Abstract
5-methylcytosine (m5C) is an abundant RNA modification that’s presence is reported in a wide variety of RNA species, including cytoplasmic and mitochondrial ribosomal RNAs (rRNAs) and transfer RNAs (tRNAs), as well as messenger RNAs (mRNAs), enhancer RNAs (eRNAs) and a number of non-coding RNAs. In eukaryotes, C5 methylation of RNA cytosines is catalyzed by enzymes of the NOL1/NOP2/SUN domain (NSUN) family, as well as the DNA methyltransferase homologue DNMT2. In recent years, substrate RNAs and modification target nucleotides for each of these methyltransferases have been identified, and structural and biochemical analyses have provided the first insights into how each of these enzymes achieves target specificity. Functional characterizations of these proteins and the modifications they install have revealed important roles in diverse aspects of both mitochondrial and nuclear gene expression. Importantly, this knowledge has enabled a better understanding of the molecular basis of a number of diseases caused by mutations in the genes encoding m5C methyltransferases or changes in the expression level of these enzymes.
Keywords: RNA methyltransferase, RNA modification, epitranscriptome, 5-methylcytosine, mitochondria, ribosome, transfer RNA (tRNA), messenger RNA (mRNA), gene expression
1. Introduction
Chemical modification of nucleic acids is a key cellular process that occurs in all three domains of life. The spectrum of different modifications detected in DNA is relatively limited (six), while the range of modifications present in RNA is much higher, with more than 140 types of modification reported so far [1]. In eukaryotes, 5-methylcytosine in DNA (5mC) and its oxidized derivatives (5-hydroxymethylcytosine (5hmC), 5-formylcytosine (5fC), and 5-carboxylcytosine (5caC)) are the most prominent modifications and have been suggested to contribute to epigenetic gene regulation through a variety of different mechanisms (reviewed in [2]). However, 5-methylcytosine is also present in diverse RNA species (m5C; Table 1; reviewed in [3]), where it has emerged as an important regulator of many aspects of gene expression, including RNA export, ribosome assembly, translation, and RNA stability. The development of a number of 5mC/m5C mapping approaches, such as bisulfite sequencing, anti-m5C-crosslinking, and immunoprecipitation (CLIP), Aza-IP, and methylated iCLIP (miCLIP) has enabled the positions of many such modified nucleotides to be precisely defined in both the genome and transcriptome. The enzymes responsible for installation of 5mC in DNA and the functions of these epigenetic marks have been described in several recent reviews (see for example [4]). Here we discuss the current knowledge on the human RNA m5C modification machinery, focusing on the mechanisms of action of m5C methyltransferases, the cellular functions of these enzymes, and the modifications they install, as well as the implications of defects in such enzymes in disease.
Table 1.
Overview of human m5C methyltransferases and their RNA targets. Abbreviations: ribosomal RNA – rRNA, transfer RNA – tRNA, mitochondrial – mt, enhancer RNA – eRNA.
| Methyl- transferase | Subcellular localization | Target RNA(s) | Modification installed | Ref. |
|---|---|---|---|---|
| NSUN1 | Nucleolus | 28S rRNA | m5C4413 | [5] |
| NSUN2 | Nucleus/Nucleolus | Pre-tRNALeu(CAA) | m5C34 | [6] |
| tRNAAla(AGC/CGC/UGC)/His(GUG)/Ile(AAU)/ Leu(CAA/AAG/CAG/UAA/UAG)/Lys(CUU)/ Met(CAU)/Ser(AGA/CGA/GCU/UGA)/Thr(CGT/UGU)/Tyr(GUA) |
m5C48 | [7,8] | ||
| tRNAAsp(GUC)/Gln(CUG/UUG)/Lys(UUU)/Phe(GAA)/ Thr(AGU)/Val(AAC/CAC/UAC) |
m5C48, 49 | [7,8] | ||
| tRNAGlu(CUC/UUC)/Gly(CCC/GCC/UCC)/Pro(AGG/CGG/UGG) | m5C48, 49, 50 | [7,8] | ||
| vtRNA1.1 | m5C69 | [9] | ||
| vtRNA1.2 | m5C27, 591 | [9] | ||
| vtRNA1.3 | m5C15, 27, 59 | [9] | ||
| mRNA | various | [10,11,12,13] | ||
| NSUN3 | Mitochondria | mt-tRNAMet | m5C34 | [14,15,16] |
| NSUN4 | Mitochondria | mt-12S rRNA | m5C8412 | [17] |
| NSUN5 | Nucleolus | 28S rRNA | m5C3761 | [18,19,20] |
| NSUN6 | Cytoplasm/Golgi | tRNACys/Thr | m5C72 | [21,22] |
| NSUN7 | Nucleus | eRNA (Pfk1/Sirt5/Hmox2/Idh3b) | various | [23] |
| DNMT2 | Cytoplasm/Nucleus | tRNAAsp(GUC)/ Gly(GCC)/ Val(AAC) | m5C38 | [24,25] |
Note: 1 Detected by miCLIP but not bisulfite, 2 by analogy to mouse.
2. Eukaryotic m5C RNA Methyltransferases and Their Catalytic Mechanisms
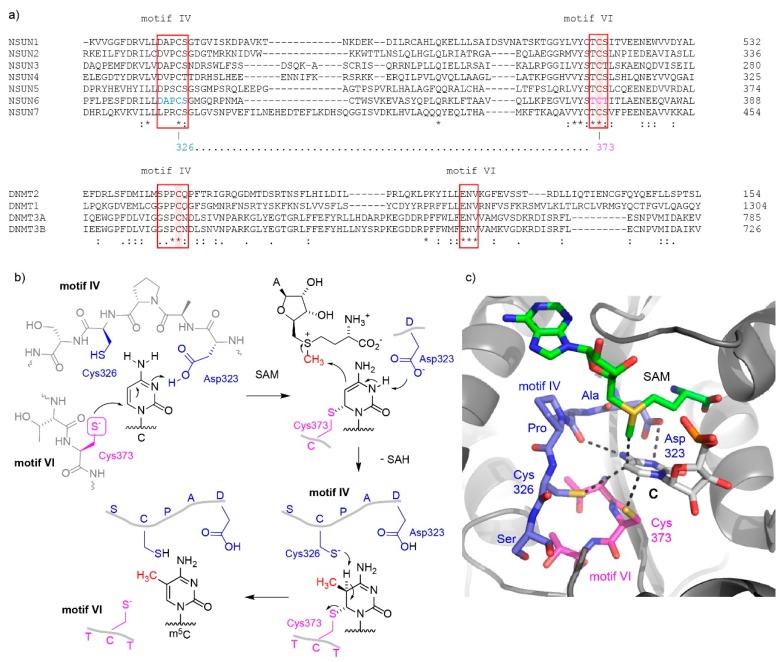
It is known that m5Cs in RNAs are introduced by members of the NOL1/NOP2/SUN domain (NSUN) family of proteins, which contains seven members (NSUN1-7) in humans [26], as well as the DNA methyltransferase (DNMT) homologue DNMT2. While NSUN1, NSUN2, and NSUN5 are conserved throughout eukaryotes (in Saccharomyces cerevisiae, named Nop2, Trm4, and Rcm1, respectively), the remaining NSUN proteins are only present in higher eukaryotes. The NSUN proteins are (putative) S-adenosylmethionine (SAM)-dependent methyltransferases that are typified by an RNA-recognition motif (RRM) and Rossman-fold catalytic core that accommodates the SAM cofactor. Mechanistically, it is proposed that NSUN proteins use two catalytic cysteines in the active site, whereas DNMT2 acts more like DNA methyltransferases that use a single active site cysteine [27,28]. In both mechanisms, a covalent intermediate is formed between a cysteine of the protein and the cytosine in RNA, in order to activate the electron-deficient pyrimidine heterocycle for the nucleophilic attack of carbon 5 on the methyl group of SAM. Interestingly, the nucleophilic cysteine that forms the covalent intermediate with the nucleoside is located in different conserved protein motifs in NSUN and DNMT proteins (Figure 1a; conserved motifs IV and VI are marked with a red box, the nucleophilic cysteine is highlighted with a magenta background).
Figure 1.
Mechanisms of C5-methylation of cytosine by m5C RNA methyltransferases. (a) Amino acid sequence alignment of regions forming the active sites of human m5C methyltransferases. Top: NSUN family of m5C RNA methyltransferases, bottom DNMT family containing DNMT2 as RNA methyltransferase and DNMT1 and DNMT3A/B as DNA methyltransferases. The conserved motifs IV and VI are boxed. The catalytic cysteine that forms a covalent bond with C6 of the target cytosine is marked with magenta background, and is located in motif VI in NSUN methyltransferases, and in motif IV in DNMT methyltransferases. (b) The catalytic mechanism is depicted in detail for NSUN6 (see text for description). (c) The active site in the crystal structure of NSUN6 with target RNA is presented, showing the arrangement and key contacts between the amino acids in the active site and the target cytosine (C72) in RNA (PDB 5WWS).
The NSUN family enzymes use the cysteine located in amino acid motif VI for the nucleophilic attack on carbon 6 of the target cytosine in RNA [27]. In all seven human NSUN variants, the catalytic cysteine is preceded by threonine. Hydrogen bonding with the backbone carbonyl of proline and the aspartate sidechain in motif IV orients the base in the active site and assists bond formation by transient protonation of the endocyclic N3 of cytidine (Figure 1b,c; residue numbers shown for NSUN6 based on the crystal structure [21]). The activated nucleobase then accepts a methyl group from the properly positioned SAM cofactor, resulting in the formation of a carbon-carbon bond and generation of S-adenosylhomocysteine (SAH). To complete the reaction, the covalently bound methylated RNA has to be released from the protein. This elimination is assisted by the cysteine located in motif IV of NSUN proteins. This cysteine is located next to a partially conserved proline and acts as a base to deprotonate the tetrahedral carbon and initiate the elimination reaction that restores the unsaturated m5C heterocycle. The catalytic mechanism is supported by extensive mutational analyses. For a yeast orthologue of the NSUN1 protein, Nop2, it has been shown that the cysteine in motif VI next to threonine is essential for function [29]. Additionally, it was shown for Nop2 as well as for human NSUN2 and NSUN3 that mutation of the cysteine in motif IV to alanine or serine resulted in a stable covalent intermediate [7,12,30,31].
In contrast to the NSUN proteins, methyltransferases of the DNMT family do not contain a cysteine in motif VI and instead use the cysteine in motif IV as the nucleophile for attack at carbon 6. A conserved glutamate in motif VI takes the role of aspartate in motif IV of NSUN enzymes to facilitate the covalent bond formation by protonation of N3 [32]. Thus, the roles of motifs IV and VI seem to be switched in NSUN and DNMT methyltransferase families. The covalent intermediate containing the 5,6-dihydropyrimidine was characterized by structural studies using mechanism-based inhibitors, such as 5-fluoropyrimidine substrate analogs, which form a stable complex with the enzyme [33,34]. Alternatively, 5-azacytosine was used as suicide inhibitor that leads to a stable covalent crosslink between the nucleic acid and the enzyme [15,35].
3. Cellular Functions of m5C RNA Methyltransferases and the Modifications They Install
3.1. NSUN1 and NSUN5 Modify Cytoplasmic Ribosomal RNAs
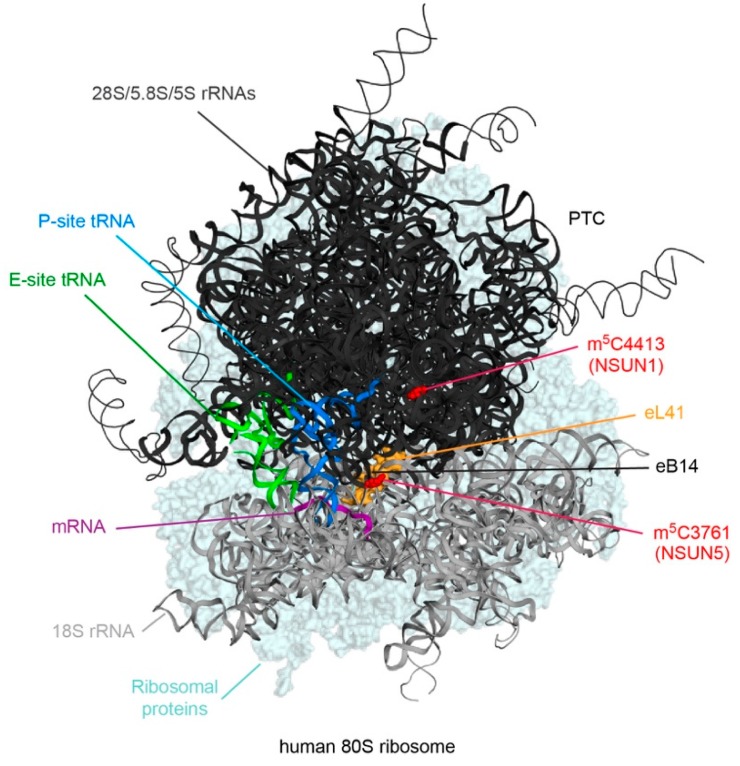
Eukaryotic cytoplasmic ribosomes are large ribonucleoprotein complexes, which are responsible for the production of all cellular proteins, and are composed of four ribosomal RNAs (rRNAs) and approximately 80 ribosomal proteins. During their maturation, the rRNAs are decorated with a cornucopia of chemical modifications, the majority of which are 2’-O-ribose methylations or pseudouridines, introduced by small nucleolar RNPs (snoRNPs) [36,37]. The eukaryotic rRNAs also contain a number of base modifications, including two m5Cs at positions 3761 (human)/2870 (yeast) and 4413 (human)/2278 (yeast) of the 28S/25S rRNA (Table 1). C5 of 28S-C3761/25S-C2278 is methylated by NSUN5 (human)/Rcm1 (yeast), whereas NSUN1 (human)/Nop2 (yeast) targets 28S-C4413/25S-C2870 [5,18,19,20]. Within the mature ribosome, these modifications lie in close proximity to the peptidyltransferase center (PTC; 28S-C4413/25S-C2870) within the large ribosomal subunit (LSU) and at the inter-subunit bridge eB14 (28S-C3761/25S-C2278; Figure 2).
Figure 2.
Schematic views of the positions of m5C modifications in cytoplasmic rRNAs. A cryo-EM structure of the human 80S ribosome is shown with the positions of 28S-m5C3761 and 28S-m5C4413, the enzymes that install them as well as key ribosomal features indicated. The ribosomal protein eL41 is highlighted in yellow, tRNAs in the P-site and E-site in blue and green, respectively, and an mRNA fragment in magenta. PTC – peptidyl transferase center; DS – decoding site; eB – eukaryotic inter-subunit bridge.
On a molecular level, m5C stabilizes RNA structures by promoting base stacking and by increasing the thermal stability of hydrogen bonding with guanine [38,39]. It is likely, therefore, that the m5Cs present in the rRNAs serve to help stabilize rRNA folding within these functionally important regions of the ribosome. Consistent with this, in yeast, loss of Rcm1 influences the structural conformation of helix 69/70 of the 25S rRNA in oxidative stress conditions [18], and the combined loss of both 25S-m5C2278 and 2’-O-methylation of the nearby 25S-G2288 dramatically destabilizes the pre-LSU indicated by failure to recruit many LSU ribosomal proteins [19]. The m5C modification installed by Rcm1/NSUN5 is further suggested to influence ribosome function, as reporter assays have revealed that deletion of Rcm1 from yeast promotes read-through of premature termination codons [18]. Within mature yeast ribosomes, m5C2278 is directly contacted by the ribosomal protein eL41 (RPL41), which acts as a pivot for small subunit (SSU) rotation during translation [40], perhaps providing a mechanistic basis for how the modification influences translation. Substoichiometric modification of rRNA nucleotides is suggested to be an important source of ribosome heterogeneity. Interestingly, although quantitative mass spectrometric analysis of rRNA modification in yeast demonstrated that in vivo, 25S-C2278 and 25S-C2870 are typically 100 and >95% methylated, respectively [41], lack of Rcm1-mediated 25S-m5C2278 promotes the recruitment of a specific subset of mRNAs coding for proteins involved in the oxidative stress response to the ribosome. This may suggest that 25S-m5C2278/28S-m5C3761 contributes to the regulation of cytoplasmic translation and is in line with the observation that Rcm1/NSUN5 contributes to stress resistance and longevity in several model organisms [18]. Less is known about the precise function(s) of the rRNA 28S-m5C4413/25S-m5C2870 modification. Although NSUN1/Nop2 is known to be required for biogenesis of the LSU [42,43], several rRNA modification enzymes are suggested to have additional functions beyond catalyzing their target modifications [44,45], and it remains unclear whether the presence of NSUN1 or 28S-m5C3761 is important for LSU assembly.
3.2. Cytoplasmic Transfer RNAs are Methylated by NSUN2, NSUN6 and DNMT2
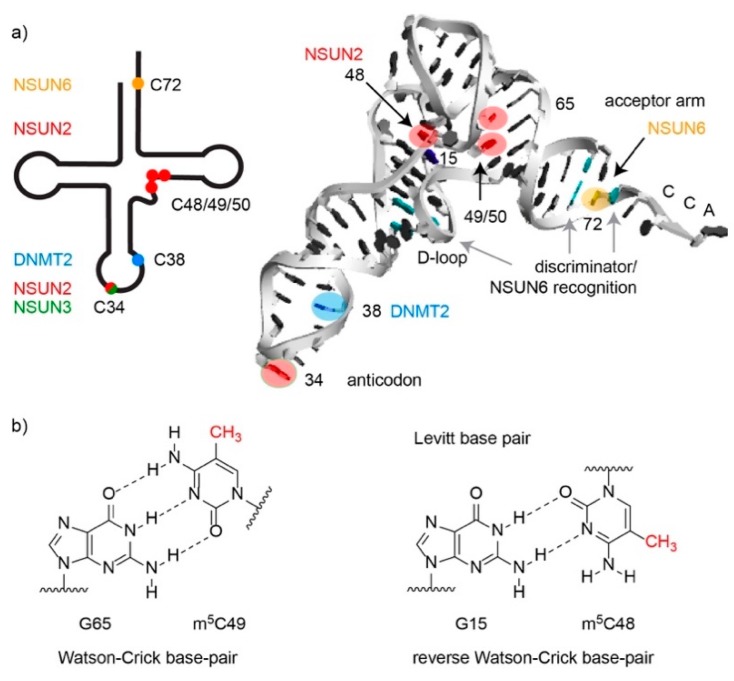
Transfer RNAs (tRNAs) are the most extensively modified cellular RNAs, and three m5C methyltransferases, NSUN2, NSUN6, and DNMT2, have been shown to act on cytoplasmic tRNAs. While NSUN6 and DNMT2 specifically methylate C72 and C38 of particular tRNAs respectively [22,24], NSUN2 has a much broader target spectrum and is able to modify several positions (C34, C40, C48, C49, and C50) in a number of different tRNAs [6,7,8], as well as other RNA substrates (see below; Table 1 and Figure 3a).
Figure 3.
The m5C modifications in cytoplasmic and mitochondrial tRNAs. (a) Schematic secondary structure and three-dimensional L-shape structure of a tRNA with the positions of m5C modifications and the cognate methyltransferases responsible for installing them marked. The interaction sites of NSUN6 with the discriminator base and additional base pairs in the acceptor stem and the D-loop are indicated as observed by X-ray crystallography. (b) Chemical structures of m5C-containing base pairs in Watson-Crick orientation (with the D-stem G65-m5C49) and the reverse-Watson-Crick orientation of the Levitt base pair G15:m5C48.
In the nucleus, pre-tRNAs are processed to remove 5’ leader, 3’ trailer, and intron sequences, and three non-templated nucleotides, CCA, are added to the 3’ end, which is a pre-requisite for aminoacylation. Although modification of tRNAs occurs at different stages of tRNA biogenesis, the majority of tRNA modification enzymes are nuclear, suggesting that most modifications occur during the early stages of tRNA biogenesis. In line with this, NSUN2 localizes predominantly in the nucleus, and NSUN2-mediated methylation of C34 of tRNALeu(CAA) has been shown to occur exclusively on intron-containing tRNA precursors [6]. Notably, in humans and Drosophila melonagaster, DNMT2 is present in both the nucleus and cytoplasm [24,46], suggesting that installation of m5C38 modifications could also occur during the later stages of tRNA biogenesis. Notably, NSUN6 localizes to the cytoplasm and appears enriched in proximity to the golgi aparatus and pericentriolar matrix [22], indicating that methylation of C72 residues is a late maturation event that takes place after nuclear export.
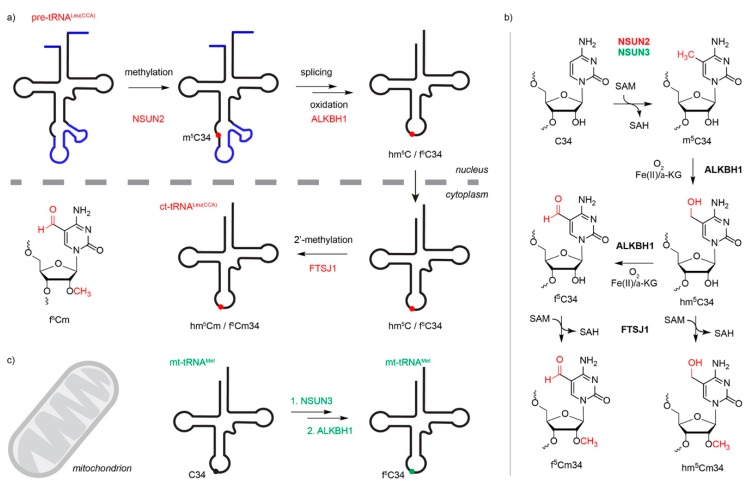
In general, modifications that lie within the tRNA core are suggested to either influence tRNA structure, stability, or both, while modifications within, or close to, the anticodon, instead contribute to tRNA function by affecting codon-anticodon interactions; tRNALeu(CCA) is the only cytoplasmic tRNA that is modified by an m5C methyltransferase within the anticodon [6]. However, tRNALeu(CAA)-m5C34 is an intermediate in the formation of a hypermodification at this position; following intron-removal, the m5C is oxidized by the α-ketogluterate- and Fe2+-dependent dioxygenase ALKBH1 to produce 5-hydroxymethylcytosine (hm5C), 5-formylcytosine (f5C), or both at this position, and then after export of the tRNA to the cytoplasm, 2’-O ribose methylation by FTSJ1 takes place to generate 5-hydroxymethyl-2’-O-methylcytidine (hm5Cm), 5-formyl-2’-O-methylcytidine (f5Cm), or both (Figure 4a,b, [47,48]). Interestingly, wobble base modification of tRNALeu(CCA) is implicated in regulating translation [49]. In yeast, C34 of tRNALeu(CCA) is substoichiometrically modified under normal conditions, but the extent of methylation is increased upon exposure to oxidative stress. This leads to enhanced translation of mRNAs enriched in UUG codons, such as the ribosomal protein eL22a (Rpl22a), which is required for the oxidative stress response [49]. Methylation of position C38 within the anticodon loop of tRNAAsp(GUC) by DNMT2 also promotes translation of a specific subset of genes, but in this case, the modification promotes association of the aspartyl-tRNA synthetase leading to more efficient aminoacylation and enhanced translation of poly-Asp-containing proteins [50]. DNMT2-mediated C38 modification has also been suggested to affect translation accuracy by facilitating discrimination between cognate and near-cognate codons; lack of tRNAAsp-m5C38 decreases the ability of tRNAAsp to compete with near cognate tRNAs (e.g., tRNAGlu), leading to greater amino acid mis-incorporation rates [51]. Interestingly, in various species, substitution of G34 of tRNAAsp(GUC) for queosine (Q) strongly increases Dnmt2-mediated m5C38 modification, indicating cross-talk between these two modifications [52,53] The functional significance of this interdependence is not yet fully understood, but as eukaryotes are not able to synthesize queuine and rather salvage it from their environment, the cross-talk between these modifications could suggest a mechanism by which translation regulation can be coupled with nutritional status [53,54]. Interestingly, DNMT2 re-localizes to stress granules following heat-shock and lack of m5C38 modification leads to increased production of tRNA fragments, suggesting that DNMT2-mediated tRNA modification plays a role in the cellular stress response [25].
Figure 4.
Hypermodification of m5C34 in anticodon of cytoplasmic and mitochondrial tRNAs. (a) During the maturation of the cytoplasmic tRNALeu(CAA), an m5C34 modification is installed by NSUN2. The methylated RNA is spliced, followed by oxidation to hm5C and f5C, which occurs in the nucleus. The oxidation is catalyzed by ALKBH1. The RNA is exported to the cytoplasm, where the methyltransferase FTSJ1 installs an additional methyl group on the ribose 2’-OH to produce hm5Cm and f5Cm. (b) Structures of modified nucleotides at position 34 and the modification pathway. (c) Modification of mt-tRNAMet by NSUN3 and ALKBH1 occurs in the mitochondria. SAM = S-adenosylmethionine, SAH = S-adenosylhomocysteine, α-KG = alpha-ketoglutarate.
All other m5C modifications in cytoplasmic tRNAs are present outside the anticodon loop (Table 1 and Figure 3a), and are therefore likely to primarily influence tRNA structure and stability. The m5C-48/49/50 modifications installed by NSUN2 cluster within the variable loop at the junction with the T-stem. A “Levitt pair” interaction between C48 and G15 in the D-loop is critical for formation of the characteristic L-shaped tertiary fold of most tRNAs [55], and it is suggested that the presence of m5C at position 48 increases the hydrophobicity of the base, increasing base stacking, and thereby helping stabilize this interaction and the tRNA tertiary fold (Figure 3b) [56]. Notably, NSUN2-mediated methylations within the variable loop have also been shown to protect tRNAs against stress-induced, angiogenin-mediated endonucleolytic cleavage. tRNAs lacking m5C48/49/50 modifications are bound more tightly by angiogenin, leading to accumulation of 5’ tRNA-derived small RNA fragments, which trigger cellular stress and are implicated in disease (see below) [7]. In contrast to the NSUN2-mediated modifications, the m5C72 modifications installed by NSUN6 lie within the acceptor stem, and currently, the precise role of these modifications remains elusive. Given the close proximity of C72 to the 3’ end of the tRNA and the recognition of specific nucleotides within the acceptor stem by aminoacyl-tRNA synthetases, it is tempting to speculate that NSUN6-mediated methylation of C72 may influence tRNA charging, but this was recently found not to be the case for the Pyrococcus horikoshii (Ph)NSUN6 homologue [57]. Instead, m5C72 was reported to promote the thermal stability of PhtRNAs [57].
3.3. NSUN3 and NSUN4 Install m5Cs in Mitochondrial RNAs
Mitochondrial gene expression is essential for the production of components of the oxidative phosphorylation system. While the majority of mitochondrial messenger RNA (mt-mRNAs) coding for these proteins, as well as the 12S and 16S mt-rRNA and 22 mt-tRNAs, are transcribed from the mitochondrial genome, assembly of the mitochondrial translation machinery requires numerous nuclear-encoded proteins. Two of the seven NSUN proteins (NSUN3 and NSUN4) are synthesized on cytoplasmic ribosomes but localize to mitochondria. In the case of NSUN4, a 25 amino acid mitochondrial leader peptide that is cleaved after mitochondrial import has been identified, suggesting that NSUN4 is imported via the TOM-TIM23 pathway [58,59]. While NSUN3 has been shown to localize to the mitochondrial matrix [15], its mitochondrial import has not been investigated.
NSUN4 is responsible for installing an m5C modification at position 911 of the mouse 12S rRNA (equivalent to 12S-m5C841 in humans) [17], which lies within the decoding site of the small mitochondrial ribosomal subunit (mt-SSU). NSUN4, which in contrast to other NSUN proteins lacks an RNA recognition motif, forms a heterodimeric complex with the mitochondrial transcription factor MTERF4 [58]. MTERF4 is not, however, required for C5 methylation of mt-12S-C911 by NSUN4, suggesting that this is an independent function of the methyltransferase. Instead, MTERF4 is responsible for recruitment of the methyltransferase to the late pre-mt-LSU complexes, where the complex is required for assembly of the mature SSU and LSU into monosomes. It is not yet clear how NSUN4-MTERF4 regulate subunit joining, but this function does not require the catalytic activity of NSUN4 and it is suggested that either the heterodimer physically blocks subunit interaction or that the binding of NSUN4-MTERF4 to a specific site in the mt-16S rRNA influences activation of the mt-LSU [17]. It is possible that the dual functionality of NSUN4 in 12S rRNA methylation and mt-LSU biogenesis acts as a quality control mechanism to ensure that only fully mature mt-SSU and mt-LSU can be assembled into functional mitochondrial ribosomes. Such a model would suggest an important role for mt-12S-m5C911 in mitoribosome function, but while lack of NSUN4 impairs mitochondrial translation [17], this may reflect the lack of monosome production and the precise molecular role of the m5C modification currently remains elusive.
In contrast to NSUN4, NSUN3 is a mitochondrial tRNA m5C methyltransferase that specifically targets the wobble position (C34) of mt-tRNAMet [14,15,16,60]. Mt-tRNAMet-C34 is almost fully modified in vivo, and interestingly, although bisulfite and reduced bisulfite sequencing analyses indicate the presence of some m5C at this position, the majority undergoes further oxidation by ALKBH1 to generate f5C (Figure 4c) [15,48]. In contrast to the cytoplasmic translation machinery, where two alternative tRNAs mediate incorporation of methionine either during translation initiation or elongation, due to evolutionary reduction of the mitochondrial genome, mitochondria contain only a single methionine tRNA. Furthermore, mitochondria employ a specialized genetic code, in which mt-tRNAMet is required to not only recognize conventional AUG codons, but additionally is employed for decoding AUA codons during translation initiation and elongation, as well as the AUU initiation codon on the ND2 mRNA. The wobble base modification(s) installed by NSUN3 and ALKBH1 likely serve to expand codon recognition by mt-tRNAMet, enabling it to fulfil these diverse functions. Structural studies indicate that the presence of the formyl group may help stabilize non-conventional base pairing of f5C34 with the adenosine in the third position of the AUA codon [61,62]. Consistent with this, lack of NSUN3 (or ALKBH1) impairs mitochondrial translation, leading to decreased cell proliferation [14,15,16].
3.4. m5C Marks in Messenger RNAs
Alongside the long-known cap-proximal 2’-O-methylations, a number of other modifications, such as N6-methyladenosine (m6A), pseudouridine (Ψ) and N1-methyladenosine (m1A), have recently been detected in messenger RNAs (mRNAs) [63,64,65]. These modifications are implicated in regulating diverse aspects of the mRNA life cycle, including pre-mRNA splicing, mRNA export, translation, and mRNA stability. Although the presence of m5C in eukaryotic mRNAs was first reported almost 50 years ago [66], the recent development of m5C mapping techniques has prompted more extensive analysis. In a seminal study performed using bisulfite sequencing of RNAs derived from HeLa cells, more than 10,000 m5C sites in approximately 8500 mRNAs were reported [67]. Subsequently, m5C detection approaches have been applied to RNAs from diverse organisms and cell types, including mouse embryonic stem cells (ESC) [68,69], various mouse tissues (small intestine, heart, muscle, brain, kidney, and liver) [10,68], plants (Arabidopsis thaliana) [70], yeast (Saccaromyces cerevisiae) [71], and archaebacteria (Sulfolobus solfataricus) [71]. Collectively, these studies support the presence of m5C in mRNA, and suggest that m5C sites are enriched in 5’ and 3’ untranslated regions (UTRs) and are especially prominent in proximity to the translation start codon. However, the number and positions of m5Cs detected in these studies vary considerably, and it has also been suggested that mRNAs carry either no, or very few, m5Cs. While the presence of cell type-specific modifications may partly explain this variation, it is also likely that some of the observed differences arise due to limitations and biases of the currently available m5C mapping approaches. The extent of m5C in mRNA is therefore still controversially discussed, and further work will be required to resolve these issues.
The methyltransferase(s) responsible for installing potential m5C modifications in mRNAs have not yet been confirmed and the possible functions of m5Cs in mRNAs largely remain elusive. Several lines of evidence link NSUN2 to mRNA methylation. On a global level, depletion, overexpression, or expression of catalytically inactive forms of NSUN2, but not NSUN1, NSUN5, or NSUN6, was reported to alter the total amount of m5C detected in the mRNA pool [10]. Through in vitro methylation assays and reporter assays, NSUN2 has also been suggested to install modifications in specific mRNAs (e.g., p27 (KIPI), CDK1, p21, SHC, ICAM, p53, E2F3 and ErbB2) and the presence of these modifications was proposed to influence mRNA translation [11,12,13], however, evidence supporting the presence of these modification in endogenous mRNAs is lacking. Interestingly, it has been suggested that m5C modifications in mRNAs may exert their effects by influencing RNA-protein interactions. Consistent with this, the nuclear export factor ALYREF was recently show to preferentially bind m5C-containing RNAs, and depletion of this m5C “reader” protein causes nuclear retention of m5C methylated transcripts [10].
3.5. Modification of Other RNA Species by m5C Methyltransferases
The transcriptome-wide nature of the available m5C mapping approaches has indicated the presence of this modification in diverse non-coding RNA species, including vault RNAs (vtRNAs), enhancer RNAs (eRNAs), long non-coding RNAs (lncRNAs; e.g., XIST and HOTAIR), and small cajal body-specific RNAs (scaRNAs, SCARNA2). While the presence of these modifications often requires further confirmation, and the functions and enzymes responsible for introducing these methylations largely remain unknown, in some cases, these modifications have been analyzed in detail. The vault ribonucleoprotein complex, which is implicated in multidrug resistance, nucleocytoplasmic transport, and has been suggested to act as a scaffold for essential cell signaling pathways, is composed of three proteins and three vtRNAs. While miCLIP data from cells lacking NSUN2 identified specific sites in all three vtRNAs as targets of this methyltransferase, bisulfite sequencing only confirmed the presence of m5C in vtRNA1.1 and vtRNA1.3 (Table 1) [9]. Interestingly, lack of m5C69 modification in vtRNA1.1 was shown to affect its processing into a small RNA (svRNA4). Furthermore, svRNA4 acts analogous to a microRNA and a concomitant increase in the levels of the svRNA4 target mRNAs CACNG7 and CACNG8 was observed in NSUN2-/- cells [9], demonstrating the functional importance of these m5C marks. In contrast to NSUN2, NSUN7 has been suggested to target eRNAs, which are short, non-coding RNAs that are linked to transcription regulation. NSUN7 was reported to methylate the Pfk1, Sirt5, Idh3b, and Hmox2 eRNAs in the context of a physical association with the transcriptional co-activator PGC-1α [23]. Depletion of NSUN7 causes significant decreases in the levels of these eRNAs and their cognate mRNAs, implying that the presence of m5C, if confirmed, may stabilize these transcripts, thereby promoting mRNA production. The observations that NUSN7 expression and eRNA methylation are upregulated during starvation [23] suggest that the methylation activity of NSUN7 may contribute to the adaptation of gene expression during the stress response. In addition to its well characterized role as a tRNA methyltransferase, Me-RIP experiments indicated a decrease in m5C methylation of the non-coding RNA, 7SK, in cells lacking DNMT2, suggesting that this enzyme could also have additional RNA substrates [72].
4. Substrate Recognition by m5C RNA Methyltransferases and Regulation of Their Activity
The identification of methylation targets for each of the human m5C methyltransferases, together with structural information on several of these proteins, allows insights into the ways in which these methyltransferases interact with their substrates and achieve methylation specificity. The broad-spectrum methyltransferase NSUN2 has been suggested to recognize different features in its diverse substrate RNAs. The reported NSUN2-mediated m5C modifications in mRNAs typically lie within highly GC-rich regions [10], suggesting that the enzyme may preferentially bind such sequences. However, all the known NSUN2-mediated m5C modifications in vtRNAs lie within a UCG motif [9], and mutagenic analysis of the NSUN2 target pre-tRNALeu revealed a consensus sequence of C/A/U32-U/A33-m5C34-A35-A36-G37 [6]. Interestingly, the NSUN2-mediated m5C modifications in the variable loops of its numerous cytoplasmic tRNA targets lie within diverse sequence contexts [7], suggesting that in this context, NSUN2 may recognize this structural feature of its non-intron-containing tRNA substrates, rather than a specific nucleotide sequence. The recognition of RNA secondary structures by NSUN2 is further supported by the finding that disruption of the elongated anticodon stem of pre-tRNALeu impedes methylation of C34 [6]. The presence of a stable anticodon stem was similarly found to be essential for formation of m5C34 of mt-tRNAMet by NSUN3 [15].
Recent structural and biochemical analyses revealed that NSUN6 forms extensive contacts with its substrate tRNAs. The catalytic core and RRM domain interact with nucleotides surrounding the modification target (C72) [21], implying that they contribute to target specificity. U73, which has been termed the “discriminator base”, is critical for substrate recognition by NSUN6 (Figure 3a), and a flexible base pair (A:U or U:A) at positions 2:71 as well as a rigid base pair (C:G or G:C) formed between positions 3:70 are preferred [73]. While the binding pocket of human NSUN6 specifically accommodates U73, structural differences in PhNSUN6 enable the archaeal enzyme to bind tRNAs containing either U73 or G73, thereby broadening its target spectrum compared to its human homologue [57]. Importantly, binding of NSUN6 disrupts base pairing within the tRNA acceptor stem and promotes base-flipping of C71 to make the C5 atom of the C72 nucleotide, which is normally base paired with G1, accessible for methylation [21]. Interestingly, NSUN6 also has a PUA domain that binds to the D-stem region of substrate tRNAs (Figure 3a), as well as the non-genomically encoded CCA 3’ end [21]. Consistent with this binding mode, the presence of the CCA was found to be an essential pre-requisite for methylation of tRNACys and tRNAThr by NUSN6 [21,22]. Recognition of this post-transcriptional feature by the PUA domain may help regulate the timing of C72 modification relative to other aspects of tRNA maturation, or serve as a quality control mechanism ensuring that only correctly processed tRNAs are methylated.
Several lines of evidence suggest that DNMT2, which specifically methylates position 38 of its substrate tRNAs, recognizes the local sequence context of its modification target. The anticodon loop sequences of tRNAAsp(GUC), the canonical target of DNMT2, are perfectly conserved in species that express DNMT2 homologues, while various sequence diversions are observed in species that lack DNMT2. This evolutionary conservation strongly suggests the importance of elements within the anticodon loop for recognition or methylation by DNMT2 [24]. This model is further supported by the observation that m5C38 in other DNMT2 substrate tRNAs (tRNAGly(GCC) and tRNAVal(AAC)) also lie within a 5’-CAm5CGCG-3’ sequence context [8,25]. Furthermore, in addition to tRNAs, the Dictyostelium discoideum DNMT2 homologue binds to the U2 small nuclear RNA, which contains two stem-loop structures containing cytosines in equivalent sequence contexts to C38 within the anticodon loop of tRNAAsp [74]. Interestingly, mutations within the variable loop of DNMT2-substrate tRNAs were found to reduce C38 methylation, suggesting that this structural feature also contributes to enzyme binding or substrate specificity [75].
The fact that only single (mt)-rRNA nucleotides have been identified as NSUN1, NSUN5, and NSUN4 substrates, together with the challenges of mutagenic studies on rRNAs, means less is known about how these enzymes recognize their targets. In the case of NSUN4, preferential binding to double stranded RNA substrates was observed in vitro [17]. However, as the modifications introduced by these enzymes occur within large ribonucleoprotein complexes, it is possible that protein-protein, as well as protein-RNA interactions, contribute to their recruitment to their sites of action. Indeed, the RNA-binding protein MTERF4 is suggested to act as a cofactor for NSUN4 [58], which in contrast to the other NSUN proteins, lacks an RRM domain. Structural analysis of the NSUN4-MTERF heterodimer identified a putative RNA-binding groove that could contribute to correct positioning of the substrate RNA in the active site of NSUN4 [76,77].
5. Roles of m5C RNA Methyltransferases in Development and Disease
Consistent with the important roles that m5C methyltransferases play in RNA metabolism, mutations in the genes encoding these enzymes have been linked to various human diseases and changes in expression levels of m5C methyltransferases have been observed in various cancers. Loss of function mutations in NSUN2 underlie several neurodevelopmental disorders (reviewed in [78]). A homozygous mutation in the NSUN2 gene that leads to the substitution of glycine 679 for arginine (p.Gly679Arg) in the protein has been detected in individuals with autosomal-recessive intellectual disability [79]. This amino acid substitution is suggested to impede NSUN2 function by preventing localization of the protein to its site of action in the nucleolus. NSUN2 has also been linked to Dubowitz syndrome, which is characterized by microcephaly, growth and mental retardation, eczema, and characteristic facial features; a homozygous mutation in the canonical splice acceptor of exon 6 leads to use of a cryptic splice donor, instability of the NSUN2 mRNA, a significant decrease in protein levels, and reduced methylation of NSUN2 target RNAs (m5C47/48 of tRNAAsp(GUC) [80]. In mice, the accumulation of 5’ tRNA fragments caused by lack of NSUN2-mediated tRNA methylation has been found to impair neurogenesis leading to decreased production of upper-layer neurons and reduced brain development [81], perhaps suggesting a mechanistic basis for the neurodevelopmental disorders observed in humans with impaired NSUN2 function.
Mutations in NSUN3 that lead to either aberrant splicing and frameshifting (p.Glu42Valfs*11) or the introduction of a premature stop codon (c.295C>T/p.Arg99*) have been detected in patients with a mitochondrial deficiency disorder characterized by developmental disability microcephaly, failure to thrive, recurrent increased lactate levels in plasma, muscular weakness, proximal accentuated, external ophthalmoplegia, and convergence nystagmus [14]. Furthermore, mitochondrial disease-associated point mutations with the gene encoding mt-tRNAMet that lead to A37G and C39U substitutions have been shown to impede methylation of C34 by NSUN3 [15,16]. In both cases, lack of NSUN3-mediated modification impairs mitochondrial translation, leading to reduced mitochondrial function. Interestingly, lack of NSUN3 impedes the differentiation of mouse embryonic stem cells towards the neuroectoderm lineage, implying that reduced mitochondrial translation affects the normal differentiation program [82].
Studies in mice show that during development, NSUN7 is expressed in a broad range of tissues [83], but in adults, is predominantly present in testis cells, especially spermatocytes and haploid spermatids. Furthermore, a chemically-induced mutation that leads to conversion of glutamine 333 to a stop codon (p.Gln333*) was shown to cause reduced sperm motility leading to sterility or subfertility [84]. Likewise, point mutations in exon 4 and exon 7 of NSUN7 that convert valine 157 to a premature stop codon (p.Val157*) and induce a serine to alanine exchange have been identified in asthenospermic men [85,86]. While NSUN7, therefore, appears to be important for male fertility, it remains unknown whether the methyltransferase activity of NSUN7 on its eRNA targets is involved or if NSUN7 has additional cellular functions that are perturbed by these mutations.
So far, no specific disease-linked mutations have been identified in the genes encoding the rRNA m5C methyltransferases, NSUN1, NSUN5, and NSUN4. However, abolition of NSUN4 is embryonically lethal and a conditional NSUN4 knockout in mouse heart tissue was found to cause cardiomyopathy [17]. NSUN5 lies within the Williams-Beuren Syndrome critical region, an approximately 1.5 Mb deletion at chromosome 7q11.23, raising the possibility that lack of NSUN5 or the 28S-m5C3761 may contribute to this multisystemic disorder [87]. Furthermore, expression of the Drosophila melanogaster and Caenorhabditis elegans NSUN5 homologs is decreased in senescent cells. This reduction of NSUN5 and its cognate RNA modification is proposed to contribute to increasing organism lifespan by promoting the translation of stress-related mRNAs, thereby negating aging-associated effects [18].
6. Conclusions and Outlook
The identification of RNA substrates of the seven NSUN proteins and DNMT2 has propelled forward understanding of the roles of m5C modifications in the regulation of gene expression by revealing important roles in cytoplasmic and mitochondrial ribosome assembly and translation, as well as in regulating tRNA stability, mRNA export, and transcription. While the development of various m5C mapping approaches has significantly expanded the repertoire of potential m5C sites within the transcriptome, the true extent and precise positions of m5C modifications in low abundance RNA species still requires further clarification. Although quantitative analyses suggest that the m5C target sites in (mt-)rRNAs and (mt-)tRNAs are typically fully modified, it is likely that m5Cs in other RNA species are present at sub-stoichiometric levels. This highlights the potential for differential m5C modification to be used to regulate the fate of particular RNA species in different conditions. This concept is further supported by the identification of ALYREF as the first m5C “reader” protein as well as the discovery that like 5mC modifications in DNA, m5Cs in RNAs can be intermediates in the generation of other modifications. The oxidized derivative of m5C, 5-hydroxymethylcytosine (hm5C), has recently been identified in D. melanogaster RNAs, where it was suggested to promote translation of specific mRNAs involved in basic cellular processes and embryogenesis [88]. Furthermore, 5-formylcytosine (f5C) has a well-established role in expanding codon recognition by mt-tRNAMet during mitochondrial translation [61,62], and its presence has recently been reported in yeast mRNAs [89]. It remains to be determined if, in these contexts, m5C merely represents a transient intermediate or if a dynamic equilibrium between m5C and its oxidized products has functional relevance. In the future, it will be important to understand how the action of m5C methyltransferases is coordinated with other m5C-interacting proteins or enzymes.
Author Contributions
Conceptualization, K.E.B. and M.T.B.; writing—original draft preparation, K.E.B., C.H., and M.T.B; writing—review and editing, K.E.B., C.H., and M.T.B.; funding acquisition, M.T.B and C.H.
Funding
This research was funded by Deutsche Forschungsgemeinschaft (SPP1784: BO3442/2-2 and HO4436/2-2) and the University Medical Center Göttingen. Publication of this manuscript was funded by the COST action, EPITRAN CA16120.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Boccaletto P., Machnicka M.A., Purta E., Piatkowski P., Baginski B., Wirecki T.K., de Crecy-Lagard V., Ross R., Limbach P.A., Kotter A., et al. MODOMICS: a database of RNA modification pathways. 2017 update. Nucleic Acids Res. 2018;46:D303–D307. doi: 10.1093/nar/gkx1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breiling A., Lyko F. Epigenetic regulatory functions of DNA modifications: 5-methylcytosine and beyond. Epigenetics Chromatin. 2015;8:24. doi: 10.1186/s13072-015-0016-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trixl L., Lusser A. The dynamic RNA modification 5-methylcytosine and its emerging role as an epitranscriptomic mark. Wiley Interdiscip. Rev. RNA. 2019;10:e1510. doi: 10.1002/wrna.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lyko F. The DNA methyltransferase family: a versatile toolkit for epigenetic regulation. Nat. Rev. Genet. 2018;19:81–92. doi: 10.1038/nrg.2017.80. [DOI] [PubMed] [Google Scholar]
- 5.Bourgeois G., Ney M., Gaspar I., Aigueperse C., Schaefer M., Kellner S., Helm M., Motorin Y. Eukaryotic rRNA modification by yeast 5- methylcytosine-methyltransferases and human proliferation-associated antigen p120. PLoS One. 2015;10:1–16. doi: 10.1371/journal.pone.0133321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brzezicha B., Schmidt M., Makałowska I., Jarmołowski A., Pieńkowska J., Szweykowska-Kulińska Z. Identification of human tRNA: m5C methyltransferase catalysing intron-dependent m5C formation in the first position of the anticodon of the pre-tRNA(CAA)Leu. Nucleic Acids Res. 2006;34:6034–6043. doi: 10.1093/nar/gkl765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blanco S., Dietmann S., Flores J.V., Hussain S., Kutter C., Humphreys P., Lukk M., Lombard P., Treps L., Popis M., et al. Aberrant methylation of tRNAs links cellular stress to neuro-developmental disorders. EMBO J. 2014;33:2020–2039. doi: 10.15252/embj.201489282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tuorto F., Liebers R., Musch T., Schaefer M., Hofmann S., Kellner S., Frye M., Helm M., Stoecklin G., Lyko F. RNA cytosine methylation by Dnmt2 and NSun2 promotes tRNA stability and protein synthesis. Nat. Struct. Mol. Biol. 2012;19:900–905. doi: 10.1038/nsmb.2357. [DOI] [PubMed] [Google Scholar]
- 9.Hussain S., Sajini A.A., Blanco S., Dietmann S., Lombard P., Sugimoto Y., Paramor M., Gleeson J.G., Odom D.T., Ule J., et al. NSun2-mediated cytosine-5 methylation of vault noncoding RNA determines its processing into regulatory small RNAs. Cell Rep. 2013;4:255–261. doi: 10.1016/j.celrep.2013.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang X., Yang Y., Sun B.-F., Chen Y.-S., Xu J.-W., Lai W.-Y., Li A., Wang X., Bhattarai D.P., Xiao W., et al. 5-methylcytosine promotes mRNA export - NSUN2 as the methyltransferase and ALYREF as an m(5)C reader. Cell Res. 2017;27:606–625. doi: 10.1038/cr.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang H., Fan X., Xing J., Liu Z., Jiang B., Dou Y., Gorospe M., Wang W. NSun2 delays replicative senescence by repressing p27 (KIP1) translation and elevating CDK1 translation. Aging (Albany. NY). 2015;7:1143–1158. doi: 10.18632/aging.100860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xing J., Yi J., Cai X., Tang H., Liu Z., Zhang X., Martindale J.L., Yang X., Jiang B., Gorospe M., et al. NSun2 Promotes Cell Growth via Elevating Cyclin-Dependent Kinase 1 Translation. Mol. Cell. Biol. 2015;35:4043–4052. doi: 10.1128/MCB.00742-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Q., Li X., Tang H., Jiang B., Dou Y., Gorospe M., Wang W. NSUN2-Mediated m5C Methylation and METTL3/METTL14-Mediated m6A Methylation Cooperatively Enhance p21 Translation. J. Cell. Biochem. 2017;118:2587–2598. doi: 10.1002/jcb.25957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Haute L., Dietmann S., Kremer L., Hussain S., Pearce S.F., Powell C.A., Rorbach J., Lantaff R., Blanco S., Sauer S., et al. Deficient methylation and formylation of mt-tRNA(Met) wobble cytosine in a patient carrying mutations in NSUN3. Nat. Commun. 2016;7:12039. doi: 10.1038/ncomms12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haag S., Sloan K.E., Ranjan N., Warda A.S., Kretschmer J., Blessing C., Hübner B., Seikowski J., Dennerlein S., Rehling P., et al. NSUN3 and ABH1 modify the wobble position of mt-tRNAMet to expand codon recognition in mitochondrial translation. Embo J. 2016;35:2104–2119. doi: 10.15252/embj.201694885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakano S., Suzuki T., Kawarada L., Iwata H., Asano K., Suzuki T. NSUN3 methylase initiates 5-formylcytidine biogenesis in human mitochondrial tRNAMet. Nat. Chem. Biol. 2016;12:546–551. doi: 10.1038/nchembio.2099. [DOI] [PubMed] [Google Scholar]
- 17.Metodiev M.D., Spahr H., Loguercio Polosa P., Meharg C., Becker C., Altmueller J., Habermann B., Larsson N.-G., Ruzzenente B. NSUN4 is a dual function mitochondrial protein required for both methylation of 12S rRNA and coordination of mitoribosomal assembly. PLoS Genet. 2014;10:e1004110. doi: 10.1371/journal.pgen.1004110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schosserer M., Minois N., Angerer T.B., Amring M., Dellago H., Harreither E., Calle-Perez A., Pircher A., Gerstl M.P., Pfeifenberger S., et al. Methylation of ribosomal RNA by NSUN5 is a conserved mechanism modulating organismal lifespan. Nat. Commun. 2015;6:6158. doi: 10.1038/ncomms7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gigova A., Duggimpudi S., Pollex T., Pollex T.I.M., Schaefer M. A cluster of methylations in the domain IV of 25S rRNA is required for ribosome stability A cluster of methylations in the domain IV of 25S rRNA is required for ribosome stability. RNA. 2014;20:1632–1644. doi: 10.1261/rna.043398.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma S., Yang J., Watzinger P., Kötter P., Entian K.D. Yeast Nop2 and Rcm1 methylate C2870 and C2278 of the 25S rRNA, respectively. Nucleic Acids Res. 2013;41:9062–9076. doi: 10.1093/nar/gkt679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu R.-J., Long T., Li J., Li H., Wang E.-D. Structural basis for substrate binding and catalytic mechanism of a human RNA:m5C methyltransferase NSun6. Nucleic Acids Res. 2017;45:6684–6697. doi: 10.1093/nar/gkx473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haag S., Warda A.S., Kretschmer J., Günnigmann M.A., Höbartner C., Bohnsack M.T. NSUN6 is a human RNA methyltransferase that catalyzes formation of m5C72 in specific tRNAs. RNA. 2015;21:1532–1543. doi: 10.1261/rna.051524.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aguilo F., Li S., Balasubramaniyan N., Sancho A., Benko S., Zhang F., Vashisht A., Rengasamy M., Andino B., Chen C.-H., et al. Deposition of 5-Methylcytosine on Enhancer RNAs Enables the Coactivator Function of PGC-1alpha. Cell Rep. 2016;14:479–492. doi: 10.1016/j.celrep.2015.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goll M.G., Kirpekar F., Maggert K.A., Yoder J.A., Hsieh C.-L., Zhang X., Golic K.G., Jacobsen S.E., Bestor T.H. Methylation of tRNAAsp by the DNA methyltransferase homolog Dnmt2. Science. 2006;311:395–398. doi: 10.1126/science.1120976. [DOI] [PubMed] [Google Scholar]
- 25.Schaefer M., Pollex T., Hanna K., Tuorto F., Meusburger M., Helm M., Lyko F. RNA methylation by Dnmt2 protects transfer RNAs against stress-induced cleavage. Genes Dev. 2010;24:1590–1595. doi: 10.1101/gad.586710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reid R., Greene P.J., Santi D.V. Exposition of a family of RNA m(5)C methyltransferases from searching genomic and proteomic sequences. Nucleic Acids Res. 1999;27:3138–3145. doi: 10.1093/nar/27.15.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y., Santi D.V. m5C RNA and m5C DNA methyl transferases use different cysteine residues as catalysts. Proc. Natl. Acad. Sci. U. S. A. 2000;97:8263–8265. doi: 10.1073/pnas.97.15.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King M.Y., Redman K.L. RNA methyltransferases utilize two cysteine residues in the formation of 5-methylcytosine. Biochemistry. 2002;41:11218–11225. doi: 10.1021/bi026055q. [DOI] [PubMed] [Google Scholar]
- 29.King M., Ton D., Redman K.L. A conserved motif in the yeast nucleolar protein Nop2p contains an essential cysteine residue. Biochem. J. 1999;337:29–35. doi: 10.1042/bj3370029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Redman K.L. Assembly of protein-RNA complexes using natural RNA and mutant forms of an RNA cytosine methyltransferase. Biomacromolecules. 2006;7:3321–3326. doi: 10.1021/bm051012l. [DOI] [PubMed] [Google Scholar]
- 31.Hussain S., Benavente S.B., Nascimento E., Dragoni I., Kurowski A., Gillich A., Humphreys P., Frye M. The nucleolar RNA methyltransferase Misu (NSun2) is required for mitotic spindle stability. J. Cell Biol. 2009;186:27–40. doi: 10.1083/jcb.200810180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeltsch A. Beyond Watson and Crick: DNA methylation and molecular enzymology of DNA methyltransferases. Chembiochem. 2002;3:274–293. doi: 10.1002/1439-7633(20020402)3:4<274::AID-CBIC274>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 33.Cheng X. Structure and function of DNA methyltransferases. Annu. Rev. Biophys. Biomol. Struct. 1995;24:293–318. doi: 10.1146/annurev.bb.24.060195.001453. [DOI] [PubMed] [Google Scholar]
- 34.Osterman D.G., DePillis G.D., Wu J.C., Matsuda A., Santi D.V. 5-Fluorocytosine in DNA is a mechanism-based inhibitor of HhaI methylase. Biochemistry. 1988;27:5204–5210. doi: 10.1021/bi00414a039. [DOI] [PubMed] [Google Scholar]
- 35.Gabbara S., Bhagwat A.S. The mechanism of inhibition of DNA (cytosine-5-)-methyltransferases by 5-azacytosine is likely to involve methyl transfer to the inhibitor. Biochem. J. 1995;307:87–92. doi: 10.1042/bj3070087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watkins N.J., Bohnsack M.T. The box C/D and H/ACA snoRNPs: Key players in the modification, processing and the dynamic folding of ribosomal RNA. Wiley Interdiscip. Rev. RNA. 2012;3:397–414. doi: 10.1002/wrna.117. [DOI] [PubMed] [Google Scholar]
- 37.Sloan K.E., Warda A.S., Sharma S., Entian K.-D., Lafontaine D.L.J., Bohnsack M.T. Tuning the ribosome: The influence of rRNA modification on eukaryotic ribosome biogenesis and function. RNA Biol. 2017;14:1138–1152. doi: 10.1080/15476286.2016.1259781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hayrapetyan A., Grosjean H., Helm M. Effect of a quaternary pentamine on RNA stabilization and enzymatic methylation. Biol. Chem. 2009;390:851–861. doi: 10.1515/BC.2009.096. [DOI] [PubMed] [Google Scholar]
- 39.Motorin Y., Helm M. tRNA stabilization by modified nucleotides. Biochemistry. 2010;49:4934–4944. doi: 10.1021/bi100408z. [DOI] [PubMed] [Google Scholar]
- 40.Sharma S., Lafontaine D.L.J. “View From A Bridge”: A New Perspective on Eukaryotic rRNA Base Modification. Trends Biochem. Sci. 2015;40:560–575. doi: 10.1016/j.tibs.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 41.Taoka M., Nobe Y., Yamaki Y., Yamauchi Y., Ishikawa H., Takahashi N., Nakayama H., Isobe T. The complete chemical structure of Saccharomyces cerevisiae rRNA: Partial pseudouridylation of U2345 in 25S rRNA by snoRNA snR9. Nucleic Acids Res. 2016;44:8951–8961. doi: 10.1093/nar/gkw564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hong B., Brockenbrough J.S., Wu P., Aris J.P. Nop2p is required for pre-rRNA processing and 60S ribosome subunit synthesis in yeast. Mol. Cell. Biol. 1997;17:378–388. doi: 10.1128/MCB.17.1.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sloan K.E., Bohnsack M.T., Watkins N.J. The 5S RNP Couples p53 Homeostasis to Ribosome Biogenesis and Nucleolar Stress. Cell Rep. 2013;5:237–247. doi: 10.1016/j.celrep.2013.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meyer B., Wurm J.P., Kotter P., Leisegang M.S., Schilling V., Buchhaupt M., Held M., Bahr U., Karas M., Heckel A., et al. The Bowen-Conradi syndrome protein Nep1 (Emg1) has a dual role in eukaryotic ribosome biogenesis, as an essential assembly factor and in the methylation of Psi1191 in yeast 18S rRNA. Nucleic Acids Res. 2011;39:1526–1537. doi: 10.1093/nar/gkq931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haag S., Kretschmer J., Bohnsack M.T. WBSCR22/Merm1 is required for late nuclear pre-ribosomal RNA processing and mediates N7-methylation of G1639 in human 18S rRNA. RNA. 2015;21:180–187. doi: 10.1261/rna.047910.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schaefer M., Steringer J.P., Lyko F. The Drosophila cytosine-5 methyltransferase Dnmt2 is associated with the nuclear matrix and can access DNA during mitosis. PLoS One. 2008;3:e1414. doi: 10.1371/journal.pone.0001414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pais de Barros J.P., Keith G., El Adlouni C., Glasser A.L., Mack G., Dirheimer G., Desgres J. 2’-O-methyl-5-formylcytidine (f5Cm), a new modified nucleotide at the “wobble” of two cytoplasmic tRNAs Leu (NAA) from bovine liver. Nucleic Acids Res. 1996;24:1489–1496. doi: 10.1093/nar/24.8.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kawarada L., Suzuki T., Ohira T., Hirata S., Miyauchi K., Suzuki T. ALKBH1 is an RNA dioxygenase responsible for cytoplasmic and mitochondrial tRNA modifications. Nucleic Acids Res. 2017;45:7401–7415. doi: 10.1093/nar/gkx354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chan C.T.Y., Pang Y.L.J., Deng W., Babu I.R., Dyavaiah M., Begley T.J., Dedon P.C. Reprogramming of tRNA modifications controls the oxidative stress response by codon-biased translation of proteins. Nat. Commun. 2012;3:937. doi: 10.1038/ncomms1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shanmugam R., Fierer J., Kaiser S., Helm M., Jurkowski T.P., Jeltsch A. Cytosine methylation of tRNA-Asp by DNMT2 has a role in translation of proteins containing poly-Asp sequences. Cell Discov. 2015;1:15010. doi: 10.1038/celldisc.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tuorto F., Herbst F., Alerasool N., Bender S., Popp O., Federico G., Reitter S., Liebers R., Stoecklin G., Grone H.-J., et al. The tRNA methyltransferase Dnmt2 is required for accurate polypeptide synthesis during haematopoiesis. EMBO J. 2015;34:2350–2362. doi: 10.15252/embj.201591382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muller M., Hartmann M., Schuster I., Bender S., Thuring K.L., Helm M., Katze J.R., Nellen W., Lyko F., Ehrenhofer-Murray A.E. Dynamic modulation of Dnmt2-dependent tRNA methylation by the micronutrient queuine. Nucleic Acids Res. 2015;43:10952–10962. doi: 10.1093/nar/gkv980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tuorto F., Legrand C., Cirzi C., Federico G., Liebers R., Muller M., Ehrenhofer-Murray A.E., Dittmar G., Grone H.-J., Lyko F. Queuosine-modified tRNAs confer nutritional control of protein translation. EMBO J. 2018;37:e99777. doi: 10.15252/embj.201899777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ehrenhofer-Murray A.E. Cross-Talk between Dnmt2-Dependent tRNA Methylation and Queuosine Modification. Biomolecules. 2017;7:14. doi: 10.3390/biom7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Levitt M. Detailed molecular model for transfer ribonucleic acid. Nature. 1969;224:759–763. doi: 10.1038/224759a0. [DOI] [PubMed] [Google Scholar]
- 56.Vare V.Y.P., Eruysal E.R., Narendran A., Sarachan K.L., Agris P.F. Chemical and Conformational Diversity of Modified Nucleosides Affects tRNA Structure and Function. Biomolecules. 2017;7:29. doi: 10.3390/biom7010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li J., Li H., Long T., Dong H., Wang E.-D., Liu R.-J. Archaeal NSUN6 catalyzes m5C72 modification on a wide-range of specific tRNAs. Nucleic Acids Res. 2018 doi: 10.1093/nar/gky1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cámara Y., Asin-Cayuela J., Park C.B., Metodiev M.D., Shi Y., Ruzzenente B., Kukat C., Habermann B., Wibom R., Hultenby K., et al. MTERF4 regulates translation by targeting the methyltransferase NSUN4 to the mammalian mitochondrial ribosome. Cell Metab. 2011;13:527–539. doi: 10.1016/j.cmet.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 59.Dudek J., Rehling P., van der Laan M. Mitochondrial protein import: common principles and physiological networks. Biochim. Biophys. Acta. 2013;1833:274–285. doi: 10.1016/j.bbamcr.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 60.Sloan K.E., Hobartner C., Bohnsack M.T. How RNA modification allows non-conventional decoding in mitochondria. Cell Cycle. 2017;16:145–146. doi: 10.1080/15384101.2016.1235860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bilbille Y., Gustilo E.M., Harris K.A., Jones C.N., Lusic H., Kaiser R.J., Delaney M.O., Spremulli L.L., Deiters A., Agris P.F. The human mitochondrial tRNAMet: Structure/function relationship of a unique modification in the decoding of unconventional codons. J. Mol. Biol. 2011;406:257–274. doi: 10.1016/j.jmb.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cantara W.A., Murphy F.V., Demirci H., Agris P.F. Expanded use of sense codons is regulated by modified cytidines in tRNA. Proc. Natl. Acad. Sci. U. S. A. 2013;110:10964–10969. doi: 10.1073/pnas.1222641110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carlile T.M., Rojas-Duran M.F., Zinshteyn B., Shin H., Bartoli K.M., Gilbert W.V. Pseudouridine profiling reveals regulated mRNA pseudouridylation in yeast and human cells. Nature. 2014;515:143–146. doi: 10.1038/nature13802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dominissini D., Moshitch-Moshkovitz S., Schwartz S., Salmon-Divon M., Ungar L., Osenberg S., Cesarkas K., Jacob-Hirsch J., Amariglio N., Kupiec M., et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201–206. doi: 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- 65.Safra M., Sas-Chen A., Nir R., Winkler R., Nachshon A., Bar-Yaacov D., Erlacher M., Rossmanith W., Stern-Ginossar N., Schwartz S. The m1A landscape on cytosolic and mitochondrial mRNA at single-base resolution. Nature. 2017;551:251–255. doi: 10.1038/nature24456. [DOI] [PubMed] [Google Scholar]
- 66.Dubin D.T., Taylor R.H. The methylation state of poly A-containing messenger RNA from cultured hamster cells. Nucleic Acids Res. 1975;2:1653–1668. doi: 10.1093/nar/2.10.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Squires J.E., Patel H.R., Nousch M., Sibbritt T., Humphreys D.T., Parker B.J., Suter C.M., Preiss T. Widespread occurrence of 5-methylcytosine in human coding and non-coding RNA. Nucleic Acids Res. 2012;40:5023–5033. doi: 10.1093/nar/gks144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Amort T., Rieder D., Wille A., Khokhlova-Cubberley D., Riml C., Trixl L., Jia X.-Y., Micura R., Lusser A. Distinct 5-methylcytosine profiles in poly(A) RNA from mouse embryonic stem cells and brain. Genome Biol. 2017;18:1. doi: 10.1186/s13059-016-1139-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Legrand C., Tuorto F., Hartmann M., Liebers R., Jacob D., Helm M., Lyko F. Statistically robust methylation calling for whole-transcriptome bisulfite sequencing reveals distinct methylation patterns for mouse RNAs. Genome Res. 2017;27:1589–1596. doi: 10.1101/gr.210666.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.David R., Burgess A., Parker B., Li J., Pulsford K., Sibbritt T., Preiss T., Searle I.R. Transcriptome-Wide Mapping of RNA 5-Methylcytosine in Arabidopsis mRNAs and Noncoding RNAs. Plant Cell. 2017;29:445–460. doi: 10.1105/tpc.16.00751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Edelheit S., Schwartz S., Mumbach M.R., Wurtzel O., Sorek R. Transcriptome-wide mapping of 5-methylcytidine RNA modifications in bacteria, archaea, and yeast reveals m5C within archaeal mRNAs. PLoS Genet. 2013;9:e1003602. doi: 10.1371/journal.pgen.1003602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ghanbarian H., Wagner N., Polo B., Baudouy D., Kiani J., Michiels J.-F., Cuzin F., Rassoulzadegan M., Wagner K.-D. Dnmt2/Trdmt1 as Mediator of RNA Polymerase II Transcriptional Activity in Cardiac Growth. PLoS One. 2016;11:e0156953. doi: 10.1371/journal.pone.0156953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Long T., Li J., Li H., Zhou M., Zhou X.-L., Liu R.-J., Wang E.-D. Sequence-specific and Shape-selective RNA Recognition by the Human RNA 5-Methylcytosine Methyltransferase NSun6. J. Biol. Chem. 2016;291:24293–24303. doi: 10.1074/jbc.M116.742569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Muller S., Windhof I.M., Maximov V., Jurkowski T., Jeltsch A., Forstner K.U., Sharma C.M., Graf R., Nellen W. Target recognition, RNA methylation activity and transcriptional regulation of the Dictyostelium discoideum Dnmt2-homologue (DnmA) Nucleic Acids Res. 2013;41:8615–8627. doi: 10.1093/nar/gkt634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shanmugam R., Aklujkar M., Schafer M., Reinhardt R., Nickel O., Reuter G., Lovley D.R., Ehrenhofer-Murray A., Nellen W., Ankri S., et al. The Dnmt2 RNA methyltransferase homolog of Geobacter sulfurreducens specifically methylates tRNA-Glu. Nucleic Acids Res. 2014;42:6487–6496. doi: 10.1093/nar/gku256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Spahr H., Habermann B., Gustafsson C.M., Larsson N.-G., Hallberg B.M. Structure of the human MTERF4-NSUN4 protein complex that regulates mitochondrial ribosome biogenesis. Proc. Natl. Acad. Sci. U. S. A. 2012;109:15253–15258. doi: 10.1073/pnas.1210688109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yakubovskaya E., Guja K.E., Mejia E., Castano S., Hambardjieva E., Choi W.S., Garcia-Diaz M. Structure of the essential MTERF4:NSUN4 protein complex reveals how an MTERF protein collaborates to facilitate rRNA modification. Structure. 2012;20:1940–1947. doi: 10.1016/j.str.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Blanco S., Frye M. Role of RNA methyltransferases in tissue renewal and pathology. Curr. Opin. Cell Biol. 2014;31:1–7. doi: 10.1016/j.ceb.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Khan M.A., Rafiq M.A., Noor A., Hussain S., Flores J.V., Rupp V., Vincent A.K., Malli R., Ali G., Khan F.S., et al. Mutation in NSUN2, which encodes an RNA methyltransferase, causes autosomal-recessive intellectual disability. Am. J. Hum. Genet. 2012;90:856–863. doi: 10.1016/j.ajhg.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Martinez F.J., Lee J.H., Lee J.E., Blanco S., Nickerson E., Gabriel S., Frye M., Al-Gazali L., Gleeson J.G. Whole exome sequencing identifies a splicing mutation in NSUN2 as a cause of a Dubowitz-like syndrome. J. Med. Genet. 2012;49:380–385. doi: 10.1136/jmedgenet-2011-100686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Flores J.V., Cordero-Espinoza L., Oeztuerk-Winder F., Andersson-Rolf A., Selmi T., Blanco S., Tailor J., Dietmann S., Frye M. Cytosine-5 RNA Methylation Regulates Neural Stem Cell Differentiation and Motility. Stem cell reports. 2017;8:112–124. doi: 10.1016/j.stemcr.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Trixl L., Amort T., Wille A., Zinni M., Ebner S., Hechenberger C., Eichin F., Gabriel H., Schoberleitner I., Huang A., et al. RNA cytosine methyltransferase Nsun3 regulates embryonic stem cell differentiation by promoting mitochondrial activity. Cell. Mol. Life Sci. 2018;75:1483–1497. doi: 10.1007/s00018-017-2700-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chi L., Delgado-Olguin P. Expression of NOL1/NOP2/sun domain (Nsun) RNA methyltransferase family genes in early mouse embryogenesis. Gene Expr. Patterns. 2013;13:319–327. doi: 10.1016/j.gep.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 84.Harris T., Marquez B., Suarez S., Schimenti J. Sperm motility defects and infertility in male mice with a mutation in Nsun7, a member of the Sun domain-containing family of putative RNA methyltransferases. Biol. Reprod. 2007;77:376–382. doi: 10.1095/biolreprod.106.058669. [DOI] [PubMed] [Google Scholar]
- 85.Khosronezhad N., Colagar A.H., Jorsarayi S.G.A. T26248G-transversion mutation in exon7 of the putative methyltransferase Nsun7 gene causes a change in protein folding associated with reduced sperm motility in asthenospermic men. Reprod. Fertil. Dev. 2015;27:471–480. doi: 10.1071/RD13371. [DOI] [PubMed] [Google Scholar]
- 86.Khosronezhad N., Hosseinzadeh Colagar A., Mortazavi S.M. The Nsun7 (A11337)-deletion mutation, causes reduction of its protein rate and associated with sperm motility defect in infertile men. J. Assist. Reprod. Genet. 2015;32:807–815. doi: 10.1007/s10815-015-0443-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Doll A., Grzeschik K.H. Characterization of two novel genes, WBSCR20 and WBSCR22, deleted in Williams-Beuren syndrome. Cytogenet. Cell Genet. 2001;95:20–27. doi: 10.1159/000057012. [DOI] [PubMed] [Google Scholar]
- 88.Delatte B., Wang F., Ngoc L.V., Collignon E., Bonvin E., Deplus R., Calonne E., Hassabi B., Putmans P., Awe S., et al. Transcriptome-wide distribution and function of RNA hydroxymethylcytosine. Science. 2016;351:282–285. doi: 10.1126/science.aac5253. [DOI] [PubMed] [Google Scholar]
- 89.Tardu M., Lin Q., Koutmou K.S. N4-acetylcytidine and 5-formylcytidine are present in Saccharomyces cerevisiae mRNAs. bioRxiv. 2018 [Google Scholar]