Abstract
Ikuko Yuyama, Takashi Nakamura, Tomihiko Higuchi, and Michio Hidaka (2016) Reef-building corals are often associated with multiple clades of symbiotic dinoflagellate Symbiodinium spp., where the relative composition of Symbiodinium can alter the phylogenetic properties (e.g., stress responsiveness, growth rate) of the host coral. The genus Symbiodinium contains nine clades, some of which behave differently in response to strong light and/or temperature stresses, for example, clade D Symbiodinium are thermally tolerant. However, previous studies are based on corals present in the field, and it is possible that the corals used in previous experiments did not contain single Symbiodinium clades. For an accurate assessment of the effects of each Symbiodinium clade on host thermal stress resistance, monoclonal cultures of clades C1 and D were inoculated into aposymbiotic juvenile polyps. Photosynthetic efficiency (maximum quantum yield: Fv/Fm) showed a decline at 30°C than at 25°C in both clades. Symbiodinium clade C1 showed a consistently higher rETRmax with larger fluctuations than clade D, with a lower survival rate of juveniles during thermal stress treatment. Under strong light exposure, corals containing clade C1 showed a greater decline in Fv/Fm (-74%), compared to decline in corals associated with clade D (-50%) after 3 hours. This is the first study to assess stress tolerances of juvenile corals in association with the monoclonal Symbiodinium clades C and D, and our results indicated greater tolerance of corals associated with clade D to strong light (500 μmol m-2 s-1). However, it is difficult to determine the impact of high-temperature stress on coral-algae symbiosis from photosynthetic activity. At high temperatures, clade C1 Symbiodinium exhibited high photosynthetic activity, but host survival rates were higher in corals associated with clade D Symbiodinium. Since clade C1 has a relatively high photosynthetic activity under high temperatures, clade C1 symbiosis at high temperatures might have a negative impact on corals compared with clade D.
Keywords: Endosymbiosis, PAM, Acropora, Symbodinium, Clade C1, Clade D
BACKGROUND
Corals can suffer from high temperature stress that often triggers a collapse of the relationship with their symbiotic dinoflagellate algae, composed of the genus Symbiodinium. This breakdown is known as “coral bleaching”, in which corals suffer a considerable loss of numbers and quality of their Symbiodinium. The response to bleaching stress is accompanied by a steep reduction in photosynthetic efficiency of the endosymbiotic algae; known as “photoinhibition” of photosynthesis (Warner et al. 1999; Takahashi et al. 2004). Even in the absence of elevated temperature stress, high irradiance can induce the production of reactive oxygen species, which in turn cause critical damage to several cellular targets, including Photosystem II (PSII). When PSII is severely damaged, photosynthetic efficiency is downregulated (Gorbunov et al. 2001; Lesser and Shick 1989), and high temperature conditions inhibit the repair of the damaged PSII at the site of de novo synthesis of the photosystem II reaction center (D1 protein), resulting in increased damage to the photosynthetic abilities of the symbionts (Takahashi et al. 2004). On the other hand, corals have anti-stress defenses, including antioxidant enzymes to avoid bleaching (Lesser 1997); mycosporine-like amino acids (MAAs) can act as a sunscreen against ultraviolet radiation (Dunlap and Shick 1998). These defenses are particularly critical for juvenile corals in subtropical to tempe- rate regions where juvenile corals must grow during the summer season under high temperature stress.
Genetic differences exist among the Symbidodinium, which are currently classified into nine clades (A-I) containing multiple sub-clades (Baker 2003; Coffroth and Santos 2005; Pochon and Gates 2010) that influence the susceptibility of corals to bleaching (Baker et al. 2004). Symbiodinium clade C is the most commonly found in corals and has the highest genetic diversity (Coffroth and Santos 2005), whereas clade D is often found in corals after bleaching events (Jones et al. 2008). Relative abundance of clade D tends to be higher in corals that experienced unusually high temperatures (Baker et al. 2013; Stat et al. 2013). Several studies have reported seasonal changes in the composition of Symbiodinium clades in several coral species (Chen et al. 2005; Thornhill et al. 2006; Suwa et al. 2008). This shuffling of the dominant Symbiodinium clade is thought to be an adaptation mechanism to high temperature conditions (Berkelmans and van Oppen 2006).
Symbiodinium clade D has been reported to be more tolerant of stress compared with clade C. In pocilloporid corals, a temperature of 32°C decreased Fv/Fm in clade C associations, but this increased in clade D associations (Rowan 2004). Berkelmans and van Oppen (2006) reported that an adult colony of Acropora millepora with a sub- clade C2 symbiont bleached after 15 days at 31°C, whereas transplants with D-type symbionts were all healthy. Juveniles of A. millepora associating with sub-clades C1 or D were also used in a stress experiment; at 32.5°C there was an earlier and stronger reduction in Fv/Fm for corals containing C1 than for those with D (Mieog et al. 2009). On the other hand, clade D was reported to be more sensitive to heat stress than clade C, as Abrego et al. (2008) showed that juveniles of A. tenuis, which associated with sub-clade C1, exhibited a higher thermal tolerance than those associated with clade D. However, previous reports were based on corals present in the field, and there is the possibility that these corals did not contain a single Symbiodinium clade, but instead contained a mix of clades. The impact of monoclonal symbiont clades on host- tolerance under stress has not yet been fully investigated.
To date, no studies have compared stress responses using corals associated with monoclonal Symbiodinium of clades C and D, since it is difficult to produce corals associated with a monoclonal Symbiodinium. As mentioned above, corals in a natural habitat may contain multiple clades of Symbiodinium, and their dominant clade could have been replaced through environmental changes over time. To accurately assess the effects of each Symbiodinium clade on host thermal stress resistance, corals associated with monoclonal Symbiodinium are needed. Recently, sub-clade C1 and clade D monoclonal cultures were successfully inoculated into juvenile corals (Yuyama and Higuchi 2014), and it was possible to test whether the coral thermal stress response was directly derived from its particular clade of endosymbiotic algae. To this end, juvenile corals of A. tenuis harboring Symbioidinium clades C1 or D were incubated at two different temperatures (25°C and 30°C) to monitor the photosynthetic parameters (Fv/Fm and rETRmax), as well as the survival rate. Secondly, the independent effect of high irradiance on the photosynthetic parameter (Fv/Fm) of each endosymbiotic Symbiodinium was investigated. An understanding of the variation in the stress responses of juveniles could provide a comprehensive insight into the impacts of global warming on newly recruited corals.
MATERIALS AND METHODS
Algae and coral juveniles
The monoclonal Symbiodinium strains CCMP2466 (sub-clade C1) and CCMP 2556 (clade D) were obtained from the Bigelow Laboratory for Ocean Sciences (West Boothbay Harbor, ME, USA; https://ccmp.bigelow.org/) and cultured in IMK medium (Wako Chemicals, Osaka, Japan) at 25°C under a 12-h light (50 μmol m-2 s-1): 12-h dark cycle. Acropora tenuis larvae were collected just after spawning and fertilization at the Akajima Marine Science Laboratory (Okinawa, Japan), and were shipped to Ryukyu University. The larvae were exposed to 2 μM Hym 248 to induce metamorphosis (Iwao et al. 2002). After metamorphosis, Symbiodinium cells (approximately 2000 cells mL-1) were introduced into polyps. Juvenile polyps were cultured in plastic petri dishes (55-mm diameter) at 25°C under a 12-h light (50 μmol m-2 s-1): 12-h dark cycle. Each dish contained 10-20 juveniles in 40 mL of filtered seawater (FSW), which was renewed daily. Seawater was filtered through a membrane filter with a 0.22-μm pore size (Millipore, Billerica, MA, USA). Each container was covered with plastic wrap to prevent cross-contamination of clades and salinity changes due to evaporation during the experiment. To confirm the endosymbiotic algal clades, restriction fragment length polymorphism (RFLP) was performed using 4-month-old symbiotic corals after inoculation with Symbiodinium (Yuyama and Higuchi 2014). Symbiotic corals of approximately 4 months old after inoculation with Symbiodinium were also used in the stress experiments since these symbionts increased to the same density in this time (Yuyama and Higuchi 2014).
High temperature stress
Four months after the introduction of Symbiodinium into the juveniles, the Symbiodinium cells had spread throughout the polyps (Yuyama and Higuchi 2014). The juvenile corals were used in the thermal stress experiments in November, 2010. Juveniles were maintained in petri dishes filled with FSW (mesh size 0.22 μm). Temperature conditions were controlled by an incubator (LH- 70CCFL-CT, NK System, Tokyo, Japan) with a gradual increase in water temperature during the stress treatments (0.5°C per day) from 25- 30°C, while the control corals were maintained at 25°C (Fig. 1). The light intensity in the incubator was 60 μmol m-2 s-1. The Fv/F>m of each coral was measured at the start of the experiment. Eighteen corals with sub-clades C1 or D were divided into six containers filled with 100 mL FSW. Three replicate petri dishes containing three juvenile corals per container (a total of nine corals) were used in each treatment. The survival rate was estimated during incubation based on the presence of intact tissues (dead corals were determined by detachment of the tissue from the skeleton).
Fig. 1.
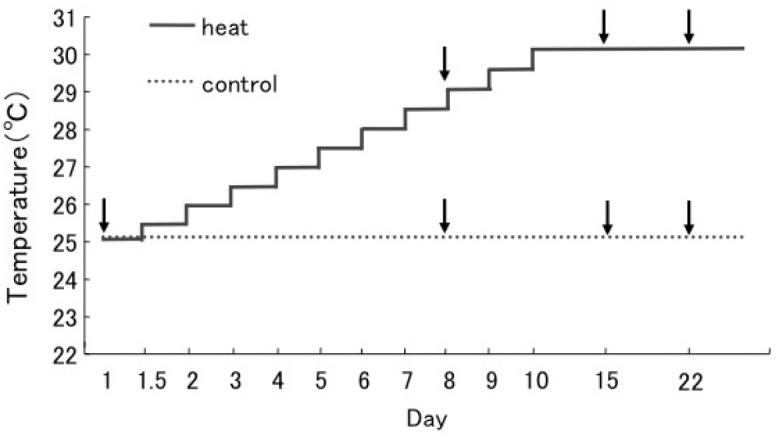
Fig. 1. Schematic representation of the experimental design. In the temperature treatment experiment, the water temperature was gradually increased from 25°C by 0.5°C daily to 30°C (approx. 30°C: the maximum water temperature around Aka Island). We have confirmed that water temperatures in containers were actually increased according to plan. The control set (...line) was maintained at 25°C throughout the experiment. Sampling times are indicated by arrows.
High irradiance stress
The light stress experiment assessed the short-term impact of strong irradiance on the photosynthetic efficiency of the endosymbiotic algae. To compare differences between two clades of symbiotic algae, 4-5 juvenile corals associated with sub-clade C1, and 5-9 corals associated with clade D were used in each treatment (no experimental replicates in this experiment). These experiments were performed in November 2013. One container filled with 100 mL of FSW was used for each treatment. Juvenile corals were exposed to three light treatments: 50 (control), 100 and 500 μmol m-2 s-1. The Fv/F>m of each coral was measured at the start of the experiment and after 3, 6, and 9 h. All light treatments took place at 25°C. The corals were illuminated (50, 100 and 500 μmol m-2 s-1) with white LED lamps PLD-60 (Tetra, Tokyo, Japan) during the experiment.
Chlorophyll fluorescence measurements
Chlorophyll α fluorescence of the in hospite Symbiodinium was measured using a Diving- PAM underwater fluorometer (Walz, Effeltrich, Germany). Dark-adapted Fv/F>m is a reliable measure of the maximum photochemical efficiency of PSII (Demmig and Björkman 1987). In this study, Fv/F>m and rETRmax were used to assess photodamage in the endosymbiotic algae. Measurements were taken on days 0, 8, 15 and 22, as shown in figure 1. All Fv/F>m measurements were performed after a 15 min dark adaptation period. Each juvenile coral was placed directly onto the PAM probe to obtain maximum fluorescence (the basal portions of the corals were attached to the surface of the PAM probe).
To determine the maximum relative electron transfer rate (rETRmax) for each sample, the rapid light curve (RLC) protocol was selected on the PAM control software (WinControl, Waltz). Samples were dark-adapted for 15 min prior to the start of the experiment then, minimum fluorescence (Fo) of the chlorophyll was determined using 3 μs pulses of a light-emitting diode (measuring light, LED, peak emission at 650 nm). The maximum fluorescence (Fm) of each dark-adapted sample was measured by a 0.8 s saturation light pulse (~4000 μmol photons m-2 s-1) right after the dark-adapt period. Fv/Fm, the ratio of variable fluorescence (Fv, where Fv = Fm - Fo) to Fm was determined as such. Subsequently, saturation light pulses were applied to obtain Fv’/Fm’ (effective quantum yield of PSII) at the end of each light exposure step (0 (dark-adapted), 75, 100, 160, 270, 350, 740, 1270 μmol Eq; 15 sec per step). In addition to pre-exposure of samples to ambient light condition prior to dark-adapt period, light- adaptation was successively achieved through the step-wise increment of actinic light intensity during the RLC measurement procedure. Fv’/Fm’ value of each light exposure step was used to calculate the rETR (effective quantum yield *PAR*0.5) to estimate the dynamic changes of rETR in response to the increasing light exposure conditions for each RLC measurement. The rETR values for each light exposure step were then fitted to form an exponential curve, following Platt et al. (1980) to calculate the rETRmax.
Statistical analysis
Physiological parameters for corals associated with clades C1 and D, including Fv/Fmand rETRmax were compared for treatments. Student’s t-test (JMP8.0; SAS Institute, Cary, NC, USA) was used for comparisons of the high temperature stress results. A two-way repeated- measures ANOVA with time and light intensity was used for the Fv/Fmdata. Post hoc differences were tested for using a Turkey-Kramer HSD (honestly significant difference) test.
RESULTS
Effect of high temperature stress on Fv/Fm
Juvenile corals hosting either sub-clade C1 (C1 coral) or clade D Symbiodinium (D coral) were incubated under different temperature regimes, and their photosynthetic efficiencies were compared. The Fv/F>m values were higher in D corals (0.464 ± 0.007, mean ± SE; n = 27) compared with C1 corals (0.409 ± 0.007; n = 27) on day 1 (Fig. 2). Corals (n = 18) were selected for further experiments and were divided into two treatment groups. The Fv/Fm values initially increased until day 8 under all conditions. At days 8 and 16, the Fv/F>m values of the C1 corals were significantly higher (p < 0.01, t-test) at 25°C than at 30°C. However, there was no difference at day 22 (0.585±0.018,n=9at25°C,0.583±0.021,n=7 at 30°C). On the other hand, D corals showed a significant difference between temperature treatments on days 15 and 22. The difference in the Fv/F>m of D corals was greater at day 22 (Fv/Fm in D coral was 0.607 ± 0.014 at 25°C, and 0.513 ± 0.014 at 30°C, n = 9)
Fig. 2.
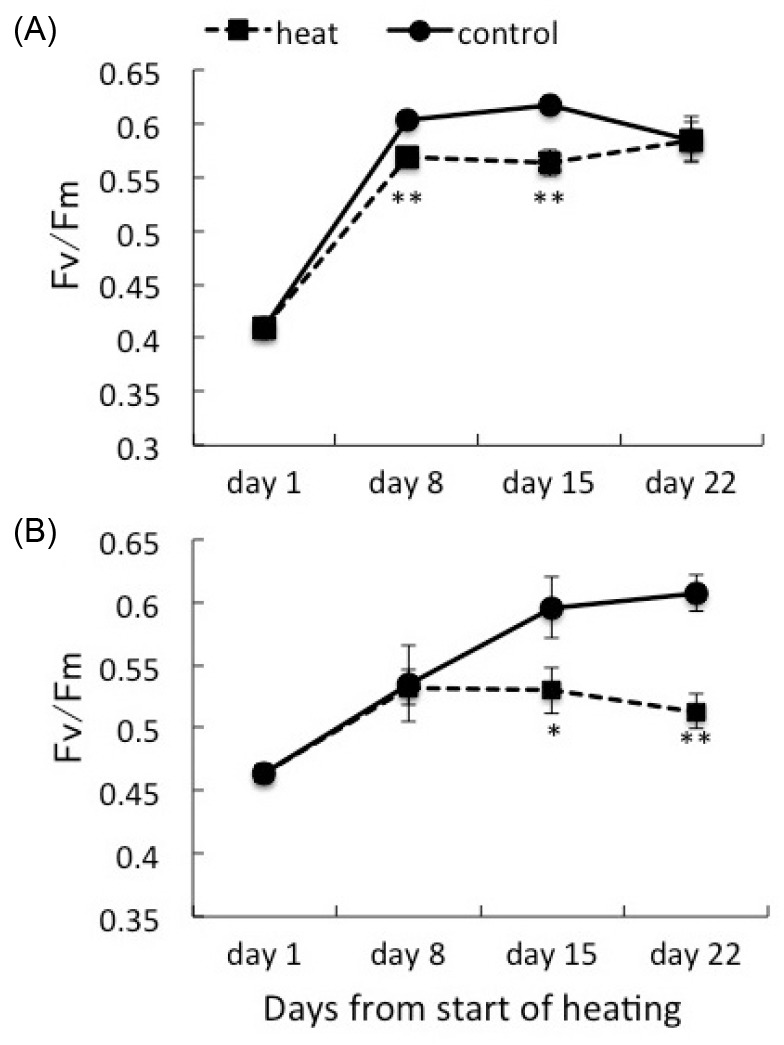
Fig. 2. Maximum quantum yield (Fv/Fm) of corals associating with Symbiodinium clade C1 (A) and corals with clade D (B) under heat treatment and the control. Values are means ± se. * p < 0.05, ** p < 0.01, t-test.
The maximum relative ETR values (rETRmax) of each coral on day 1 were 5.260 ± 0.170 (n = 27) for C1corals and 5.119 ± 0.146 (n = 27) for D corals (Fig. 3). The rETRmax value of the C1 corals increased dramatically during days1-8. The rETRmax values at 30°C were tend to higher than those at 25°C in C1 and D corals. But there was no significant difference between two treatments. C1 corals consistently maintained higher rETRmax values than D corals during the experimental period.
Fig. 3.
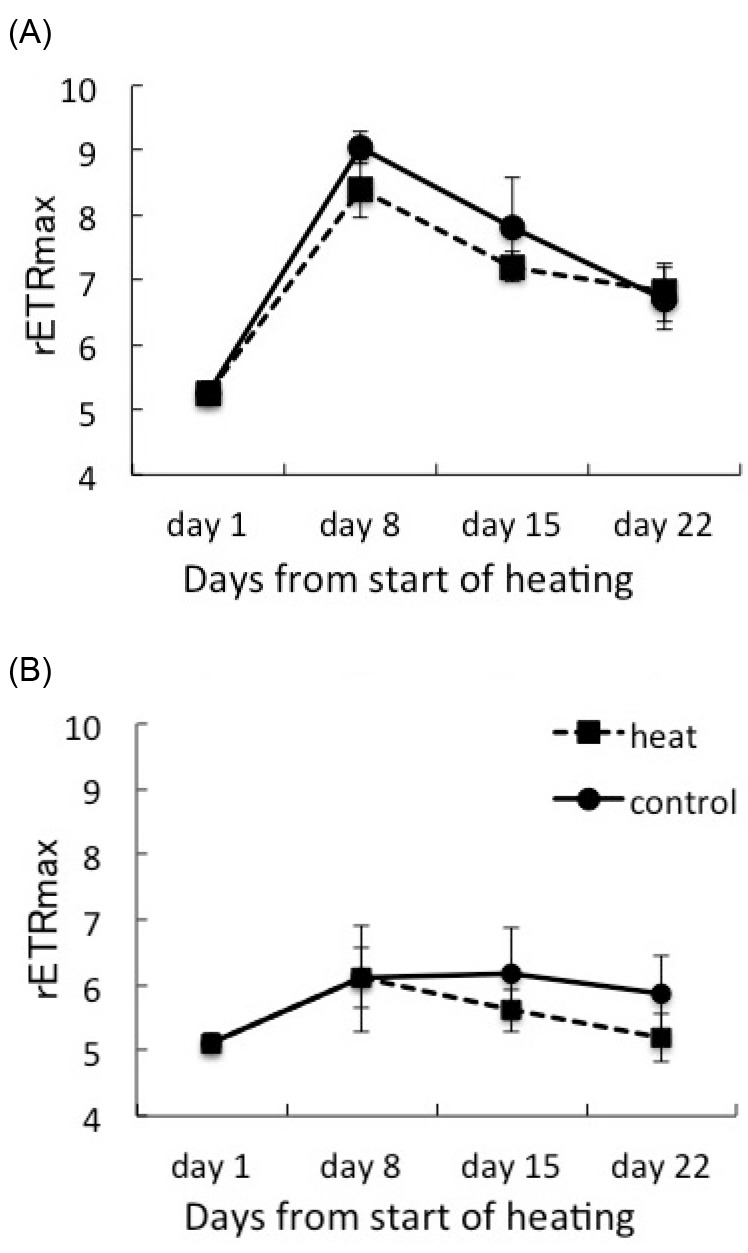
Fig. 3. Maximum relative electron transport rate (rETRmax) of corals hosting Symbiodinium clade C1 (A) and clade D (B) under heat treatment and the control. Values are means ± SE.
Survival rate during heat stress
The survival rate of D corals was 100% during the experimental period under both temperature conditions. The survival rate of C1 corals declined to 77.7% in the 30°C treatment, and all the mortality was recorded within days 1-8 (Fig. 4). No significant decline of Fv/Fmwas observed in the juvenile corals prior to their mortality.
Fig. 4.
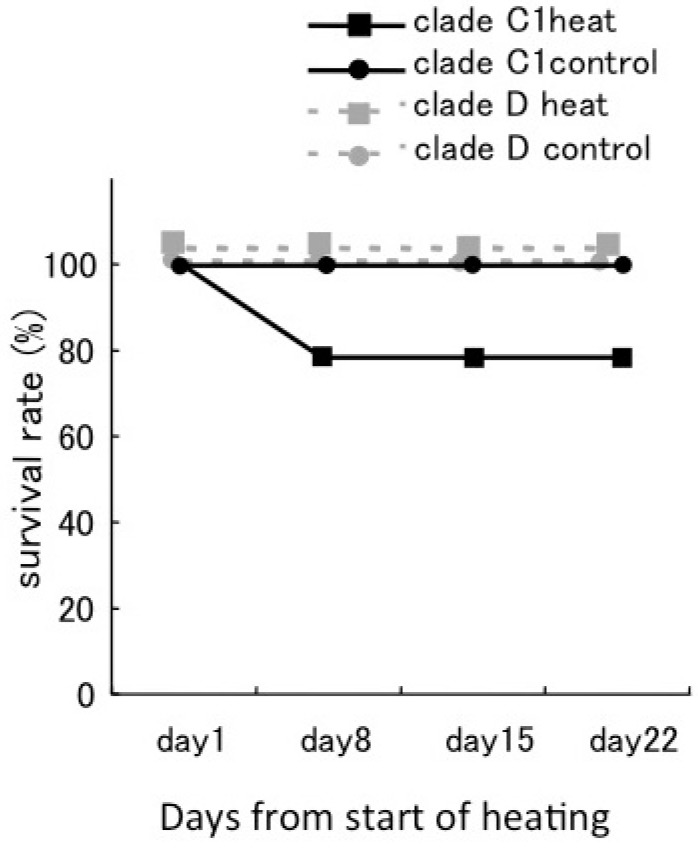
Fig. 4. Survival rate of corals during the experimental period. The survival rates of corals associating with Symbiodinium clade C1 are shown as black lines, and for those associating with clade D are shown as grey dotted lines. Nine corals were used for each treatment. The survival rate of all corals with clade D was 100%. The survival rate of corals with clade C1 decreased with increasing temperature.
Effect of strong irradiance on Fv/Fm
The Fv/Fm values of C1 corals and D corals were significantly reduced by the strongest light treatment (p < 0.01, ANOVA). A light exposure of 500 μmol m-2 s-1 induced a reduction in Fv/Fm in the juvenile corals associated with both clades within 3 h. The Fv/Fm dropped to 0.141 ± 0.041, 0.118 ± 0.029, and 0.103 ± 0.023 in C1 corals (n = 5), while it decreased to 0.300 ± 0.034, 0.347 ± 0.040 and 0.283 ± 0.035 in D corals (n = 9), after 3, 6 and 9 h, respectively. C1 corals showed a significant decline in Fv/Fm over time compared with D corals under 500 μmol m-2 s-1 (p < 0.01, HSD). Light treatments of 100 μmol m-2 s-1 and 50 μmol m-2 s-1 light did not result in significant declines of Fv/Fm in either clade.
DISCUSSION
The sensitivity of corals to heat and light stress varies depending on their associated endosymbiotic Symbiodinium clades, with clade D providing the greatest stress tolerance (Baker et al. 2004). However, many questions remain regarding the differences between associations with clades C and D. In this study, the results of survival rate indicated that D corals might have a better chance of survival compared with C1 corals under thermally stressful conditions. Our results also highlighted the dynamics of the photosynthetic efficiency (Fv/Fm) and maximum relative electron transport rate (rETRmax) of Symbiodinium clades C1 and D in hospite in the early stages of the coral life history. Differences observed between C1 and D corals indicated the different abilities of Symbiodinium in hospite to response to various temperature and light conditions.
The results showed that a gradual tempe- rature increase to 30°C resulted in 22.3% mortality of C1 corals. Dead corals were observed only during the first 8 days. During this period, the Fv/Fmof both clades gradually increased. This might have been affected by environmental changes in the pre-experiment containers, because the Fv/Fmof the control (25°C) corals also increased gradually. In hospite, clades C1 and D tended to show lower photosynthetic efficiencies at 30°C compared with 25°C. A significant difference in Fv/Fmbetween two temperature treatments was observed after 8 days for C1 corals, and after 15 days for D corals. By day 22, a significant decline due to heat stress was observed in clade D, but not in clade C1, suggesting that clade C1 had acclimated to the heat stress. While the survival rate data showed that D corals had higher resistance to thermal stress, Fv/Fmdata suggested that the clade C1 symbiont had higher stress resistance than the clade D symbiont. Other studies showed that colonies that predominantly contained clade C symbionts suffered high mortality from coral bleaching compared to clade D-predominant colonies (Baker et al. 2004; Jones et al. 2008), and our results in the juveniles agreed with these findings. On the other hand, photosynthetic responses of each clade to tempe- rature stress appeared to vary. Abrego et al. (2008) observed that a decline in Fv/Fmobserved in A. tenuis juveniles associating with clade D was greater than juveniles with clade C at 32°C. Rowan (2004) reported that a temperature of 32°C decreased Fv/Fmin pocilloporid corals with clade C symbionts, whereas those with clade D maintained an elevated Fv/Fm. These differences may be due to differences in the life stage and species of the corals observed. Our results were similar to the result of Abrego et al. (2008), who reported that the clade C symbiont appeared to have higher stress tolerance than the clade D symbiont in Acropora tenuis juveniles. It is likely that the decrease in Fv/Fmafter exposure to 30°C in this study reflected the response to moderate heat stress, or different levels of acclimation to higher temperatures. Dead juveniles were observed only during the initial temperature increase within the first 8 days of the incubation period, suggesting that the temperature change had become intolerable, especially to juveniles with clade C1 symbionts.
Our results also showed a lower maximum electron transport rate (rETRmax) in clade D than in clade C1 during the experiments, while there was no significant effect of higher temperatures on the rETRmax of both in hospite clades. The different level of rETRax between clade C and clade D might indicate that the electron transport rate in clade D was lower by a slower Calvin Benson cycle. Our result is consistent with observations made by Cantin et al. (2009) and Jones and Berkelmans (2012), in which clade C1 Symbiodinium hosted by A. millepora juveniles had a greater rETRmax than clade D. They also reported a two-fold higher rate of 14C photosynthate incorporation into A. millepora juvenile corals harboring clade C compared with those harboring clade D (Cantin et al. 2009). A. tenuis juveniles exhibited different growth rates according to their associated symbiont types. Variation in rETRmax between Symbiodinium clades might affect the utilization of photosynthetic products as well as the growth of corals. From these photosynthetic parameters, we were unable to determine the cause of the difference in survival rates between C1 and D corals. However, it is possible that clade C might become a burden for corals at high temperatures, because clade C1 exhibited a relatively high Fv/Fmand rETR at high temperatures. The survival rate differential may be associated with different levels of oxidative stress from endosymbiotic algae (Yakovleva et al. 2009). To determine the cause of coral death under high temperature stress, it will be necessary to investigate levels of oxidative stress and antioxidant enzyme activities in corals.
Exposing corals to high light (500 μmol m-2 s-1) caused a significant reduction in Fv/Fmin both C1 and D corals. However, the extent of the reduction in Fv/Fmdiffered between each clade. C1 corals showed a greater decline in Fv/Fm(-74%), compared to a decline in D corals (-50%) after 3 h (Fig. 5). The damage to the function of PSII under high light conditions appeared to differ between clades. D corals were able to maintain greater photosynthetic activity than were C1 corals under strong light exposure. Field studies indicated that corals predominantly hosted clade D in shallow water (Mostafavi et al. 2007; Keshavmurthy et al. 2014), and clade D may provide a physiological advantage to host corals under conditions of greater irradiation. The reduction in Fv/Fmwas also affected by high temperature during high light exposure (Ferrier-Pages et al. 2007; Bhagooli and Hidaka 2003). When C1 corals are exposed to the combined stresses of high temperature and strong irradiance, the photosynthetic system of clade C1 may suffer more damage than that of clade D. Potential explanations for the observed differences in the photoinhibitory responses of clades C1 and D are (1) differences in reactive oxygen species produced (Weis 2008), (2) differences in photoprotective proteins such as MAA (Dunlap and Shick 1998; Yakovleva et al. 2009) in different hosts, and (3) differences in the rate of repair of damaged PSII (Takahashi et al. 2012). It will be important to explain these differences between clades under photoinhibitory conditions.
Fig. 5.
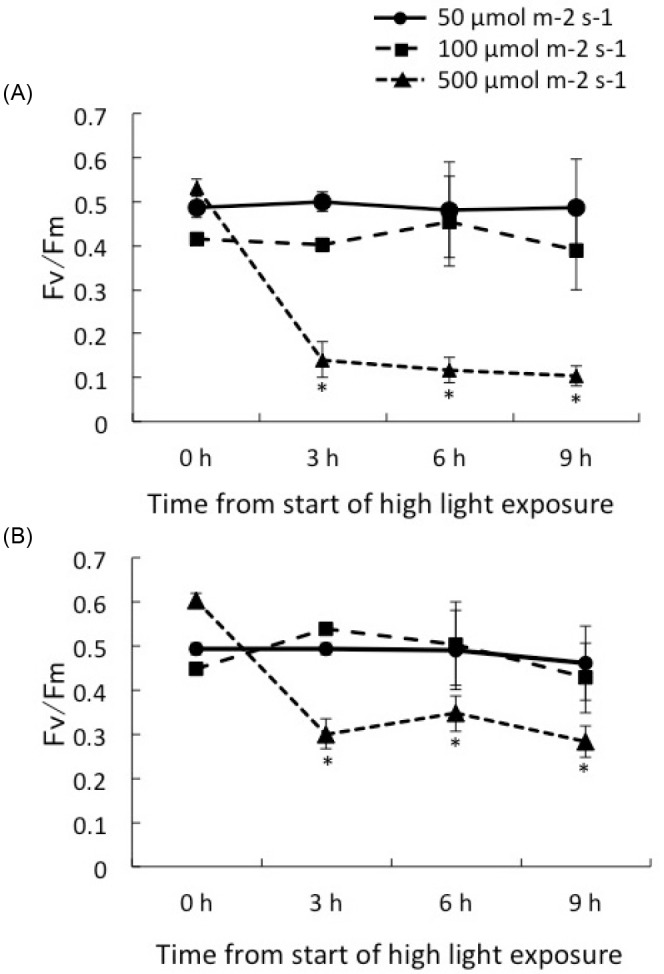
Fig. 5. Maximum quantum yield (Fv/Fm) of corals hosting Symbiodinium clade C1 (A) and clade D (B) under irradiance of 50, 100, 500 μmol m-2 s-1. Values are means ± se for each Symbiodinium type. * P < 0.05 compared to the value of < 500 μmol m-2 s-1 according to the Tukey-Kramer HSD test.
CONCLUSIONS
We showed the differences in light/temp- erature stress responses in juveniles hosting either clade C1 or clade D monoclonal Symbiodinium that can alter the fate of the host corals at an early growth stage. At high temperatures, in hospite clade C1 Symbiodinium had a higher photosynthetic rate, compared to clade D, but the survival rate of corals with clade C1 was lower than that of corals with clade D. Generally strong light can induce a photoinhibitory response in both clades C and D. However, the extent of the reduction in Fv/Fmwas more severe in clade C compared to clade D.
Acknowledgments
Acknowledgments: We are grateful to Mr. Kenji Iwao (the Akajima Marine Science Laboratory) and Dr. Saki Harii (The university of Rryukyu) for provision of Acropora tenuis larvae. We also thank Dwi Haryanti and Dr. Hiroki Tsuta and Sho Nakaema for help during incubation of juvenile coralss. This work was supported in part by a Grant-in-Aid for Scientific Research on Innovative Areas (# 20121001), from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan, and (#26-40135) to I.Y. from Japan Society for the promotion of Science.
References
- Abrego D R, Ulstrup K E, Willis B L, Oppen Van. Species -specific interactions between algal endosymbionts and coral hosts define their bleaching response to heat and light stress. Proc of Roy Soc B. 275:2273–2282. doi: 10.1098/rspb.2008.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker A C, Mcclanahan T R, Starger C J, Boonstra R K. Long-term monitoring of algal symbiont communities in corals reveals stability is taxon dependent and driven by site-specific thermal regime. Mar Ecol Prog Ser. 2013;479:85–97. [Google Scholar]
- Baker A C, Starger C J, Mcclanahan T R, Glynn P W. Corals' adaptive response to climate change. Nature; 2004. 430. [DOI] [PubMed] [Google Scholar]
- Baker A C. Flexibility and specificity in coral-algal symbiosis: diversity, ecology and biogeography of Symbiodinium. Annu Rev Ecol Evol Syst. 34:661–689. [Google Scholar]
- Berkelmans R, Van Oppen Mjh. The role of zooxanthellae in the thermal tolerance of corals: a 'nugget of hope' for coral reefs in an era of climate change. Proc Biol Sci. 273:2305–2312. doi: 10.1098/rspb.2006.3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagooli H, Hidaka M. Photoinhibition, bleaching susceptibility and mortality in tow scleractinian corals, Platygyra ryukyuensis and Stylophora pistillata, in response to thermal and light stresses. Comp Biochem Physiol A. 137:547–555. doi: 10.1016/j.cbpb.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Cantin N E, Van Oppen Mjh, Willis B L, Mieog J C, Negri A P. Juvenile corals can acquire more carbon from highperformance algal symbionts. Coral Reefs. 28:405–414. [Google Scholar]
- Wang J T, Fang L S, Yang Y W. Fluctuating algal symbiont communities in Acropora palifera (Scleractinia: Acroporidae) from Taiwan. Mar Ecol Prog Ser. 295:113–121. [Google Scholar]
- Coffroth M A, Santos S R. Genetic diversity of symbiotic dinoflagellates in the genus Symbiodinium. Protist. 156:19–34. doi: 10.1016/j.protis.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Demmig B, Björkman O. Comparison of the effect of excessive light on chlorophyll fluorescence (77 K) and photon yield of O, evolution in leaves of higher plants. Planta. 171:171–184. doi: 10.1007/BF00391092. [DOI] [PubMed] [Google Scholar]
- Dunlap W C, Shick J M. Ultraviolet radiation-absorbing mycosporine-like amino acids in coral reef organisms: A biochemical and environmental perspective. J Phycol. 34:418–430. [Google Scholar]
- Ferrier-Pages C, Richard C, Forcioli D, Allemand D, Pichon M, Shick J M. Effects of temperature and UV radiation increases on the photosynthetic efficiency in four scleractinian coral species. Biol Bull. 213:76–87. doi: 10.2307/25066620. [DOI] [PubMed] [Google Scholar]
- Gorbunov M Y, Kolber Z S, Lesser M P, Falkowski P G. Photosynthesis and photoprotection in symbiotic corals. Limnol Oceanogr. 46:75–85. [Google Scholar]
- Iwao K, Fujisawa T, Hatta M. A cnidarian neuropeptide of the GLWamide family induces metamorphosis of reef-building corals in the genus Acropora. Coral Reefs. 21:127–129. [Google Scholar]
- Jones A, Berkelmans R. The photokinetics of thermaltolerance in Symbiodinium. Mar Ecol. 33:490–498. [Google Scholar]
- Jones A M, Berkelmans R, Van Oppen Mjh, Mieog J C, Sinclair W. A community change in the algal endosymbionts of a scleractinian coral following a natural bleaching event: field evidence of acclimatization. Proc Biol Sci. 22:1359–1365. doi: 10.1098/rspb.2008.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavmurthy S, Meng P J, Wang J T, Kuo C Y, Yang S Y, Hsu C M, Gan C H, Dai C F, Chen C H. Can resistant coralSymbiodinium associations enable coral communities to survive climate change? A study of a site exposed to longterm hot water input. PeerJ; 2014. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesser M P. Oxidative stress causes coral bleaching during exposure to elevated temperatures. Coral Reefs. 16:187–192. [Google Scholar]
- Lesser M P, Shick J M. Effects of visible and ultraviolet radiation on photoadaptation in the zooxanthellae of Aiptasia pallida: primary production, photoinhibition and enzymatic defenses against oxygen toxicity. Mar Bull. 102:243–255. [Google Scholar]
- Mieog J C, Olsen J L, Berkelmans R, Bleuler-Martinez S A, Willis B L, Van Oppen Mjh. The role and interactions of symbiont, host and environment in defining coral fitness. PlosOne; 2009. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafavi P G, Fatemi M R, Shahhosseiny M H, Hoeghguldberg O, Loh Wkw. Predominance of clade D Symbiodinium in shallow-water reef-building corals off Kish and Larak Islands. Mar Biol. 153:25–34. [Google Scholar]
- Platt T, Gallegos C, Harrison W G. Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J Mar Res. 28:687–701. [Google Scholar]
- Pochon X, Gates R D. A new Symbiodinium clade (Dinophyceae) from sorites foraminifera in Hawaii. Mol Phyl Evol. 56:492–497. doi: 10.1016/j.ympev.2010.03.040. [DOI] [PubMed] [Google Scholar]
- Rowan R. Thermal adaptations in reef coral symbionts. Nature; 2004. 430. [DOI] [PubMed] [Google Scholar]
- Stat M, Pochon X, Franklin E C, Bruno J F, Casey K S, Selig E R, Gates R D. The distribution of the thermally tolerant symbiont lineage (Symbiodinium clade D) in corals from Hawaii: correlations with host and the history of ocean thermal stress. Ecol Evol. 3:1317–1329. doi: 10.1002/ece3.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwa R, Hirose M, Hidaka M. Seasonal fluctuation in zooxanthellae genotype composition and photophysiology in the corals Pavona divaricata and P. decussata. Mar Ecol Prog Ser. 361:129–137. [Google Scholar]
- Takahashi S, Yoshioka-Nishimura M, Nanba D, Badger M R. Thermal acclimation of the symbiotic algae Symbiodinium alleviates photobleaching under heat stress. Plant Physiol. 161:477–485. doi: 10.1104/pp.112.207480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Nakamura T, Sakamizu M, Woesik R V, Yamasaki H. Repair machinery of symbiotic photosynthesis as the primary target of heat stress for reef-building corals. Plant cell physiol. 45:251–255. doi: 10.1093/pcp/pch028. [DOI] [PubMed] [Google Scholar]
- Thornhill D J, Lajeunesse T C, Kemp D W, Fitt W K, Schmidt G W. Multi-year, seasonal genotypic surveys of coral-algal symbioses reveal prevalent stability or postbleaching reversion. Mar Biol. 148:711–722. [Google Scholar]
- Warner M E, Fitt W K, Schmidt G W. Damage to photosystem II in symbiotic dinoflagellates: a determinant of coral bleaching. Proc Natl Acad Sci. 96:8007–8012. doi: 10.1073/pnas.96.14.8007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis V M. Cellular mechanisms of cnidarian bleaching: Stress causes the collapse of symbiosis. Jour Exp Bio. 211:3059–3066. doi: 10.1242/jeb.009597. [DOI] [PubMed] [Google Scholar]
- Yakovleva I M, Baird A H, Yamamoto H H, Bhagooli R, Nonaka M, Hidaka M. Algal symbionts increase oxidative damage and death in coral larvae at high temperatures. Mar Ecol Prog Ser. 378:105–112. [Google Scholar]
- Yuyama I, Higuchi T. Comparing the effects of symbiotic algae (Symbiodinium) clade C1 and D on early growth stage of Acropora tenuis. PlosOne; 2014. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]


