% ± 1% for Dorsomorphin) (F).
(G–I) Replacing CHIR99021 with its functional analog ARA014418 (G) or SB216763 (H) yielded less numbers of neurons (57% ± 4% for ARA014418 and 76% ± 3% for SB216763) (I).
(J–L) Replacing DAPT with its functional analog BMS906024 (J) or RO4929097 (K) also achieved similar conversion efficiency (81% ± 1% for BMS906024 and 95% ± 5% for RO4929097) (L).
(M–O) Replacing the four core drugs (M) with their corresponding functional analogs RDSRo (N, SB431542 replaced by Repsox, LDN193189 by Dorsomorphin, CHIR99021 by SB216763, and DAPT by RO4929097) resulted in lower reprogramming efficiency (66% ± 16%) (O).
∗∗∗p < 0.001, ∗∗∗∗p < 0.0001, one-way ANOVA followed with Dunnett's multiple comparison test. Data are represented in means ± SEM. N = 3 batches. Scale bar, 50 μm.
Further Down-Selection of Small Molecules for Chemical Reprogramming
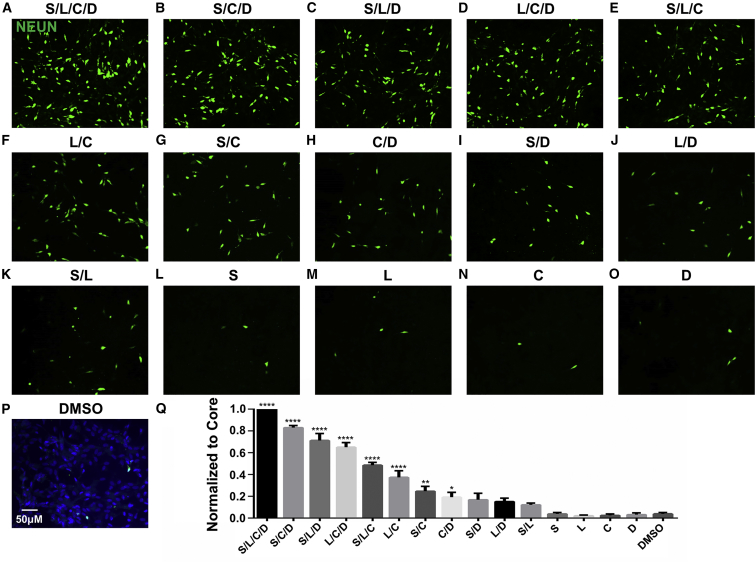
To investigate whether the four-drug cocktail (SLCD) is the minimal combination capable of reprogramming HAs into neurons, we further tested all possible combinations of three drugs, two drugs, and each individual drug, among the four-drug cocktail (SLCD). Interestingly, all three-drug combinations were capable of reprogramming HAs into neurons but with lower efficiency compared with the four-drug group (Figures 6A–6E). Notably, the combination of three drugs SB431542/CHIR99021/DAPT reached >80% of the four-drug efficiency (Figure 6Q, One-way ANOVA followed with post hoc Dunnett's multiple comparison test, n ≥ 3 batches). On the other hand, all combinations of two drugs showed significantly diminished reprogramming efficiency, with the highest one (LDN193189/CHIR99021) still <40% of that induced by the four-drug cocktail (Figures 6F–6K, quantified in Figure 6Q). None of the individual drugs among the four core drugs showed any significant difference from the DMSO control (Figure 6L–6P, quantified in Figure 6Q). real-time PCR analysis also showed that the core drugs and combinations of three or two drugs all downregulated astrocytic genes such as S100β and Glast (SLC1A3) (Figures S6H–S6I). These studies demonstrate that HAs may be chemically reprogrammed into neurons by simultaneously modulating at least three or two signaling pathways, but not by changing a single signaling pathway.
Figure 6.
A Systematic Investigation of Chemical Reprogramming Efficiency among Various Combinations of Fewer Core Drugs
(A) Core drug (S for SB431542, L for LDN193189, C for CHIR99021, or D for DAPT)-induced chemical reprogramming of HA into neurons (NEUN, green) as a control. For all experiments in this figure, immunostaining was performed at 14 days after initial drug treatment.
(B–E) Three-drug combinations, including S/C/D (B), S/L/D (C), L/C/D (D), and S/L/C (E), among the four core drugs could also reprogram a significant number of HAs into neurons, although with lower efficiency compared with the four core drugs.
(F–K) Two-drug combinations, including L/C (F), S/C (G), C/D (H), S/D (I), L/D (J), and S/L (K), also induced some neurons, especially those combinations containing CHIR99021 (F–H).
(L–P) Few neurons were found when treated with each individual drug, including S (L), L (M), C (N), and D (O), compared with the DMSO control (P). Scale bar, 50 μm in (A–P).
(Q) Quantification of all combinations among the four core drugs. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗∗p < 0.0001, one-way ANOVA followed with Dunnett's multiple comparison test. Data are represented in means ± SEM. N = 3 batches.
Regulation of Adult Neurogenesis through In Vivo Administration of Small Molecules
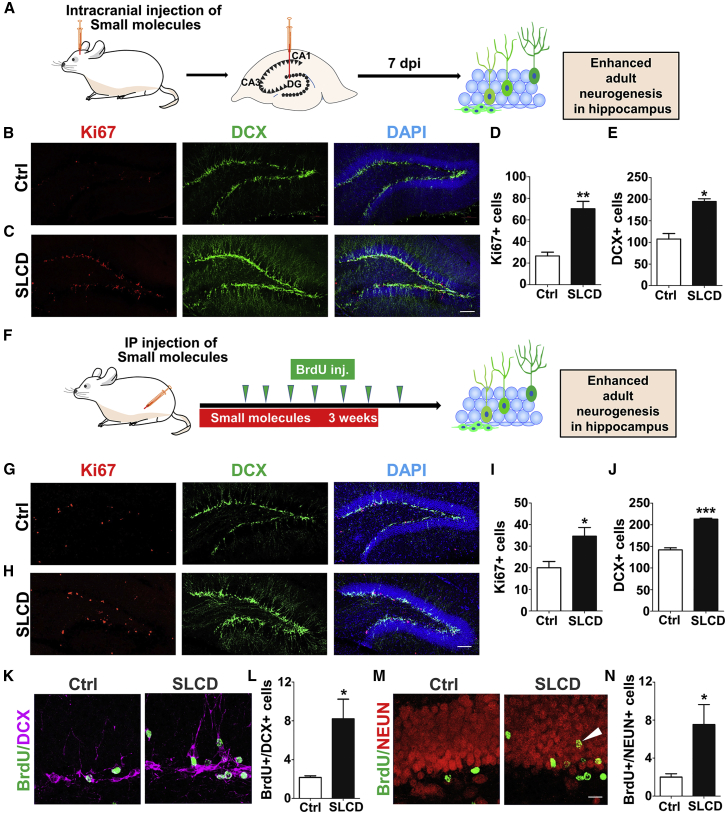
With successful conversion of cultured HAs into neurons in vitro, we then injected the core drugs into mouse brains to test whether they can convert astrocytes into neurons in vivo. After spending several years using various methods to deliver small molecules in the mouse brain in vivo, we have not achieved definitive success of chemical conversion inside mouse brains despite the observation of a few neurons after chemical treatment. This is rather disappointing, but we are still continuously trying direct in vivo chemical conversion in the mouse brain. The biggest challenge for in vivo chemical conversion is how to maintain a constant concentration of small molecules inside the brain without causing a severe invasive damage to the brain. We have tried using biomaterial to encapsulate small molecules, but, perhaps because our small molecules are too small or we have not found the right biomaterial for such small molecules, the small molecules we applied might not stay for a long time inside the brain. We also tried an osmotic minipump (Alzet) but the tip of the insertion caused significant tissue damage inside the brain, and the injury induced many DCX+ cells that were mainly reactive astrocytes 2 weeks after drug treatment (Figures S7I–S7K). On the other hand, during our vigorous testing of in vivo chemical reprogramming, we accidentally found that core drugs significantly increased adult neurogenesis in the mouse hippocampus (Figure 7). We initially injected core drugs through intracranial injection into the hippocampus and sacrificed the mice 7 days later (Figure 7A). We observed remarkable increase of DCX-labeled newborn neurons together with Ki67-labeled proliferative cells in the dentate granule layer (Figures 7B–7E). When separating the four core drugs into two-drug combinations (CHIR99021 + DAPT; and SB431542 + LDN193189), we found that the two-drug combinations could also increase adult hippocampal neurogenesis (Figures S7A–S7H). However, the four drugs together were more potent in promoting neuronal maturation, as shown by more complex dendritic morphology and more neurons migrated into the outer granule layer (Figures S7A–S7H).
Figure 7.
Significant Increase of Adult Neurogenesis after In Vivo Application of Core Drugs
(A) Schematic drawing illustrating a single dose of core drug injection into the dentate gyrus of mouse brains. After 7 days, the mice were sacrificed for immunostaining.
(B–E) Representative images (B and C) and quantitative analyses (D and E) showing that core drugs significantly increased the number of dividing cells (Ki67, red) and newborn neurons (DCX, green). Student's t test, ∗p < 0.01, ∗∗p < 0.001, N = 4 mice. Scale bar, 100 μm.
(F) Schematic drawing illustrating intraperitoneal injection of core drugs (SB 431542, 165 μM; LDN193189, 8.25 μM; CHIR99021, 49.5 μM; and DAPT, 165 μM; all in 20% captisol, 10 mL/kg) daily for 23 days. BrdU was administrated every 3–4 days from day 5 to day 26. The mice were sacrificed at day 32 for immunostaining.
(G–J) Representative images (G and H) and quantitative analyses (I and J) showing that core drugs significantly increased the cell number of dividing cells (Ki67, red) and newborn neurons (DCX, green) compared with vehicle control with 20% captisol (CPTS) injection (Student's t test, ∗p < 0.01, ∗∗∗p < 0.0001, N = 4 mice). Scale bar, 100 μm. The total cell number of Ki67+ or DCX+ cells in one side of the dentate gyrus was quantified based on 10 stacked images with a total thickness of 18 μm and a volume of ∼0.014 mm3.
(K and L) Representative images (K) and quantitative analysis (L) showing the number of cells colabeled by BrdU (green) and DCX (magenta) was enhanced by core drug treatment (Student's t test, ∗p < 0.01, N = 4 mice).
(M and N) Representative images (M) and quantitative analysis (N) showing the number of cells colabeled by BrdU (green) and NEUN (red) was increased in granular layer by core drugs (Student's t test, ∗p < 0.01, N = 4 mice). Scale bar, 20 μm.
We next further tested whether our core drugs can pass through blood-brain barrier (BBB) to regulate adult neurogenesis through intraperitoneal (i.p.) administration (daily i.p. injection of core drugs for 3 weeks, and bromodeoxyuridine (BrdU) was i.p. injected every 3–4 days from day 5 to day 26 to identify newborn cells) (Figure 7F). It is important to note that the intracranial injection of core drugs was one-time injection, whereas the i.p. injection was repeat injections over 3 weeks. Interestingly, i.p. injection of core drugs also resulted in a significant increase of DCX-labeled newborn neurons and Ki67-positive cells in the dentate granule layer (Figures 7G–7J). Moreover, BrdU-labeled cells that were dually immunopositive for DCX or NEUN increased significantly after i.p. injection of core drugs (Figures 7K–7N), with more newborn neurons migrating toward the outer layer of the dentate granule layer than in control group (Figure 7M, arrowhead), suggesting an accelerated neuronal maturation after core drug treatment. These results suggest that core drugs can regulate adult neurogenesis in vivo.
Discussion
In this work, we identified a chemical formula using only three to four small molecules to reprogram HAs into neurons. Through replacement of different functional analogs, we demonstrate that modulating multiple signaling pathways, but not a single signaling pathway, is important for chemical reprogramming of astrocytes into neurons. The chemically converted iNs can survive more than 7 months and are highly functional with bursts of synaptic activities. Besides upregulation of neural transcription factors such as NEUROD1 and NGN2, we found that the neural suppressor gene REST was significantly downregulated, while MECP2 was upregulated during chemical conversion. During our attempt to chemically convert astrocytes into neurons in the mouse brain in vivo, we accidentally discovered that our core drugs can potently regulate adult neurogenesis in the mouse hippocampus. Together, our chemical formula with only three to four small molecules to reprogram HAs into neurons brings us one step closer toward a potential drug therapy for brain repair.
One discovery made in this study is the synergistic modulation of multiple signaling pathways that are critical for chemical reprogramming. Modulating a single signaling pathway alone is not sufficient to change the cell fate. For example, while CHIR99021 alone increased NGN2 by 120-fold, it did not convert astrocytes into neurons, suggesting that modulating a single signaling pathway is not sufficient for chemical conversion. Nevertheless, CHIR99021 is one of the most widely used small molecules in chemical reprogramming studies (Hu et al., 2015, Li et al., 2015, Zhang et al., 2015). Application of SB431542 and LDN193189 together has been reported to induce neurogenesis from stem cells (Chambers et al., 2009), but they are not sufficient to reprogram HAs into neurons, suggesting that HAs, as terminally differentiated cells, are very different from neural stem cells in terms of reprogrammability. By substituting each core drug with its functional analogs, we further demonstrate that it is not the specific drugs (SLCD) per se but rather the four signaling pathways (Notch, GSK-3β, TGF-β, and BMP) that are regulated by the four core drugs are critical for chemical reprogramming. Interestingly, modulating any three out of the four signaling pathways can successfully reprogram HAs into neurons, suggesting that these four pathways may have some overlapping functions. It is worth mentioning that, during the progress of this study, Dr. Pei's group reported that human adult astrocytes isolated from patients with brain tumor can be reprogrammed into neurons by a six-small-molecule cocktail VCRFBI (VPA, CHIR99021, Repsox, forskolin, i-Bet151, and ISX-9) (Gao et al., 2017). This is a very interesting work because it represents another major step toward a future clinical application to directly convert adult HAs into neurons using a drug approach. On the other hand, it should be cautioned that any astrocytes isolated from the brain and put into Petri dishes may lose their in vivo identity. Therefore, it is still pivotal to test whether small molecules can convert HAs directly in a brain circuit in vivo. Interestingly, we have unexpectedly found that in vivo injection of the core drugs can significantly increase adult neurogenesis in the mouse hippocampus. This finding of small molecules passing through the BBB and regulating adult neurogenesis may have important implications for future development of drug therapies.
Another discovery of this study is that, regardless of nine- or four-molecule cocktails, a common outcome of our chemical reprogramming protocols is the upregulation of neural transcription factors NEUROD1 and NGN2. Consistent with our discovery, recent work on chemical reprogramming of cultured fibroblasts or astrocytes into neurons also reported an upregulation of NEUROD1 and NGN2 together with other transcription factors (Cheng et al., 2015, Gao et al., 2017, Li et al., 2015). Therefore, it seems that our chemical reprogramming approach is an upstream process that may trigger the upregulation of neural transcription factors, which then carry on the reprogramming process. Furthermore, we and other groups have reported that overexpression of neural transcription factors NEUROD1 (Guo et al., 2014) and NGN2 (Heinrich et al., 2010) can both convert astrocytes into glutamatergic neurons. Interestingly, both our nine- and four-molecule cocktails convert HAs mainly into glutamatergic neurons as well (Zhang et al., 2015), possibly through the upregulation of NEUROD1 and NGN2. Dr. Pei's group recently also reported that a six-drug cocktail (VCRFBI) converted adult HAs into glutamatergic neurons associated with upregulation of neural transcription factors including NEUROD1 and NGN2 (Gao et al., 2017). Notably, our nine-drug cocktail generated a small percentage of GABAergic neurons but a four-drug cocktail did not, suggesting that the small molecules removed in this current study (TTNPB, SAG/Purmo, VPA, TZV) might be responsible for the small number of GABAergic neurons. Further work is needed to find various chemical cocktails that can reprogram HAs into other neuronal subtypes such as GABAergic and dopaminergic neurons. In addition, besides the four signaling pathways identified in this study, other signaling pathways that may also be important for chemical reprogramming of astrocytes into neurons, or to reprogram other glial cells such as NG2 glia into neurons, should be investigated.
In summary, this study reveals a drug formula that uses only three or four small molecules to efficiently reprogram HAs into functional neurons. Such a simple chemical reprogramming protocol makes it possible to develop a potentially useful drug therapy for neuroregeneration and brain repair, although great challenges such as chemical toxicity and CNS drug delivery are yet to be solved in future studies.
Experimental Procedures
Cell Culture
Human cortical astrocytes purchased from ScienCell (1800, San Diego, CA) and Lonza (cc-2565) were subcultured as described previously (Guo et al., 2014, Zhang et al., 2015). In brief, HAs were cultured in a medium (HA medium) containing DMEM/F12 (Gibco), 10% FBS (Gibco), B27 supplements (Gibco), 3.5 mM Glucose (Sigma), 10 ng/mL fibroblast growth factor 2 (FGF2) (Alomone Labs), 10 ng/mL epidermal growth factor (Alomone Labs), and penicillin/streptomycin (Gibco). The human cortical astrocytes used in this study were subjected to 10–15 passages after purchasing from ScienCell, and 6–10 passages after purchasing from Lonza.
To examine whether HAs (ScienCell, 1800) contain any neural progenitor cells (NPCs), we cultured the cells in neuronal differentiation medium for 1 month and then performed immunostaining on different cell markers. As a positive control, human NPCs (gift from Dr. Fred Gage) were cultured on the coverslips coated with 1% Matrigel (Gibco), in NPC proliferation medium containing DMEM/F12, penicillin/streptomycin, B27 supplement, N2 supplement, and FGF2 (20 ng/mL).
Reprogramming HAs to Neurons
HAs were seeded onto coverslips (12 mm) pre-coated with poly-D-lysine (Sigma) in 24-well plates (BD Biosciences) and cultured in HA medium until reaching ∼90% confluence. One day before reprogramming, half of the HA medium was changed to N2 medium, which contained DMEM/F12, N2 supplements (Gibco), and penicillin/streptomycin. The following day, the culture medium would be totally changed to N2 medium, together with small molecules or vehicle control (0.2% DMSO). The culture medium and drugs were refreshed three times every 2 days. In the core drug together group, SB431542, LDN193189, CHIR99021, and DAPT were always applied together to HAs. In the core drug sequential group, SB431542 and LDN193189 were added for 2 days, and then replaced by CHIR99021 and DAPT for another 4 days. In both groups, at 6 days after drug treatment, the culture medium was changed to neuronal differentiation medium consisting of DMEM/F12, N2, B27, 0.5% FBS, 1 μM Y-27632 (Tocris), 5 μg/mL vitamin C, and penicillin/streptomycin (Gibco). To promote long-term neuronal maturation, brain-derived neurotrophic factor (BDNF) (10 ng/mL, Invitrogen), neurotrophin-3 (NT3) (10 ng/mL, Invitrogen) and insulin growth factor 1 (IGF-1) (20 ng/mL, Invitrogen) were added into the neuronal differentiation medium. Importantly, 200 μL medium with BDNF, NT3, and IGF-1 was added to each well every week to prevent the osmolarity change caused by medium evaporation and to keep the total medium volume around 2 mL in 24-well plates.
Statistical Analysis
Data were analyzed using Student's t test for two-group comparison, and one-way ANOVA for multiple-group comparison followed by Dunnett's post hoc test in GraphPad. Dunnett's post hoc was chosen here because of its power in comparing every mean with a control mean. All data are represented as means ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
Author Contributions
G.C. conceived and supervised the entire project, analyzed the data, and wrote the manuscript. J.-C.Y. performed the majority of the experiments, analyzed the data, and participated in writing the manuscript. L.Z. performed the experiments on MECP2 and REST, and, together with Y.W., X.-Y.H., and Z.-F.L., did in vivo work. N.-X.M., G.L., and F.-P.D. performed real-time PCR experiments on regional markers, transcriptional regulation, and pathway targets during chemical reprogramming. G.-Y.W. participated in the discussion and designing of some of the experiments.
Acknowledgment
This work was supported by grants from NIH (AG045656) and Alzheimer's Association (ZEN-15-321972), as well as Charles H. “Skip” Smith Endowment Fund at Pennsylvania State University to G.C. G.C. is a co-founder of NeuExcell Therapeutics.
Published: February 7, 2019
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, seven figures, two tables and one video and can be found with this article online at https://doi.org/10.1016/j.stemcr.2019.01.003.
Accession Numbers
Our own RNA-seq data on human fetal astrocytes are available at the GEO (GEO: GSE123397).
Previous datasets published by other labs (Modrek et al., 2017, Zhang et al., 2016b) and used for comparison here are: hNSCs (GSE94962) and human fetal and adult astrocytes (GSE73721).
More Supplemental Experimental Procedures can be found in the Supplemental Information.
Supplemental Information
References
- Ballas N., Grunseich C., Lu D.D., Speh J.C., Mandel G. REST and its corepressors mediate plasticity of neuronal gene chromatin throughout neurogenesis. Cell. 2005;121:645–657. doi: 10.1016/j.cell.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Berninger B., Costa M.R., Koch U., Schroeder T., Sutor B., Grothe B., Gotz M. Functional properties of neurons derived from in vitro reprogrammed postnatal astroglia. J. Neurosci. 2007;27:8654–8664. doi: 10.1523/JNEUROSCI.1615-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burda J.E., Sofroniew M.V. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron. 2014;81:229–248. doi: 10.1016/j.neuron.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao N., Huang Y., Zheng J.S., Spencer C.I., Zhang Y., Fu J.D., Nie B.M., Xie M., Zhang M.L., Wang H.X. Conversion of human fibroblasts into functional cardiomyocytes by small molecules. Science. 2016;352:1216–1220. doi: 10.1126/science.aaf1502. [DOI] [PubMed] [Google Scholar]
- Chambers S.M., Fasano C.A., Papapetrou E.P., Tomishima M., Sadelain M., Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L., Gao L., Guan W., Mao J., Hu W., Qiu B., Zhao J., Yu Y., Pei G. Direct conversion of astrocytes into neuronal cells by drug cocktail. Cell Res. 2015;25:1269–1272. doi: 10.1038/cr.2015.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L., Hu W.X., Qiu B.L., Zhao J., Yu Y.C., Guan W.Q., Wang M., Yang W.Z., Pei G. Generation of neural progenitor cells by chemical cocktails and hypoxia. Cell Res. 2014;24:665–679. doi: 10.1038/cr.2014.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano C.A., Chambers S.M., Lee G., Tomishima M.J., Studer L. Efficient derivation of functional floor plate tissue from human embryonic stem cells. Cell Stem Cell. 2010;6:336–347. doi: 10.1016/j.stem.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Filous A.R., Silver J. Targeting astrocytes in CNS injury and disease: a translational research approach. Prog. Neurobiol. 2016;144:173–187. doi: 10.1016/j.pneurobio.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L., Guan W., Wang M., Wang H., Yu J., Liu Q., Qiu B., Yu Y., Ping Y., Bian X. Direct generation of human neuronal cells from adult astrocytes by small molecules. Stem Cell Reports. 2017;8:538–547. doi: 10.1016/j.stemcr.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grande A., Sumiyoshi K., Lopez-Juarez A., Howard J., Sakthivel B., Aronow B., Campbell K., Nakafuku M. Environmental impact on direct neuronal reprogramming in vivo in the adult brain. Nat. Commun. 2013;4:2373. doi: 10.1038/ncomms3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z.Y., Zhang L., Wu Z., Chen Y.C., Wang F., Chen G. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer's disease model. Cell Stem Cell. 2014;14:188–202. doi: 10.1016/j.stem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich C., Blum R., Gascon S., Masserdotti G., Tripathi P., Sanchez R., Tiedt S., Schroeder T., Gotz M., Berninger B. Directing astroglia from the cerebral cortex into subtype specific functional neurons. PLoS Biol. 2010;8:e1000373. doi: 10.1371/journal.pbio.1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirabayashi Y., Itoh Y., Tabata H., Nakajima K., Akiyama T., Masuyama N., Gotoh Y. The Wnt/beta-catenin pathway directs neuronal differentiation of cortical neural precursor cells. Development. 2004;131:2791–2801. doi: 10.1242/dev.01165. [DOI] [PubMed] [Google Scholar]
- Hu W.X., Qiu B.L., Guan W.Q., Wang Q.Y., Wang M., Li W., Gao L.F., Shen L., Huang Y., Xie G.C. Direct conversion of normal and Alzheimer's disease human fibroblasts into neuronal cells by small molecules. Cell Stem Cell. 2015;17:204–212. doi: 10.1016/j.stem.2015.07.006. [DOI] [PubMed] [Google Scholar]
- Inman G.J., Nicolas F.J., Callahan J.F., Harling J.D., Gaster L.M., Reith A.D., Laping N.J., Hill C.S. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol. Pharmacol. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- Kishi N., Macklis J.D. MECP2 is progressively expressed in post-migratory neurons and is involved in neuronal maturation rather than cell fate decisions. Mol. Cell. Neurosci. 2004;27:306–321. doi: 10.1016/j.mcn.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Kriks S., Shim J.W., Piao J., Ganat Y.M., Wakeman D.R., Xie Z., Carrillo-Reid L., Auyeung G., Antonacci C., Buch A. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Chen G. In vivo reprogramming for CNS repair: regenerating neurons from endogenous glial cells. Neuron. 2016;91:728–738. doi: 10.1016/j.neuron.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Zuo X.H., Jing J.Z., Ma Y.T., Wang J.M., Liu D.F., Zhu J.L., Du X.M., Xiong L., Du Y.Y. Small-molecule-driven direct reprogramming of mouse fibroblasts into functional neurons. Cell Stem Cell. 2015;17:195–203. doi: 10.1016/j.stem.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Li Y., Hibbs M.A., Gard A.L., Shylo N.A., Yun K. Genome-wide analysis of N1ICD/RBPJ targets in vivo reveals direct transcriptional regulation of Wnt, SHH, and hippo pathway effectors by Notch1. Stem Cells. 2012;30:741–752. doi: 10.1002/stem.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie D.C., Colamarino S.A., Song H.J., Desire L., Mira H., Consiglio A., Lein E.S., Jessberger S., Lansford H., Dearie A.R., Gage F.H. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- Liu Y.G., Miao Q.L., Yuan J.C., Han S.E., Zhang P.P., Li S.L., Rao Z.P., Zhao W.L., Ye Q., Geng J.L. Ascl1 converts dorsal midbrain astrocytes into functional neurons in vivo. J. Neurosci. 2015;35:9336–9355. doi: 10.1523/JNEUROSCI.3975-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrek A.S., Golub D., Khan T., Bready D., Prado J., Bowman C., Deng J.J., Zhang G.A., Rocha P.P., Raviram R. Low-grade astrocytoma mutations in IDH1, P53, and ATRX cooperate to block differentiation of human neural stem cells via repression of SOX2. Cell Rep. 2017;21:1267–1280. doi: 10.1016/j.celrep.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa M., Koinuma D., Tsutsumi S., Vasilaki E., Kanki Y., Heldin C.H., Aburatani H., Miyazono K. ChIP-seq reveals cell type-specific binding patterns of BMP-specific Smads and a novel binding motif. Nucleic Acids Res. 2011;39:8712–8727. doi: 10.1093/nar/gkr572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu W., Zang T., Zou Y., Fang S., Smith D.K., Bachoo R., Zhang C.L. In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nat. Cell Biol. 2013;15:1164–1175. doi: 10.1038/ncb2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekny M., Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- Skene P.J., Illingworth R.S., Webb S., Kerr A.R., James K.D., Turner D.J., Andrews R., Bird A.P. Neuronal MeCP2 is expressed at near histone-octamer levels and globally alters the chromatin state. Mol. Cell. 2010;37:457–468. doi: 10.1016/j.molcel.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z.D., Niu W.Z., Liu M.L., Zou Y.H., Zhang C.L. In vivo conversion of astrocytes to neurons in the injured adult spinal cord. Nat. Commun. 2014;5:3338. doi: 10.1038/ncomms4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X., Zhou L., Wagner A.M., Marchetto M.C.N., Muotri A.R., Gage F.H., Chen G. Astroglial cells regulate the developmental timeline of human neurons differentiated from induced pluripotent stem cells. Stem Cell Res. 2013;11:743–757. doi: 10.1016/j.scr.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torper O., Ottosson D.R., Pereira M., Lau S., Cardoso T., Grealish S., Parmar M. In vivo reprogramming of striatal NG2 Glia into functional neurons that integrate into local host circuitry. Cell Rep. 2015;12:474–481. doi: 10.1016/j.celrep.2015.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban N., Guillemot F. Neurogenesis in the embryonic and adult brain: same regulators, different roles. Front. Cell Neurosci. 2014;8:396. doi: 10.3389/fncel.2014.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrecchia F., Chu M.L., Mauviel A. Identification of novel TGF-beta/Smad gene targets in dermal fibroblasts using a combined cDNA microarray/promoter transactivation approach. J. Biol. Chem. 2001;276:17058–17062. doi: 10.1074/jbc.M100754200. [DOI] [PubMed] [Google Scholar]
- Yang N., Ng Y.H., Pang Z.P.P., Sudhof T.C., Wernig M. Induced neuronal cells: how to make and define a neuron. Cell Stem Cell. 2011;9:517–525. doi: 10.1016/j.stem.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiu G., He Z. Glial inhibition of CNS axon regeneration. Nat. Rev. Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Yang X.Y., Yang S.Y., Zhang J.N. The Wnt/beta-catenin signaling pathway in the adult neurogenesis. Eur. J. Neurosci. 2011;33:1–8. doi: 10.1111/j.1460-9568.2010.7483.x. [DOI] [PubMed] [Google Scholar]
- Zhang L., Yin J.C., Yeh H., Ma N.X., Lee G., Chen X.A., Wang Y.M., Lin L., Chen L., Jin P. Small molecules efficiently reprogram human astroglial cells into functional neurons. Cell Stem Cell. 2015;17:735–747. doi: 10.1016/j.stem.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M.L., Lin Y.H., Sun Y.J., Zhu S.Y., Zheng J.S., Liu K., Cao N., Li K., Huang Y.D., Ding S. Pharmacological reprogramming of fibroblasts into neural stem cells by signaling-directed transcriptional activation. Cell Stem Cell. 2016;18:653–667. doi: 10.1016/j.stem.2016.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Sloan S.A., Clarke L.E., Caneda C., Plaza C.A., Blumenthal P.D., Vogel H., Steinberg G.K., Edwards M.S.B., Li G. Purification and characterization of progenitor and mature HA reveals transcriptional and functional differences with mouse. Neuron. 2016;89:37–53. doi: 10.1016/j.neuron.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Zhao T., Guan J., Zhang X., Fu Y., Ye J., Zhu J., Meng G., Ge J., Yang S. A XEN-like state bridges somatic cells to pluripotency during chemical reprogramming. Cell. 2015;163:1678–1691. doi: 10.1016/j.cell.2015.11.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.