Abstract
Quorum sensing (QS) is closely associated with the production of multiple virulence factors in bacterial pathogens. N-acyl homoserine lactones (AHLs) are important QS signal molecules that modulate the virulence of gram-negative pathogenic bacteria. Enzymatic degradation of AHLs to interrupt QS, termed quorum quenching (QQ), has been considered a novel strategy for reduction of pathogenicity and prevention of bacterial disease. However, the low expression levels of QQ proteins in the original host bacteria has affected the applications of these proteins. Previously, we identified a novel marine QQ enzyme, named MomL, with high activity and promising biocontrol function. In this study, we linked the target fragment momL to pNCMO2, which provided a basis for the first heterologous expression of MomL in the antifungal and anti-gram-positive-bacteria biocontrol strain Bacillus brevis, and obtaining the recombinant strain named BbMomL. The QQ activity of BbMomL was confirmed using a series of bioassays. BbMomL could not only degrade the exogenous signal molecule C6-HSL, but also the AHL signal molecules produced by the gram-negative pathogens Pectobacterium carotovorum subsp. carotovorum (Pcc) and Pseudomonas aeruginosa PAO1. In addition, BbMomL significantly reduced the secretion of pathogenic factors and the pathogenicity of Pcc and P. aeruginosa PAO1. We tested the biocontrol function of BbMomL for prevention of plant diseases in vitro. The result indicates that BbMomL has a broad antibacterial spectrum. Compared with wild-type B. brevis, BbMomL not only inhibited fungi and gram-positive bacterial pathogens but also considerably inhibited gram-negative bacterial pathogens. Moreover, the Bacillus brevis expression system has good application prospects and is an ideal host for expression and secretion of foreign proteins.
Keywords: quorum sensing, quorum quenching enzyme, N-acylhomoserine lactones, Bacillus brevis expression system, biological control
1. Introduction
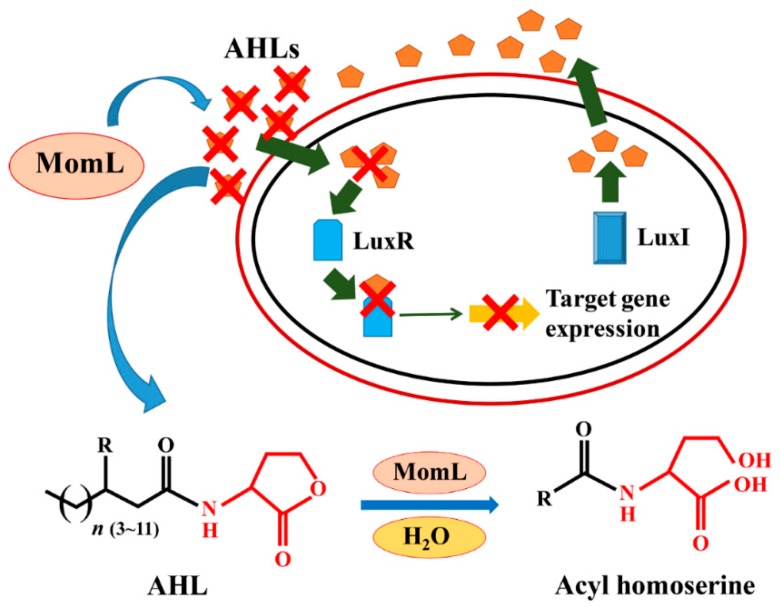
Microbial quorum sensing (QS), also known as self-induction, is a self-sensing system in which microorganisms perceive population density by diffusion of secreted small molecules (self-inducers) between cells, and these small molecules regulate the expression of a series of genes [1]. The pathogenicity of many pathogens is mediated by the QS systems. For example, Pectobacterium carotovorum subsp. carotovorum (Pcc) can cause a soft rot of various plants, such as carrot and potato [2,3]; and Pantoea stewartii subsp. stewartii can cause symptoms of corn leaf blight [4]. Quorum quenching (QQ) is environmental protection, and a disease prevention strategy, that interferes with QS between microbial cells and blocks QS-dependent gene expression to prevent pathogenic infection [5]. N-acyl homoserine lactones (AHLs) are used by gram-negative pathogenic bacteria as autoinducers for intraspecies communications, and AHL lactonase can produce the corresponding acyl homoserine via cleavage of the lactone ring, thereby blocking the communication among bacteria [6]. MomL, which was identified from Muricauda olearia Th120, is a QQ enzyme with high AHL degradation activity [7]. This enzyme belongs to the metallo-β-lactamase family, in which QQ activity can be achieved via opening the lactone ring moiety (Figure 1) [8]. MomL shares 24.5% identity with AiiA, but the degradation efficiency of C6-HSL is approximately 10 times that of AiiA [9]. Previous research has shown that MomL significantly attenuated the virulence of Pseudomonas aeruginosa in a Caenorhabditis elegans infection model and has the potential for further development and application [7].
Figure 1.
Mechanism of MomL-mediated degradation of N-acyl homoserine lactones (AHL) signal molecules.
The selection of a suitable heterologous host, as well as efficient cloning, are key factors associated with successful heterologous expression [10]. Currently, the commonly used expression systems are the Escherichia coli expression system, the Bacillus subtilis expression system and the yeast expression system. In this paper, we use a prokaryotic expression system, namely, the Bacillus brevis expression system [11,12,13,14]. The B. brevis expression system, characterized by the high-efficiency secretory expression, offers excellent protein production characteristics, allowing the production of a large number of heterologous proteins. Moreover, B. brevis can secrete a variety of active substances, such as chitinase and gramicidin, which have strong inhibitory effects on many pathogenic fungi and especially on gram-positive bacteria, but the effect on gram-negative bacteria was weak [15,16]. Tostadin, a novel small antibacterial peptide, was obtained from the liquid culture of Brevibacillus brevis XDH, which is a broad-spectrum antagonistic bacterium. The study showed that this peptide had a strong inhibitory effect on many pathogens both in vivo and in vitro [15]. Gramicidin S and polymyxins are small cationic cyclic peptides that act as effective antibiotics against pathogenic bacteria by disrupting the integrity of bacterial membranes [16]. AiiA, the first identified AHL lactonase with AHL degradation activity, has been identified in several strains of Bacillus species [17,18]. Previous reports have suggested that the AiiA in gram-positive bacteria plays a greater role in detoxification than in quenching [19]. Bacillus sp. do not produce AHL signal molecules. In theory, the heterologous expression of the AHL lactonase MomL in B. brevis does not affect intraspecific information exchange in this organism. Examples of protein production using this system are listed in Table 1. High expression levels have been achieved for a variety of proteins (enzymes, antigens and cytokines) regardless of gene origin (bacteria, archaea and eukaryotes).
Table 1.
Examples of heterologous protein expression with the Bacillus brevis expression system.
| Protein | Origin | Quantity of Expression (g/L) | References |
|---|---|---|---|
| Enzymes | |||
| Alpha-Amylase | B. licheniformis | 3.7 | [20] |
| Sphingomyelinase | B. cereus | 3.0 | [21] |
| Xylanase | B. halodurans | 0.2 | [21] |
| CGTase | B. macerans | 1.5 | [22] |
| Chitosanase | B. circulans | 1.4 | [23] |
| Hyperthermophilic protease | A. pernix | 0.1 | [24] |
| Hyperthermophilic nuclease | P. horikoshii | 0.7 | [25] |
| PDI | Human | 1.0 | [26] |
| Antigens | |||
| Surface antigen | E. rhusiopathiae | 0.9 | [21] |
| Surface antigen | T. pallidum | 0.8 | [21] |
| Cytokines | |||
| EGF | Human | 1.5 | [27] |
| IL-2 | Human | 0.6 | [28] |
| NGF | Mouse | 0.2 | [21] |
| IFN-γ | Chicken | 0.5 | [29] |
| TNF-α | Cow | 0.4 | [30] |
| GM-CSF | Cow | 0.2 | [21] |
| GH | Flounder | 0.2 | [31] |
In this study, we selected pNCMO2 and B. brevis as the shuttle expression vector and the heterologous host cell, respectively, to achieve highly efficient expression of the marine-derived QQ enzyme MomL. The P2 promoter, derived from a cell wall protein of the host bacterium, was used as the promoter for pNCMO2 expression. The virulence factors of the plant pathogen Pcc are controlled by cell density-dependent regulation, and the QQ enzymes are expected to provide a new tool for attenuation of the development of soft rot in plants. By conduction signal molecule degradation experiments and in vitro detection of the control of plant soft rot by the recombinant strain BbMomL, we found that the recombinant strain BbMomL can effectively degrade the AHL molecules produced by pathogenic bacteria, block the QS system and reduce the pathogenicity of the bacteria. Our results provide a biological strategy for disruption of bacterial QS and further expand the potential applications of B. brevis in biological control.
2. Results
2.1. Construction of the Recombinant Expression Strain BbMomL and Detection of AHL Degradation Activity
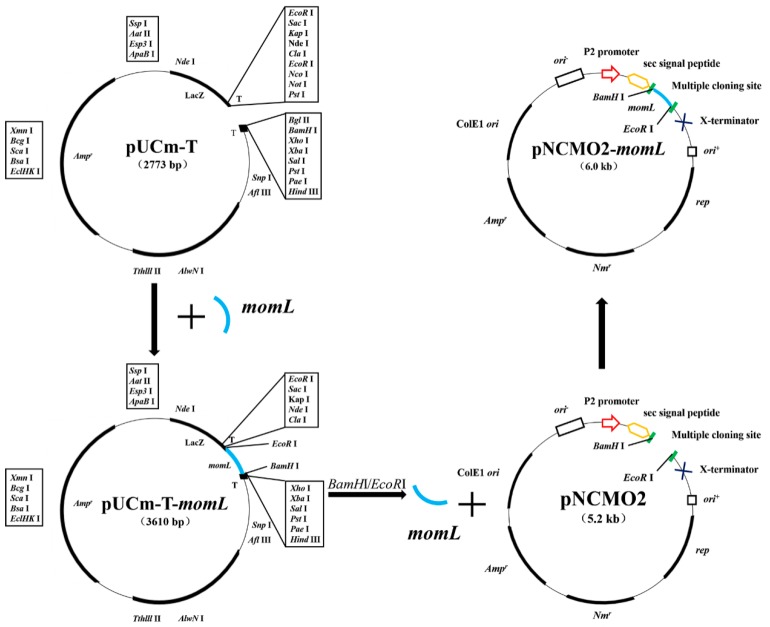
To improve the transformation efficiency and increase the possibility of successful transformation, we prepared the recombinant plasmid pUC-T-momL and transformed it into E. coli JM109. Then, we constructed the expression plasmid pNCMO2-momL using pNCMO2 as a vector, and the constructed recombinant plasmid contained an 837-bp DNA fragment of encoding the marine QQ enzyme MomL (Figure 2).
Figure 2.
Flow chart of the construction of the gene expression vector pNCMO2-momL.
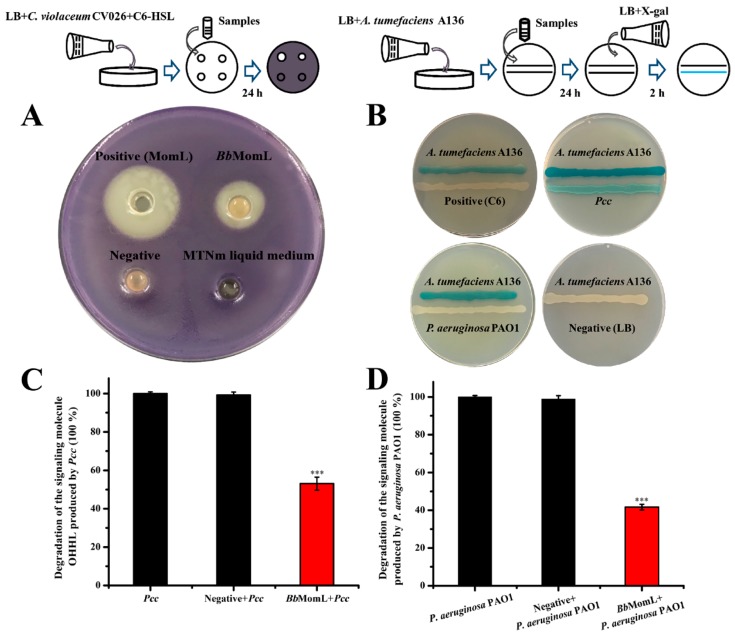
In this experiment, Chromobacterium violaceum CV026 and Agrobacterium tumefaciens A136 were used as indicator strains [32,33]. The C6-HSL, a kind of AHL signal molecule, is one of the most widely studied QS molecules. In the presence of C6-HSL, C. violaceum CV026 is capable of producing violacein, and when QQ enzymes are added, the production of violacein is inhibited. Specifically, C. violaceum CV026 did not produce violacein when the purified protein MomL or BbMomL was added, indicating that the recombinant strain BbMomL exhibits QQ activity and degrades exogenous C6-HSL (Figure 3A). The concentration of purified MomL was higher than the supernatant of BbMomL, therefore, the QQ activity of BbMomL was significantly lower than the pure MomL protein on the indicator plate. The indicator strain A. tumefaciens A136 expresses β-galactosidase and decomposes X-gal under the induction of AHL signaling molecule to produce a blue substrate. In this study, we found that A. tumefaciens A136 turned into blue under the C6-HSL, Pcc or P. aeruginosa PAO1, while the negative control did not show this phenomenon (Figure 3B). The result showed that both Pcc and P. aeruginosa PAO1 could produce AHL signaling molecules, and BbMomL had significant degradation effect on them. The results further confirmed that the marine-derived QQ enzyme MomL could be expressed in B. brevis and confer a new property to this bacterium, namely, the ability to degrade AHL signal molecules (Figure 3C,D).
Figure 3.
Detection of the quorum quenching (QQ) activity of BbMomL. (A) Degradation of the exogenous signal molecule C6-HSL by BbMomL. The concentration of the pure MomL protein was 1.363 mg/mL. (B) Determination of whether Pcc and P. aeruginosa PAO1 can generate AHL signal molecules. (C) Degradation of the 3-oxo-hexanoyl-homoserine-lactone (OHHL) signal molecule by BbMomL. (D) Degradation of the signal molecule produced by P. aeruginosa PAO1 by BbMomL. The negative control was B. brevis (pNCMO2 transformant). The results shown are representative of biological duplicates. Error bars represent the standard deviations of three replicates. A t-test of unpaired unequal variance was performed for testing differences between groups. For statistical analysis, ***, **, and * indicate P < 0.001, P < 0.01, and P < 0.05, respectively.
2.2. Effects of BbMomL on the Growth of Pcc and P. aeruginosa PAO1
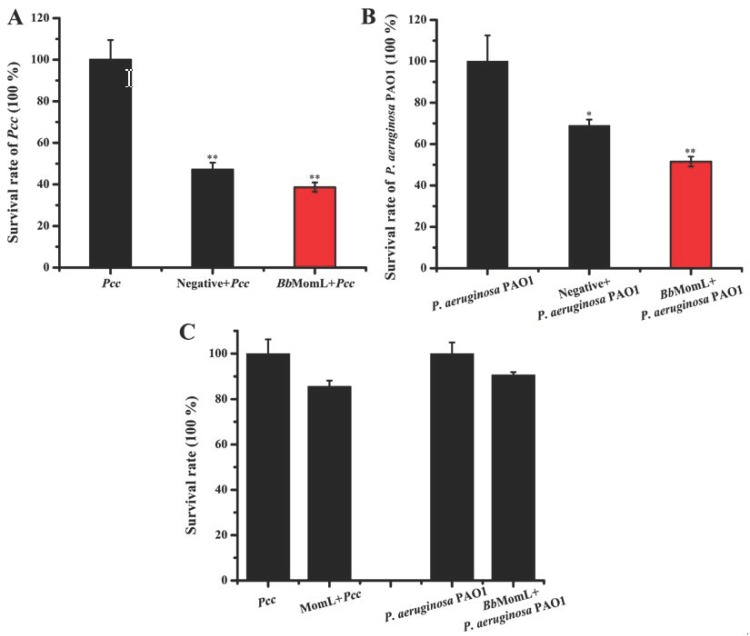
We determined the impact of MomL on the survival rate of Pcc and P. aeruginosa PAO1 using the dilution plate count method. The results showed that the survival rate of Pcc and P. aeruginosa PAO1 decreased significantly after coculture with BbMomL and B. brevis (pNCMO2 transformant), respectively (Figure 4A,B). However, it is not clear whether the decrease of the survival rate is caused by the expression of the momL gene. Therefore, subsequent supplementary experiments were carried out. We found that the survival rates of Pcc and P. aeruginosa PAO1 after coculture with pure MomL protein were not significantly reduced (Figure 4C). This finding indicates that the QQ enzyme MomL does not directly inhibit bacterial growth and does not exert a selection pressure on bacterial survival.
Figure 4.
Effects of BbMomL on the survival rates of Pcc and P. aeruginosa PAO1. (A) Effect of BbMomL on the survival rate of Pcc. (B) Effect of BbMomL on the survival rate of P. aeruginosa PAO1. (C) Effects of the recombinant MomL protein on the survival rates of Pcc and P. aeruginosa PAO1. The concentration of the pure MomL protein was 1.363 mg/mL. The negative control was B. brevis (pNCMO2 transformant). The results shown are representative of biological duplicates. Error bars represent the standard deviations of three replicates. A t-test of unpaired unequal variance was performed for testing differences between groups. For statistical analysis, ** and * indicate P < 0.01 and P < 0.05, respectively.
2.3. Isolation of the BbMomL Extracellular Protein and Analysis of AHL Degradation Activity
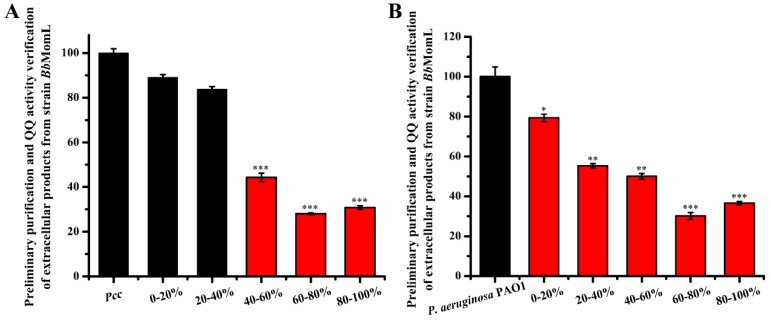
Bacillus brevis, as a promising heterologous expression system, can achieve extracellular expression of MomL. In this study, the extracellular protein was isolated by the ammonium sulfate step-precipitation method, and the A. tumefaciens A136 plate-based method was used to detect the QQ activity of different components. The components with degradation activity toward the signal molecules produced by Pcc were concentrated mainly in the ammonium sulfate concentration (w/v) ranges of 40–60%, 60–80% and 80–100%, and the QQ activity reached up to 50%, while the other components did not exhibit QQ activity (Figure 5A).
Figure 5.
Effects of different components of the BbMomL extracellular protein on the signal molecules produced by Pcc and P. aeruginosa PAO1. (A) Effects of different components of BbMomL extracellular protein on signal molecules produced by Pcc. (B) Effects of different components of the BbMomL extracellular protein on signal molecules produced by P. aeruginosa PAO1. The X-axis refers to different protein components isolated from 0–20%, 20–40%, 40–60%, 60–80%, and 80–100% ammonium sulfate. The Y-axis refers to the QQ activity of different protein components separated from the culture supernatant. The results shown are representative of biological duplicates. Error bars represent the standard deviations of three replicates. A t-test of unpaired unequal variance was performed for testing differences between groups. For statistical analysis, ***, ** and * indicate P < 0.001, P < 0.01, and P < 0.05, respectively.
Upon verification of the degradation of the signal molecules produced by P. aeruginosa PAO1 by different protein components, we found that the degradation efficiency of the 0–20% ammonium sulfate component toward the AHL signal molecule was only 20%. The degradation efficiencies of the 20–40% and 40–60% ammonium sulfate components reached up to 40%, and the highest degradation efficiencies were observed for the 60–80% and 80–100% ammonium sulfate components, reached 60% (Figure 5B). The above results indicated that the BbMomL extracellular protein was separated into different components, which exhibited different degradation efficiencies toward the signal molecules produced by Pcc and P. aeruginosa PAO1.
2.4. In Vitro Experiments to Evaluate the Ability of BbMomL to Inhibit the Virulence Factors of Pathogenic Bacteria
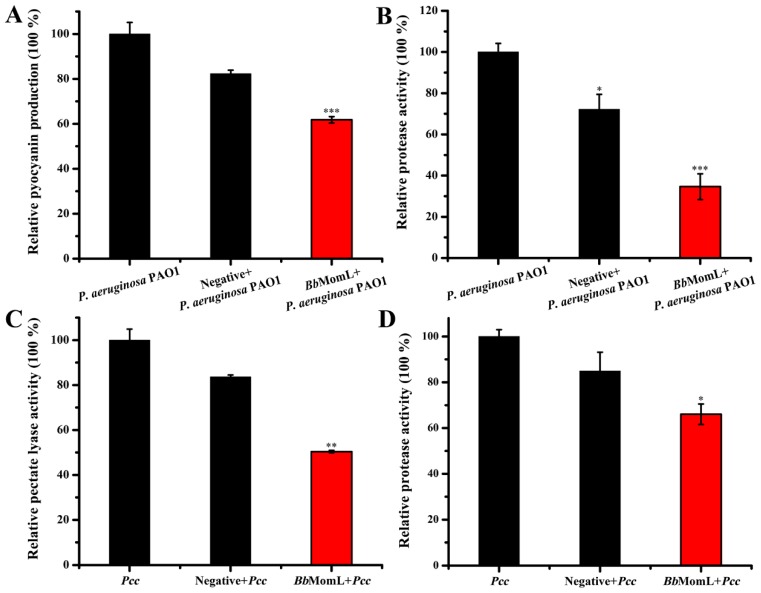
The inhibitory effect of BbMomL on the virulence factors of pathogens was detected. We found that after coculture with the recombinant strain BbMomL, the secretion of pyocyanin and extracellular protease of P. aeruginosa PAO1 and extracellular proteases and pectate lyase of Pcc was significantly reduced. The recombinant strain BbMomL could significantly reduce the secretion of pathogenic factors of P. aeruginosa PAO1 and Pcc, so we suggested that it could reduce the pathogenicity (Figure 6). At the same time, we found that the negative control also slightly inhibited the virulence factors, which may be due to some unidentified active substances produced by B. brevi.
Figure 6.
Inhibitory effect of BbMomL on pathogenic factors produced by Pcc and P. aeruginosa PAO1. (A) Relative production of pyocyanin of P. aeruginosa PAO1. (B) Relative protease activity of P. aeruginosa PAO1. (C) Relative pectate lyase activity of Pcc. (D) Relative protease activity of Pcc. The negative control was B. brevis (pNCMO2 transformant). The results shown are representative of biological duplicates. Error bars represent the standard deviations of three replicates. A t-test of unpaired unequal variance was performed for testing differences between groups. For statistical analysis, ***, ** and * indicate P < 0.001, P < 0.01, and P < 0.05, respectively.
2.5. Analysis of Growth and Protein Content of BbMomL in Different Media and Secretion of the Target Protein at Different Times
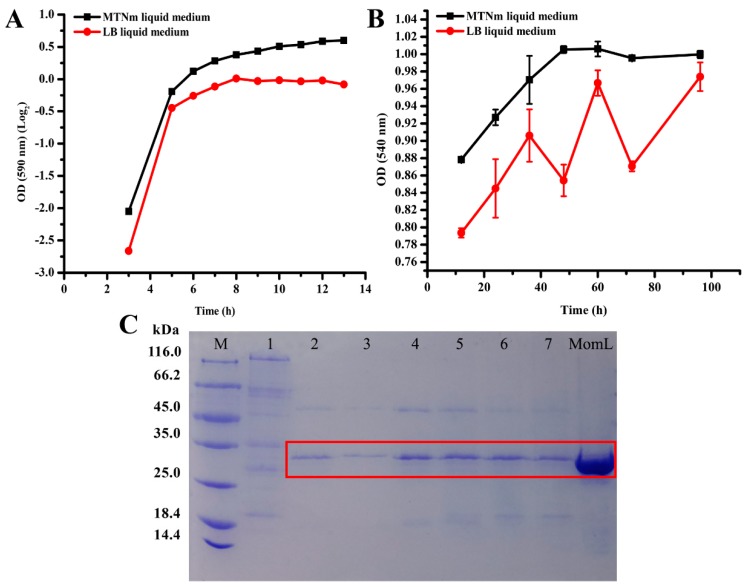
It has been reported that B. brevis can grow in different media. In this experiment, we compared the growth and protein secretion activity of BbMomL in two different media, namely Luria-Bertani (LB) and MTNm media. The growth rate of BbMomL in MTNm medium was higher than that in LB medium (Figure 7A). Moreover, the protein secretion activity of BbMomL in MTNm liquid medium was higher and more stable than that in LB (Figure 7B). To verify the change in the secretion of the target protein MomL at different time points in MTNm medium, we performed sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The SDS-PAGE analysis showed that the intensity of the protein bands increased with culture time, indicating that the level of the target protein MomL in the culture supernatant also increased with incubation time (Figure 7C).
Figure 7.
Analysis of the growth and protein content of BbMomL in different media and by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). (A) Analysis of BbMomL growth in LB and MTNm. (B) Analysis of protein secretion by BbMomL in LB and MTNm. (C) The SDS-PAGE analysis of secretion of the target protein MomL at different culture time points. M: Marker; 1: Negative control; 2: 12 h; 3: 24 h; 4: 48 h; 5: 60 h; 6: 84 h; 7: 96 h; MomL: Positive control. The results shown are representative of biological duplicates. Error bars represent the standard deviations of three replicates.
2.6. Inhibitory Effect of BbMomL on Bacterial Soft Rot of Plants
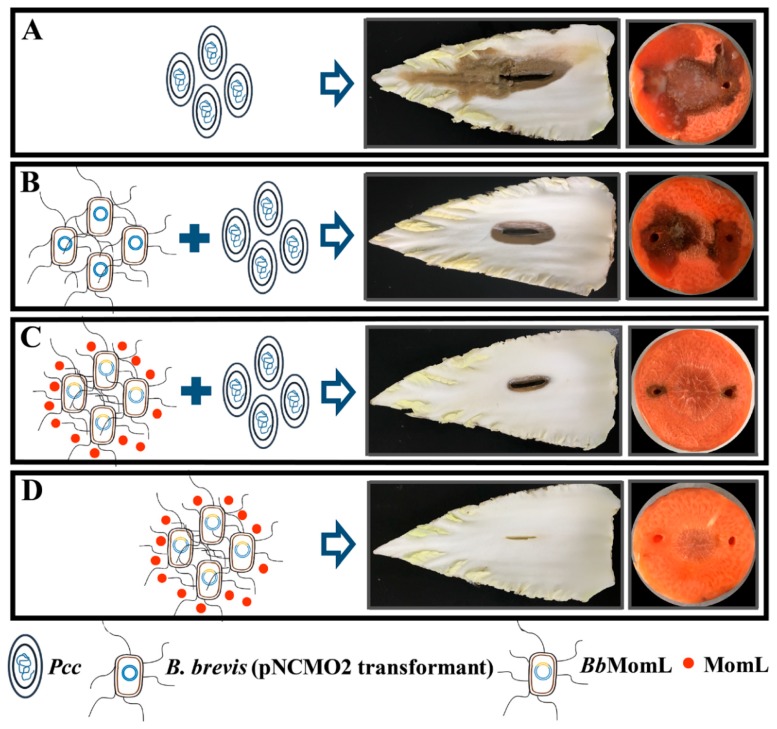
Pcc is one of the main pathogens that cause plant bacterial soft rot, affecting various vegetables, cash crops and ornamental plants. To verify the application potential of BbMomL to treat plant soft rot caused by Pcc, we infected root tissues of Chinese cabbage and carrot in vitro. Inoculation of Chinese cabbage and carrot root tissues with the pathogenic bacteria Pcc alone caused obvious symptoms of bacterial soft rot. The rotten area of the plant tissue was significantly reduced, and the symptoms of bacterial soft rot in the plants were improved after coinoculation with Pcc and the recombinant BbMomL strain, while symptoms of tissue decay were only slightly improved by coinoculation with Pcc and a negative control (Figure 8). This phenomenon may be due to the antagonistic effect between Pcc and B. brevis (pNCMO2 transformant). According to the results, we speculated that one possible reason why BbMomL inhibited the soft rot in Chinese cabbage and carrot was that BbMomL inhibited the production of pectate lyase and protease in Pcc. In addition, when only BbMomL was used to infect Chinese cabbage and carrot tissues, there were no soft rot symptoms in the root tissues, indicating that BbMomL had no toxic effects on the plant tissues.
Figure 8.
In vitro control efficacy of BbMomL against Pcc soft rot of Chinese cabbage and carrot root tissues. (A) Pcc; (B) Pcc with B. brevis (pNCMO2 transformant); (C) Pcc with the BbMomL strain; (D) the BbMomL strain.
3. Discussion
Quorum sensing (QS) is a population-dependent behavior that enables bacteria to sense and communicate with other neighbors and then to regulate multiple genes in response to external environmental changes [1,34,35,36,37,38,39]. Among them, QS induced by AHLs (QSA) was identified in multiple bacterial species, most of which were common pathogens existing in various environments. Previous studies have shown that QSA is closely related to the pathogenicity, virulence factor production and biofilm formation in multiple pathogens, including many antibiotic-resistant microorganisms [40,41,42,43,44,45]. Traditionally, antibiotic therapy is recognized as an effective way for the control of bacterial pathogens. However, the overdose of antibiotics has accelerated the emergence of multidrug-resistant bacteria [46,47,48]. The dilemma has prompted the research of novel antibacterial strategies. Interfering with QSA systems via quorum quenching (QQ) represents a promising strategy for the treatment of bacterial diseases [5]. Theoretically, QQ could decrease the expression of virulence factors produced by any pathogens under the control of QSA. Many QQ agents have been discovered, and their mechanism of action has also been well studied [7,49,50,51]. However, it is largely unknown about how to efficiently utilize these QQ agents and express them in ideal microorganism hosts.
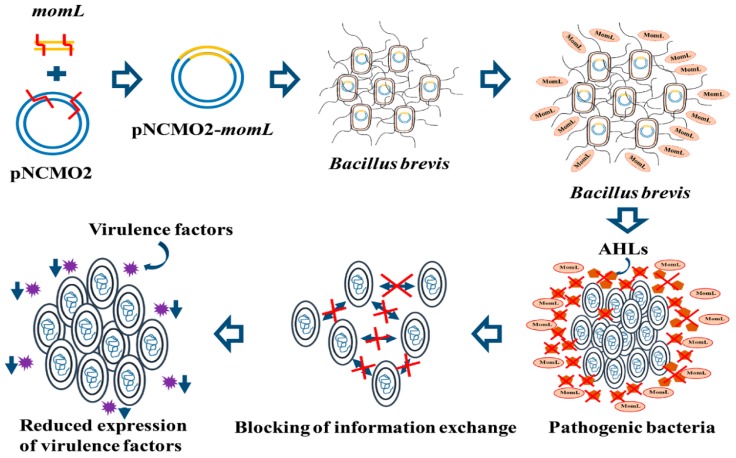
In this study, we carried out heterologous expression of the marine-derived QQ agent MomL in B. brevis, which is an antifungal and anti-gram-positive-bacteria biocontrol bacterial microorganism, and obtained the recombinant strain named BbMomL. This is novel that B. brevis was used as a heterologous expression vector for marine QQ enzyme. Moreover, the study of QQ agents’ heterologous expression is at the initial stage, and this is the first time MomL was expressed in Bacillus genus. Our result showed that BbMomL could degrade both the exogenous signal molecule C6-HSL and the AHLs produced by the gram-negative pathogens Pcc and P. aeruginosa PAO1. In addition, BbMomL significantly reduced the production of pathogenic factors and the pathogenicity of Pcc and P. aeruginosa PAO1. The reason we chose Pcc and P. aeruginosa PAO1 as the study materials was that these two kinds of bacteria are widely existed and have serious pathogenicity to animals and crops respectively. More importantly, previous studies have shown that the production of virulence factors of these two pathogens are closely related to AHL-mediated quorum sensing process. The heterologous expression of MomL in B. brevis can lead to the production of MomL with biological activity. MomL can degrade AHL signal molecules produced by pathogenic bacteria and interrupt the communication among pathogenic bacteria. Therefore, it has an obvious biological control effect (Figure 9). Besides, our result indicated that BbMomL had a broad antibacterial spectrum. BbMomL not only inhibited fungi and gram-positive bacterial pathogens, but also considerably inhibited gram-negative bacterial pathogens. This result raises our thinking that if we express MomL or other QQ agents in other beneficial bacteria, will they also improve the antibacterial activity of the bacteria? The mechanism that MomL enhanced the antimicrobial ability of beneficial bacteria is an interesting scientific question deserved further study. In the study, we also found an interesting phenomenon that BbMomL has a significant inhibitory effect on plant soft rot caused by Pcc, while B. brevis also showed a slight therapeutic effect. We speculated that the reason for this phenomenon was some unidentified active substances secreted by B. brevis had a certain therapeutic effect on soft rot, meanwhile, they also had a certain effected on the survival of pathogenic bacteria. In addition, we found that MomL could reduce the survival rate of Pcc and P. aeruginosa PAO1, which was not consistent with the theory that MomL cannot directly kill bacteria. We speculated that MomL indirectly affected the survival rate of the pathogenic bacteria. MomL can cause pathogenic bacteria to lose their survival advantage in the presence of other alien bacteria. Several studies have shown that AHL-mediated QS affect a series of physiological behaviors of bacteria, such as the production of pathogenic factors, biofilm formation, antibiotic resistance and stress resistance. MomL resulted in the loss of AHL and then these physiological behaviors were affected, which in turn affected the viability of bacteria.
Figure 9.
Schematic diagram of the mechanism of action of MomL.
The heterologous expression has received increased attention because of the low expression of target proteins in the original host bacteria. Now there are a variety of heterologous expression systems, which allows us to select the most appropriate one for different target proteins. A series of heterologous expression systems have their own advantages and disadvantages features. For example, although the E. coli expression system is easy to operate, the expressed products readily form inclusion bodies and lose biological activity. Meanwhile, the extracellular expression of protease may hydrolyze the proteins of the host bacteria and cause host bacterial death [11]. In recent years, there have been many successful examples of studies on the secretion and expression of foreign proteins in Bacillus. A series of effective expression systems have been established, such as B. subtilis and B. brevis expression systems [52]. Currently, the B. subtilis expression system is among the most important prokaryotic expression systems, following E. coli, and has been successfully used a host species in industrial applications [12,53,54,55]. However, as an expression host, B. subtilis also exhibits some limitations that restrict the application of this species in industrial production, such as high extracellular protease activity and high degradation susceptibility of the expression products [53]. Compared with B. subtilis, the B. brevis expression system has not only a strong ability to secrete proteins, but also low extracellular protease activity. For example, the extracellular protease activity level of B. brevis 47 is only 1.6% that of B. subtilis, and the protease activity of B. brevis HPD31 is almost undetectable [56,57]. In addition, disulfide bond oxidoreductase (Dsb) and peptide proline cis-trans isomerase (PPIase), which can promote the correct folding of polypeptides, have also been detected in B. brevis culture supernatant [58]. Therefore, B. brevis has the natural advantage of being able to secrete and express exogenous proteins. There have been successful reports on the use of B. brevis for construction of a secretion system for foreign proteins. The production of a mouse/human chimeric antibody against human prourokinase by B. brevis reached 100 mg/L by a simple shake flask culture method [59]. Peng et al. [60] also successfully expressed α-amylase in B. brevis. These results all show the considerable application potential of B. brevis in the secretion and expression of exogenous proteins.
4. Materials and Methods
4.1. Strains, Plasmids and Culture Conditions
The bacterial strains and plasmids used in this study are described in Table S1. C. violaceum CV026 and A. tumefaciens A136 were used as indicator strains in the AHL activity bioassay. M. Th120, C. violaceum CV026, Pcc and P. aeruginosa PAO1 were routinely grown on LB agar medium at 28 °C. When the effects of BbMomL on in vitro pyocyanin production in P. aeruginosa PAO1 were determined, the cells were cultured in Pseudomonas broth (PB medium) [61]. The recombinant strain BbMomL was cultured in MTNm liquid medium at 37 °C [62]. The media components are described in Table S2. When required, antibiotics were used at the following concentrations: 10 μg/mL neomycin for BbMomL; and 4.5 μg/mL tetracycline and 50 μg/mL spectamycin for A. tumefaciens A136. C6-HSL was purchased from Sigma-Aldrich (St. Louis, MO, USA) and prepared in dimethyl sulfoxide (DMSO). Genomic DNA of M. olearia Th120 was extracted by the phenol-chloroform method. Plasmids and DNA fragment extraction were performed following the instructions included with the kits purchased from OMEGA (Plasmid Mini Kit I and Gel Extraction Kit, Omega Bio-Tek, Norcross, GA, USA). The Bacillus brevis expression system (cat. #HB200), PrimeSTAR GXL DNA polymerase (Code No. R050A), Solution I (cat. #6022-1) and restriction enzymes were purchased from TaKaRa (TaKaRa Bio Group, Shiga, Japan). PCR primers were synthesized by Tsingke Biological Technology Company (Qingdao, China).
4.2. Plasmids Construction
The B. brevis expression system was used for the expression of native MomL according to the manufacturer’s instructions. To improve the transformation efficiency, we linked the target gene momL with pUC-T vector and transformed it into E. coli JM109. Insertion of the lac operators into pNCMO2 can weaken the activity of promoters in E. coli, and thus makes it necessary to use the E. coli JM109 host which F factor (lacIq) has been integrated. Briefly, an 837-bp DNA fragment encoding MomL was obtained using M. olearia Th120 genomic DNA as a template. Based on the momL sequence, a His-tag was included at the C-terminus. Polymerase chain reaction (PCR) was carried out using specific primers (forward primer, 5′-CGCGGATCCAAAAAGGAAGCT-3′; reverse primer, 5′-CCGGAATTCGTGGTGGTGGTG-3′) with BamH I and EcoR I restriction sites for directional cloning of MomL. PCR was conducted as follows: Denaturation at 95 °C for 2 min followed by 30 cycles of 95 °C for 10 s, 60 °C for 15 s and 68 °C for 1 min and an elongation step of 72 °C for 2 min (PrimeSTAR GXL DNA polymerase can amplify 1 kb every 10 s, so the extension time was set according to the length of the target fragment). The amplified DNA was purified, digested with BamH I and EcoR I, and cloned into the pUCm-T vector (previously digested with the same enzymes) by Solution I to construct the recombinant pUCm-T-momL plasmid. This plasmid was then transformed into E. coli JM109 cells. The positive clones were screened by blue-white selection on LB plates containing 50 μg/mL ampicillin and then sequenced to confirm the identity of the amplicon. The pUCm-T-momL plasmid was extracted with kits, digested with the restriction endonuclease BamH I and EcoR I, and cloned into the pNCMO2 vector (previously digested with the same enzymes) with Solution I to construct the recombinant pNCMO2-momL [62]. This construct was transformed into E. coli JM109 cells, grown overnight at 37 °C in plates with LB agar medium containing ampicillin-neomycin (10 μg/mL), and colonies were screened for the presence of the pNCMO2-momL construct. Next, the correct positive transformant plasmid was extracted, and the target plasmid was introduced into B. brevis competent cells according to the manufacturer’s instructions and grown overnight at 37 °C in MTNm plates containing neomycin (10 μg/mL) to screen the positive transformants and obtain BbMomL.
4.3. Bioassay for AHL Degradation Activity
The AHL degradation activity of BbMomL was detected using the strain C. violaceum CV026 according to the method described by McClean [32]. Detection of QQ activity was performed based on the inhibition of violacein production by the C. violaceum CV026 strain in culture medium supplemented with an exogenous QS signal molecule, namely, C6-HSL [63]. Fifteen milliliters of molten semisolid LB agar (1%, w/v) was seeded with 1 mL of an overnight LB culture of C. violaceum CV026. Then, 7.5 µL of C6-HSL (DMSO, 1 mM) was added before the agar was poured over the plates. When the agar solidified in the screening plates, the solidified semisolid medium was perforated using an Oxford cup. Then, 150 μL of the culture was added to the well, and 10 μL of the purified recombinant MomL protein (1.363 mg/mL) was used as the positive control. The plates were incubated overnight at 28 °C.
To determine whether Pcc and P. aeruginosa PAO1 can produce AHL signal molecules, we performed experiments using the indicator strain A. tumefaciens A136. The A. tumefaciens A136 and test strains were streaked on an LB plate in the form of a “=” symbol, and the plate was incubated at 28 °C for 12 h. Then, X-gal (100 μg/mL) was added to LB semisolid medium (1% agar), mixed and poured into the above plate, which was then covered and incubated at 28 °C for 2 h.
We detected the degradation of AHL signal molecules produced by Pcc and P. aeruginosa PAO1 by BbMomL using the methods described above. The bacterial supernatants of BbMomL coculture with Pcc and P. aeruginosa PAO1 were separately placed in the plate containing the strain C. violaceum CV026, and cultured overnight at 28 °C to observe the results.
4.4. Effects of BbMomL on the Survival of Pcc and P. aeruginosa PAO1
Pcc or P. aeruginosa PAO1 and BbMomL were separately cultured to the exponential phase and mixed at a ratio of 1:1. The cultures were cultivated on a shaker (170 rpm) at 28 °C for 8 h. The bacterial suspensions were diluted with fresh LB and plated on LB agar. After 15 h of incubation at 28 °C, colonies were counted. The number of colonies on the Pcc and P. aeruginosa PAO1 plates were set to 100%. Each experiment involved three repetitions.
4.5. Isolation and Purification of Recombinant Enzymes and AHL Bioassay of Pcc and P. aeruginosa PAO1
The extracellular protein of BbMomL was isolated and purified by the ammonium sulfate step-precipitation. Based on the volume of the extracellular product, ammonium sulfate was added to achieve a saturation of 20%. When the ammonium sulfate was completely dissolved, the extracellular product was centrifuged at 12,000× g for 30 min at 4 °C to obtain a protein precipitate. The above steps were repeated, and ammonium sulfate was sequentially added to the supernatant to achieve saturation levels of 40%, 60%, 80% and 100%, and the protein precipitates were collected by centrifugation. The protein precipitates were dissolved in PBS and dialyzed for 24 h in the same solution. Using strain A. tumefaciens A136 as an indicator strain, the QQ activity of the extracellular protein was detected by the Oxford cup method.
4.6. Effects of BbMomL on Virulence Factor Production in Pcc and P. aeruginosa PAO1
The inhibitory effect of BbMomL on the synthesis of pyocyanin in P. aeruginosa PAO1 was measured as described by Essar [64]. The total volume of each experimental group was 5 mL. BbMomL and P. aeruginosa PAO1 with the same cell density were mixed at a ratio of 1:1 and cultivated at 170 rpm for 24 h at 28 °C. One milliliter of the culture solution was removed, and 800 μL of chloroform was added for extraction. After centrifugation at 13,000× g for 2 min, 500 μL was extracted from the lower chloroform layer and uniformly mixed with 1.5 mL of 200 mM hydrochloric acid, and then, the OD520 was measured. Each experiment involved three repetitions.
The effect of BbMomL on the extracellular protease activity of Pcc and P. aeruginosa PAO1 was determined according to the method described by Hentzer [65,66]. The coculture samples were incubated at 28 °C for 24 h, and the OD590 was measured. After centrifugation at 13,000× g for 10 min at 4 °C, 100 μL of the supernatant was mixed with a 50 mM Tris/HCl solution (pH = 7.8) containing 2% azocasein and incubated at 37 °C for 2 h. The reaction was stopped by addition of 200 μL of 10% trichloroacetic acid solution, allowed to stand for 2 min, and centrifuged at 13,000× g for 5 min. A total of 100 μL of the supernatant was removed and mixed with 100 μL of 525 mM sodium hydroxide solution, and then, 200 μL of the mixture was placed in a 96-well plate. The OD415 was measured, and the protease activity of each sample was calculated according to the formula OD415/(OD590 × 100 μL).
This experiment was performed to determine the inhibitory effect of BbMomL on pectate lyase in Pcc. The absorbance at 590 nm of the mixtures was determined. Then 600 μL of the coculture supernatant, 100 μL of CaCl2 solution and 800 μL of glycine-sodium hydroxide buffer (50 mM, pH = 9.5) containing 0.2% (w/v) polygalacturonic acid were uniformly mixed. The reaction was stopped by the addition of 500 μL of HCl (50 mM) after 5 min. The OD235 was measured, and the relative activity of pectate lyase was determined according to the formula OD235/OD590.
4.7. Comparison of the Growth and Protein Content of BbMomL in Different Media and by SDS-PAGE Analysis
BbMomL was cultured separately in LB and MTNm liquid medium containing 10 μg/mL neomycin at 37 °C with shaking at 170 rpm. The absorbance values at 590 nm was determined at different time points, and a curve was generated. The concentration of the total proteins was determined by the Bradford method in this study [67]. The Bradford assay relies on the binding of the dye Coomassie Blue G250 to protein in cell-free supernatants. The cell-free culture supernatants at different time points were mixed with the dye Coomassie Blue G250 solution and its absorbance at 540 nm was determined. Three parallel sample sets were examined for each set of experiments. The supernatants of the recombinant BbMomL cultured in MTNm medium were collected by centrifugation, and equal amounts of total protein were loaded onto SDS-PAGE gels to detect the secretion of the target protein MomL at different time points [68].
4.8. Inhibition of Plant Bacterial Soft Rot
To examine the biocontrol effect of BbMomL on soft rot disease caused by Pcc, an in vitro disease control efficacy test was performed using Chinese cabbage and carrot roots. Coculture suspensions of BbMomL with Pcc were prepared as previously described. Washed Chinese cabbage leaves were disinfected with 70% ethanol for 30 s and dried on a clean bench. A 2 cm long wound was made with a sterile razor in the center of the leaves, and 30 μL of the mixed bacterial suspension was applied to the wound. A Pcc bacterial suspension was used as a positive control. The compound-treated Chinese cabbage leaves were incubated for 48 h at 28 °C in a plastic bag with filter paper moistened with 5 mL of sterile water to prevent excessive drying, and then, the lesions were examined. This experiment was conducted three times independently with three replicates in each trial. The infection experiment with carrot root tissues was performed as described previously with slight modifications [63,69]. The surface-sterilized carrot roots were cut into 0.7 cm thick sliced, and two wells (5 mm diameter) were made in each slice. Then 30 μL of the coculture suspension was applied to the wells, while the control group was inoculated with only pathogenic or antagonistic bacterial suspension. The compound-treated carrot slices were placed on Petri dishes with wet filter paper to prevent excessive drying and incubated for 48 h at 28 °C, and then, the lesions were examined. This experiment was conducted three times independently, with three replicates in each trial.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-3397/17/2/128/s1, Table S1: Bacteria and plasmids used in the study. Table S2: Medium compositions used in this study.
Author Contributions
Conceptualization, J.Z. and Y.W.; Formal analysis, J.Z., J.W., T.F. and Y.W.; Methodology, J.Z., J.W., T.F., R.D. and X.T.; Supervision, Y.W. and X.-H.Z.; Writing—original draft, J.Z.; Writing—review and editing, Y.W. and X.-H.Z.
Funding
This work was supported by the National Natural Science Foundation of China (No. 31870023, 31571970 and 41506160), the Young Elite Scientists Sponsorship Program by CAST (No. YESS20160009) and the Marine S&T Fund of Shandong Province for Pilot National Laboratory for Marine Science and Technology (Qingdao) (No. 2018SDKJ0406-4).
Conflicts of Interest
The authors declare no competing interests.
References
- 1.Whiteley M., Diggle S.P., Greenberg E.P. Progress in and promise of bacterial quorum sensing research. Nature. 2017;551:313–320. doi: 10.1038/nature24624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corbett M., Virtue S., Bell K., Birch P., Burr T., Hyman L., Lilley K., Poock S., Toth I., Salmond G. Identification of a new quorum-sensing-controlled virulence factor in Erwinia carotovora subsp. atroseptica secreted via the type II targeting pathway. Mol. Plant Microbe Interact. 2005;18:334–342. doi: 10.1094/MPMI-18-0334. [DOI] [PubMed] [Google Scholar]
- 3.Burr T., Barnard A.M., Corbett M.J., Pemberton C.L., Simpson N.J., Salmond G.P. Identification of the central quorum sensing regulator of virulence in the enteric phytopathogen, Erwinia carotovora: The VirR repressor. Mol. Microbiol. 2006;59:113–125. doi: 10.1111/j.1365-2958.2005.04939.x. [DOI] [PubMed] [Google Scholar]
- 4.Koutsoudis M.D., Tsaltas D., Minogue T.D., von Bodman S.B. Quorum-sensing regulation governs bacterial adhesion, biofilm development, and host colonization in Pantoea stewartii subspecies stewartii. Proc. Natl. Acad. Sci. USA. 2006;103:5983–5988. doi: 10.1073/pnas.0509860103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clatworthy A.E., Pierson E., Hung D.T. Targeting virulence: A new paradigm for antimicrobial therapy. Nat. Chem. Biol. 2007;3:541–548. doi: 10.1038/nchembio.2007.24. [DOI] [PubMed] [Google Scholar]
- 6.Li Q.Q., Ni H., Meng S., He Y., Yu Z.N., Li L. Suppressing Erwinia carotovora pathogenicity by projecting N-acyl homoserine lactonase onto the surface of Pseudomonas putida cells. J. Microbiol. Biotechnol. 2011;21:1330–1335. doi: 10.4014/jmb.1107.07011. [DOI] [PubMed] [Google Scholar]
- 7.Tang K., Su Y., Brackman G., Cui F., Zhang Y., Shi X., Coenye T., Zhang X.H. MomL, a novel marine-derived N-acyl homoserine lactonase from Muricauda olearia. Appl. Environ. Microbiol. 2015;81:774–782. doi: 10.1128/AEM.02805-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ulrich R.L. Quorum quenching: Enzymatic disruption of N-acylhomoserine lactone-mediated bacterial communication in Burkholderia thailandensis. Appl. Environ. Microbiol. 2004;70:6173–6180. doi: 10.1128/AEM.70.10.6173-6180.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong Y.H., Wang L.H., Xu J.L., Zhang H.B., Zhang X.F., Zhang L.H. Quenching quorum-sensing-dependent bacterial infection by an N-acyl homoserine lactonase. Nature. 2001;411:813–817. doi: 10.1038/35081101. [DOI] [PubMed] [Google Scholar]
- 10.Wenzel S.C., Muller R. Recent developments towards the heterologous expression of complex bacterial natural product biosynthetic pathways. Curr. Opin. Biotechnol. 2005;16:594–606. doi: 10.1016/j.copbio.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Baneyx F. Recombinant protein expression in Escherichia coli. Curr. Opin. Biotechnol. 1999;10:411–421. doi: 10.1016/S0958-1669(99)00003-8. [DOI] [PubMed] [Google Scholar]
- 12.Bolhuis A., Tjalsma H., Smith H.E., de Jong A., Meima R., Venema G., Bron S., van Dijl J.M. Evaluation of bottlenecks in the late stages of protein secretion in Bacillus subtilis. Appl. Environ. Microbiol. 1999;65:2934–2941. doi: 10.1128/aem.65.7.2934-2941.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seok J.H., Kim H.S., Hatada Y., Nam S.W., Kim Y.H. Construction of an expression system for the secretory production of recombinant alpha-agarase in yeast. Biotechnol. Lett. 2012;34:1041–1049. doi: 10.1007/s10529-012-0864-0. [DOI] [PubMed] [Google Scholar]
- 14.Kashima Y., Udaka S. High-level production of hyperthermophilic cellulase in the Bacillus brevis expression and secretion system. Biosci. Biotechnol. Biochem. 2004;68:235–237. doi: 10.1271/bbb.68.235. [DOI] [PubMed] [Google Scholar]
- 15.Song Z., Liu Q., Guo H., Ju R., Zhao Y., Li J., Liu X. Tostadin, a novel antibacterial peptide from an antagonistic microorganism Brevibacillus brevis XDH. Bioresour. Technol. 2012;111:504–506. doi: 10.1016/j.biortech.2012.02.051. [DOI] [PubMed] [Google Scholar]
- 16.Mogi T., Kita K. Gramicidin S and polymyxins: The revival of cationic cyclic peptide antibiotics. Cell. Mol. Life Sci. 2009;66:3821–3826. doi: 10.1007/s00018-009-0129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong Y.-H., Zhang X.-F., Xu J.-L., Zhang L.-H. Insecticidal Bacillus thuringiensis silences Erwinia carotovora virulence by a new form of microbial antagonism, signal interference. Appl. Environ. Microbiol. 2004;70:954–960. doi: 10.1128/AEM.70.2.954-960.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong Y.-H., Xu J.-L., Li X.-Z., Zhang L.-H. AiiA, an enzyme that inactivates the acylhomoserine lactone quorum-sensing signal and attenuates the virulence of Erwinia carotovora. Proc. Natl. Acad. Sci. USA. 2000;97:3526–3531. doi: 10.1073/pnas.97.7.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grandclément C., Tannières M., Moréra S., Dessaux Y., Faure D. Quorum quenching: Role in nature and applied developments. FEMS Microbiol. Rev. 2015;40:86–116. doi: 10.1093/femsre/fuv038. [DOI] [PubMed] [Google Scholar]
- 20.He P., Zhang Z., Cai D., Chen Y., Wang H., Wei X., Li S., Chen S. High-level production of alpha-amylase by manipulating the expression of alanine racamase in Bacillus licheniformis. Biotechnol. Lett. 2017;39:1389–1394. doi: 10.1007/s10529-017-2359-5. [DOI] [PubMed] [Google Scholar]
- 21.Mizukami M., Hanagata H., Miyauchi A. Brevibacillus expression system: Host-vector system for efficient production of secretory proteins. Curr. Pharm. Biotechnol. 2010;11:251–258. doi: 10.2174/138920110791112031. [DOI] [PubMed] [Google Scholar]
- 22.Takano T., Miyauchi A., Takagi H., Kadowaki K., Yamane K., Kobayashi S. Expression of the Cyclodextrin Glucanotransferase Gene of Bacillus macerans in Bacillus brevis. Biosci. Biotechnol. Biochem. 1992;56:808–809. doi: 10.1271/bbb.56.808. [DOI] [PubMed] [Google Scholar]
- 23.Tomita M., Kikuchi A., Kobayashi M., Yamaguchi M., Ifuku S., Yamashoji S., Ando A., Saito A. Characterization of antifungal activity of the GH-46 subclass III chitosanase from Bacillus circulans MH-K1. Antonie Van Leeuwenhoek. 2013;104:737–748. doi: 10.1007/s10482-013-9982-5. [DOI] [PubMed] [Google Scholar]
- 24.Li Z., Su L., Duan X., Wu D., Wu J. Efficient Expression of Maltohexaose-Forming α-Amylase from Bacillus stearothermophilus in Brevibacillus choshinensis SP3 and Its Use in Maltose Production. Biomed. Res. Int. 2017;2017:5479762. doi: 10.1155/2017/5479762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wasels F., Ferreira N.L., Collas F., Contreras A.L. Genetic Tool for the Transformation of Clostridium Bacteria. WO2017064439. Google Patents. 2017 Apr 12;
- 26.Tojo H., Asano T., Kato K., Udaka S., Horiuchi R., Kakinuma A. Production of human protein disulfide isomerase by Bacillus brevis. J. Biotechnol. 1994;33:55–62. doi: 10.1016/0168-1656(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 27.Yamagata H., Nakahama K., Suzuki Y., Kakinuma A., Tsukagoshi N., Udaka S. Use of Bacillus brevis for efficient synthesis and secretion of human epidermal growth factor. Proc. Natl. Acad. Sci. USA. 1989;86:3589–3593. doi: 10.1073/pnas.86.10.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takimura Y., Kato M., Ohta T., Yamagata H., Udaka S. Secretion of human interleukin-2 in biologically active form by Bacillus brevis directly into cultute medium. Biosci. Biotechnol. Biochem. 1997;61:1858–1861. doi: 10.1271/bbb.61.1858. [DOI] [PubMed] [Google Scholar]
- 29.Yashiro K., Lowenthal J.W., O’Neil T.E., Ebisu S., Takagi H., Moore R.J. High-level production of recombinant chicken interferon-γ by Brevibacillus choshinensis. Protein Expr. Purif. 2001;23:113–120. doi: 10.1006/prep.2001.1481. [DOI] [PubMed] [Google Scholar]
- 30.Mukai H., Takahashi M., Watanabe Y. Potential usefulness of Brevibacillus for bacterial cancer therapy: Intratumoral provision of tumor necrosis factor-α and anticancer effects. Cancer Gene Ther. 2018;25:47–57. doi: 10.1038/s41417-017-0009-7. [DOI] [PubMed] [Google Scholar]
- 31.Ando A., Saito A., Arai S., Usuda S., Furuno M., Kaneko N., Shida O., Nagata Y. Molecular characterization of a novel family-46 chitosanase from Pseudomonas sp. A-01. Biosci. Biotechnol. Biochem. 2008;72:2074–2081. doi: 10.1271/bbb.80175. [DOI] [PubMed] [Google Scholar]
- 32.McClean K.H., Winson M.K., Fish L., Taylor A., Chhabra S.R., Camara M., Daykin M., Lamb J.H., Swift S., Bycroft B.W., et al. Quorum sensing and Chromobacterium violaceum: Exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology. 1997;143:3703–3711. doi: 10.1099/00221287-143-12-3703. [DOI] [PubMed] [Google Scholar]
- 33.Zhu J., Beaber J.W., More M.I., Fuqua C., Eberhard A., Winans S.C. Analogs of the autoinducer 3-oxooctanoyl-homoserine lactone strongly inhibit activity of the TraR protein of Agrobacterium tumefaciens. J. Bacteriol. 1998;180:5398–5405. doi: 10.1128/jb.180.20.5398-5405.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davies D.G., Parsek M.R., Pearson J.P., Iglewski B.H., Costerton J.W., Greenberg E.P. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280:295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 35.Rakoff-Nahoum S., Foster K.R., Comstock L.E. The evolution of cooperation within the gut microbiota. Nature. 2016;533:255. doi: 10.1038/nature17626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pader V., Hakim S., Painter K.L., Wigneshweraraj S., Clarke T.B., Edwards A.M. Staphylococcus aureus inactivates daptomycin by releasing membrane phospholipids. Nat. Microbiol. 2016;2:16194. doi: 10.1038/nmicrobiol.2016.194. [DOI] [PubMed] [Google Scholar]
- 37.Bandyopadhaya A., Tsurumi A., Maura D., Jeffrey K.L., Rahme L.G. A quorum-sensing signal promotes host tolerance training through HDAC1-mediated epigenetic reprogramming. Nat. Microbiol. 2016;1:16174. doi: 10.1038/nmicrobiol.2016.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee J.H., Lee J. Indole as an intercellular signal in microbial communities. Fems Microbiol. Rev. 2010;34:426–444. doi: 10.1111/j.1574-6976.2009.00204.x. [DOI] [PubMed] [Google Scholar]
- 39.Piewngam P., Zheng Y., Nguyen T.H., Dickey S.W., Joo H.-S., Villaruz A.E., Glose K.A., Fisher E.L., Hunt R.L., Li B., et al. Pathogen elimination by probiotic Bacillus via signalling interference. Nature. 2018;562:532–537. doi: 10.1038/s41586-018-0616-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bjarnsholt T., Jensen P.O., Burmolle M., Hentzer M., Haagensen J.A., Hougen H.P., Calum H., Madsen K.G., Moser C., Molin S., et al. Pseudomonas aeruginosa tolerance to tobramycin, hydrogen peroxide and polymorphonuclear leukocytes is quorum-sensing dependent. Microbiology. 2005;151:373–383. doi: 10.1099/mic.0.27463-0. [DOI] [PubMed] [Google Scholar]
- 41.Dou Y., Song F., Guo F., Zhou Z., Zhu C., Xiang J., Huan J. Acinetobacter baumannii quorum-sensing signalling molecule induces the expression of drug-resistance genes. Mol. Med. Rep. 2017;15:4061–4068. doi: 10.3892/mmr.2017.6528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koch G., Nadal-Jimenez P., Reis C.R., Muntendam R., Bokhove M., Melillo E., Dijkstra B.W., Cool R.H., Quax W.J. Reducing virulence of the human pathogen Burkholderia by altering the substrate specificity of the quorum-quenching acylase PvdQ. Proc. Natl. Acad. Sci. USA. 2014;111:1568–1573. doi: 10.1073/pnas.1311263111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sokol P.A., Sajjan U., Visser M.B., Gingues S., Forstner J., Kooi C. The CepIR quorum-sensing system contributes to the virulence of Burkholderia cenocepacia respiratory infections. Microbiology. 2003;149:3649–3658. doi: 10.1099/mic.0.26540-0. [DOI] [PubMed] [Google Scholar]
- 44.Lynch M.J., Swift S., Kirke D.F., Keevil C.W., Dodd C.E.R., Williams P. The regulation of biofilm development by quorum sensing in Aeromonas hydrophila. Environ. Microbiol. 2002;4:18–28. doi: 10.1046/j.1462-2920.2002.00264.x. [DOI] [PubMed] [Google Scholar]
- 45.Swift S., Karlyshev A.V., Fish L., Durant E.L., Winson M.K., Chhabra S.R., Williams P., Macintyre S., Stewart G.S. Quorum sensing in Aeromonas hydrophila and Aeromonas salmonicida: Identification of the LuxRI homologs AhyRI and AsaRI and their cognate N-acylhomoserine lactone signal molecules. J. Bacteriol. 1997;179:5271–5281. doi: 10.1128/jb.179.17.5271-5281.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harms A., Maisonneuve E., Gerdes K. Mechanisms of bacterial persistence during stress and antibiotic exposure. Science. 2016;354:aaf4268. doi: 10.1126/science.aaf4268. [DOI] [PubMed] [Google Scholar]
- 47.Alekshun M.N., Levy S.B. Molecular Mechanisms of Antibacterial Multidrug Resistance. Cell. 2007;128:1037–1050. doi: 10.1016/j.cell.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 48.Defoirdt T., Sorgeloos P., Bossier P. Alternatives to antibiotics for the control of bacterial disease in aquaculture. Curr. Opin. Microbiol. 2011;14:251–258. doi: 10.1016/j.mib.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 49.Mayer C., Romero M., Muras A., Otero A. Aii20J, a wide-spectrum thermostable N-acylhomoserine lactonase from the marine bacterium Tenacibaculum sp. 20J, can quench AHL-mediated acid resistance in Escherichia coli. Appl. Microbiol. Biotechnol. 2015;99:9523–9539. doi: 10.1007/s00253-015-6741-8. [DOI] [PubMed] [Google Scholar]
- 50.Liu D., Momb J., Thomas P.W., Moulin A., Petsko G.A., Fast W., Ringe D. Mechanism of the quorum-quenching lactonase (AiiA) from Bacillus thuringiensis. 1. Product-bound structures. Biochemistry. 2008;47:7706–7714. doi: 10.1021/bi800368y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Momb J., Wang C., Liu D., Thomas P.W., Petsko G.A., Guo H., Ringe D., Fast W. Mechanism of the quorum-quenching lactonase (AiiA) from Bacillus thuringiensis. 2. Substrate modeling and active site mutations. Biochemistry. 2008;47:7715–7725. doi: 10.1021/bi8003704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Y.-H., Lu F.-P., Li Y., Yin X.-B., Wang Y., Gao C. Characterisation of mutagenised acid-resistant alpha-amylase expressed in Bacillus subtilis WB600. Appl. Microbiol. Biotechnol. 2008;78:85–94. doi: 10.1007/s00253-007-1287-z. [DOI] [PubMed] [Google Scholar]
- 53.Sarvas M. Gene Expression in Recombinant Bacillus. Marcel Dekker Inc.; New York, NY, USA: 1995. [PubMed] [Google Scholar]
- 54.Liu G., Xing M., Yu S. High-effective expression of thermostable alpha-amylase from a bacterial phage based recombinant Bacillus subtilis. Chin. J. Appl. Environ. Biol. 2005;11:368. [Google Scholar]
- 55.Schallmey M., Singh A., Ward O.P. Developments in the use of Bacillus species for industrial production. Can. J. Microbiol. 2004;50:1–17. doi: 10.1139/w03-076. [DOI] [PubMed] [Google Scholar]
- 56.Udaka S., Tsukagoshi N., Yamagata H. Bacillus brevis, a host bacterium for efficient extracellular production of useful proteins. Biotechnol. Genet. Eng. Rev. 1989;7:113–146. doi: 10.1080/02648725.1989.10647857. [DOI] [PubMed] [Google Scholar]
- 57.Udaka S., Yamagata H. Protein secretion in Bacillus brevis. Antonie Van Leeuwenhoek. 1993;64:137–143. doi: 10.1007/BF00873023. [DOI] [PubMed] [Google Scholar]
- 58.Udaka S., Yamagata H. High-level secretion of heterologous proteins by Bacillus brevis. Methods Enzym. 1993;217:23–33. doi: 10.1016/0076-6879(93)17053-8. [DOI] [PubMed] [Google Scholar]
- 59.Inoue Y., Ohta T., Tada H., Iwasa S., Udaka S., Yamagata H. Efficient production of a functional mouse/human chimeric Fab′ against human urokinase-type plasminogen activator by Bacillus brevis. Appl. Microbiol. Biotechnol. 1997;48:487–492. doi: 10.1007/s002530051084. [DOI] [PubMed] [Google Scholar]
- 60.Peng Q., Zhang W., Zhu H. The construction of shuttle vectors of Brevibacillus brevis-Escherichia coli. Sheng Wu Gong Cheng Xue Bao (Chin. J. Biotechnol.) 2002;18:438–441. [PubMed] [Google Scholar]
- 61.Vandeputte O.M., Kiendrebeogo M., Rajaonson S., Diallo B., Mol A., El Jaziri M., Baucher M. Identification of catechin as one of the flavonoids from Combretum albiflorum bark extract that reduces the production of quorum-sensing-controlled virulence factors in Pseudomonas aeruginosa PAO1. Appl. Environ. Microbiol. 2010;76:243–253. doi: 10.1128/AEM.01059-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pedro A.Q., Bonifacio M.J., Queiroz J.A., Maia C.J., Passarinha L.A. A novel prokaryotic expression system for biosynthesis of recombinant human membrane-bound catechol-O-methyltransferase. J. Biotechnol. 2011;156:141–146. doi: 10.1016/j.jbiotec.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 63.Kang J.E., Han J.W., Jeon B.J., Kim B.S. Efficacies of quorum sensing inhibitors, piericidin A and glucopiericidin A, produced by Streptomyces xanthocidicus KPP01532 for the control of potato soft rot caused by Erwinia carotovora subsp. atroseptica. Microbiol. Res. 2016;184:32–41. doi: 10.1016/j.micres.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 64.Essar D.W., Eberly L., Hadero A., Crawford I.P. Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: Interchangeability of the two anthranilate synthases and evolutionary implications. J. Bacteriol. 1990;172:884–900. doi: 10.1128/jb.172.2.884-900.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hentzer M., Riedel K., Rasmussen T.B., Heydorn A., Andersen J.B., Parsek M.R., Rice S.A., Eberl L., Molin S., Hoiby N., et al. Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology. 2002;148:87–102. doi: 10.1099/00221287-148-1-87. [DOI] [PubMed] [Google Scholar]
- 66.Ayora S., Gotz F. Genetic and biochemical properties of an extracellular neutral metalloprotease from Staphylococcus hyicus subsp. hyicus. Mol. Gen. Genet. 1994;242:421–430. doi: 10.1007/BF00281792. [DOI] [PubMed] [Google Scholar]
- 67.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 68.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 69.Jafra S., Przysowa J., Czajkowski R., Michta A., Garbeva P., van der Wolf J.M. Detection and characterization of bacteria from the potato rhizosphere degrading N-acyl-homoserine lactone. Can. J. Microbiol. 2006;52:1006–1015. doi: 10.1139/w06-062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.