Abstract
The β-carbonic anhydrase (CA, EC 4.2.1.1) from the pathogenic protozoan Entamoeba histolytica, EhiCA, was investigated for its activation with a panel of natural and non-natural amino acids and amines. EhiCA was potently activated by D-His, D-Phe, D-DOPA, L- and D-Trp, L- and D-Tyr, 4-amino-L-Tyr, histamine and serotonin, with KAs ranging between 1.07 and 10.1 µM. The best activator was D-Tyr (KA of 1.07 µM). L-Phe, L-DOPA, L-adrenaline, L-Asn, L-Asp, L-Glu and L-Gln showed medium potency activation, with KAs of 16.5–25.6 µM. Some heterocyclic- alkyl amines, such as 2-pyridyl-methyl/ethyl-amine and 4-(2-aminoethyl)-morpholine, were devoid of EhiCA activating properties with KAs > 100 µM. As CA activators have poorly been investigated for their interaction with protozoan CAs, our study may be relevant for an improved understanding of the role of this enzyme in the life cycle of E. histolytica.
Keywords: Entamoeba histolytica, carbonic anhydrase, metalloenzymes, protozoan, amine, amino acid, activator
1. Introduction
Recently, we have reported [1,2] the cloning, purification and characterization of a β-carbonic anhydrase (CA, EC 4.2.1.1) present in the genome of the pathogenic protozoan Entamoeba histolytica, the etiological agents provoking amebiasis, an endemic disease in developing countries and also affecting travelers returning from risk zones [3,4,5]. In addition, invasive forms of E. histolytica infection were reported to lead to liver cysts, associated frequently with complications such as pleural effusion due to the rupture of the cysts as well as dissemination to extra-intestinal organs, e.g., the brain or pericardium, which occasionally may have fatal consequences [3,6]. In the previous work [1,2] we also investigated the inhibition profile of the new enzyme (nominated EhiCA) with the main classes of CA inhibitors (CAIs) [7,8,9,10], the sulfonamides and the inorganic anions [11,12,13,14]. Our main scope was to identify agents that by interference with the activity of this enzyme, might lead to anti-infectives with a novel mechanism of action, considering the fact that many CAs are essential in the life cycle of microorganisms belonging to the bacteria, fungal or protozoan domains [15,16,17]. As β-CAs are not present in mammals [18,19], effective EhiCA inhibitors may represent an alternative therapeutic option for this protozoan infection. In fact, in the previous work we have shown that inhibition of other protozoan CAs, such as the β-class enzyme from Leishmania donovani [20,21] or the α-CA from Trypanosoma cruzi [20,22,23], has important antiparasitic effects in vitro and in vivo [21].
Indeed, various pathogenic organisms belonging to the bacteria, fungal or protozoan domains encode for CAs, which have been investigated in some detail ultimately, in the search of anti-infectives with a diverse mechanism of action [7,8,9,10,14,15,16,17,18,19,20,21,22,23]. CAs catalyze the reaction between CO2 and water, with formation of bicarbonate (HCO3-) and protons (H+), and are highly effective catalysts, among the most efficient known so far in nature [7,8,9,10]. CAs are involved in various biochemical and metabolic processes, among which are acid-base homeostasis, respiration, biosynthesis of various metabolites (urea, glucose, fatty acids, carbamoyl phosphate), electrolytes secretion, etc. [7,8,9,10,11,12]. Seven distinct CA families are known to date, the α, β, γ, δ, ζ, ƞ and θ class CAs, which are widespread all over the phylogenetic tree, from simple organisms, such as bacteria and Archaea, to more complex ones, such as vertebrates [7,8,9,10,24,25,26,27,28]. These diverse CA genetic families do not share significant sequence homology or structural identity, being an interesting example of convergent evolution at the molecular level [7,8,9,10]. In humans, as in many other vertebrates, only α-CAs are present, and their inhibition has been exploited from the pharmacological viewpoint for decades, for drugs such as diuretics [29], anticonvulsants [29,30], antiobesity [30] and more recently, antitumor agents [31]. However, these enzymes may also be activated [32] but the CA activators (CAAs) have seen fewer applications up until now. However, recent studies [33] pointed out to the possible application of CAAs targeting human enzymes for the enhancement of cognition. The nonvertebrate CAs were on the other hand only in the last few years investigated in some detail [34,35,36,37]. Here we report the first activation study of the β-CA from E. histolytica with a panel of amines and amino acid derivatives. As CAAs have poorly been investigated for their interaction with protozoan CAs, our study may be relevant for an improved understanding of the role of this enzyme in the life cycle of E. histolytica.
2. Results and Discussion
The catalytic activity of the recombinant EhiCA (for the CO2 hydration reaction), has been recently reported [1,2], being measured by using a stopped flow technique [38]. EhiCA showed a significant catalytic activity for the physiologic, CO2 hydration reaction, with the following kinetic parameters: kcat = 6.7 × 105 s−1 and kcat/Km = 8.9 × 107 M−1 × s−1. Thus, EhiCA is 1.8 times more effective as a catalyst compared to the slow human (h) isoform hCA I (considering the kcat/Km values) or 3.35 times more effective than hCA I (considering only the kinetic constant kcat) [1,2]. EhiCA activity was also inhibited by the standard, clinically used sulfonamide CA inhibitor acetazolamide (AZA, 5-acetamido-1,3,4-thiadiazole-2-sulfonamide), with a KI of 509 nM (data not shown here) [1,2].
Similar to all β-CAs investigated to date, EhiCA has a catalytically crucial zinc ion and its conserved protein ligands, which for this enzyme are: Cys50, His103 and Cys106 [1,2]. The fourth metal ion ligand is a water molecule/hydroxide ion, which acts as nucleophile in the catalytic cycle (Equation (1) below). A catalytic dyad constituted by the pair Asp52-Arg54 [1,2], conserved in all enzymes belonging to the β-class is also present in EhiCA, presumably with the role to enhance the nucleophilicity of the zinc-coordinated water molecule [18,19,20]. However, the rate-determining step for many CAs is the generation of the nucleophilic species of the enzyme, represented by Equation (2) below:
| H2O |
| EZn2+—OH− + CO2 ⇔ EZn2+—HCO3- ⇔ EZn2+—OH2 + HCO3 | (1) |
| EZn2+— -OH2 ⇔ EZn2+—OH− + H+ | (2) |
In most CAs, this step (Equation (2)) is assisted by amino acid residues from the active site [32], becoming an intramolecular step (instead of an intermolecular one), which is favored thermodynamically. Furthermore, the activators (CAAs) may participate in this step, as outlined in Equation (3):
| EZn2+— -OH2 + A ⇔ [EZn2+— -OH2 − A] ⇔ [EZn2+—OH− − AH+] ⇔ EZn2+—OH− + AH+ | (3) |
| enzyme - activator complexes |
The enzyme forms with the activator complexes (E-A complexes, where E stands for enzyme and A for activator), in which the proton transfer step from the zinc-coordinated water to the environment is intramolecular and thus, more efficient than the corresponding intermolecular process shown schematically in Equation (2) [32]. In fact, X-ray crystal structures are available for many CAs to which activators are bound within the active site [32,39,40,41], but only for α-class enzymes these structures have been reported to date. The activator binding site for the α-CAs is situated at the entrance of the active site cavity not far away from His64, which acts as proton shuttle residue in the process described by Equation (2) [32,39,40,41].
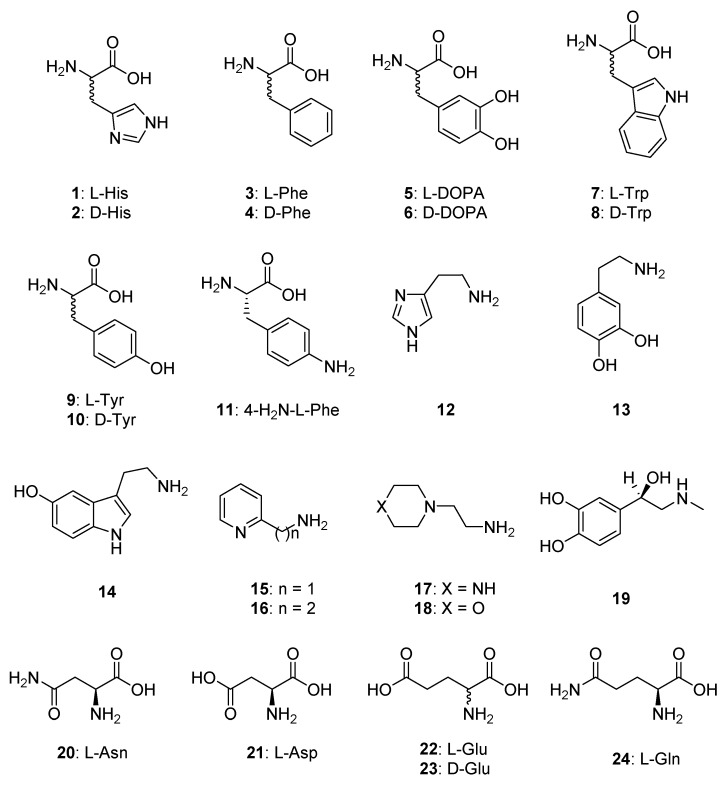
We have performed detailed kinetic measurements of EhiCA activity in the presence of amine and amino acid activators (Figure 1), such as for example L-Trp (Table 1). Data of Table 1 show that the presence of L-Trp does not change the KM, both for the α-class enzymes hCA I/II as well as the β-CA, EhiCA, investigated here. Interestingly, it has an effect on the kcat, which at 10-µM concentration of activator leads to a 2.83 times enhancement of the kinetic constant for the protozoan enzyme, from 6.7 × 105 s−1 to 1.9 × 106 s−1 (Table 1).
Figure 1.
CAAs of type 1–24 used in the present study.
Table 1.
Activation of human carbonic anhydrase (hCA) isozymes I, II, and EhiCA with L-Trp at 25 °C for the CO2 hydration reaction [38].
| Isozyme | kcat * | KM * | (kcat)L-Trp ** | KA *** (µM) |
|---|---|---|---|---|
| (s−1) | (mM) | (s−1) | L-Trp | |
| hCA I a | 2.0 × 105 | 4.0 | 3.4 × 105 | 44.0 |
| hCA II a | 1.4 × 106 | 9.3 | 4.9 × 106 | 27.0 |
| LdCA | 9.35 × 105 | 15.8 | 1.9 × 106 | 4.02 |
| EhiCA b | 6.7 × 105 | 7.5 | 1.9 × 106 | 5.24 |
* Observed catalytic rate without activator. KM values in the presence and the absence of activators were the same for the various CAs (data not shown).; ** Observed catalytic rate in the presence of 10 µM activator; *** The activation constant (KA) for each enzyme was obtained by fitting the observed catalytic enhancements as a function of the activator concentration [41]. Mean from at least three determinations by a stopped-flow, CO2 hydrase method [38]. Standard errors were in the range of 5–10% of the reported values (data not shown); a Human recombinant isozymes, from ref. [32]; b Protozoan recombinant enzyme, this work.
In order to obtain an activation profile of EhiCA with a wide range of amino acid and amine activators of types 11–24, we performed dose response curves of the activation of EhiCA in the presence of increasing concentrations of activators, in order to determine the activation constants KA-s (see Materials and Methods for details). We included in our study the amino acids and amines which were investigated as activators of CAs belonging to various classes from diverse organisms [32,33,34,35,36,37,40,41,42]. These activation data are reported in Table 2, in which, for comparison reasons, the activation of the human isoforms hCA I and II and of the protozoan β-CA from Leishmania donovani chagasi are also presented.
Table 2.
Activation constants of hCA I, hCA II and the protozoan enzymes LdcCA (L. donovani chagasi) and EhiCA (E. histolytica) with amino acids and amines 1–24. Data for hCA I and II are from [32] and for LdcCA from [42].
| No. | Compound | KA (mM) * | |||
|---|---|---|---|---|---|
| hCA I a | hCA II a | LdcCA b | EhiCA c | ||
| 1 | L-His | 0.03 | 10.9 | 8.21 | 78.7 |
| 2 | D-His | 0.09 | 43 | 4.13 | 9.83 |
| 3 | L-Phe | 0.07 | 0.013 | 9.16 | 16.5 |
| 4 | D-Phe | 86 | 0.035 | 3.95 | 10.1 |
| 5 | L-DOPA | 3.1 | 11.4 | 1.64 | 16.6 |
| 6 | D-DOPA | 4.9 | 7.8 | 5.47 | 4.05 |
| 7 | L-Trp | 44 | 27 | 4.02 | 5.24 |
| 8 | D-Trp | 41 | 12 | 6.18 | 4.95 |
| 9 | L-Tyr | 0.02 | 0.011 | 8.05 | 4.52 |
| 10 | D-Tyr | 0.04 | 0.013 | 1.27 | 1.07 |
| 11 | 4-H2N-L-Phe | 0.24 | 0.15 | 15.9 | 8.12 |
| 12 | Histamine | 2.1 | 125 | 0.74 | 7.38 |
| 13 | Dopamine | 13.5 | 9.2 | 0.81 | 30.8 |
| 14 | Serotonin | 45 | 50 | 0.62 | 4.94 |
| 15 | 2-Pyridyl-methylamine | 26 | 34 | 0.23 | >100 |
| 16 | 2-(2-Aminoethyl)pyridine | 13 | 15 | 0.012 | >100 |
| 17 | 1-(2-Aminoethyl)-piperazine | 7.4 | 2.3 | 0.009 | 43.8 |
| 18 | 4-(2-Aminoethyl)-morpholine | 0.14 | 0.19 | 0.94 | >100 |
| 19 | L-Adrenaline | 0.09 | 96 | 4.89 | 25.6 |
| 20 | L-Asn | 11.3 | >100 | 4.76 | 23.8 |
| 21 | L-Asp | 5.2 | >100 | 0.3 | 23.9 |
| 22 | L-Glu | 6.43 | >100 | 12.9 | 25.5 |
| 23 | D-Glu | 10.7 | >100 | 0.082 | 30.3 |
| 24 | L-Gln | >100 | >50 | 2.51 | 20.1 |
The structure–activity relationship (SAR) for the activation of EhiCA with compounds 1–24 revealed the following observations:
-
(i)
Some heterocyclic-alkyl amines, such as 2-pyridyl-methyl/ethyl-amine 15, 16 and 4-(2-aminoethyl)-morpholine, were devoid of EhiCA activating properties up to 100 µM concentration of activator in the assay system. All these compounds are structurally related, possessing a heterocyclic ring and aminomethyl/aminoethyl moieties in their molecules.
-
(ii)
L-His, dopamine, 1-(2-aminoethyl)-piperazine and D-Glu were poor EhiCA activators, with activation constants ranging between 30.3 and 78.7 µM (Table 2). There is no strong structural correlation between these three compounds.
-
(iii)
Many of the compounds investigated here showed medium potency efficacy as EhiCA activators, with KAs ranging between 16.5 and 25.6 µM. They include L-Phe, L-DOPA, L-adrenaline, L-Asn, L-Asp, L-Glu and L-Gln. It may be observed that there are no remarkable differences of activity between the pairs L-Asp/L-Asn and L-Glu/L-Gln, whereas D-Glu was more ineffective compared to L-Glu. This is in fact the exception, as for other L-/D-enantiomeric amino acids investigated here, the D-enantiomer was the most effective activator (see later in the text).
-
(iv)
Effective EhiCA activating properties were detected for the following amino acids/amines: D-His, D-Phe, D-DOPA, L- and D-Trp, L- and D-Tyr, 4-amino-L-Tyr, histamine and serotonin, which showed KAs ranging between 1.07 and 10.1 µM. The best activator was D-Tyr (KA of 1.07 µM). In fact for all aromatic amino acids investigated here, the D-enantiomer was more effective as EhiCA activator compared to the corresponding L-enantiomer. For the Phe-Tyr-DOPA subseries, the activity increased by hydroxylation of the Phe, achieving a maximum for Tyr and then slightly decreased with the introduction of an additional OH moiety in DOPA, but always the D-enantiomers were better activators compared to the L-ones. The loss of the carboxyl moiety, such as in histamine and serotonin, did not lead to important changes of activity compared to the corresponding D-amino acids, but in the case of dopamine, the activating efficacy was much lower compared to those of both L- and D-DOPA.
-
(v)
The activation profile of EhiCA with amino acid and amine derivatives is rather different from those of other CAs, among which the protozoan β-CA from Leishmania donovani chagasi (LdcCA) or the α-class human CAs, isoforms hCA I and II. For example 17 was a nanomolar activator for LdcCA whereas its affinity for EhiCA was of only 43.8 µM. For the moment, no EhiCA-selective activators were detected.
3. Materials and Methods
3.1. EhiCA Production and Purification
The protocol described in [1,2] has been used to obtain purified recombinant EhiCA. All activators were commercially available from Sigma-Aldrich (Milan, Italy) and were of the highest purity available.
3.2. CA activity and Activation Measurements
An Sx.18Mv-R Applied Photophysics (Oxford, UK) stopped-flow instrument has been used to assay the catalytic activity of various CA isozymes for CO2 hydration reaction [38]. Phenol red (at a concentration of 0.2 mM) was used as indicator, working at the absorbance maximum of 557 nm, with 10 mM Hepes (pH 7.5, for α-CAs) or TRIS (pH 8.3, for β-CAs) as buffers, 0.1 M NaClO4 (for maintaining constant ionic strength), following the CA-catalyzed CO2 hydration reaction for a period of 10 s at 25 °C. The CO2 concentrations ranged from 1.7 to 17 mM for the determination of the kinetic parameters and inhibition constants. For each activator at least six traces of the initial 5–10% of the reaction have been used for determining the initial velocity. The uncatalyzed rates were determined in the same manner and subtracted from the total observed rates. Stock solutions of activators (at 0.1 mM) were prepared in distilled-deionized water and dilutions up to 1 nM were made thereafter with the assay buffer. Enzyme and activator solutions were pre-incubated together for 15 min prior to assay, in order to allow for the formation of the enzyme–activator complexes. The activation constant (KA), defined similarly with the inhibition constant KI, can be obtained by considering the classical Michaelis–Menten equation (Equation (4)), which has been fitted by nonlinear least squares by using PRISM 3:
| v = vmax/{1 + (KM/[S]) (1 + [A]f/KA)} | (4) |
where [A]f is the free concentration of activator.
Working at substrate concentrations considerably lower than KM ([S] << KM), and considering that [A]f can be represented in the form of the total concentration of the enzyme ([E]t) and activator ([A]t), the obtained competitive steady-state equation for determining the activation constant is given by Equation (5):
| v = v0 · KA/{KA + ([A]t − 0.5{([A]t + [E]t + KA) − ([A]t + [E]t + KA)2 − 4[A]t · [E]t)1/2}} | (5) |
where v0 represents the initial velocity of the enzyme-catalyzed reaction in the absence of activator [32,41,42].
4. Conclusions
We report the first activation study of the β-CA from the protozoan parasite Entamoeba histolytica, EhiCA, with a panel of amino acids and amines, some of which are important autacoids. The enzyme was potently activated by D-His, D-Phe, D-DOPA, L- and D-Trp, L- and D-Tyr, 4-amino-L-Tyr, histamine and serotonin, with KAs ranging between 1.07 and 10.1 µM. The best activator was D-Tyr (KA of 1.07 µM). L-Phe, L-DOPA, L-adrenaline, L-Asn, L-Asp, L-Glu and L-Gln showed medium potency activation, with KAs of 16.5–25.6 µM. Some heterocyclic-alkyl amines, such as 2-pyridyl-methyl/ethyl-amine and 4-(2-aminoethyl)-morpholine, were devoid of EhiCA activating properties with KAs > 100 µM. The X-ray crystal structure of this enzyme is not known for the moment, and in addition, no adducts of other parasite enzymes complexed with activators are available so far in order to rationalize our results. However, as CAAs have poorly been investigated for their interaction with protozoan CAs, our study may be relevant for an improved understanding of the role of this enzyme in the life cycle of E. histolytica.
Acknowledgments
The authors acknowledge the Tampere Facility of Protein Services (PS) for their service. The work has been supported by grants from the Academy of Finland, Sigrid Juselius Foundation and Jane and Aatos Erkko Foundation.
Author Contributions
S.B., S.H. and M.K. performed the experiments. S.P. and C.T.S. designed the experiments, evaluated the data and wrote the manuscript. All authors participated to the writing of the work.
Funding
Academy of Finland, Sigrid Juselius Foundation and Jane and Aatos Erkko Foundation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Bua S., Haapanen S., Kuuslahti M., Parkkila S., Supuran C.T. Sulfonamide Inhibition Studies of a New β-Carbonic Anhydrase from the Pathogenic Protozoan Entamoeba histolytica. Int. J. Mol. Sci. 2018;19:E3946. doi: 10.3390/ijms19123946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haapanen S., Bua S., Kuuslahti M., Parkkila S., Supuran C.T. Cloning, Characterization and Anion Inhibition Studies of a β-Carbonic Anhydrase from the Pathogenic Protozoan Entamoeba histolytica. Molecules. 2018;23:E3112. doi: 10.3390/molecules23123112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shirley D.T., Farr L., Watanabe K., Moonah S. A Review of the Global Burden, New Diagnostics, and Current Therapeutics for Amebiasis. Open Forum Infect. Dis. 2018;5:ofy161. doi: 10.1093/ofid/ofy161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hashmey N., Genta N., White N., Jr. Parasites and Diarrhea. I: Protozoans and Diarrhea. J. Travel Med. 1997;4:17–31. doi: 10.1111/j.1708-8305.1997.tb00769.x. [DOI] [PubMed] [Google Scholar]
- 5.Loftus B., Anderson I., Davies R., Alsmark U.C., Samuelson J., Amedeo P., Roncaglia P., Berriman M., Hirt R.P., Mann B.J., et al. The genome of the protist parasite Entamoeba histolytica. Nature. 2005;433:865–868. doi: 10.1038/nature03291. [DOI] [PubMed] [Google Scholar]
- 6.Andrade R.M., Reed S.L. New drug target in protozoan parasites: The role of thioredoxin reductase. Front. Microbiol. 2015;6:975. doi: 10.3389/fmicb.2015.00975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Supuran C.T. Structure and function of carbonic anhydrases. Biochem. J. 2016;473:2023–2032. doi: 10.1042/BCJ20160115. [DOI] [PubMed] [Google Scholar]
- 8.Supuran C.T. Carbonic Anhydrases and Metabolism. Metabolites. 2018;8:E25. doi: 10.3390/metabo8020025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capasso C., Supuran C.T. An overview of the alpha-, beta- and gamma-carbonic anhydrases from Bacteria: Can bacterial carbonic anhydrases shed new light on evolution of bacteria? J. Enzyme Inhib. Med. Chem. 2015;30:325–332. doi: 10.3109/14756366.2014.910202. [DOI] [PubMed] [Google Scholar]
- 10.Supuran C.T., Capasso C. An Overview of the Bacterial Carbonic Anhydrases. Metabolites. 2017;7:E56. doi: 10.3390/metabo7040056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Supuran C.T. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 2008;7:168–181. doi: 10.1038/nrd2467. [DOI] [PubMed] [Google Scholar]
- 12.Neri D., Supuran C.T. Interfering with pH regulation in tumours as a therapeutic strategy. Nat. Rev. Drug Discov. 2011;10:767–777. doi: 10.1038/nrd3554. [DOI] [PubMed] [Google Scholar]
- 13.Supuran C.T. Carbonic Anhydrase Inhibition and the Management of Hypoxic Tumors. Metabolites. 2017;7:E48. doi: 10.3390/metabo7030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Supuran C.T. Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Expert Opin. Drug Discov. 2017;12:61–88. doi: 10.1080/17460441.2017.1253677. [DOI] [PubMed] [Google Scholar]
- 15.Nishimori I., Onishi S., Takeuchi H., Supuran C.T. The α and β-Classes Carbonic Anhydrases from Helicobacter pylori as Novel Drug Targets. Curr. Pharm. Des. 2008;14:622–630. doi: 10.2174/138161208783877875. [DOI] [PubMed] [Google Scholar]
- 16.Supuran C.T., Capasso C. New light on bacterial carbonic anhydrases phylogeny based on the analysis of signal peptide sequences. J. Enzyme Inhib. Med. Chem. 2016;31:1254–1260. doi: 10.1080/14756366.2016.1201479. [DOI] [PubMed] [Google Scholar]
- 17.Supuran C.T., Capasso C. Biomedical applications of prokaryotic carbonic anhydrases. Expert Opin. Ther. Pat. 2018;28:745–754. doi: 10.1080/13543776.2018.1497161. [DOI] [PubMed] [Google Scholar]
- 18.Zolfaghari Emameh R., Barker H., Hytönen V.P., Tolvanen M.E.E., Parkkila S. Beta carbonic anhydrases: Novel targets for pesticides and anti-parasitic agents in agriculture and livestock husbandry. Parasites Vect. 2014;7:403. doi: 10.1186/1756-3305-7-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Syrjänen L., Parkkila S., Scozzafava A., Supuran C.T. Sulfonamide inhibition studies of the β carbonic anhydrase from Drosophila melanogaster. Bioorg. Med. Chem. Lett. 2014;24:2797–2801. doi: 10.1016/j.bmcl.2014.04.117. [DOI] [PubMed] [Google Scholar]
- 20.Vermelho A.B., Capaci G.R., Rodrigues I.A., Cardoso V.S., Mazotto A.M., Supuran C.T. Carbonic anhydrases from Trypanosoma and Leishmania as anti-protozoan drug targets. Bioorg. Med. Chem. 2017;25:1543–1555. doi: 10.1016/j.bmc.2017.01.034. [DOI] [PubMed] [Google Scholar]
- 21.Da Silva Cardoso V., Vermelho A.B., Ricci Junior E., Almeida Rodrigues I., Mazotto A.M., Supuran C.T. Antileishmanial activity of sulphonamide nanoemulsions targeting the β-carbonic anhydrase from Leishmania species. J. Enzyme Inhib. Med. Chem. 2018;33:850–857. doi: 10.1080/14756366.2018.1463221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vermelho A.B., da Silva Cardoso V., Ricci Junior E., Dos Santos E.P., Supuran C.T. Nanoemulsions of sulfonamide carbonic anhydrase inhibitors strongly inhibit the growth of Trypanosoma cruzi. J. Enzyme Inhib. Med. Chem. 2018;33:139–146. doi: 10.1080/14756366.2017.1405264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Menezes Dda R., Calvet C.M., Rodrigues G.C., de Souza Pereira M.C., Almeida I.R., de Aguiar A.P., Supuran C.T., Vermelho A.B. Hydroxamic acid derivatives: A promising scaffold for rational compound optimization in Chagas disease. J. Enzyme Inhib. Med. Chem. 2016;31:964–973. doi: 10.3109/14756366.2015.1077330. [DOI] [PubMed] [Google Scholar]
- 24.Rowlett R.S. Structure and catalytic mechanism of the β-carbonic anhydrases. Biochim. Biophys. Acta Prot. Proteom. 2010;1804:362–373. doi: 10.1016/j.bbapap.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Covarrubias A.S., Bergfors T., Jones T.A., Högbom M. Structural mechanics of the pH-dependent activity of beta-carbonic anhydrase from Mycobacterium tuberculosis. J. Biol. Chem. 2006;281:4993–4999. doi: 10.1074/jbc.M510756200. [DOI] [PubMed] [Google Scholar]
- 26.Murray A.B., Aggarwal M., Pinard M., Vullo D., Patrauchan M., Supuran C.T., McKenna R. Structural Mapping of Anion Inhibitors to β-Carbonic Anhydrase psCA3 from Pseudomonas aeruginosa. Chem. Med. Chem. 2018;13:2024–2029. doi: 10.1002/cmdc.201800375. [DOI] [PubMed] [Google Scholar]
- 27.Zimmerman S.A., Ferry J.G., Supuran C.T. Inhibition of the archaeal beta-class (Cab) and gamma-class (Cam) carbonic anhydrases. Curr. Top. Med. Chem. 2007;7:901–908. doi: 10.2174/156802607780636753. [DOI] [PubMed] [Google Scholar]
- 28.De Simone G., Supuran C.T. (In) organic anions as carbonic anhydrase inhibitors. J. Inorg. Biochem. 2012;111:117–129. doi: 10.1016/j.jinorgbio.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 29.Supuran C.T. Applications of carbonic anhydrases inhibitors in renal and central nervous system diseases. Expert Opin. Ther. Pat. 2018;28:713–721. doi: 10.1080/13543776.2018.1519023. [DOI] [PubMed] [Google Scholar]
- 30.Supuran C.T. Carbonic anhydrase inhibitors and their potential in a range of therapeutic areas. Expert Opin. Ther. Pat. 2018;28:709–712. doi: 10.1080/13543776.2018.1523897. [DOI] [PubMed] [Google Scholar]
- 31.Nocentini A., Supuran C.T. Carbonic anhydrase inhibitors as antitumor/antimetastatic agents: A patent review (2008–2018) Expert Opin Ther Pat. 2018;28:729–740. doi: 10.1080/13543776.2018.1508453. [DOI] [PubMed] [Google Scholar]
- 32.Supuran C.T. Carbonic anhydrase activators. Future Med. Chem. 2018;10:561–573. doi: 10.4155/fmc-2017-0223. [DOI] [PubMed] [Google Scholar]
- 33.Canto de Souza L., Provensi G., Vullo D., Carta F., Scozzafava A., Costa A., Schmidt S.D., Passani M.B., Supuran C.T., Blandina P. Carbonic anhydrase activation enhances object recognition memory in mice through phosphorylation of the extracellular signal-regulated kinase in the cortex and the hippocampus. Neuropharmacology. 2017;118:148–156. doi: 10.1016/j.neuropharm.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 34.Angeli A., Kuuslahti M., Parkkila S., Supuran C.T. Activation studies with amines and amino acids of the α-carbonic anhydrase from the pathogenic protozoan Trypanosoma cruzi. Bioorg. Med. Chem. 2018;26:4187–4190. doi: 10.1016/j.bmc.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 35.Angeli A., Del Prete S., Alasmary F.A.S., Alqahtani L.S., AlOthman Z., Donald W.A., Capasso C., Supuran C.T. The first activation studies of the η-carbonic anhydrase from the malaria parasite Plasmodium falciparum with amines and amino acids. Bioorg. Chem. 2018;80:94–98. doi: 10.1016/j.bioorg.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Stefanucci A., Angeli A., Dimmito M.P., Luisi G., Del Prete S., Capasso C., Donald W.A., Mollica A., Supuran C.T. Activation of β- and γ-carbonic anhydrases from pathogenic bacteria with tripeptides. J. Enzyme Inhib. Med. Chem. 2018;33:945–950. doi: 10.1080/14756366.2018.1468530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Angeli A., Alasmary F.A.S., Del Prete S., Osman S.M., AlOthman Z., Donald W.A., Capasso C., Supuran C.T. The first activation study of a δ-carbonic anhydrase: TweCAδ from the diatom Thalassiosira weissflogii is effectively activated by amines and amino acids. J. Enzyme Inhib. Med. Chem. 2018;33:680–685. doi: 10.1080/14756366.2018.1447570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khalifah R.G. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J. Biol. Chem. 1971;246:2561–2573. [PubMed] [Google Scholar]
- 39.Briganti F., Mangani S., Orioli P., Scozzafava A., Vernaglione G., Supuran C.T. Carbonic anhydrase activators: X-ray crystallographic and spectroscopic investigations for the interaction of isozymes I and II with histamine. Biochemistry. 1997;36:10384–10392. doi: 10.1021/bi970760v. [DOI] [PubMed] [Google Scholar]
- 40.Clare B.W., Supuran C.T. Carbonic anhydrase activators. 3: Structure-activity correlations for a series of isozyme II activators. J. Pharm. Sci. 1994;83:768–773. doi: 10.1002/jps.2600830603. [DOI] [PubMed] [Google Scholar]
- 41.Temperini C., Scozzafava A., Vullo D., Supuran C.T. Carbonic anhydrase activators. Activation of isoforms I, II, IV, VA, VII, and XIV with L- and D-phenylalanine and crystallographic analysis of their adducts with isozyme II: Stereospecific recognition within the active site of an enzyme and its consequences for the drug design. J. Med. Chem. 2006;49:3019–3027. doi: 10.1021/jm0603320. [DOI] [PubMed] [Google Scholar]
- 42.Angeli A., Donald W.A., Parkkila S., Supuran C.T. Activation studies with amines and amino acids of the β-carbonic anhydrase from the pathogenic protozoan. Leishmania donovani chagasi. Bioorg. Chem. 2018;78:406–410. doi: 10.1016/j.bioorg.2018.04.010. [DOI] [PubMed] [Google Scholar]