Abstract
Introduction
Topical ivermectin is an effective treatment for inflammatory papulopustular rosacea in adults. Positive therapeutic effects of ivermectin due to its potential anti-inflammatory properties could be achieved in the other facial dermatoses.
Aim
To assess the efficacy of topical ivermectin 1% cream therapy in mild and moderate perioral dermatitis (PD), seborrheic dermatitis (SD) and acne vulgaris (AV).
Material and methods
The study comprising 20 patients diagnosed with PD (8), SD (8) and AV (4) was conducted between November 2016 and July 2017. Two scales were applied to establish efficacy of the treatment: Investigator Global Assessment score (IGA) and Patient Global Assessment of Treatment (PGA).
Results
All patients responded to the treatment with topical ivermectin very well with a gradual reduction in inflammatory skin lesions. Complete or almost complete clearance (IGA score 0–1) was achieved in 20 cases. Four patients with PD achieved IGA 0–1 after 4 weeks of treatment, 1 patient after 5 weeks, 2 patients after 6 weeks and 1 patient after 12 weeks. In the total group of 8 patients with SD, 4 presented IGA 0 after 4 weeks of therapy, while 4 patients demonstrated IGA 1 after 6 weeks. Patients with AV required 8 and 10 weeks to obtain IGA 1. Nineteen patients of the studied group reported “very good” or “excellent” response to the therapy, only one patient with AV assessed therapy with topical ivermectin as “good”. The adverse events were transient and manifested as mild-moderate desquamation, stinging and burning in 2 patients with PD.
Conclusions
Topical ivermectin was well tolerated and beneficial for treatment of mild and moderate PD, SD and AV.
Keywords: ivermectin, topical, perioral dermatitis, seborrheic dermatitis, acne vulgaris
Introduction
Ivermectin is a broad-spectrum parasiticide in widespread systemic use discovered in 1970 and used in medicine since 1987 in the oral form [1, 2]. In parasites, paralysis and death was observed due to blocking chemical transmission and nerve impulse conduction across γ-aminobutyric acid-gated chloride channels [3, 4].
In December 2014, the US Food and Drug Administration approved 1% ivermectin cream for treatment of rosacea in adults while the first European approval was obtained in March 2015.
Ivermectin in the topical form is a new, effective and well-tolerated treatment for inflammatory papulopustular rosacea in adults [5–7]. The rationale for treating rosacea with ivermectin is based on its anti-inflammatory properties and anti-parasitic effect. Ivermectin suppressed the lipopolysaccharide-induced production of inflammatory cytokines (interleukin 1 (IL-1)and 6 (IL-6), tumor necrosis factor α (TNF-α)) in vitro in a murine study, and this was mediated at least in part by suppression of the nuclear factor κB pathway) [3, 4]. Ivermectin has also been shown to inhibit multiple pro-inflammatory cytokines which are downstream of LL-37 activation, including IL-6 and TNF-α [4]. However, the effect of ivermectin on IL-8, the pro-inflammatory cytokine responsible for neutrophil recruitment, is yet to be elucidated [8]. The recent study has shown that ivermectin is able to inhibit kallikrein 5 (KLK5) and LL-37 gene expression and protein secretion in normal human epidermal keratinocytes (NHEK) stimulated with calcitriol [9]. The anti-inflammatory effects of ivermectin were associated with an inhibition of IL-8, IL-6 and MCP-1 (CCL2) secretion from NHEK cells [9]. These results suggest that ivermectin can prevent the inflammatory effects of rosacea triggered by abnormal LL-37 processing, through the inhibition of KLK5 gene expression in the epidermis [9]. Inhibition of LL-37 has been suggested to correlate directly with beneficial therapeutic activity in patients with rosacea. Azelaic acid, commonly used in the treatment of rosacea, perioral dermatitis (PD) and acne has the ability to directly inhibit KLK5 and cathelicidin gene expression [10]. Ivermectin has a similar structure to macrolide antibiotics, however, its use is not associated with the antibiotic resistance development [11].
Topical ivermectin 1% cream is registered for therapy of rosacea [12]. We searched the following databases up to March 2017: Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, SCOPUS and the Science Citation Index and concluded that there is one report in the literature regarding possible application of topical ivermectin in the treatment of PD and no reports of other inflammatory facial dermatoses: seborrheic dermatitis (SD) and acne vulgaris (AV). Despite different pathophysiologic mechanisms involved in the development of these disorders we decided to apply the symptomatic treatment assuming the potential anti-inflammatory activity of ivermectin.
Treatment of PD can be challenging in children and adults. Demodex mites, although are a part of the normal-appearing skin microbiome, are more numerous in rosacea and PD than in the healthy skin [1]. In contrast to rosacea and perioral dermatitis, the colonization with Demodex folliculorum in acne and SD is comparable with healthy population. Considering the increasing rate of antibiotic resistance in AV patients, new therapeutic options should be estimated. The mechanism of action of topical ivermectin should be also efficient in reduction of inflammatory lesions in AV as well as SD.
Aim
The aim of our study was to assess the benefit and tolerability of topical ivermectin therapy in mild and moderate perioral dermatitis, SD and AV.
Material and methods
We present 20 cases of different facial inflammatory dermatoses (8 cases of PD, 8 cases of SD and 4 cases of papulopustular acne vulgaris) treated in our centers between November 2016 and May 2017. All patients, 16 females and 4 males, were aged 18 and over (ranged between 18 and 54 years at the diagnosis, with average age: 32.4 years), duration of the disease was 2–36 months (mean duration of 21.56 ±1.11 months).
Patients signed informed consent on the day of first consultation and topical ivermectin treatment introduction. Detailed anamnesis, clinical diagnosis and photographs were obtained from the whole studied group. Following data were collected: demographics (date of birth, gender and the duration of the disease) and treatment scheme (duration of treatment, side effects, disease response). Patients had been diagnosed, controlled and treated by a single observer. 1% ivermectin in an oil-in-water base cream was applied once a day for 1 to 3 months depending on the clinical response. To assess disease severity at baseline and after treatment, we applied two scales: Investigator Global Assessment score by retrospectively reviewing clinical charts and photographs and Patient Global Assessment of Treatment based on 5-point Likert scale. For IGA we rated overall severity on a 0-to-4 scale as 0 1/4 clear, 1 1/4 almost clear, 2 1/4 mild, 3 1/4 moderate, and 4 1/4 severe. We considered treatment to be successful when lesions cleared or almost cleared. Possible recurrence was documented when a disease flare required a further course of therapy. For PGA responses choices were: poor, fair, good, very good and excellent.
Results
All patients responded to the treatment with topical ivermectin very well. Gradual decrease in inflammatory skin lesions was observed during the study period. Complete or almost complete clearance (IGA score 0–1) was achieved in 8 cases with PD treated with topical ivermectin. Onset of treatment effect can be seen as early as 2 weeks after treatment initiation with 1% ivermectin cream in monotherapy. Four patients with PD achieved IGA 0-1 after 4 weeks of treatment, 1 patient after 5 weeks (Figures 1 A, B), 2 patients after 6 weeks and 1 patient after 12 weeks. In the total group of 8 patients with seborrheic dermatitis, 4 presented IGA 0 after 4 weeks of therapy, while 4 patients demonstrated IGA 1 after 6 weeks (Figures 2 A, B). Patients with AV required 8 weeks (1 patient) and 10 weeks (3 patients) to obtain IGA 1 (Figures 3 A, B).
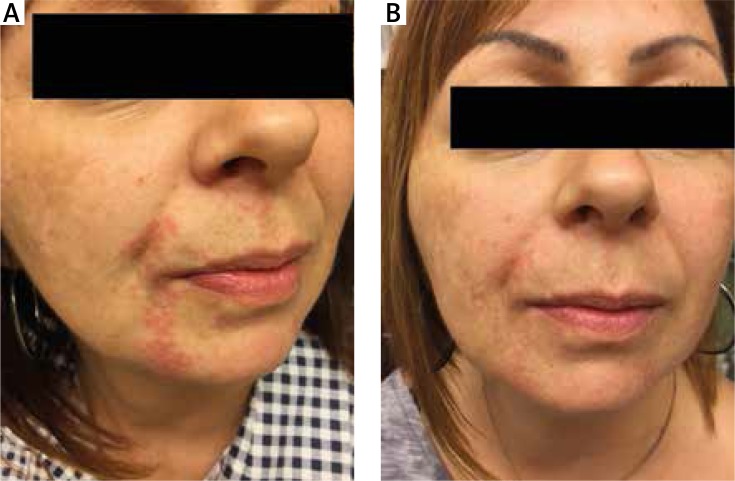
Figure 1.
Patient with perioral dermatitis treated with 1% ivermectin. A – Before treatment, B – 5 weeks after treatment
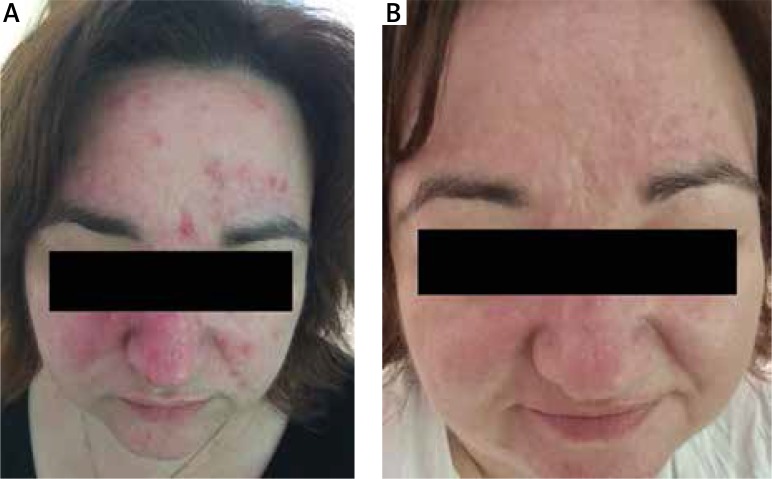
Figure 2.
Patient with seborrheic dermatitis treated with 1% ivermectin. A – Before treatment, B – 4 weeks after treatment
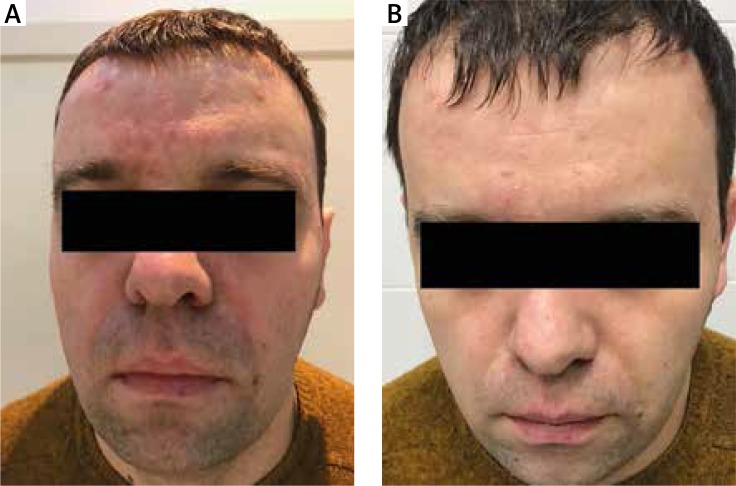
Figure 3.
Patient with acne vulgaris treated with 1% ivermectin. A – Before treatment, B – 8 weeks after treatment
The only adverse event in our series was transient, mild desquamation in one patient and moderate desquamation with burning and stinging in one patient with PD between 2 and 3 week of treatment. It may be hypothesized that desquamation has been a result of a Mazzotti-like reaction resulting from an immunological reaction to dying Demodex, as observed in systemic parasitoses after ivermectin therapy [13, 14].
The results of the treatment with ivermectin 1% cream were also very satisfactory to patients in the studied group. Overall 19 patients reported “very good” and “excellent” improvement (8 with PD, 8 with SD and 3 with acne). Only 1 patient with AV assessed treatment results as “good”. We did not notice disease flares during 18-week observation period in any of our patients.
Discussion
Overall, the safety and efficacy of a topical 1% ivermectin treatment in PD, SD and AV make this medication a potentially attractive candidate for use in the skin diseases mentioned.
Firstly, ivermectin is able to suppress production of the inflammatory mediators NO and prostaglandin E2 (PGE2), and reduce inducible NO synthase (iNOS) and cyclooxygenase-2 (COX2) mRNA expression levels by inhibiting phosphorylation of the mitogen activated protein kinases (MAPK) p38, extracellular – signal-regulated kinase (ERK)n1/2, and c-Jun N-terminal kinase (JNK). Inhibition of NO and PGE2 production by ivermectin results from the inhibition of iNOS and COX2 gene expression [15]. Secondly, the anti-inflammatory effects of topical ivermectin have been demonstrated in clinical trials as shown by decreased counts of inflammatory lesions [5, 6]. This medication can induce burning sensation, pruritus and dryness in 0.7% to 1.8% of patients, which corresponds to our results. Onset of treatment effect can be seen as early as 2 weeks after treatment initiation with 1% ivermectin cream in monotherapy. Having known that there is no risk of systemic accumulation of this cream after 52 weeks of use, the duration of treatment can be planned individually in each patient [16, 17].
Considering the mode of action and results obtained in our research, topical ivermectin seems to be efficient in the treatment of mild and moderate dermatitis, SD and AV. The efficacy of ivermectin in periorificial dermatitis has been recently proved by Noguera-Morel et al. [18].
Anti-parasitic activity of topical ivermectin was studied in the clinical trial estimating safety and efficacy of three concentrations of this medication in the lotion form as a treatment for head lice infestation. A single application of a 0.5% concentration of this ivermectin lotion was the most effective in eliminating head lice in most subjects [19]. Miyajima et al. proposed a novel administration method of ivermectin for scabies treatment in a whole-body bathing method. The results showed that this method can deliver ivermectin to the human stratum corneum without systemic exposure or serious adverse events in healthy volunteers, and at concentrations that would be adequate for scabies treatment [20].
There are no published data about the treatment with the use of topical and systemic ivermectin of AV nor SD in the literature. Based on documented anti-inflammatory properties of ivermectin we decided to introduce the therapy in particular cases of patients with these two conditions. Our patients with SD had no satisfactory response to conventional treatment (topical imidazoles, calcineurin inhibitors and corticosteroids) with frequent flares. Acne patients were treated for 2 and 3 years (consecutively) with topical medications (retinoids, benzoyl peroxide, antibiotics) with transient remission and refused any systemic treatment. All patients with AV included into the study presented a papulopustular and nodular type of acne with a relatively small number of comedones. Interestingly, patients with SD and PD achieved improvement during a shorter period of time (4–6 weeks) than patients with AV (8–10 weeks). The only exception was the patient with PD who responded to the treatment after 12 weeks.
We assumed that positive response of the applied therapy in patients with AV could be achieved due to a potential anti-inflammatory mechanism of action of the ivermectin without any additional direct antimicrobial effect. It probably could explain longer expectancy for improvement in patients with AV compared to patients with PD, but the small number of treated patients did not allow to draw definite conclusions.
One of the greatest therapeutic problems in patients with PD, SD and AV is the tendency to relapse and short time of remission. Well-documented efficacy of ivermectin 1% cream in papulopustular rosacea in 12–16-week randomized studies was confirmed in 36-week extension study evaluating the length of the remission and time of first relapse in patients treated with ivermectin and metronidazole 0.75% cream [7]. The study showed that the initial successful treatment with ivermectin significantly extended remission of rosacea compared with initial treatment with metronidazole following treatment cessation [7]. The median time to first relapse was significantly longer (115 days vs. 85 days) and relapse rates at the end of the study period were significantly lower (62.7% vs. 68.4%) for patients initially successfully treated with ivermectin than with metronidazole [7]. The median number of days free of treatment was higher for ivermectin compared with metronidazole (196 days vs. 169.5 days) [7]. Interestingly, we did not notice any relapse in our patients during 18 weeks’ observation, but obviously a longer period of time is needed to compare the results.
Our pilot study has a few limitations. The study sample (20 patients) was small and the time of observation after achieving remission was not sufficient. A well-designed prospective study with a large number of patients is necessary to confirm our results.
Conclusions
Topical ivermectin was well tolerated and beneficial for treatment of mild and moderate PD, SD and acne vulgaris.
Acknowledgments
Wioletta Barańska-Rybak and Elżbieta Kowalska-Olędzka have equally contributed to this work.
Conflict of interest
The authors are the members of the Advisory Board of Galderma.
References
- 1.Burg RW, Miller BM, Baker EE, et al. Avermectin, new family of potent anthelmintic agents: producing organism and fermentation. Antimicrob Agents Chemother. 1979;15:361–7. doi: 10.1128/aac.15.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crump A, Omura S. Ivermectin, ”wonder drug”, from Japan: the human use perspective. Proc Jpn Acad Ser B Phys Biol Sci. 2011;87:13–28. doi: 10.2183/pjab.87.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dourmishev AL, Dourmishev LA, Schwartz RA. IvermectIn: pharmacology and application in dermatology. Int J Dermatol. 2005;44:981–8. doi: 10.1111/j.1365-4632.2004.02253.x. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Song Y, Ci X, et al. Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice. Inflamm Res. 2008;57:524–9. doi: 10.1007/s00011-008-8007-8. [DOI] [PubMed] [Google Scholar]
- 5.Stein Gold L, Kircik L, Fowler J, et al. Efficacy and safety of ivermectin 1% cream in treatment of papulopustular rosacea: results of two randomized, double blind vehicle controlled pivotal studies. J Drugs Dermatol. 2014;13:316–23. [PubMed] [Google Scholar]
- 6.Taieb A, Orthonne JP, Ruzicka T, et al. Superiority of ivermectin 1% cream over metronidazole 0,75% cream in treating inflammatory lesions of rosacea: a randomized, investigator-blinded trial. Br J Dermatol. 2015;172:1103–10. doi: 10.1111/bjd.13408. [DOI] [PubMed] [Google Scholar]
- 7.Taieb A, Khemis A, Ruzicka T, et al. Maintenance of remission following successful treatment of papulopustular rosacea with ivermectin 1% cream vs. metronidazole 0,75% cream: 36-week extension of the ATTRACT randomized study. J Eur Acad Dermatol Venereol. 2016;30:829–36. doi: 10.1111/jdv.13537. [DOI] [PubMed] [Google Scholar]
- 8.Hoffmann E, Dittrich-Breiholz O, Holtmann H, et al. Multiple control of interleukin-8 gene expression. J Leukoc Biol. 2002;72:847–55. [PubMed] [Google Scholar]
- 9.Thibaut de Mènonville S, Rosignoli C, Soares E, et al. Topical treatment of rosacea with ivermectin inhibits gene expression of cathelicidin innate immune mediators, LL-37 and KLK5, in reconstructed and ex-vivo models. Dermatol Ther. 2017;7:213–25. doi: 10.1007/s13555-017-0176-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coda AB, Hata T, Miller J, et al. Cathelicidin, kallikrein 5, and serine protease activity is inhibited during treatment of rosacea with azelaic acid 15% gel. J Am Acad Dermatol. 2013;69:570–7. doi: 10.1016/j.jaad.2013.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raedler LA. Soolantra (Ivermectin) 1% cream: a novel, antibiotic-free agent approved for the treatment of patients with rosacea. Am Health Drug Benefits. 2015;8(Spec Feature):122–5. [PMC free article] [PubMed] [Google Scholar]
- 12.Galderma (Polska) Ltd Soolantra (ivermectin) 10 mg/g krem – characteristics. http://www.galderma.pl.
- 13.Elston DM. Demodex mites: facts and controversies. Clin Dermatol. 2010;28:502–4. doi: 10.1016/j.clindermatol.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Mackenzie CD, Geary TG, Gerlach JA. Possible pathogenic pathways in the adverse clinical events seen following ivermectin administration to onchocerciasis patients. Filaria J. 2003;(2 Suppl 1):S5. doi: 10.1186/1475-2883-2-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X, Song Y, Xiong H, et al. Inhibitory effects of ivermectin on nitric oxide and prostaglandin E2 production in LPS-stimulated RAW 264.7 macrophages. Int Immunopharmacol. 2009;9:354–9. doi: 10.1016/j.intimp.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 16.Deeks ED. IvermectIn: a review in rosasea. Am J Clin Dermatol. 2015;16:447–52. doi: 10.1007/s40257-015-0150-8. [DOI] [PubMed] [Google Scholar]
- 17.Zargari O, Aghazadeh N, Moeineddin F. Clinical applications of topical ivermectin in dermatology. Dermatol Online J. 2016;22 [PubMed] [Google Scholar]
- 18.Noguera-Morel L, Gerlero P, Torello A, et al. Ivermectin therapy for papulopustular rosacea and periorificial dermatitis in children: a series of 15 cases. J Am Acad Dermatol. 2017;76:567–70. doi: 10.1016/j.jaad.2016.10.034. [DOI] [PubMed] [Google Scholar]
- 19.Meinking TL, Mertz-Rivera K, Villar ME, et al. Assessment of the safety and efficacy of three concentrations of topical ivermectin lotion as a treatment for head lice infestation. Int J Dermatol. 2013;52:106–12. doi: 10.1111/j.1365-4632.2012.05629.x. [DOI] [PubMed] [Google Scholar]
- 20.Miyajima A, Hirota T, Tashiro M, et al. Pharmacokinetics of ivermectin applied topically by whole-body bathing method in healthy volunteers. J Dermatol. 2017;44:406–13. doi: 10.1111/1346-8138.13628. [DOI] [PubMed] [Google Scholar]