Summary
There is a profound need for functional, biomimetic in vitro tissue constructs of the human blood-brain barrier and neurovascular unit (NVU) to model diseases and identify therapeutic interventions. Here, we show that induced pluripotent stem cell (iPSC)-derived human brain microvascular endothelial cells (BMECs) exhibit robust barrier functionality when cultured in 3D channels within gelatin hydrogels. We determined that BMECs cultured in 3D under perfusion conditions were 10–100 times less permeable to sodium fluorescein, 3 kDa dextran, and albumin relative to human umbilical vein endothelial cell and human dermal microvascular endothelial cell controls, and the BMECs maintained barrier function for up to 21 days. Analysis of cell-cell junctions revealed expression patterns supporting barrier formation. Finally, efflux transporter activity was maintained over 3 weeks of perfused culture. Taken together, this work lays the foundation for development of a representative 3D in vitro model of the human NVU constructed from iPSCs.
Keywords: induced pluripotent stem cell, blood-brain barrier, neurovascular unit, three dimensional model, tissue engineering
Highlights
-
•
Development of a functional 3D in vitro blood-brain barrier model
-
•
Evidence showing that perfusion stabilized barrier integrity for up to 21 days
-
•
Junctional analysis shows patterns supporting barrier formation in channels
-
•
Cells exhibit stable efflux transporter activity for 3 weeks in perfused channels
Faley et al. present a 3D in vitro model of the blood-brain barrier constructed using induced pluripotent stem cell-derived human brain microvascular endothelial cells cultured in 3D channels in gelatin hydrogels. The model displays robust tight junction protein expression, restricted paracellular permeability, and active efflux transporter activity, and represents an important step toward a representative human in vitro neurovascular model.
Introduction
The neurovascular unit (NVU), composed of brain microvascular endothelial cells (BMECs) that form the blood-brain barrier (BBB), pericytes, neurons, and glial cells, tightly regulates transport of substances between the bloodstream and the brain. Abnormal BBB and NVU function is associated with a broad spectrum of neurological pathologies (Sweeney et al., 2018), and increasing evidence suggests that a number of non-neural disorders, such as diabetes (Prasad et al., 2014), are associated with compromised BBB integrity and/or functionality, often giving rise to secondary complications and cumulative neurological insults that increase the risk of additional neurodegenerative and cerebrovascular disease. Animal models (in vitro and in vivo) have historically been the gold-standard platform for investigating the complexities of human neurovascular disease. However, the difficulties in translating information gleaned from animal models to successful clinical intervention, which are exemplified by the lack of therapeutics that can effectively treat neurodegenerative diseases, highlight the need to develop a functional in vitro tissue model of the human NVU that will improve mechanistic understanding of disease progression and accelerate the development of new treatment strategies.
Recent advances in biomaterials patterning and microfluidic device fabrication have enabled a shift from standard 2D monolayer cell culture to 3D approaches that either seed cells on the surface of porous scaffolds or embed cells within hydrogel matrices. This shift has highlighted the fact that 3D culture techniques generally result in cell behavior that more closely mimics in vivo phenotypes (Huh et al., 2011, Ravi et al., 2015, Wikswo, 2014). Approaches that rely on cell-laden hydrogels are particularly attractive, as hydrogels mimic many aspects of the natural extracellular matrix (ECM) including stiffness, enzymatic degradability, and (with appropriate material choice or RGD modification) binding sites (Tibbitt and Anseth, 2009). Cell-laden hydrogels cast with thicknesses in the few hundred-micron range have allowed researchers to observe cell behavior in a more biomimetic, 3D environment. Additionally, cell-laden hydrogels can be patterned so that channels supporting fluid flow exist within the gel. Initial work in this area leveraged photolithographic and soft templating techniques (Cabodi et al., 2005, Golden and Tien, 2007, Zheng et al., 2012), and more recently many researchers have moved toward using 3D printing approaches to pattern either the gel itself or a sacrificial template that is first embedded within the gel and subsequently removed to form a channel (Bertassoni et al., 2014, Kolesky et al., 2014, Miller et al., 2012). While these approaches are still generally limited to forming channels with diameters on the 100-μm or larger scale, this advance enables new investigations into phenomena occurring within and around arteriole and larger-sized vessels. These platforms allow variation of multiple critical parameters, such as flow, shear, pressure, and soluble biochemical concentration, in a 3D geometry that mimics a natural vessel.
Accordingly, several reports have implemented advanced fabrication methods to develop more complex in vitro BBB and NVU models. Thin-film, synthetic polyethylene glycol hydrogels supporting self-assembled NVU constructs have been used for high-throughput toxicity screening (Barry et al., 2017, Pellett et al., 2015). Meanwhile, microfluidic approaches have enabled the observation and measurement of NVU function in a highly controlled, perfused environment; these range from the commercially available sym-bbb (Prabhakarpandian et al., 2013) to highly complex, organ-on-a-chip platforms that provide powerful methods for gaining critical insights into population-specific responses to environmental perturbations with multiple readout mechanisms (Brown et al., 2015, Markov et al., 2012). While there are some recent reports that have incorporated hydrogel matrices into microfluidic devices (Kim et al., 2013, Phan et al., 2017), most of these models rely on the use of solid substrates such as polydimethylsiloxane (PDMS) or glass (Cho et al., 2015). Such BBB models are well suited to high-throughput, massively parallel drug screening efforts. However, scaffolds should ideally be more biomimetic, such that the scale, biological matrix, cellular components, and organization better approximate physiological processes, including both direct and indirect cellular interactions. Of late there have only been a few studies involving tissue-scale biological scaffolds with 3D cultures of endothelial cells (Ingram et al., 2016, Jiménez-Torres et al., 2016).
Indeed, cell fidelity has often been a limiting factor for recreating the BBB portion of NVU models. Historically, BMECs have been isolated from primary animal sources (Helms et al., 2016) but, as described above, species differences can limit the predictive power of such non-human models (Deo et al., 2013). However, BMECs from primary human sources are tedious to isolate, genetically heterogeneous between donors, can only be obtained in low yield, and often come from unhealthy tissue (e.g., brain tumor resections). Conversely, immortalized human BMECs can be obtained in high yields from a clonal source but suffer from poor passive barrier properties that do not appropriately mimic the in vivo BBB (Weksler et al., 2005). In recent years, the development of protocols to differentiate human induced pluripotent stem cells (iPSCs) into BMECs has circumvented many of these issues. iPSC-derived BMECs were initially characterized by expression of representative BBB markers, active efflux transporter activity, permeability to a panel of small molecules that correlate with in vivo uptake in rodents, and modest barrier properties as determined by transendothelial electrical resistance (TEER) measurements of ∼800 Ωcm2 (Lippmann et al., 2012). Subsequently, retinoic acid (RA) was shown to boost the passive barrier properties of iPSC-derived BMECs above 3,000 Ωcm2 (Lippmann et al., 2014). Since these initial publications, others have validated the fidelity of these cells and advancements have been made toward improving the differentiation procedure (Appelt-Menzel et al., 2017, Hollmann et al., 2017, Katt et al., 2016, Wilson et al., 2015). In addition, these iPSC-derived BMECs, co-cultured with astrocytes on opposite sides of a Transwell filter, have been incorporated into a microfluidic device that maintained BBB properties over 10 days (Wang et al., 2017). However, studies of iPSC-derived BMEC performance in biomimetic 3D hydrogel scaffolds, which are a crucial step toward building representative in vivo-like NVU models that can be used for disease modeling and pre-clinical validation of drug efficacies, have been limited.
Ideally, a 3D NVU model constructed from iPSCs would be fully isogenic from a single pluripotent source, exhibit robust BBB function (including passive and active barrier properties), possess long-term stability, and be relatively simple to fabricate and implement. Herein, we describe a process to fabricate such a model, with a focus on establishing a functional iPSC-derived BMEC layer within a continuously perfused channel. Using easily accessible materials (unmodified, enzymatically crosslinked porcine gelatin) and a straightforward fabrication approach to assemble a platform that recirculates liquid through a single channel (Figures 1A–1C), we demonstrate that iPSC-derived BMECs assembled in 3D establish a robust barrier that remains stable for up to 3 weeks under continuous perfusion. Furthermore, we found that BMECs in perfused channels retain efflux transporter activity, a key functional characteristic of BBB endothelium, for over 2 weeks in culture. Together, these results validate the performance of a 3D, continuously perfused biomimetic model of brain microvasculature with long-term functional barrier properties.
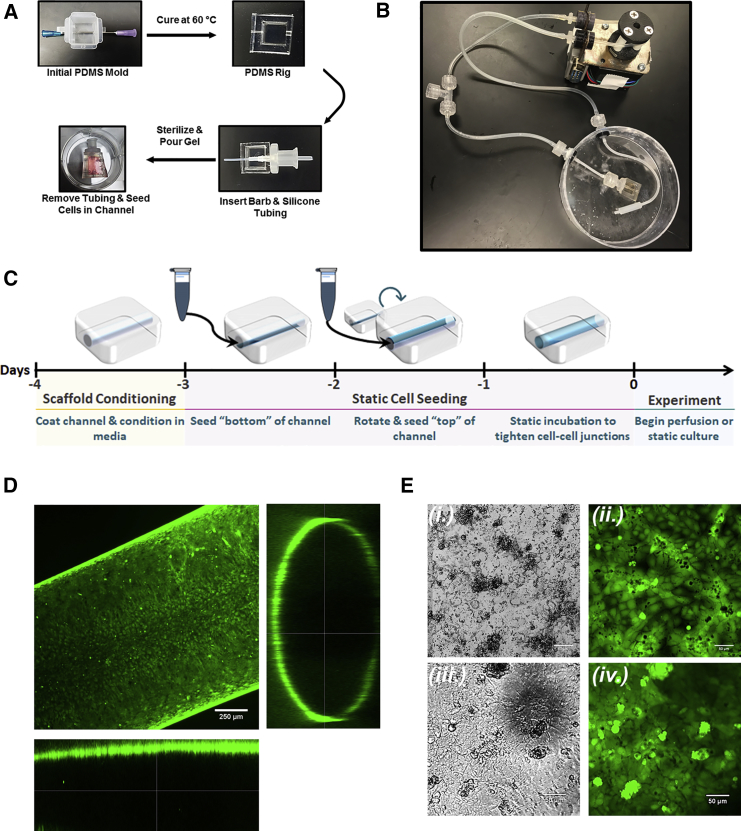
Figure 1.
Cell-Laden Scaffold Assembly and Perfusion
(A and B) Fabrication of gelatin channel within supportive PDMS rig (A) and fully assembled perfusion platform (B).
(C) Schematic for cell seeding and initiation of experiments.
(D) BMECs stained with Calcein AM Ester following 7 days of culture on the channel surface as shown by orthogonal confocal image.
(E) Morphology comparison between Calcein AM-stained BMECs cultured in 2D tissue-culture plates (i and ii) and in gelatin channels (iii and iv).
Results
Optimization of the Hydrogel Scaffold for Endothelial Adhesion
We first optimized scaffold composition and determined that iPSC-derived BMECs performed best when seeded on gelatin hydrogels coated with collagen IV and fibronectin (Figure S1), an approach determined to be effective in previously published protocols (Hollmann et al., 2017, Lippmann et al., 2014). With our intention to incorporate glial and neural cells in future NVU models, we initially explored fabricating the scaffolds using alginate/gelatin composite hydrogels based upon previous reports in the literature characterizing 3D culture of neural and glial cells (Bozza et al., 2014). We evaluated the ability of IMR90-4-derived BMECs to adhere to the surface of tissue-culture plates coated with thin hydrogel films comprising 10% gelatin or 10% gelatin/0.25% alginate composite hydrogels both with and without subsequent adsorption of the collagen/fibronectin solution commonly used in iPSC-derived BMEC cultures (Lippmann et al., 2012). As shown in Figure S1A, visual inspection of cells 24 h after seeding revealed that iPSC-derived BMECs failed to attach and grow on hydrogels containing alginate, even with additional treatment with collagen/fibronectin. Although visually indistinguishable in terms of cell morphology and viability, TEER measurements of BMECs grown on gelatin hydrogels with and without additional collagen/fibronectin coating demonstrated that gelatin treated with these additional ECM proteins yielded enhanced barrier properties (Figure S1B). Thus, we elected to proceed with all experiments using scaffolds composed of 10% gelatin and channel surfaces coated with collagen/fibronectin solution 24 h prior to cell seeding. Visual comparison of IMR90-4-derived BMECs indicates that cell morphology remains unchanged in the transition from 2D to 3D culture formats (Figures 1D and 1E).
iPSC-Derived BMECs Exhibit Robust, Long-Term Passive Barrier Function in 3D Culture
To evaluate the ability of iPSC-derived BMECs to recapitulate passive barrier function in 3D culture over the course of at least 2 weeks, we measured and compared the diffusive permeability of sodium fluorescein (molecular weight [MW] = 330 Da), 3 kDa MW dextran, and albumin (MW = 66 kDa) with human umbilical vein endothelial cells (HUVECs) and human dermal microvascular endothelial cells (μVas). The use of these tracers was intended to assess permeability to relatively small-, medium-, and large-sized molecules. In each cellular cohort, we compared samples cultured under static conditions versus continuous perfusion (100 μL/min). Results from these experiments are summarized in Figures 2 and 3, with all raw data located in Figure S2. Videos of select experiments are also provided to illustrate real-time differences in compound extravasation (Videos S1, S2, S3, and S4).
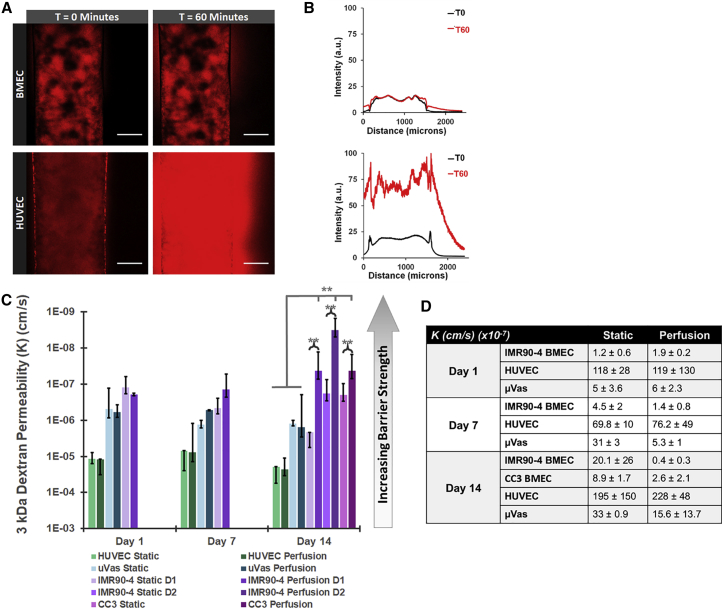
Figure 2.
Quantitative Comparison of Cell Monolayer Permeability in Perfused and Non-perfused Channels
(A) Confocal images of 3 kDa dextran (Red) diffusion in gelatin channels lined with either iPSC-derived BMECs or HUVECs. Images show samples cultured for 7 days under static conditions. Scale bars, 100 μm.
(B) Intensity profiles across channels lined with BMECs (top) or HUVECs (bottom) at 0 and 60 min of perfusion with 3 kDa dextran.
(C and D) Graphical depiction of permeability to 3 kDa dextran on days 1, 7, and 14 of culture under either static culture or continuous perfusion at 100 μL/min (C). Average permeability coefficients are listed in (D). Data are compiled from at least eight separate channel seedings, composed of cells obtained from five independent differentiations. N ≥ 3 independent biological replicates for all data points. Error bars indicate ±1 SD. ∗p < 0.05, ∗∗p < 0.01 based on one-way ANOVA. Individual values from each replicate (e.g., measurements from individual devices) are listed in Figure S2.
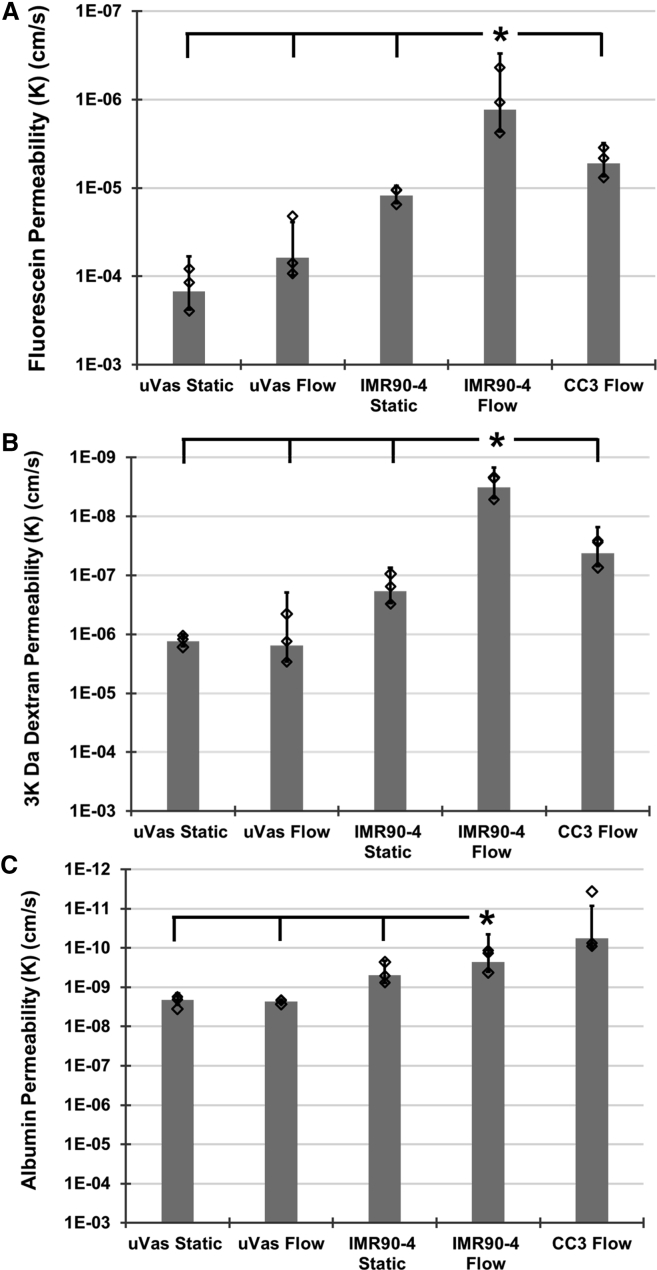
Figure 3.
Permeability Coefficients for Molecules of Varying Molecular Weights
Comparison of permeability for (A) sodium fluorescein, (B) 3 kDa dextran, and (C) albumin between μVas, IMR90-4-derived BMECs, and CC3-derived BMECs that were maintained under either static conditions or continual perfusion for 14 days. Diamonds indicate actual values of individual replicates. N ≥ 3 independent biological replicates for all data points. Error bars indicate ±1 SD. ∗p < 0.05 based on one-way ANOVA.
We initially examined the permeability of 3 kDa dextran in static versus perfused channels on days 1, 7, and 14 of culture (Figures 2A–2D). The permeability coefficients for 3 kDa dextran in IMR90-4-derived BMECs were measured at nearly 2 orders of magnitude lower than HUVECs on day 1 static (KBMEC = 1.2 × 10−7 cm/s, KHUVEC = 1.2 × 10−5 cm/s) and perfused (KBMEC = 1.9 × 10−7 cm/s, KHUVEC = 1.2 × 10−5 cm/s) samples and roughly 5-fold lower than μVas (KμVas = 5 × 10−7 cm/s) (e.g., the BMECs exhibit a >100-fold and 5-fold stronger passive barrier compared with HUVECs and μVas, respectively). These data indicate that the BMECs not only attach to the gelatin matrix, but also immediately form a robust barrier that is tighter than the non-BBB endothelial cells. In static culture, BMEC barrier function declined on day 7 (KBMEC = 4.6 × 10−7 cm/s) and day 14 (KBMEC = 2.2 × 10−6 cm/s), although it remained ∼10-fold better than HUVEC (KHUVEC = 7 × 10−6 cm/s and 2 × 10−5 cm/s on days 7 and 14, respectively) and μVas controls (KμVas = 1.3 × 10−6 cm/s and 1.2 × 10−6 cm/s on days 7 and 14, respectively). In contrast, the barrier against 3 kDa dextran in BMECs that were continuously perfused for 2 weeks was comparable with initial values (KBMEC = 4.3 × 10−8 cm/s on day 14), suggesting that exposure to shear might further stabilize, and perhaps enhance, BMEC barrier integrity over time. A similar trend was observed in μVas (KμVas = 1.5 × 10−6 cm/s on day 14), although the permeability coefficient for these cells remained more than ten times greater than BMECs. We also separated data compiled from different IMR90-4 BMECs differentiations to show biological reproducibility (all data from Figures 2 and 3 are compiled from more than five independent differentiations). The permeability to 3 kDa dextran from two separate IMR90-4 BMEC differentiations (indicated as either D1 or D2) were measured at day 14 under both static and perfused conditions. All BMEC cohorts exhibit permeability coefficients over 10-fold smaller than those for HUVEC/μVas controls. Furthermore, all BMECs under perfusion are significantly less permeable than their non-perfused counterparts, further highlighting the reproducibility of the system.
To further explore the influence of perfusion upon barrier strength in BMECs, we measured the diffusion coefficients of sodium fluorescein and albumin in addition to 3 kDa dextran after 14 days of perfused or static culture (Figure 3). To validate these results in a different iPSC line, we also measured permeability in CC3-derived BMECs (Hollmann et al., 2017) (Figures 2 and 3). Both IMR90-4- and CC3-derived BMECs subjected to perfusion for 14 days exhibited permeability to all compounds that was an order of magnitude lower than μVas controls. Additionally, IMR90-4-derived BMECs subjected to perfusion exhibited permeability that was significantly lower compared with non-perfused counterparts, confirming permeability trends across a spectrum of molecular weights. Exclusion of albumin suggests reduced vesicular transport, which is a hallmark of the BBB. To further probe this finding, we used qPCR to quantify MFSD2A and CAV1 (caveolin-1) expression. MFSD2A, which is highly expressed in brain endothelium relative to lung and liver endothelium and known to suppress endocytosis/transcytosis (Ben-Zvi et al., 2014), was not expressed at significantly different levels between BMECs and μVas (Figure S3). However, CAV1, an important component of vesicular transport that is suppressed in BMECs and activated under pathogenic conditions such as ischemia/reperfusion (Knowland et al., 2014), exhibited ∼8-fold higher expression in μVas relative to BMECs. Coupled with the permeability data demonstrating reduced albumin extravasation, these data suggest the BMECs suppress vesicular transport, although not necessarily through MFSD2A upregulation. Since these BMECs are maintained in isolation, it is possible that regulatory cues for MFSD2A provided by other cell types (e.g., pericytes) are not present. In future model iterations, inclusion of these cell types could further suppress vesicular transport.
To further examine gene expression patterns, we used qPCR to quantify OCLN (occludin) and SLC2A1 (GLUT-1) between IMR90-4-derived BMECs and μVas at day 14 under perfusion. OCLN and SLC2A1 are recognized as being relatively specific to BBB compared with peripheral endothelium (Daneman and Prat, 2015), and these genes were expressed at ∼5-fold and ∼11-fold higher levels, respectively, in BMECs (Figure S3). Next, we used RNA sequencing to compare global gene expression patterns in BMECs cultured for 1 day under static conditions and 14 days under constant perfusion (Table S1). The Pearson correlation coefficient between these samples was 0.91, which indicates a strong positive association and provides evidence that the BMEC gene expression signatures are maintained under longitudinal perfusion.
Finally, to establish appropriate correlations between 2D and 3D barrier function, we performed a similar dextran diffusion assay using CC3-derived BMECs and HUVECs cultured in Transwell filters (Figure S4A). Permeability values obtained from cells cultured in 2D were on the same order of magnitude as those obtained in 3D channels (10−7 cm/s for BMECs and 10−5 cm/s for HUVECs), thus validating the measurements made within the tissue construct. Interestingly, permeability values for both BMECs and HUVECs remained stable over time in 2D, unlike the steady increase in permeability observed in cells lining non-perfused channels, indicating a possible role for nutrient exchange in maintaining barrier integrity in the 3D construct. To further explore this potential impact of nutrient exchange, we examined the permeability in BMECs and μVas in 3D culture under stop-flow conditions, in which samples were subjected to perfusion from the medium reservoir, but for only 10 min per day; these experiments permitted nutrient exchange with minimal exposure to shear stress (we note that the scaffolds are designed such that the upper surface of the hydrogel is in direct contact with the medium reservoir, which likely provides some additional material exchange to the cells). Results from these stop-flow experiments revealed that permeability was similar to, and in some cases worse than, static conditions, suggesting that fluidic shear stress does maintain barrier integrity within the context of the 3D culture platform (Figure S4B). We note the possibility that the endothelial cells provide greater conditioning of the medium in the Transwell system relative to the hydrogel system due to volume differences (2 mL versus ∼40 mL), and that shear stress-induced effects may compensate for these trophic factors in the perfusion versus static conditions. However, because the goal of this work is to solely establish the performance of the 3D BBB culture platform, these mechanisms were not explored further.
Assessment of Junctional Integrity
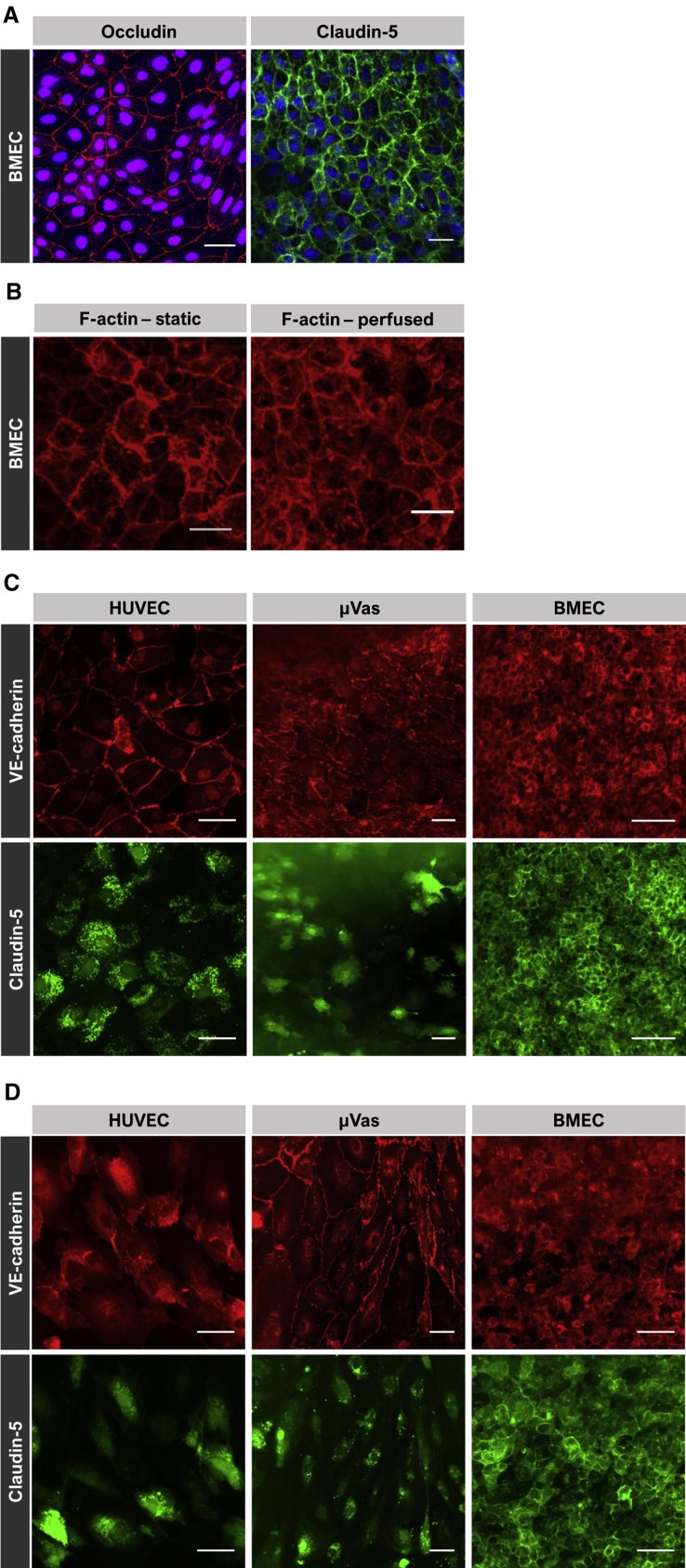
To validate that the observed permeability values corresponded to proper cell-cell junction formation, we evaluated cells for junctional and cytoskeletal markers including occludin, claudin-5, VE-cadherin, and F-actin. CC3-derived BMECs lining gelatin channels show robust expression of both occludin and claudin-5 localized to intercellular junctions (Figure 4A). F-actin expression (Figure 4B) similarly indicated strong intercellular localization in BMEC-lined channels. In addition, F-actin staining patterns demonstrate the lack of cellular elongation in cells exposed to fluidic shear, a property previously reported to be a unique characteristic of BMECs (DeStefano et al., 2017, Reinitz et al., 2015, Wang et al., 2017, Ye et al., 2014). This is particularly evident when BMEC morphology is compared with HUVECs and μVas maintained under static (Figure 4C) and perfused (Figure 4D) conditions. HUVECs and μVas also strongly expressed VE-cadherin along cell boundaries, but claudin-5 expression was generally absent at cell boundaries and instead diffusely distributed within the cells. Claudin-5 is essential for tight junction formation and regulation of BBB permeability, particularly to small compounds (Nitta et al., 2003). In contrast, claudin-5 is functionally insignificant in establishment of an umbilical vein (HUVEC) barrier (Kluger et al., 2013). When compared with the fluorescein permeability data (Figure 3), these overall data strongly suggest the formation of robust cell-cell junctions in BMECs in 3D culture.
Figure 4.
Immunofluorescent Staining
(A) IMR90-4-derived BMECs labeled for occludin and claudin-5 at day 1 under static conditions. Nuclei are counterstained with Hoechst.
(B) IMR90-4-derived BMECs labeled for F-actin at day 1 under static conditions and day 14 under perfused conditions.
(C and D) HUVECs, μVas, and IMR90-4-derived BMECs labeled for VE-cadherin and claudin-5 at day 7 under static (C) and perfused (D) conditions. Each individual image reflects a summative z projection of individual confocal images without additional processing to flatten images. For each fluorescence channel, the intensity scale is held constant across all samples. As a result, in some images there is a perceived decrease in fluorescence signal for points farthest from the objective that is reflective of channel curvature and slight variations in gelatin topology rather than inherent signal.
Scale bars, 50 μm.
Influence of Increased Shear Stress on Barrier Function
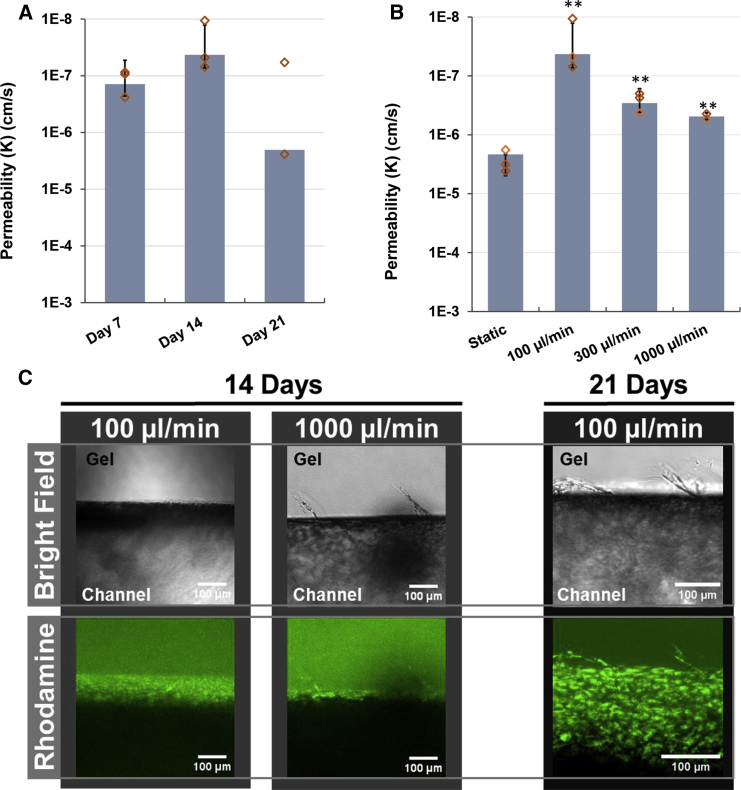
Based on the comparisons between perfusion, static, and stop-flow permeability experiments, we sought to further probe the effects of shear stress on barrier integrity. We found that permeability in channels perfused at 100 μL/min heterogeneously declined between two samples by day 21 (Figure 5A). One sample exhibited substantially worse permeability of 2.4 × 10−6 cm/s, while the other sample remained extremely tight at 5.8 × 10−8 cm/s. At this time, we observed signs of angiogenic sprouting at 21 days of perfusion (Figure 5C), which was not evident in previous time points in either static or perfused samples and could explain the decline in barrier function between samples.
Figure 5.
Relative Impact of Shear on BMEC Permeability
(A) Permeability to 3 kDa dextran in IMR90-4-derived BMECs perfused at 100 μL/min over 21 days. N ≥ 3 independent biological replicates for all datasets, except N = 2 independent biological replicates for the day-21 time point. Diamonds indicate actual values of individual replicates. Error bars indicate ±1 SD.
(B) Permeability coefficients to 3 kDa dextran in BMECs perfused for 14 days at 100, 300, and 1,000 μL/min, compared with the static control. Diamonds indicate actual values of individual replicates. N = 2 independent biological replicates for 1,000 μL/min dataset, N ≥ 3 independent biological replicates for all other cohorts. Error bars indicates ±1 SD. ∗∗p < 0.01 compared with static based upon one-way ANOVA.
(C) Images showing cell sprouting in channels perfused for 14 days (left) at 100 and 1,000 μL/min, and 21 days (right) at 100 μL/min. Top image is bright-field, bottom image shows cells stained by rhodamine 123.
Perfusion of the ∼800-μm diameter channel at a rate of 100 μL/min generates a wall shear of approximately 32 mPa (0.3 dyne/cm2), well below the physiological range of ∼1–3 Pa (10–30 dyne/cm2) estimated for brain microvasculature (Cheng et al., 2007, Cucullo et al., 2008, Garcia-Polite et al., 2017, Koutsiaris et al., 2013). Compared with the permeability of BMECs perfused at 100 μL/min, perfusion at 300 μL/min and 1,000 μL/min (wall shear of approximately 100 mPa [1 dyne/cm2] and 320 mPa [3.2 dyne/cm2], respectively) for 14 days yielded an overall reduction in passive barrier function. As shown in Figure 5B, the permeability coefficient to 3 kDa dextran for BMECs perfused at 300 μL/min and 1,000 μL/min was 2.9 × 10−7 cm/s and 4.8 × 10−7 cm/s, respectively, which is approximately ten times more permeable than BMECs perfused at 100 μL/min. This increase in permeability also corresponded to an increase in observed incidence of angiogenic sprouting observed on day 14 in channels perfused at higher flow rates and day 21 in channels perfused at 100 μL/min (Figure 5C). Importantly, these values are still 10-fold lower than BMECs maintained under static conditions, providing additional evidence that perfusion, even at subphysiological levels of shear, stabilizes barrier function over time despite minor vascular sprouting.
Assessment of Active Barrier Function
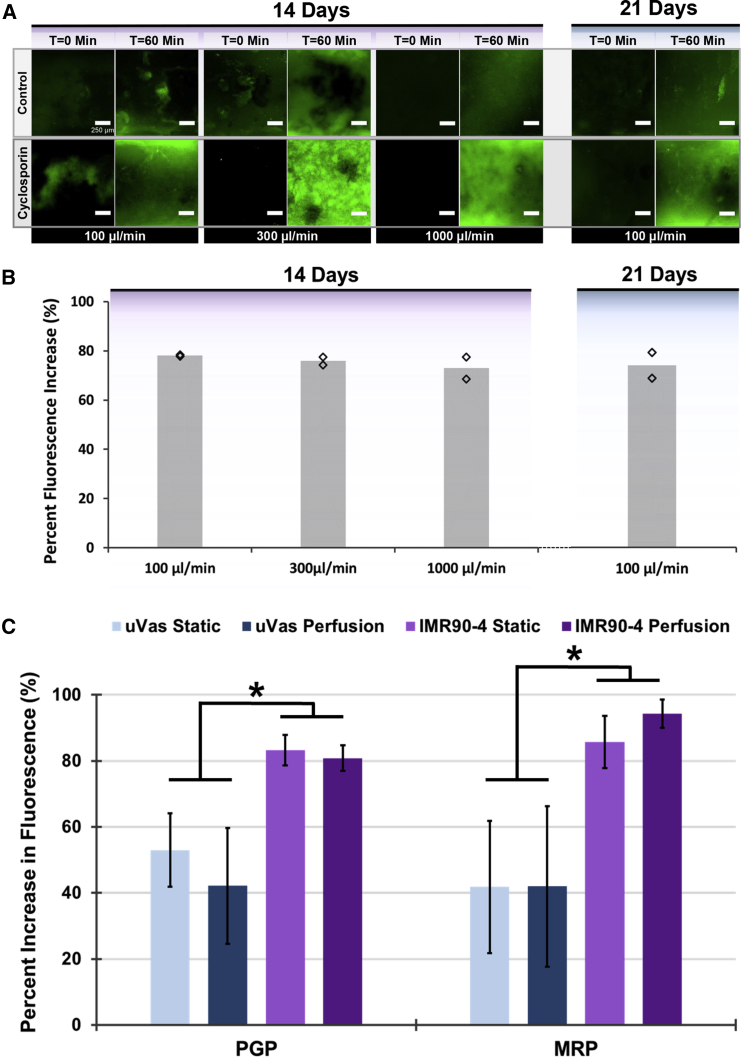
As discussed previously, a hallmark of brain endothelium is the expression of active efflux transporter such as P-glycoprotein (Pgp), breast cancer resistance protein, and multidrug resistance proteins (MRPs). Accordingly, numerous reports have demonstrated that iPSC-derived BMECs possess active efflux activity determined by substrate inhibition assays (Hollmann et al., 2017, Lippmann et al., 2012, Lippmann et al., 2014). Here, we sought to characterize this efflux activity in IMR90-4-derived BMECs under 3D perfusion culture. Intracellular accumulation of rhodamine 123 (a Pgp substrate) was evaluated in the presence or absence of cyclosporin A (a Pgp inhibitor) in BMECs after 14 days of perfusion. Similarly, MRP-associated efflux activity was assessed by measuring intracellular accumulation of H2DCFDA in the presence or absence of inhibitor MK-571. As shown in Figures 6A and 6B, BMECs possess robust Pgp activity after 14 days in culture as determined by an increase in intracellular fluorescence of inhibited samples exceeding 80%, providing supporting evidence of the long-term functionality of iPSC-derived BMECs in this 3D culture format. Pgp activity was not influenced by perfusion rate (100, 300, or 1,000 μL/min) and remained stable at 21 days of perfusion at 100 μL/min. Comparatively, μVas also exhibited increased uptake of rhodamine 123 and H2DCFDA in the presence of inhibitors (Figure 6C). However, uptake was significantly lower than for BMECs, likely indicating that BMECs have a higher efflux activity and/or expression of efflux transporters. Although peripheral endothelial cells are not commonly known for possessing efflux activity, Pgp expression has historically been observed in skin endothelial cells from papillary but not reticular dermis (Cordon-Cardo et al., 1989). Thus, some baseline efflux activity may reflect the source of these control cells.
Figure 6.
Assessment of Efflux Transporter Activity Reflects Long-Term BMEC Functionality in Perfused Channels
(A) Confocal images (z projections) of IMR90-4-derived BMECs after 14 and 21 days of perfusion at various flow rates that were imaged over the course of 1 h of perfusion with medium containing rhodamine 123 alone (control; top row) or following a 1-h pre-incubation with cyclosporin A (bottom row). Each pair of control versus cyclosporin A comparisons were conducted using separate channel devices from the same seeding and perfusion cohort. Images reflect summative z projections of confocal z stacks without any further processing to flatten images, and all images are presented using the same intensity scale.
(B) The increase in cellular rhodamine 123 accumulation resulting from Pgp inhibition is quantified in terms of cellular fluorescence intensity relative to non-inhibited controls. N ≥ 2 independent biological replicates for each dataset. Diamonds indicate actual values of individual replicates.
(C) Comparison of Pgp and MRP activity in channels lined with either μVas or BMECs after 14 days of culture. N ≥ 3 independent biological replicates for each dataset. Error bars indicate ±1 SD. ∗p < 0.05 based on one-way ANOVA.
Discussion
In this study, we demonstrate the capability of iPSC-derived BMECs to form confluent 3D monolayers that sustain barrier integrity for up to 3 weeks under continuous perfusion, as measured by low passive diffusion to a cohort of molecular tracers, tight junction localization, and efflux transporter activity. Overall, two of the most significant findings in this work are the longevity of BMEC barrier function and relative barrier properties compared with the non-BBB controls, particularly with respect to permeability of low molecular weight compounds. We note that relative to μVas, iPSC-derived BMECs were significantly less permeable to fluorescein, which is an important small-molecule tracer for qualifying BBB integrity. The absolute permeability of fluorescein is similar to results obtained from measurements in 2D Transwell platforms (10−6–10−7 cm/s) (Hollmann et al., 2017), indicating consistency in BMEC performance and robustness of the paracellular barrier in 3D culture. Meanwhile, the exclusion of albumin and decreased CAV1 expression suggest reduced vesicular transport in BMECs relative to non-BBB controls.
We further note that perfusion of BMEC-lined channels under low shear conditions has a stabilizing effect on barrier integrity over time compared with non-perfused controls, with similar performance in barrier function in BMECs derived from two separate iPSC lines. Our data indicate that perfusion helps maintain long-term barrier function through a combined effect of shear-induced mechanical cues and continual medium circulation providing improved nutrient/waste exchange. Recently, iPSC-derived BMECs co-cultured with astrocytes in perfused microfluidic channels were reported to maintain in vivo-like TEER values for 12 days, whereby the authors concluded that shear forces were not essential for the establishment of strong intercellular junctions but did provide a clear stabilizing effect upon barrier integrity over time (Wang et al., 2017), which is consistent with our findings. Another group has reported that shear forces are non-requisite for tight junction formation in iPSC-derived BMEC monolayers, but that shear positively contributes to barrier health by providing necessary mechanical cues as well as reducing reactive oxygen species-mediated degradation (DeStefano et al., 2017, Rochfort et al., 2015). In our 3D system, increased shear forces above our initially tested values did not strengthen or stabilize barrier function. However, these measurements are complicated by the observation of increased angiogenic sprouting at earlier time points associated with higher flow rates, and the cytoskeletal restructuring of angiogenic sprouting is known to increase the permeability of brain endothelium (Kutys and Chen, 2016). It is likely that the increase in interstitial flow associated with higher perfusion rates resulted in increased sprouting in BMEC monolayers, a process normally inhibited by interactions with surrounding smooth muscle cells and astrocytes (Galie et al., 2014, Partyka et al., 2017). Thus, we hypothesize that incorporation of additional NVU cell types will further stabilize barrier function by preventing angiogenic sprouting, which will be examined in future studies.
Interestingly, despite robust barrier function and expression of junction-associated VE-cadherin, claudin-5, and occludin protein, our global expression data indicate low mRNA transcript abundance for both endothelial and BBB-associated genes. Transcript levels in iPSC-derived BMECs cultured for 1 day under static conditions were similar to iPSC-derived BMECs cultured in 2D well plates (Qian et al., 2017), suggesting consistency with other models. We note that transcriptional profiling has been performed on both mouse and human brain endothelial cells, and for canonical BBB/endothelial genes (e.g., OCLN, CLDN5, CDH5, PECAM1, SLC2A1, MFSD2A, ABCB1), expression levels in mouse are consistently ∼10- to ∼100-fold higher than in human (Zhang et al., 2016). At present, it is unclear whether low transcript abundance in iPSC-derived BMEC models reflects in vitro culture conditions or a species difference, and more investigations are required in this area. We also note that despite overall similarity between day-1 static cultures and day-14 perfusion cultures, endothelial-specific genes become noticeably downregulated at the later time point. Thus, although perfusion and shear stress may help stabilize barrier function through upregulation of currently unknown signaling pathways, these factors potentially have a lower influence on maintenance of vascular identity. We hypothesize that inclusion of pericytes and future optimization of media composition will help promote maintenance of endothelial gene signatures at these later time points. These investigations and more in-depth analyses of transcript data are expected to identify more explicit differences between 2D and 3D cultures of iPSC-derived BMECs, similar to other studies (Zhang et al., 2017), as well as possible human-specific BBB gene expression signatures.
Overall, we highlight the utility of our 3D model for exploring interactions between NVU cell types, modeling neurovascular disease, and assessing treatment strategies. Since we have used gelatin with a benign enzymatic crosslinking approach compatible with cell encapsulation (Lee et al., 2016), we expect to be able to establish co-cultures that represent endogenous organization of NVU cell types. Given advancements in the production of neurovascular cells from iPSCs, including diverse subtypes of neurons, astrocytes, oligodendrocytes, microglia, pericytes, and smooth muscle, a fully isogenic 3D model is a realistic possibility in the near future. Also, given prominent vascular contributions to neurodegenerative diseases (Sweeney et al., 2018), future iterations of this biomimetic 3D NVU model could provide a useful platform for modeling disease phenotypes. Lastly, although this model is not expected to replace standard Transwell setups for screening prospective therapeutic compounds for BBB permeation, the performance of lead candidates could be further tested in these 3D NVU systems, where a more physiological microenvironment is expected to improve predictions of therapeutic efficacy.
Experimental Procedures
Cell Culture
IMR90-4 (Yu et al., 2007) and CC3 (Kumar et al., 2014) iPSCs were maintained on growth factor-reduced Matrigel (VWR) in E8 medium (produced in-house as previously described) (Hollmann et al., 2017). iPSCs were passaged every 3–4 days with Versene (Thermo Fisher) as previously described. Differentiation to BMECs was conducted as previously described. In brief, iPSCs were passaged to single cells with Accutase (Thermo Fisher) and seeded overnight on Matrigel at a density of 12,500–15,800 cells/cm2 in E8 medium containing 10 μM Y27632 (Tocris). The following day, cells were fed with E6 medium (produced in-house as previously described) (Hollmann et al., 2017), and medium was changed every day thereafter. On day 4, cells were switched to human endothelial serum-free medium (hESFM; Thermo Fisher) containing 1% platelet-poor plasma-derived serum (PDS; Alfa Aesar), 20 ng/mL basic fibroblast growth factor (bFGF; Peprotech), collectively referred to as EC culture medium, and 10 μM all-trans RA (Sigma). On day 6, cultures were collected with Accutase and either frozen in 60% EC culture medium, 10% DMSO (Sigma), 30% fetal bovine serum (FBS; Thermo Fisher), and 10 μM Y27632 as previously described (Wilson et al., 2016), or seeded directly into scaffolds. HUVECs and μVas (Angioproteomie) were cultured in DMEM/F12 medium (Thermo Fisher) supplemented with 5% FBS (Gibco), 10 mM L-glutamine (Corning), 50 μg/mL ascorbic acid (Thermo Fisher), 0.75 U/mL heparin (Thermo Fisher), 15 ng/mL insulin growth factor 1, 5 ng/mL vascular endothelial growth factor, 5 ng/mL bFGF, and 5 ng/mL epidermal growth factor (all from Peprotech).
Scaffold Fabrication
External support frames for gelatin scaffolds were generated from thin slabs of PDMS cast in small Peel-A-Way embedding molds (Fisher Scientific) transected by two intersecting needles (gauge 23 and 16, BD Biosciences). After removing the central section and bonding to a thin PDMS film base, a barbed luer fitting (Cole Parmer) was attached to the inlet to facilitate easy integration with the fluidic apparatus. Tubing (1/16th inch outer diameter, VWR) threaded through the barbed inlet served as the channel mold (Figure 1A). PDMS frames (5 × 5 × 2 mm) were washed and sterilized in 70% ethanol with sonication. Sterile solution of 10% (w/v) porcine gelatin (300 bloom, Sigma) combined 10:1 (v/v) with 10% (w/v) microbial transglutaminase (Modernist Pantry) was poured into the assembled frames and allowed to polymerize in a 37°C incubator for 4 h. The channel was formed by removal of tubing and was coated with 0.4 mg/mL collagen IV (Sigma) and 0.1 mg/mL fibronectin (Sigma) and conditioned in complete medium overnight before seeding channels with 3 × 106 cells/mL of either iPSC-derived BMECs, RFP-expressing HUVECs, or μVas. The approximate volume of the channel (∼800 μm × 5 mm) is 2.5 μL.
Cell Seeding
IMR90-4-derived BMECs were reconstituted from frozen stock, whereas CC3-derived BMECs, HUVECs, and μVas were seeded from live cultures. For quality control, TEER was measured across BMECs seeded concurrently in Transwell filters and was consistent with previous publications. Cells were suspended at 3 × 106 cells/mL in their standard medium and pipetted through the inlet fitting to coat the bottom half of a channel. Cells were incubated for either 4 h or overnight at 37°C to facilitate attachment. The process was then repeated to seed the top half of the channel. Cells were stained with 2 μM Calcein AM (Life Technologies) and imaged by confocal microscopy (LSM 710, Zeiss) to confirm a confluent cell layer throughout channel before connecting to perfusion (Figure 1C).
Scaffold Culture and Perfusion
On experiment day 0, hydrogels were placed in a Petri dish and either remained in static culture or were connected to a perfusion system (Figure 1B). Under both conditions, the hydrogels are submerged in ∼40–45 mL of medium. Based on the design of the PDMS frame, the upper surface of each hydrogel is in direct contact with the medium. For perfusion, a custom peristaltic pump (O'Grady et al., 2018) circulated culture medium through the channel at 100 μL/min. For samples perfused at rates greater than 100 μL/min, the flow rate was increased to the indicated value following an initial 24-h perfusion at 100 μL/min. As seen in Figure 1B, medium is extracted through the open tube not connected to the hydrogel, circulated through the hydrogel, and expelled back into the bulk reservoir. HUVECs were cultured in the same medium described above, whereas BMECs and μVas were both cultured in hESFM containing 1% PDS, 10 μM RA, and 10 μM Y27632. The medium was unchanged throughout the course of each experiment. Components used to construct the perfusion system are described in Figure S5.
Permeability Measurements
Permeability was measured by imaging diffusion of 2 μM sodium fluorescein (Sigma), 12.5 μg/mL 3 kDa AF680-conjugated dextran (Thermo Fisher), and 80 μg/mL Texas red-conjugated albumin (Thermo Fisher) across cell monolayers. During the course of the imaging experiment, channels were perfused at a rate of 30 μL/min for 2 h while obtaining fluorescence images every 30 s using the LSM 710 confocal microscope with pinhole set to 1 Airy unit. The resulting image intensity profiles were processed in FIJI (Schindelin et al., 2012) using a custom macro to automate extraction of fluorescence intensity values of the channel region, the diffusion region between channel and edge, and the edge of the gel. These values were then imported into a MATLAB (Mathworks) script to calculate K (cm s−1), the permeability of gelatin scaffold and cell layer combined, as detailed in Supplemental Experimental Procedures and Figure S6.
Efflux Transporter Activity Assays
Pgp activity was assessed by measuring accumulation of fluorescent dye, rhodamine 123, in samples pre-incubated with and without Pgp-specific inhibitor, cyclosporin A. MRP activity was assessed by measuring fluorescence accumulation of fluorescent dye H2DCFDA, in samples pre-incubated with and without MRP-specific inhibitor, MK-571. Specifically, non-inhibited controls were evaluated by perfusion (30 μL/min) with medium supplemented with 10 μM rhodamine 123 (Thermo Fisher) or 10 μM H2DCFDA (Thermo Fisher) and 12.5 μg/mL 3 kDa dextran. Inhibited samples were pre-incubated in medium containing 10 μM cyclosporin A (Tocris) or 10 μM MK-571 (Tocris) for 1 h, then similarly perfused with the medium supplemented with 12.5 μg/mL 3 kDa dextran and appropriate inhibitor/dye cocktail (Pgp: 10 μM cyclosporin A and 10 μM rhodamine 123; MRP: 10 μM MK-571 and 10 μM H2DCFDA). Confocal z stacks were obtained at 0 and 60 min of perfusion. For each sample, the change in intracellular fluorescence intensity was determined using FIJI by adding the cellular fluorescence from each z stack, then subtracting the cumulative fluorescence intensity of the initial z stack from the final z stack (ΔI = Ifinal − Iinitial). The percent increase in fluorescence was calculated using the formula
where ΔIinhibited is the change in fluorescence calculated from channels exposed to cyclosporin A and ΔInon-inhibited is the change in fluorescence calculated from channels not exposed to inhibitor.
Author Contributions
S.L.F., E.H.N., E.S.L., and L.M.B. designed experiments. S.L.F., E.H.N., A.M.B., C.M.W., K.M.B., and J.X.W. conducted/assisted with experiments and/or reagent preparation. All authors read and approved the manuscript.
Acknowledgments
Funding for this research was provided by NIH 4R00EB013630 (NIBIB) (L.M.B.), NSF BMAT 1506717 (L.M.B.), a NARSAD Young Investigator Award from the Brain and Behavior Research Foundation (E.S.L.), NSF CBET 1706155 (E.S.L.), and the donors of Alzheimer's Disease Research, a program of the BrightFocus Foundation (grant A20170945 to E.S.L.). E.H.N. was supported by a National Science Foundation Graduate Research Fellowship. The authors gratefully acknowledge the SyBBURE Searle Undergraduate Research Program supporting C.M.W. and J.X.W.
Published: February 14, 2019
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, six figures, two appendices, one table, and four videos and can be found with this article online at https://doi.org/10.1016/j.stemcr.2019.01.009.
Contributor Information
Ethan S. Lippmann, Email: ethan.s.lippmann@vanderbilt.edu.
Leon M. Bellan, Email: leon.bellan@vanderbilt.edu.
Accession Numbers
The RNA-sequencing data referenced in this paper were submitted to GEO under accession number GEO: GSE122588.
Supplemental Information
References
- Appelt-Menzel A., Cubukova A., Günther K., Edenhofer F., Piontek J., Krause G., Stüber T., Walles H., Neuhaus W., Metzger M. Establishment of a Human blood-brain barrier co-culture model mimicking the neurovascular unit using induced pluri- and multipotent stem cells. Stem Cell Reports. 2017;8:894–906. doi: 10.1016/j.stemcr.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry C., Schmitz M.T., Propson N.E., Hou Z., Zhang J., Nguyen B.K., Bolin J.M., Jiang P., McIntosh B.E., Probasco M.D. Uniform neural tissue models produced on synthetic hydrogels using standard culture techniques. Exp. Biol. Med. 2017;242:1679–1689. doi: 10.1177/1535370217715028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Zvi A., Lacoste B., Kur E., Andreone B.J., Mayshar Y., Yan H., Gu C. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature. 2014;509:507–511. doi: 10.1038/nature13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertassoni L.E., Cecconi M., Manoharan V., Nikkhah M., Hjortnaes J., Cristino A.L., Barabaschi G., Demarchi D., Dokmeci M.R., Yang Y. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip. 2014;14:2202–2211. doi: 10.1039/c4lc00030g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozza A., Coates E.E., Incitti T., Ferlin K.M., Messina A., Menna E., Bozzi Y., Fisher J.P., Casarosa S. Neural differentiation of pluripotent cells in 3D alginate-based cultures. Biomaterials. 2014;35:4636–4645. doi: 10.1016/j.biomaterials.2014.02.039. [DOI] [PubMed] [Google Scholar]
- Brown J.A., Pensabene V., Markov D.A., Allwardt V., Neely M.D., Shi M., Britt C.M., Hoilett O.S., Yang Q., Brewer B.M. Recreating blood-brain barrier physiology and structure on chip: a novel neurovascular microfluidic bioreactor. Biomicrofluidics. 2015;9:054124. doi: 10.1063/1.4934713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabodi M., Choi N.W., Gleghorn J.P., Lee C.S.D., Bonassar L.J., Stroock A.D. A microfluidic biomaterial. J. Am. Chem. Soc. 2005;127:13788–13789. doi: 10.1021/ja054820t. [DOI] [PubMed] [Google Scholar]
- Cheng C., Helderman F., Tempel D., Segers D., Hierck B., Poelmann R., van Tol A., Duncker D.J., Robbers-Visser D., Ursem N.T.C. Large variations in absolute wall shear stress levels within one species and between species. Atherosclerosis. 2007;195:225–235. doi: 10.1016/j.atherosclerosis.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Cho H., Seo J.H., Wong K.H.K., Terasaki Y., Park J., Bong K., Arai K., Lo E.H., Irimia D. Three-dimensional blood-brain barrier model for in vitro studies of neurovascular pathology. Sci. Rep. 2015;5:15222. doi: 10.1038/srep15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordon-Cardo C., O’Brien J.P., Casals D., Rittman-Grauer L., Biedler J.L., Melamed M.R., Bertino J.R. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc. Natl. Acad. Sci. U S A. 1989;86:695–698. doi: 10.1073/pnas.86.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucullo L., Couraud P.-O., Weksler B., Romero I.-A., Hossain M., Rapp E., Janigro D. Immortalized human brain endothelial cells and flow-based vascular modeling: a marriage of convenience for rational neurovascular studies. J. Cereb. Blood Flow Metab. 2008;28:312–328. doi: 10.1038/sj.jcbfm.9600525. [DOI] [PubMed] [Google Scholar]
- Daneman R., Prat A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015;7:a020412. doi: 10.1101/cshperspect.a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deo A.K., Theil F.-P., Nicolas J.-M. Confounding parameters in preclinical assessment of blood-brain barrier permeation: an overview with emphasis on species differences and effect of disease states. Mol. Pharm. 2013;10:1581–1595. doi: 10.1021/mp300570z. [DOI] [PubMed] [Google Scholar]
- DeStefano J.G., Xu Z.S., Williams A.J., Yimam N., Searson P.C. Effect of shear stress on iPSC-derived human brain microvascular endothelial cells (dhBMECs) Fluids Barriers CNS. 2017;14:20. doi: 10.1186/s12987-017-0068-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galie P.A., Nguyen D.-H.T., Choi C.K., Cohen D.M., Janmey P.A., Chen C.S. Fluid shear stress threshold regulates angiogenic sprouting. Proc. Natl. Acad. Sci. U S A. 2014;111:7968–7973. doi: 10.1073/pnas.1310842111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Polite F., Martorell J., Del Rey-Puech P., Melgar-Lesmes P., O’Brien C.C., Roquer J., Ois A., Principe A., Edelman E.R., Balcells M. Pulsatility and high shear stress deteriorate barrier phenotype in brain microvascular endothelium. J. Cereb. Blood Flow Metab. 2017;37:2614–2625. doi: 10.1177/0271678X16672482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden A.P., Tien J. Fabrication of microfluidic hydrogels using molded gelatin as a sacrificial element. Lab Chip. 2007;7:720–725. doi: 10.1039/b618409j. [DOI] [PubMed] [Google Scholar]
- Helms H.C., Abbott N.J., Burek M., Cecchelli R., Couraud P.-O., Deli M.A., Förster C., Galla H.J., Romero I.A., Shusta E.V. In vitro models of the blood-brain barrier: an overview of commonly used brain endothelial cell culture models and guidelines for their use. J. Cereb. Blood Flow Metab. 2016;36:862–890. doi: 10.1177/0271678X16630991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann E.K., Bailey A.K., Potharazu A.V., Neely M.D., Bowman A.B., Lippmann E.S. Accelerated differentiation of human induced pluripotent stem cells to blood-brain barrier endothelial cells. Fluids Barriers CNS. 2017;14:9. doi: 10.1186/s12987-017-0059-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh D., Hamilton G.A., Ingber D.E. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011;21:745–754. doi: 10.1016/j.tcb.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram P.N., Hind L.E., Jiminez-Torres J.A., Huttenlocher A., Beebe D.J. An accessible organotypic microvessel model using iPSC-derived endothelium. Adv. Healthc. Mater. 2016;7:1700497. doi: 10.1002/adhm.201700497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Torres J.A., Peery S.L., Sung K.E., Beebe D.J. LumeNEXT: a practical method to pattern luminal structures in ECM gels. Adv. Healthc. Mater. 2016;5:198–204. doi: 10.1002/adhm.201500608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katt M.E., Xu Z.S., Gerecht S., Searson P.C. Human brain microvascular endothelial cells derived from the BC1 iPS cell line exhibit a blood-brain barrier phenotype. PLoS One. 2016;11:e0152105. doi: 10.1371/journal.pone.0152105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Lee H., Chung M., Li Jeon N. Engineering of functional, perfusable 3D microvascular networks on a chip. Lab Chip. 2013;13:1489–1500. doi: 10.1039/c3lc41320a. [DOI] [PubMed] [Google Scholar]
- Kluger M.S., Clark P.R., Tellides G., Gerke V., Pober J.S. Claudin-5 controls intercellular barriers of human dermal microvascular but not human umbilical vein endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2013;33:489–500. doi: 10.1161/ATVBAHA.112.300893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowland D., Arac A., Sekiguchi K.J., Hsu M., Lutz S.E., Perrino J., Steinberg G.K., Barres B.A., Nimmerjahn A., Agalliu D. Stepwise recruitment of transcellular and paracellular pathways underlies blood-brain barrier breakdown in stroke. Neuron. 2014;82:603–617. doi: 10.1016/j.neuron.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesky D.B., Truby R.L., Gladman A.S., Busbee T.A., Homan K.A., Lewis J.A. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv. Mater. 2014;26:3124–3130. doi: 10.1002/adma.201305506. [DOI] [PubMed] [Google Scholar]
- Koutsiaris A.G., Tachmitzi S.V., Batis N. Wall shear stress quantification in the human conjunctival pre-capillary arterioles in vivo. Microvasc. Res. 2013;85:34–39. doi: 10.1016/j.mvr.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Kumar K.K., Lowe E.W., Aboud A.A., Neely M.D., Redha R., Bauer J.A., Odak M., Weaver C.D., Meiler J., Aschner M. Cellular manganese content is developmentally regulated in human dopaminergic neurons. Sci. Rep. 2014;4:6801. doi: 10.1038/srep06801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutys M.L., Chen C.S. Forces and mechanotransduction in 3D vascular biology. Curr. Opin. Cell Biol. 2016;42:73–79. doi: 10.1016/j.ceb.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.B., Wang X., Faley S., Baer B., Balikov D.A., Sung H.-J., Bellan L.M. Development of 3D microvascular networks within gelatin hydrogels using thermoresponsive sacrificial microfibers. Adv. Healthc. Mater. 2016;5:781–785. doi: 10.1002/adhm.201500792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippmann E.S., Azarin S.M., Kay J.E., Nessler R.A., Wilson H.K., Al-Ahmad A., Palecek S.P., Shusta E.V. Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells. Nat. Biotechnol. 2012;30:783–791. doi: 10.1038/nbt.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippmann E.S., Al-Ahmad A., Azarin S.M., Palecek S.P., Shusta E.V. A retinoic acid-enhanced, multicellular human blood-brain barrier model derived from stem cell sources. Sci. Rep. 2014;4:4160. doi: 10.1038/srep04160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markov D.A., Lu J.Q., Samson P.C., Wikswo J.P., McCawley L.J. Thick-tissue bioreactor as a platform for long-term organotypic culture and drug delivery. Lab Chip. 2012;12:4560–4568. doi: 10.1039/c2lc40304h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J.S., Stevens K.R., Yang M.T., Baker B.M., Nguyen D.-H.T., Cohen D.M., Toro E., Chen A.A., Galie P.A., Yu X. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 2012;11:768–774. doi: 10.1038/nmat3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta T., Hata M., Gotoh S., Seo Y., Sasaki H., Hashimoto N., Furuse M., Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J. Cell Biol. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Grady B., Wang J., Faley S., Balikov D., Lippmann E., Bellan L.M. A customizable, low-cost perfusion system for sustaining tissue constructs. SLAS Technol. 2018;23:592–598. doi: 10.1177/2472630318775059. [DOI] [PubMed] [Google Scholar]
- Partyka P.P., Godsey G.A., Galie J.R., Kosciuk M.C., Acharya N.K., Nagele R.G., Galie P.A. Mechanical stress regulates transport in a compliant 3D model of the blood-brain barrier. Biomaterials. 2017;115:30–39. doi: 10.1016/j.biomaterials.2016.11.012. [DOI] [PubMed] [Google Scholar]
- Pellett S., Schwartz M.P., Tepp W.H., Josephson R., Scherf J.M., Pier C.L., Thomson J.A., Murphy W.L., Johnson E.A. Human induced pluripotent stem cell derived neuronal cells cultured on chemically-defined hydrogels for sensitive in vitro detection of Botulinum Neurotoxin. Sci. Rep. 2015;5:14566. doi: 10.1038/srep14566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan D.T.T., Wang X., Craver B.M., Sobrino A., Zhao D., Chen J.C., Lee L.Y.N., George S.C., Lee A.P., Hughes C.C.W. A vascularized and perfused organ-on-a-chip platform for large-scale drug screening applications. Lab Chip. 2017;17:511–520. doi: 10.1039/c6lc01422d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakarpandian B., Shen M.-C., Nichols J.B., Mills I.R., Sidoryk-Wegrzynowicz M., Aschner M., Pant K. SyM-BBB: a microfluidic blood brain barrier model. Lab Chip. 2013;13:1093–1101. doi: 10.1039/c2lc41208j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad S., Sajja R.K., Naik P., Cucullo L. Diabetes mellitus and blood-brain barrier dysfunction: an overview. J. Pharmacovigil. 2014;2:125. doi: 10.4172/2329-6887.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian T., Maguire S.E., Canfield S.G., Bao X., Olson W.R., Shusta E.V., Palecek S.P. Directed differentiation of human pluripotent stem cells to blood-brain barrier endothelial cells. Sci. Adv. 2017;3:e1701679. doi: 10.1126/sciadv.1701679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi M., Paramesh V., Kaviya S.R., Anuradha E., Solomon F.D.P. 3D cell culture systems: advantages and applications. J. Cell. Physiol. 2015;230:16–26. doi: 10.1002/jcp.24683. [DOI] [PubMed] [Google Scholar]
- Reinitz A., DeStefano J., Ye M., Wong A.D., Searson P.C. Human brain microvascular endothelial cells resist elongation due to shear stress. Microvasc. Res. 2015;99:8–18. doi: 10.1016/j.mvr.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochfort K.D., Collins L.E., McLoughlin A., Cummins P.M. Shear-dependent attenuation of cellular ROS levels can suppress proinflammatory cytokine injury to human brain microvascular endothelial barrier properties. J. Cereb. Blood Flow Metab. 2015;35:1648–1656. doi: 10.1038/jcbfm.2015.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney M.D., Sagare A.P., Zlokovic B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018;14:133–150. doi: 10.1038/nrneurol.2017.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbitt M.W., Anseth K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009;103:655–663. doi: 10.1002/bit.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.I., Abaci H.E., Shuler M.L. Microfluidic blood–brain barrier model provides in vivo-like barrier properties for drug permeability screening. Biotechnol. Bioeng. 2017;114:184–194. doi: 10.1002/bit.26045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weksler B.B., Subileau E.A., Perrière N., Charneau P., Holloway K., Leveque M., Tricoire-Leignel H., Nicotra A., Bourdoulous S., Turowski P. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 2005;19:1872–1874. doi: 10.1096/fj.04-3458fje. [DOI] [PubMed] [Google Scholar]
- Wikswo J.P. The relevance and potential roles of microphysiological systems in biology and medicine. Exp. Biol. Med. 2014;239:1061–1072. doi: 10.1177/1535370214542068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson H.K., Canfield S.G., Hjortness M.K., Palecek S.P., Shusta E.V. Exploring the effects of cell seeding density on the differentiation of human pluripotent stem cells to brain microvascular endothelial cells. Fluids Barriers CNS. 2015;12:13. doi: 10.1186/s12987-015-0007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson H.K., Faubion M.G., Hjortness M.K., Palecek S.P., Shusta E.V. Cryopreservation of brain endothelial cells derived from human induced pluripotent stem cells is enhanced by rho-associated coiled coil-containing kinase inhibition. Tissue Eng. Part C Methods. 2016;22:1085–1094. doi: 10.1089/ten.tec.2016.0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M., Sanchez H.M., Hultz M., Yang Z., Bogorad M., Wong A.D., Searson P.C. Brain microvascular endothelial cells resist elongation due to curvature and shear stress. Sci. Rep. 2014;4:4681. doi: 10.1038/srep04681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Vodyanik M.A., Smuga-Otto K., Antosiewicz-Bourget J., Frane J.L., Tian S., Nie J., Jonsdottir G.A., Ruotti V., Stewart R. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Sloan S.A., Clarke L.E., Caneda C., Plaza C.A., Blumenthal P.D., Vogel H., Steinberg G.K., Edwards M.S.B., Li G. Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron. 2016;89:37–53. doi: 10.1016/j.neuron.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Schwartz M.P., Hou Z., Bai Y., Ardalani H., Swanson S., Steill J., Ruotti V., Elwell A., Nguyen B.K. A genome-wide analysis of human pluripotent stem cell-derived endothelial cells in 2D or 3D culture. Stem Cell Reports. 2017;8:907–918. doi: 10.1016/j.stemcr.2017.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Chen J., Craven M., Choi N.W., Totorica S., Diaz-Santana A., Kermani P., Hempstead B., Fischbach-Teschl C., López J.A. In vitro microvessels for the study of angiogenesis and thrombosis. Proc. Natl. Acad. Sci. U S A. 2012;109:9342–9347. doi: 10.1073/pnas.1201240109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.