Abstract
Agriculture and climate change are internally correlated with each other in various aspects, as climate change is the main cause of biotic and abiotic stresses, which have adverse effects on the agriculture of a region. The land and its agriculture are being affected by climate changes in different ways, e.g., variations in annual rainfall, average temperature, heat waves, modifications in weeds, pests or microbes, global change of atmospheric CO2 or ozone level, and fluctuations in sea level. The threat of varying global climate has greatly driven the attention of scientists, as these variations are imparting negative impact on global crop production and compromising food security worldwide. According to some predicted reports, agriculture is considered the most endangered activity adversely affected by climate changes. To date, food security and ecosystem resilience are the most concerning subjects worldwide. Climate-smart agriculture is the only way to lower the negative impact of climate variations on crop adaptation, before it might affect global crop production drastically. In this review paper, we summarize the causes of climate change, stresses produced due to climate change, impacts on crops, modern breeding technologies, and biotechnological strategies to cope with climate change, in order to develop climate resilient crops. Revolutions in genetic engineering techniques can also aid in overcoming food security issues against extreme environmental conditions, by producing transgenic plants.
Keywords: crop adaptation, climate change, genetic engineering, genome wide association studies (GWAS), marker-assisted selection (MSA), molecular breeding, hormone responses, physiological responses
1. Introduction
Natural systems, human health, and agricultural production have been badly affected by devastating environmental changes [1]. With the rapid increase in the world’s population, there is a corresponding increase in food demand owing to concerns about the stability of the global environment. Water availability, air pollution, and soil fertility have a large impact on agriculture productivity [2]. With abrupt changes in environmental conditions, the harsh impacts on plant productivity are progressing in great intensities owing to direct and indirect effects of abiotic stresses. Because of the continuous deforestation and excessive utilization of fossil fuels, the concentration of CO2 has escalated from 280 µmol−1 to 400 µmol−1 in the atmosphere. It is predicted that the CO2 concentration will elevate two-fold, i.e., up to 800 µmol−1 at the end of this century. Emission of dangerous gases, especially CO2, are the main factors for the greenhouse effect and warmer average global temperatures [3]. The effects of climate change and environmental variation are mainly estimated by the number of stress spells, their impact on daily life, and damage to agricultural crops [4]. In developing countries, agricultural yield is predominantly suffered due to adverse environmental conditions, therefore high temperature and excess of CO2 accumulation forced scientists to devise new strategies to cope with less predictable challenges [5]. To tackle these limitations and guaranteed food security there is a need for production of new climate-smart crop cultivars [6]. Plant growth and yield are greatly influenced by abiotic stresses. Under natural climate conditions, plants often experience numerous stresses like waterlogging, drought, heat, cold, and salinity [7,8]. The abiotic factors also include UV-B, light intensities, flooding, gas emissions, and physical and chemical factors which induce more stresses [9]. In the 21st century, the Earth’s average temperature is expected to increase from 2 to 4.5 °C. According to IPCC-2014 (http://www.ipcc.ch/), the time-span between the 19th and the 21st centuries is considered to be the period which experienced the most warming [10]. Extreme precipitation events might well cause destructions due to floods whereas the scarcity or the total absence of rainfall for a longer period of time leads to drought stresses [11]. The environment of the globe is continuously changing and industrialization is one of the main factors for temperature increase. Due to extreme weather events the frequency of global warming is expected to rise, which will ultimately disturb the ecosystem globally [12]. All living organisms such as plants, animals, fishes, and humans have been affected by the extreme environmental conditions around the globe. The danger to the world’s climate conditions has triggered anxiety among everyone because crop yield might be compromised by fluctuations in various environmental factors that can risk food security. Recent studies reported that the developed countries have more vulnerability towards climatic changes (8–11%) than developing states [13,14]. Climate change and food insecurity are the two major issues of the 21st century. Around 815 million people are affected by malnutrition, hindering sustainable development programs to achieve the universal goal of eliminating hunger by 2030 [15]. Food security and agricultural yield is considerably affected by the adverse weather. With elevation in temperature, the production of major crops has been reduced evidently around the world [16]. At the end of this century global production of crops is likely to decrease as climatic severity increases from 2.6 to 4 °C. Reduction in the productivity of these crops signifies the main threat to food security, particularly in the speedy increase in the world’s population [17]. The population is supposed to grow to about 9 billion in 2050 and food requirement are expected to escalate by about 85% [18]. Climatic influences are worsened by present cropping schemes with low variation and elevated concentration of inputs, and unstable productivity due to environmental changes in crops [19]. The increased frequency of drought and heavy rainfalls, temperature fluctuations, salinity, and insect pest attacks are anticipated to decrease crop productivity leading to higher threats of starvation [20]. Crop adaptability has suffered not only as a result of temperature variations, but also because of rainfall [21]. Currently, the main task is lessening the pressure on food security [22]. This review emphasizes the influence of weather variations on crop production. The next sections outline the overview of the climate change, stresses produced due to climate change, impacts on agricultural crops, strategies to cope with extreme environmental conditions, and some recent genetically engineered approaches to develop transgenic plants against abiotic stresses.
2. Plant Yield and Climate Change
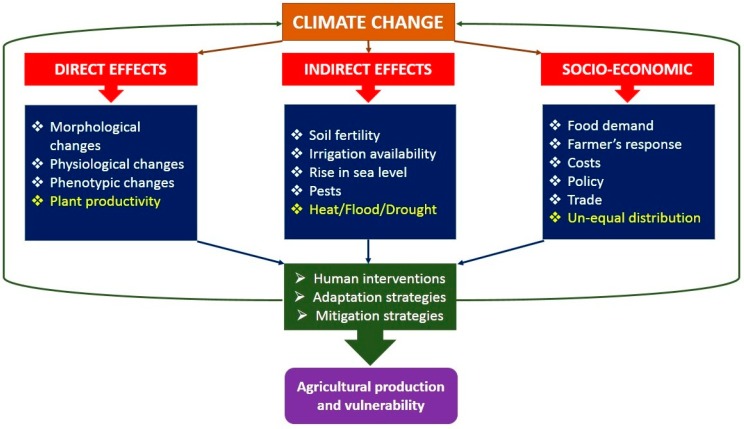
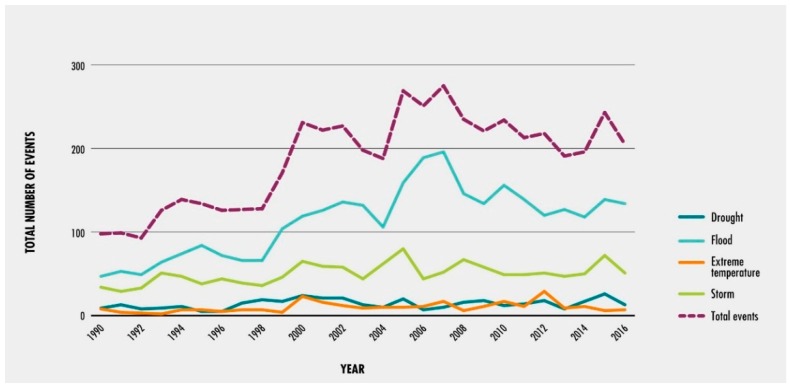
Plant physiology has been greatly influenced by climate variability by several means. Environmental extremes and climate variability enhanced the chances of numerous stresses on plants [23]. Climate change affects crop production by means of direct, indirect, and socio-economic effects as described in Figure 1. Furthermore, climate change (drought, flood, high temperature, storm etc.) events are increased dramatically as reported by Food and Agriculture Organization (FAO) and as shown in Figure 2.
Figure 1.
Direct, indirect and socio-economic effects of climate change on agricultural production.
Figure 2.
Increasing number of extreme climate-related events occurred during 1990–2016. Source: Food and Agriculture Organization (FAO) based on data from Emergency Events Database (EM-DAT) (https://www.emdat.be/) [24,25].
Boyer reported that the climate changes have reduced the crop yield up to 70% since 1982 [26]. According to the study of FAO 2007 (http://www.fao.org/home/en/), all cultivated areas in the world are affected by climatic changes and only 3.5% of areas are safe from environmental limitations (for detail look table 3.7 in http://www.fao.org/docrep/010/a1075e/a1075e00.htm) [27]. Whereas the outcomes of abiotic stresses on crop yield are hard to calculate accurately, it is believed that abiotic stresses have a substantial influence on crop production depending upon the extent of damage to the total area under cultivation. In future, the productivity of the major crops is estimated to drop in many countries of the world due to global warming, water shortage, and other environmental impacts [28,29].
Based on national crop yields and questionnaire surveys, large differences in vulnerabilities to current climate changes were detected across Europe. In Northern Europe, the short duration for crop development and cool temperature are the major concerns, while the temperature extremes and low rainfall limits the crop productivity in Southern Europe, although the most negative effects will be found for the continental climate in the Pannonian zone, which includes Hungary, Serbia, Bulgaria, and Romania [30]. It was predicted that the enhancement of greenhouse gas emissions and abrupt climatic changes will occur that may increase the crop yield in North-Western Europe and decrease the crop yield in the Mediterranean area [31]. Wheat production is heavily affected by the temperature extremes due to climate change in many countries, and may reduce the crop yield by 6% for each °C rise in temperature [32]. Drought and high temperatures are key stress factors with high impact on cereal yields [33], and Rubisco, the central enzyme of photosynthesis, is disrupted if the temperature increases from 35 °C, and stops the photosynthetic process [34]. Gong et al. (1997) reported the negative influence of heat stress on antioxidant enzymes in Zea mays [35]. The combined impact of heat and drought stresses on crop yield have been examined in sorghum, maize, and barley. It was revealed that the combined effect of heat and drought stress had more damaging outcomes as compared to individual stress [36]. Xu and Zhou (2006) subjected the Leymus Chinensis under the combined stresses of drought and heat and found that the function of Photosystem II (PSII) decreased [37].
Due to climate change, water deficit and temperature extremes influence the reproductive phase of plant growth. It was described that the flower initiation and inflorescence is badly affected by the water stress in cereals [38]. Similarly, if the temperature increase of about 30 °C during floret development it can cause sterility in cereals [39]. During the meiotic phase, wheat and rice suffered from the 35–75% reduction in grain set due to water deficit [40,41]. In rice, drought stress greatly disturbs the process of fertilization and anthesis. Due to water deficit, the harvest index is reduced to 60% and decreases the grain set [42]. The cocoa yield has been significantly reduced by the major drought spells in West Africa during the 1980s El Niño years [43]. It has been estimated that agricultural production could reduce to 25.7% by 2080 due to climate change and maize will be the most affected crop in Mexico [44]. A study based on ECHAM6 climate data was analyzed for North German Plains during two different time durations: 1981–2010 and 2041–2070. The results showed that if the yield for winter wheat is to be sustained, water availability must be guaranteed [45]. Zhao et al (2017) carried an experiment to analyze the climate change impact on major crop yields and showed considerable yield reductions of 6%, 3.2%, 3.1%, and 7.4% in wheat, rice, soybean, and maize respectively [46]. To tackle the climate change new discoveries in genomics are enabling climate-smart agriculture by developing climate resilient crops [47].
Drought stress influences wheat during all developmental stages, but grain formation and the reproductive stage are the most critical ones [48]. Wheat yield was decreased from 1% to 30% during the mild drought stress at post-anthesis while this reduction increased up to 92% in case of prolonged mild drought stress at flowering and grain formation [49,50]. Drought stress has greatly reduced the yield of important grain legumes. Mashbean (Vigna mungo L.) yield has been reduced by drought stress from 31% to 57% during the flowering stage while a 26% reduction was reported by drought stress during the reproductive phase [51]. Maleki et al. (2013) reported that the soybean yield has been largely effected by drought stress and a 42% reduction was observed during the grain filling stage of soybean [52]. Schlenker and Roberts (2009) described that maize yield was increased at an optimum temperature of 29 °C but a further increase in temperature hampered the yield of maize [53]. Every 1 °C rise in temperature was found to negatively influence the maize yield [54]. Similarly, it was reported that yield in maize decreased by 8.3% with every 1 °C rise in temperature from the optimum growth temperature [55]. Brown (2009) reported that wheat yield decreased by 10% with every 1 °C increase in temperature [56]. In another report it was revealed that a 3–4% reduction in wheat yield takes place for every 1 °C increase in temperature [57]. Easterling et al. (2007) described that a 2 °C increase in temperature cause 7% reduction in yield while a further increase in temperature to 4 °C decreased the yield by up to 34% in wheat. Similarly, rice yield decreased by 2.6% for every 1 °C rise in temperature [58]. In sorghum, yield was reduced by 7.8% due to a 1 °C increase in temperature [59]. In sorghum, water shortage is another big issue reported in most of the world’s top producer countries [60]. Schlenker and Roberts (2009) revealed that the threshold temperature for soybean is 30 °C; a rise in temperature to the optimum level increased soybean yield but after that level, further rise in temperature reduced the yield abruptly [53]. Eastburn et al. (2010) reported that the rise in ozone and CO2 concentration in the atmosphere influenced the disease type, and with a continuous rise in temperature, disease susceptibility in soybean was enhanced [61].
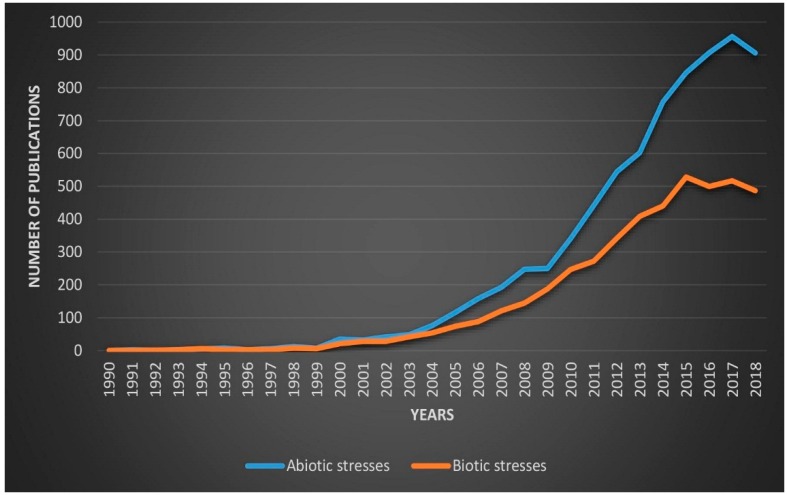
This rising concern was revealed in the growing quantity of research papers focused on abiotic problems after the crucial review by Kitano on systems biology [62]. The amount of research studies has increased dramatically related to biotic and abiotic stresses in plants by applying different strategies (Figure 3).
Figure 3.
The number of publications per year related to abiotic and biotic stresses from Jan/1990–Nov/2018. Source: PubMed (Keywords (abiotic stresses, drought, cold, heat, salinity and water-logging), (biotic stresses, bacteria, virus, fungi, insects, parasites, and weeds) used to search the number of publications in PubMed).
Climate change influences food security in a very complicated manner. It hampers the agricultural yield directly by means of disturbing the agro-ecological environment and indirectly by putting pressure on growth and circulation of income and consequently increased the necessity of agricultural products. Impacts of climate change on food security have been calculated in several ways [63]. Here we briefly discuss the potential impact of climate change and food security.
In temperate regions and humid grassland zones, a slight elevation in temperature may raise the pasture productivity. These advances have to be established to tackle amplified rate of climate changes, for example, drought and temperature extremes in the Mediterranean zone or massive rainfall spells and in temperate areas increase the risk of flooding [64]. But in the case of arid and semiarid regions, it may cause a reduction in livestock growth and enhance their death rates [65]. The extensive rate of evapotranspiration and less moisture in the soil are predicted in drier regions by various climate models [63]. Consequently, due to climate changes, many regions of cultivated land may become unsuitable for cultivation, and other tropical regions may produce more crops. Temperature instability will also provide more favorable environmental conditions for insect pests of crops to boost their capacity to stay alive in cold temperatures and then emerge in outbreaks in spring. It is very crucial to observe that in case of food accessibility, all recent calculations for food security and safety have concentrated mainly on the effects of climate changes in ways that did not measure the probability of substantial alteration in the rate of climate extremes on crop productivity. They have also not considered the situations of sudden changes in socio-economic status and climate, so all these factors have been putting negative impacts on global food security and safety [64]. Around the globe, food security is remarkably significant for human beings. Because of climate change, food quality, supply, and safety are still the biggest problems for researchers. Future studies on food security will need to incorporate climate change, crop productivity, water supply, and population to estimate food security conditions entirely and scientifically [21].
3. Crop Adaptation to Overall Extreme Climate Stresses
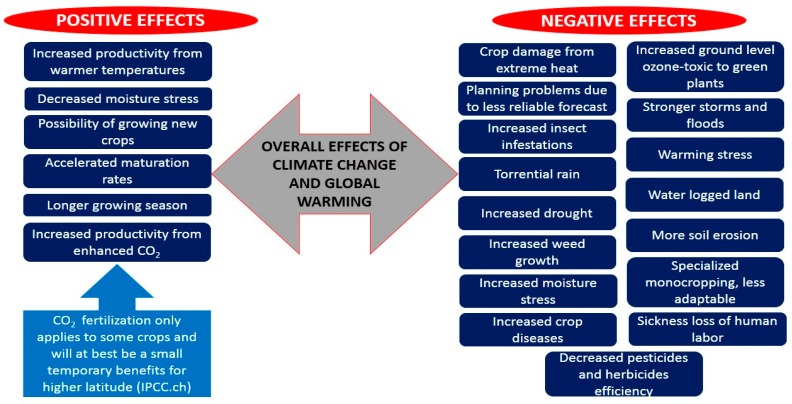
With the increase of the Earth’s temperature, the climate undergoes severe alterations and becomes abiotically stressful. Environmental changes are very damaging and pose various threats to naturally prevailing crop species [66]. Under field circumstances, drought and heat are the most predominant stresses and have a significant influence on plants [67]. It is reported that plants require an optimum temperature for their normal growth and blooming. Plant physiology is heavily influenced by temperature fluctuations [68]. As heat stress affects the grain production and yield, cold stress results in sterility, and drought stress negatively influences the morpho-physiology of plants [69,70]. These climatic problems severely distress plant development and yield, produce enormous responses, comprising molecular, biochemical, physiological, and morphological modifications [71]. Overall, global warming and climate change both have some negative and positive effects on agricultural crops as well as on humans as explained in Figure 4.
Figure 4.
Overall positive and negative effects of climate change and global warming on crops and humans.
In this context, understanding the stress-resistance processes in plants has emerged as a very difficult task for plant scientists in order to develop stress-resistant plants [72]. The chief cereal crops around the world, such as maize, rice, and wheat, are crucial to meet the daily food demand. Out of them, wheat was the leading staple crop which has been cultivated on a large scale [73]. Wheat is harvested on 38.8% of total agricultural land worldwide and provides a considerably high concentration of proteins: 15% per gram as compared to maize or rice which only supplies 2 to 3% [74]. Regardless of large growing land globally, its productivity has been predominantly less than the maize and rice [18]. Reasonable reduction was anticipated in wheat productivity with a 2 °C increase in temperature. Related research on environmental variability expected a 6% reduction in wheat yield [75]. Challinor and his colleagues described that due to the increase in temperature, the grain filling phase decrease is the major reason of crop productivity reduction in changing climatic conditions [76]. Therefore, sustaining crop yield is an important task in current agriculture, and to produce stress-tolerant crop plants [75].
4. Various Limiting Factors for Crop Development
For sustainable agriculture and food safety for an increasing population of the world, it is necessary to grow stress-tolerant plants and understand their responses under different stress conditions. In relation to various climatic stresses, the response of plants varies in the expression of genes, physiology, and metabolism. It was reported that plants have the ability to sense any variation in surrounding environmental signals but in spite of many studies, only some reputed sensors have been recognized [77]. Due to different stresses, the organs and tissues of the plants are damaged and they respond accordingly, for example, transcriptional responses against various stresses are different in specific cells or tissues of roots [78]. Stress-responsive protein creation, high levels of associated solutes, and more elevated antioxidant ratios are the cellular signals which are produced due to salinity, drought, and chemical effluence. These stresses are regarded as primary stresses and they generate secondary stresses like oxidative and osmotic stress [79].
Under drought conditions, elevated level of CO2 in leaf causes the initiation of reactive oxygen species (ROS) which trigger the multiple stresses in crops. With locked stomata, movement of CO2 inside the leaf is clogged, and ROS are produced due to enhanced levels of oxygen under drought conditions. The frequency of plant development, photosynthesis, and respiration are disturbed by membrane breakdown due to ROS production. Several cell building materials like carbohydrates, lipids, proteins, and nucleic acid are impaired by ROS in drought stress [80]. In recent studies, it was observed that Osmo-protectants have been produced under the combined stress conditions of heat and salinity in tomato plants, but do not appear in individual stresses. Another experiment demonstrated that the combined effect of heat and salt stress leads to diverse metabolomic profiling which was established with molecular and physiological statistics. For plant development, ROS has a significant role and it is considered as a crucial secondary signal for cellular metabolism: an elevated level of ROS prompts cell apoptosis. Therefore, a gentle equilibrium among ROS creation and their decontamination may occur in every oxygenated organism [81]. The adaptability of Arabidopsis to persistent water deficiency at the molecular and morpho-physiological levels was examined. Arabidopsis collected from various habitations presented alterations at the transcriptomic level [82].
Metabolic profiling of various crucial plants have been comprehensively completed under water stress, such as rice, soybean, maize, and tomato. In barley, numerous metabolomic analyses have also been conducted to understand the impact of water scarcity on the oxidative phase, abscisic acid, and free amino acids. Barley cultivars were subjected to water shortage to explore the genetic variation on the metabolomic level at grain formation phase [83]. Protein production inhibition is the initial metabolic signal against the abiotic factors [84]. Post-translational modifications and processing are also the primary responsibilities of abiotic stresses [85]. Drought stress in coffee has been studied from a wide viewpoint by assimilating the vital features of plant biochemistry and physiology. The plants subjected to multiple events of constant drought stresses have greater photosynthesis processes, in contrast to plants with only one event of drought stress imposed on them. Certainly, these plants showed advance RuBisCo control and several enzymes related to metabolism. Adaptability to various drought doses elaborated the gene expressions associated with drought resistance [86].
5. Impact on Plants’ Morpho-Biochemical and Physiological Processes
With great environmental variability, plants are suffering from unique climatic conditions that limit the plants’ ability to adapt successfully in a range of ways. Due to more spells of rainfall and warmth, plant relocation is not to be the solution to this problem. However, modifications in plant physiology have been beneficial in unique climatic conditions, but environmental variability can be risky for plants [87]. Morphological, biological, and biochemical mechanisms of plants have been severely affected by abiotic stresses. Although for expected weather conditions in the future, plant physiology reactions are predicted to propagate quickly, with minor variation in fruiting and flowering [88,89]. The ideal temperature for plant development is in the range of 10 to 35 °С. Elevation of the temperature to a specific point will permit plants to generate excess energy but a larger increase in temperature retards the plant growth and the photosynthesis rate abates to deadly levels [90].
Turgor pressure is limited by the drought stress and therefore delays cell development. Water shortage impacts the photosynthesis enzymes actions and decreases the metabolic competency and ultimately destroys photosynthetic machinery [91]. Because of environmental changes CO2 levels proliferate and retard respiration in plants and enhance temperature level. Respiration rates of the plant were elevated when the temperature was raised from 15 to 40 °C, disturbing morphological features of some crops [92,93]. During the process of photosynthesis, the enzyme Rubisco is associated with carbon fixation and translation of CO2 into a complex energy-rich compound [94]. Rubisco is activated by the Rubisco activase at an optimum temperature by abolishing secondary metabolites. A minor elevation in temperature resulted in the deactivation of Rubisco enzyme leading to the generation of xylulose-1,5-bisphosphate which is supposed to be an inhibitory compound. At an increased temperature, Rubisco did not work properly because of the Rubisco activase breakdown and was unable to activate Rubisco [95]. ROS containing OH, H2O2, and singlet oxygen are derivatives of metabolisms and are regulated by antioxidant defense mechanism. ROS are mostly formed in minimum amount under optimum conditions but with the increase in concentration environmental stress triggered [96].
6. Plant Hormone Responses in Abiotic Stresses
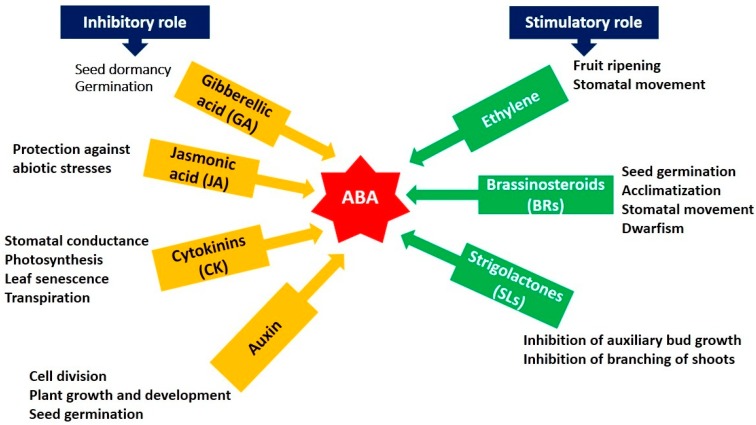
Under different abiotic stresses, hormones are very crucial for regulating many signaling pathways and responses such as salicylic acid (SA), abscisic acid (ABA), and ethylene [97]. The major role is played by ABA in the regulation of stress responses by the interactions with some other hormones as shown in crosstalk (Figure 5). The most important and vital hormone for regulating the climatic stresses in the plant is ABA. ABA plays a major role in different stages of plant development particularly in stomata opening and closing, drought stress, seed germination, and dormancy. PYR/PYL/RCAR-PP2C-SnRK2 is recognized as a signaling cascade generated by ABA and controls the seed dormancy efficiently. Under drought conditions, the plant growth is severely retarded and it increases the ABA concentration in cells. ABA accumulation during drought stress controls transpiration and inhibit stomatal disclosure [98]. ABA also triggers many physiological mechanisms in plants such as water scarcity, regulates stomata to close down, and produces many stress-responsive genes in this period [99]. ABA signaling machinery have been investigated recently and their mechanism of operation was elucidated. The signaling cascade consisted of 3 units, SnRK2/OST1 (Protein kinase), PP2C (protein phosphatases) and PYR/PYL/RCAR proteins [100].
Figure 5.
Hormonal crosstalk related to different stresses.
Two different group of scientists found the ABA PYR/PYL/RCAR receptors [101,102]. PP2C was first observed in Arabidopsis knockouts of abi1-1 and abi2-1 and is regarded as the negative controller of ABA [103]. Similarly, the protein kinase was collected and separated as SnRK2 and it is the activator of ABA [104]. Salicylic acid also has regulated numerous physiological processes in plants under stress climatic condition. It was identified that acetylsalicylic acid can encourage protoplast cluster development in corn, controlling cell cycle regulation [105]. The role of SA was discovered by a group of scientists working on cell cultures of tobacco, they found that SA regulates the bud development and flowering initiation [106]. Malamy and his colleagues were pioneers in studying the Tobacco Mosaic Virus and established the part of SA in plant-pathogen interaction [107]. Recent studies on SA described its impacts on fruit productivity, legumes nodulation, temperature resistance, stomata closing, respiration, genes related to senescence, and cell growth [107]. Salicylic acid regulations in these events might be secondary because they control the production of further plant stress-responsive hormones [108,109].
Phytohormones play a vital part in stress response by modulating different signal transduction mechanisms under climatic variability. One of the most important members of phytohormones is ethylene. It is found in gaseous form and thus enables plant-to-plant connections. A century ago, ethylene was discovered and since then many research studies were carried out to reveal its biosynthesis. Ethylene has a function in the control of seed germination, ripening, leaf growth, and senescence under different abiotic and biotic climatic stresses. It is supposed that ethylene acts as signaling pathway among plant growth and weather variations. Abiotic stresses such as salinity, water logging, high temperature, frost, heavy metal contact, nutrient deficiency, and drought are the reasons which modulate the synthesis of ethylene [110]. Ethylene response factors (ERFs) in plant ethylene belong to a massive transcription factors (TFs) family and are activated during the different physiological and environmental stresses. There have been extensive investigations on ERF proposing its role in abiotic stresses but until now there is no evidence of any specific signaling pathway under abiotic stresses. In a recent study, ERFs in tomato were subjected to drought, salinity, heat, cold, and excessive water conditions for their expression profiling [111].
7. Approaches to Combat Climate Changes
Variation in the environment has a long-lasting influence on agriculture and food security globally. Food security and safety are threatened by the severe weather conditions and it is not a recent problem. But formerly, no consideration was adopted to tackle this problem. Therefore, to cope with these weather variations is the most urgent demand worldwide. For crops to adapt to changing environmental stresses subsequent approaches are required.
7.1. Cultural Methodologies
Recently some experiments reported investigations of the strategies trialled by farmers to tackle the climatic variation for plant adaptation. There are many useful approaches adopted by farmers, including abiotic factors such as altering planting and harvesting time, a collection of crops with short life cycles, crop rotation, irrigation techniques, and variation in cropping schemes. Under climatic stress conditions, all of these approaches are very beneficial for crop adaptability [112,113,114,115]. Modification in sowing time, application of drought resistant cultivars, and the cultivation of new crops are some important strategies to lessen the climatic variability danger and provide better adaptability to crop plants for assuring food safety and security [116]. Another plant adaptability approach is by means of crop-management techniques that have the ability to enhance crop development under various environmental stresses. The choice of sowing time, planting density, and optimum irrigation practices are crucial techniques to tackle weather stresses [117]. Fertilizers are also very vital to reduce the effect of global warming and support the plant for better adaptability. It provides substantial energy to plants and is beneficial to maintain the fertility of the soil and increase productivity. Hence, the importance of fertilizer in nourishing the world is undeniable [118].
7.2. Conventional Techniques
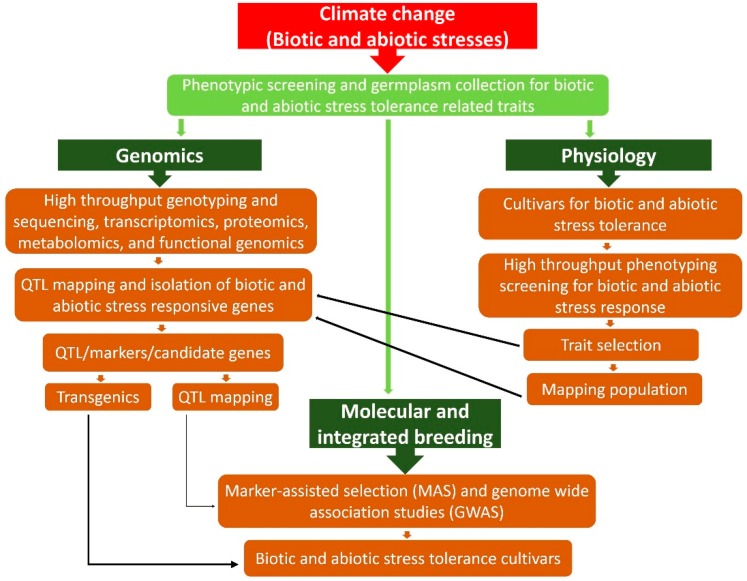
Under various environmental stresses, plant breeding shows dynamic techniques in crop development and betterment. It gives a way to potentially guarantee food security and safety under harsh weather variations and help plants escape from various stresses through a crucial phase of plant growth by developing stress resistant cultivars [119]. Genetic divergence analysis is used for polymorphism, inbreeding, assessment, assortment, and recombination to attain plant perfection, and is amongst the main aspects for defining accomplished inbreeding. Genetic divergence analysis is considered a very important method for the development of new cultivars based on genetic distance and similarities [120,121]. For genetic studies landraces are a significant source, for example, a wheat landrace kept in data bank comprises broader genetic variance and is a valuable basis for stress resistance as it contains cultivars adjustable to diverse environmental stress [122]. Figure 6 demonstrates how molecular and integrated plant breeding are useful to develop the biotic and abiotic stress tolerance cultivars using genomics approaches like marker-assisted selection (MAS) and genome wide associated studies (GWAS).
Figure 6.
A step-wise presentation of physiological, molecular breeding and genomics approaches to develop biotic and abiotic stress tolerance cultivars.
7.3. Genetics and Genomics Strategies
7.3.1. Omics-Led Breeding and Marker-Assisted Selection (MAS)
Omics approaches provide beneficial resources to elucidate biological functions of any genetic information for crop upgrading and development [123]. Different molecular markers are studied in population genomics across the environment in many individuals to find out novel variation patterns and help to find if the genes have functions in significant ecological traits [124]. In many crops, the breeding program is coupled with genomic approaches to achieve great heights in molecular breeding and to screen elite germplasms with multi-trait assembly [125] For the identification of phenotypes under different environmental variation associations, genetics and transcriptomic analysis are used [126] Genomics also enables investigation of the molecular mechanisms underlying the abiotic stress resistance. These approaches aid in the development of climate smart crops for better yield and production under different climate changes [127]. With the advent of high throughput sequencing and phenotyping, genomic-led breeding paved the way for identifying different stresses that are expected to adversely affect crop yield. Furthermore, the data available on environmental extremes, DNA fingerprinting, and quantitative trait loci (QTL) mapping allows the screening of elite germplasm under abiotic stresses [128]. QTL dissection of yield-related traits in crops under stress conditions permits the development of novel cultivars with better adaptability in abiotic stress [129]. Molecular plant breeding is an essential approach to enhancing crop yield and production in the presence of various biotic and abiotic stresses [130]. For speedy breeding progression marker-assisted selection (MAS) presents a crucial part in the betterment of crop traits and yield. With the advancement in crop genomics, DNA markers have been identified which are valuable for marker-assisted breeding [131].
The introduction of novel sequencing tools greatly eased the difficulty in researching genomic variants and lead towards the identification of huge amounts of DNA polymorphism, particularly single nucleotide polymorphism (SNPs) markers [132] Precision of QTL mapping enhanced on average from 10–30 centimogran to <1 cM [133,134] with the advancement of linkage maps [135]. The high-throughput phenomics approach is also contributing to increasing the accuracy of QTL mapping [136]. The association among genotypic and phenotypic data is crucial for enlightening the genetic basis of multiple traits [137]. By applying QTL mapping Haley and his colleagues successfully developed a wheat variety called “Ripper” which has the ability to withstand the drought conditions of Colorado, without affecting its grain yield and quality [138]. In 2009 Badu-Aparku and Yallou performed QTL mapping to screen elite maize germplasm with high yield under drought stress [139]. Merchuk-Ovant et al. (2016) conducted marker assisted selection studies on bread wheat (Triticum aestivum L.) and durum wheat (Triticum turgidum L.) to identify the QTLs related to drought stress [140]. Barley is cultivated on a wide range of land across the world but it is severely affected by drought stresses globally. QTL mapping of two novel barley cultivars that have been totally different in their response to drought stresses were selected and QTL mapping study performed for malting characters in double haploid. QTL investigation showed that MSA are specific reliable genomic sections regulating the malting feature which can be helpful [141]. Similarly, a recent QTL study was carried out to explore the epistatic mechanism and physiology of QTL for the elucidation of the targeted gene. Under drought stresses, 3 QTL were recognized such as qDTY6.2, qDTY6.1, and qDTY3.1, which have a considerable impact on grain productivity [142]. To be resistant under hyper temperature conditions three vital points on the genome of bread wheat have been predicted: 7D, 7B, and 2B [47]. Tahmasebi et al. (2016) performed QTL mapping for a recombinant inbred lines (RILs) population of wheat under different stress conditions of flooding, drought, heat, and a combination of both heat and drought simultaneously. QTL mapping showed a 19.6% variation in grain yield under these stress conditions. The authors concluded from this research that the molecular markers could be exploited to explore the unique allelic variations in wheat to enhance the potential to screen drought tolerant cultivars [143].
7.3.2. Genome Wide Association Studies (GWAS) for Stress Tolerance
Genome wide association studies (GWAS) is a powerful tool for understanding the complete set of genetic variants in different crop cultivars to recognize allelic variant linked with any specific trait [144]. GWAS generally highlight linkage among SNPs and traits and based on GWAS design, genotyping tools, statistical models for examination, and results interpretation [145]. In many crops GWAS has been carried out to exploit the genetic process responsible for genetic resistance under climate change [146]. In plants, GWAS has widespread applications related to biotic and abiotic stresses. GWAS have been applied to describe drought tolerance [147], salt tolerance [148], and heat tolerance [149].
In Arabidopsis thaliana GWAS study was carried out by Verslues et al. (2013) aided by reverse genetic approaches to elucidate unique genes that accumulate proline under drought stress. The linkage among SNPs of both genotypic and phenotypic data were examined, and specific regions regulating proline accumulation were recognized. Similarly, different proteins controlling the pro-accumulation such as aMADS box protein, Universal Stress Protein A domain proteins, protein phosphatase 2A subunit A3, thioredoxins, ribosomal protein RPL24A, and mitochondrial protease LON1 were identified by using reverse genetics. This research gave insights for proline accumulation under drought stress conditions [150]. Aegilops tauschii is reported to have many resistance genes regulating the abiotic stresses [151]. A significant knowledge is required for the breeders to understand the genetic architecture of Aegilops tauschii to improve drought resilience. Qin et al. (2016) investigated 373 different varieties of A. tauschii to examine 13 traits controlling drought stress. For GWAS 7185 SNPs were designated to study the phenotypic behavior and carried out mixed linear model and general linear model to find the association between SNPs with phenotypic traits [152].
QTLs related to salinity resistance in plants were studied by using GWAS. Kumar et al. (2015) reported various genes regulating the salinity tolerance in rice by using infinium high-throughput SNPs arrays. Six thousand genotype-based SNP were constructed for genes related to stress and linkage among SNPs and phenotypic data were interpreted. QTLs for salt tolerance by genomic regions were mapped on chromosome numbers 1, 4, 6, and 7. A novel QTL present on chromosome number 1 was reported and was called “Saltol” which is associated with salt tolerance at seedling stage [153]. Lafarge et al. (2017) performed GWAS for genotyping 167 rice varieties for spikelet sterility (SPKST), and panicle micro-nutrient by applying 3 techniques of haplotype regression, single marker regression, and co-fitting of all markers to analyze the impact of heat during anthesis process. A significant association was present between SPKST, secondary traits, and 14 loci. These loci were investigated for functions related to heat shock proteins, controlling plant responses, development of gametophyte, cell division, and detecting abiotic stresses [149]. Chopra et al. (2017) reported various stress-tolerant genes in Sorghum bicolor associated with heat and cold stresses. GWAS was conducted for genotyping and phenotyping analysis. Thirty SNPs were identified for genes related to anthocyanin expression and carbohydrate metabolism, which are powerfully associated with cold stress at the seedling growth phase of sorghum. Similarly, 12 SNPs were discovered for heat stress at the seedling stage and controlled by the genes having functions in ion transport mechanism and sugar metabolism [154]. In another study Chen et al. (2017) examined Sorghum bicolor for heat tolerant traits such as leaf firing (LF) and leaf blotching (LB) at the vegetative phase of growth. To identify the association among SNPs with genotype and heat tolerance, GWAS was performed. Nine SNPs were closely linked with LF and five SNPs were identified for LB traits. Furthermore, 14 genes associated with SNPs were discovered that have stress-responsive expression to abiotic stresses [155].
7.3.3. Genome Selection (GS) for Crop Improvement
Genomic selection (GS) is the exciting tool to revolutionize the crop improvement by using high-throughput phenotyping and marker densities to screen the elite germplasm, improving the polygenic traits and economical breeding line development [156]. Currently, the prospective of genomic selection (GS) to fast-track the speed of genetic achievements in main crops has stimulated the development of multi-environment designs for genomic estimation. Burgueño et al. (2012) proposed the first statistical design by applying a linear mixed model to G × E model [157]. Jarquín et al. (2014) suggested a system of modeling connections among an elevated dimensional combination of markers and environment that integrates with each other (G × E) [158]. Another model (GBLUP-type model) was proposed by Lopez-Cruz et al. (2015) in which regression of phenotypes was used for the interaction of marker × environment (M × E) [159]. The modern multi-environment model for genomic prediction was proposed by Cuevas et al. (2017) based on Bayesian model. These methods are applied on 4 wheat and 1 maize cultivars and CIMMYT data bank revealed that the G × E model have high significance rates and better genomic predictions as compared to other models [160].
Around 40 research studies based on GS have been published so far. Wheat is the most studied crop with 29 genomic selection studies. Moreover barley, oat, and durum wheat have 5, 2, and 1 research paper published. Diversity Array Technology (DArT) was the most promising maker used in GS followed by single nucleotide polymorphism (SNP) and genotyping by sequencing (GBS). These experiments showed that GS could be magnificently used in cereal breeding [161]. Genomic Selection (GS) designs were extensively developed for wheat to reveal the germplasms that have better ability to adapt in climate changes [162]. Crain et al. (2018) studied the different GS techniques to detect phenotypic data from high throughput phenotyping. At CIMMYT, heat and drought stresses were examined in 1000 elite wheat cultivars by using a high throughput phenomics approach [163].
7.3.4. Genetic Engineered Plants for Stress Tolerance
Biotechnology is an influential approach for genetic manipulation of the genome for the betterment of human beings. The genetic modification through biotechnology is a powerful strategy. Encouraging data is collected from genetics which can be exploited significantly to various biotic and abiotic stresses such as salinity, drought, heat, and cold. Identification of stress-responsive TFs are powerful findings to develop stress-resistant crop cultivars. These TFs can control the phenotypes of genes in genetic engineered crops associated with various stresses [164]. There are numerous transgenic plants which have been established by genetic engineering to tackle the biotic and abiotic stresses. These genetically engineered plants demonstrate significant resistance against climatic variations compared to normal plants [165,166].
Various transcriptions factor (TFs) are recognized as plant-specific TFs which includes AP2/ERFBP group [167]. This family of AP2/ERFBP TFs is responsible for many plant growth pathways and has functions in biotic and abiotic stress responses [168]. AP2/EREBP TFs are categorized into 4 sub-groups based on their similarity and numbers. The subfamilies consist of ERF TFs, DREB (dehydration-responsive element-binding protein), AP2 (Apetala 2), TFs, and RAV (related to ABI3/VP1). DREB and ERF are two major subfamilies which have been widely examined due to their role in plant biotic and abiotic responses [169]. The DREB TFs have significant regulating ability in various water deficit and cold stress conditions [170]. DREB TFs have been investigated in response to stresses in various plants species such as wheat, barley, maize, soybean, rice, tomato, and Arabidopsis [171,172,173]. In numerous experiments DREB1 has been studied in rice and Arabidopsis for its controlling mechanism in cold stress while DREB2 functions in drought, salinity, and high temperature stresses [174,175,176]. The DREB1 TFs were over-expressed to develop transgenic Arabidopsis with a better ability to withstand salinity, drought, and freezing stresses [177,178]. Similarly, DREB1 genes were introduced into the rapeseed, rice, tomato, and tobacco for cold stress resistance [179,180,181,182]. Many of the DREB1 genes have also been purified from wheat, rye, maize, rice, and oilseed rape and have been transformed to develop transgenic crops against different abiotic stress [183,184]. Qin et al. (2007) isolated the ZmDREB2A gene from maize and over-expressed in Arabidopsis to developed transgenic Arabidopsis with improved resistance against drought stress [185]. Similarly Chen et al. (2007) revealed that the transgenic plants with over-expression of GmDREB2 gene extracted from soybean showed significant tolerance against salt and drought resistance [186]. Mallikarjuna et al. (2011) successfully developed transgenic rice with the improved resistance against salinity and drought stresses by over-expressing the OsDREB2A gene [187].
The larger subfamily of AP2/EREBP TFs are ERF [188] and are responsible to regulate stress-tolerance genes in plants [189]. Under abiotic stresses these ERF genes are induced to hyper-express [190] results in the better tolerance against stresses in transgenic plants. Additionally, some ERF TFs are also involved both in biotic and abiotic stress tolerance due to their ability to regulate numerous hormonal biosynthesis pathways [191]. Zhu et al. (2014) studied that the introduction of TaPIE1 in wheat has successfully improved the tolerance ability against chilling stress and resistance to pathogens [192]. Further study was carried out to develop transgenic tobacco by an over-expressed GmERF3 gene. This transgenic tobacco has increased resistance against TMV, dehydration, and also toleration of salinity stress [193].
MYB TFs family called the myeloblastosis oncogene is a huge group of TFs discovered in eukaryotes and is extensively distributed in plants [194,195]. Various MYB TFs have been identified to regulate numerous biochemical and physiological pathways such as the cell cycle, hormonal biosynthesis, and primary and secondary metabolism. These TFs are also known to have functions in biotic and abiotic stress responses [196]. Li et al. (2015) have summarized various MYB TFs related to abiotic stress tolerance in plants and Arabidopsis [195]. Some of the MYB TFs such as AtMYB61, AtMYB60, and AtMYB44 were identified to enhance the drought resistance in transgenic Arabidopsis by controlling the movement of stomata [197,198,199]. The AtMYB96 gene was expressed in Arabidopsis as a regulator, either by ABA signaling pathways to impart drought resistance [200] or by controlling the biosynthesis process of cuticle wax [201]. Yang et al. (2012) developed transgenic rice by over-expressing the OsMYB2 gene to improve the resistance of rice against chilling, salinity, and dehydration [202]. The GmMYB76 gene isolated from soybean was successfully transformed into Arabidopsis for salinity and freeze resistance [203]. MdMYB121 from apple was significantly transformed into apple and tomato to develop transgenic plants with enhanced drought and salt tolerance [204]. Chen et al. (2017) reported that the ZmMYB30 gene isolated from maize was transformed into Arabidopsis to enhanced tolerance against salinity [205]. Similarly, the MdSIMYB1 gene from apple was used to developed transgenic tobacco and apple with improved resistance against cold, drought, and salt stresses [206]. The TaPIMP1 gene was expressed to develop transgenic wheat and it showed remarkable tolerance against drought and fungal pathogen Bipolaris sorokiniana. Hyper-expression of TaPIMP1 was confirmed by microarray analysis [207]. In another experiment TaPIMP1 TF was investigated for its regulating ability to tackle biotic and abiotic stresses in transgenic tobacco. Transgenic tobacco showed resistance against Ralstonia solanacearum, salinity, and drought stresses [208].
Another important family of TFs are WRKY which are extensively distributed in relation to abiotic [209] and biotic stresses in plants [210]. In transgenic plants WRKY genes were over-expressed to increase the abiotic stress tolerance such as in transgenic rice OsWRKY11 gene was introduced to enhance its tolerance to heat and drought stresses [211]. Zhou et al. (2008) conducted an experiment on Arabidopsis to make it resistant against different stress conditions. GmWRKY21 and GmWRKY54 were over-expressed in transgenic Arabidopsis to improved resistance to cold and drought stress respectively [212]. Niu et al. (2012) investigated the TaWRKY19 gene over-expression in transgenic wheat to freezing, drought, and salinity stresses [213]. Similarly, TaWRKY1 and TaWRKY33 isolated from wheat was used to develop transgenic Arabidopsis against drought and heat tolerance [214]. ZmWRKY33 genes in maize have been induced for salinity, drought, freeze, and ABA stresses. Under salinity stress conditions transgenic Arabidopsis with over-expression of ZmWRKY33 genes showed significant tolerance [215]. Dehydration tolerance was increased in transgenic Chrysanthemum with the over-expression of CmWRKY1 [216].
NAC TFs have significant importance in many processes, such as flower growth, cell division, and stress-responsive regulation in the plant due to biotic and abiotic stresses [217,218]. Numerous NAC TFs have been discovered in a wide range of plants with sequenced genomes such as in rice with 151, in Arabidopsis 117 [219], in maize 152 [220], and in soybean 152 [221] NAC TFs have been identified. Additionally, a large number of NAC TFs have been reported to have direct association in abiotic stresses, such as in transgenic Arabidopsis 31 NAC genes, which have been identified for salinity tolerance [222], and in rice 40 NAC genes were identified against drought tolerance [223]. In sorghum the expression of SbSNAC1 gene was induced by salt and drought stress and over-expression of this gene in transgenic Arabidopsis showed drought resistance [224]. Zheng et al. (2009) developed transgenic rice by over-expression of OsNAC045 which conferred drought and salt tolerance [225]. Over-expression of OsNAC1 in transgenic rice was studied for salinity and drought tolerance [226].
7.4. Genome Editing Strategies
Genome editing (GE) is the most powerful strategy to manipulate the plant genome by means of sequence-specific nucleases. GE for crop improvement has the remarkable ability to tackle food insecurity and develop a climate-smart agriculture system globally [227]. In traditional plant breeding approaches, genes are discovered to be associated with various important traits by means of mutation and conventional breeding strategies, which has recognized as a significant technique for the development of elite and high yielding germplasm [228]. Genetic diversity of various elite varieties has been substantially decreased due to the exploitation of important crops extensively that has, in various circumstances, been associated with the enhancement of the susceptibility to several abiotic and biotic stresses [229,230]. Plant breeding approaches have been greatly influenced by the GE tools and exploring new strategies for fast and accurate manipulations in crop genomes to protect them against different stresses and improve crop yield [231]. Thus, developing the novel modifications in the gene pool of various plant germplasm is required under abiotic and biotic stresses for the improvement of elite crop varieties with great ability to produce high yielding crops [232]. In genome editing technology site specific endonucleases are used comprising of zinc-finger nucleases (ZFNs), transcription activator like effector nucleases (TALENs), and CRISPR-Cas9 [233]. In contrast to the ZENs and TALENs genome editing tools, the CRISPR/Cas9 system is emerging as the most powerful GE strategy because it is economical, rapid, accurate, and enables multiple site specific editing within the genome [234].
CRISPR/Cas9 System for Crop Advancement
CRISPR/Cas9 is a modern genome editing strategy based on the prokaryotic defense mechanism triggered by type II RNA organization that offers protection to prokaryotes against attacking viruses [146,235,236]. Genome editing has been modernized by CRISPR-Cas9 assembly, by producing candidate gene mutants and knock down single nucleotides in a genome [237]. As compared to other genome editing tools like TALENs/ZFNs, CRISPR-based strategies have been tremendously exploited in plant genomes [238]. Moreover, it has great potential to aid crop breeding to establish high yielding and stress-resistant varieties [234]. Most significantly, the CRISPR/Cas9 tool is converting into a comprehensible environmentally friendly technique for the establishment of genome edited non-transgenic plants to tackle environmental extremes and guarantee food security [239]. A model of CRISPR/Cas9 based genome engineering to develop transgenic plants or abiotic stress tolerance cultivars is explained in Figure 7.
Figure 7.
A model of CRISPR/Cas9 based genome engineering to develop transgenic plants or abiotic stress tolerance cultivars.
CRISPR/Cas9 has been extensively carried out for plant genome editing to cope with abiotic and biotic stresses [240]. A study was conducted to disrupt the gene TaERF3 and TaDREB2 to produce abiotic stress resistance by using the CRISPR genome editing tool [241]. Similarly, 21 KUP genes have been identified in cassava which were hyper-expressed under abiotic stresses. Differential expression analysis of KUP genes revealed that they induced drought resistance [242]. For drought tolerance studies MAPKKK genes have been investigated by means of genome-wide analysis [243]. CRISPR/Cas9 was applied in rice for producing triplet mutants. Genes TGW6, GW5, and GW2 have a function in regulating the seed size. By mutating this gene, the size of the seed was increased by 30% [244]. CRISPR technology has been adopted for a mutation in Brassica napus by knocking down the gene CLVTA3 which resulted in more seed production. A similar strategy was applied to increase the wheat seed size by knocking down the TaGW2 gene which has the ability to limit the seed size to increase [245]. Without any transformation of a gene, wheat has been developed with a low gluten level by using CRISPR/Cas9 technique [246]. Wang et al. (2017) studied the protein kinases 3 (slmapk3) gene to investigate its regulating mechanism to confer drought resistance in tomato. The CRISPR/Cas9 strategy was used to develop tomato mutant lines which showed a considerably enhanced concentration of proline, malondialdehyde, and H2O2. The tomato mutant lines were susceptible to more oxidative stress in drought. The outcomes of this study revealed the importance of the SlMAPK3 gene in drought tolerance mechanisms and over-expression of this gene by genetic engineering provide improved drought tolerance [247]. The SlAGL6 gene was knocked down by Klap et al. (2017) by using the CRISPR/Cas9 system to develop parthenocarpic fruits in response to heat stress. The resulting mutated tomato lines were the same as the normal plants having same shape and weight [248]. Shen et al. (2017) studied japonica rice by knocking out the Osann3 gene. CRISPR/Cas9 technology was applied for mutant rice lines, resulting in the enhancement of tolerance under cold stress. This study showed the ability of the OsANN3 gene in cold tolerance and that it could be a potential gene for transgenic rice with increased cold tolerance [249]. Herbicide tolerance was developed by knocking down PmCDA1 gene in mutant rice lines with the help of CRISPR/Cas9 [250].
8. Conclusions
Climate changes are alarming the world by hampering agriculture and its products. Industrialization and poisonous gases cause global warming, which ultimately disturbs the world’s environment. Climate change has devastating effects on plant growth and yield. Abiotic stresses are the major type of stresses that plants suffer. To understand the plant responses under different abiotic conditions the most pressing current need is to explore the genetic basis underlying these mechanisms. Some bottleneck molecular and physiological challenges present in plants need to be resolved for better plant adaptation under abiotic conditions. Temperature fluctuations and variations in rainfall spells are a very crucial indicators of environmental stresses. Weather variations collectively have positive and negative outcomes but the negative effects are more thought-provoking. It is very difficult to overcome the imbalance in agriculture by climate change. How to tackle this problem and what strategies we should apply are still ambiguous. Hence, researchers need to focus on optimizing plant growth and development in abiotic stresses. For crop resistance against biotic and abiotic stresses, propagating novel cultural methods, implementing various cropping schemes, and different conventional and non-conventional approaches will be adopted to save agriculture in the future. Breeding approaches will help to develop climate resilient crops with better adaptability under drought and heat. Genome wide association studies (GWAS), genomic selection (GS) with high throughput phenotyping, and genotyping strategies are significant in identifying the different genes for crop improvement under climate change. Genetic engineering approaches have been significantly applied to develop transgenic plants with enhanced resistance against different biotic and abiotic stress responses. In future, we have to make eco-friendly genome edited crops through a CRISPR/Cas9 mediated genome editing to battle against climate change.
Acknowledgments
Authors are grateful to all members of Oil Crops Research Institute, Chinese Academy of Agricultural Sciences (CAAS), Wuhan, China for their support and encouragement.
Author Contributions
A.R. (Ali Raza) and A.R. (Ali Razzaq) wrote the manuscript, S.S.M. and X.Z. (Xiling Zou) helped in literature; Y.L. helped in making figures; J.X. and X.Z. (Xuekun Zhang) proofread the manuscript. All authors have read and approved the manuscript.
Funding
This research was funded by the National Basic Research Program of China (2017YFD0101701) and Agricultural Science and Technology Innovation Program of CAAS and the APC was funded by the National Basic Research Program of China (2017YFD0101701) and Agricultural Science and Technology Innovation Program of CAAS.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Arunanondchai P., Fei C., Fisher A., McCarl B.A., Wang W., Yang Y. The Routledge Handbook of Agricultural Economics. Routledge; Abingdon-on-Thames, UK: 2018. How does climate change affect agriculture. [Google Scholar]
- 2.Noya I., González-García S., Bacenetti J., Fiala M., Moreira M.T. Environmental impacts of the cultivation-phase associated with agricultural crops for feed production. J. Clean. Prod. 2018;172:3721–3733. doi: 10.1016/j.jclepro.2017.07.132. [DOI] [Google Scholar]
- 3.Vaughan M.M., Block A., Christensen S.A., Allen L.H., Schmelz E.A. The effects of climate change associated abiotic stresses on maize phytochemical defenses. Phytochem. Rev. 2018;17:37–49. doi: 10.1007/s11101-017-9508-2. [DOI] [Google Scholar]
- 4.FAO. UNICEF. WFP. WHO . The State of Food Security and Nutrition in the World 2017: Building Resilience for Peace and Food Security. Food and Agriculture Organization of the United Nations (FAO); Rome, Italy: 2018. [Google Scholar]
- 5.Rosenzweig C., Elliott J., Deryng D., Ruane A.C., Müller C., Arneth A., Boote K.J., Folberth C., Glotter M., Khabarov N. Assessing agricultural risks of climate change in the 21st century in a global gridded crop model intercomparison. Proc. Natl. Acad. Sci. USA. 2014;111:3268–3273. doi: 10.1073/pnas.1222463110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wheeler T., Von Braun J. Climate change impacts on global food security. Science. 2013;341:508–513. doi: 10.1126/science.1239402. [DOI] [PubMed] [Google Scholar]
- 7.Ashraf M.A., Akbar A., Askari S.H., Iqbal M., Rasheed R., Hussain I. Advances in Seed Priming. Springer; Berlin/Heidelberg, Germany: 2018. Recent Advances in Abiotic Stress Tolerance of Plants Through Chemical Priming: An Overview; pp. 51–79. [Google Scholar]
- 8.Benevenuto R.F., Agapito-Tenfen S.Z., Vilperte V., Wikmark O.-G., Van Rensburg P.J., Nodari R.O. Molecular responses of genetically modified maize to abiotic stresses as determined through proteomic and metabolomic analyses. PLoS ONE. 2017;12:e0173069. doi: 10.1371/journal.pone.0173069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suzuki N., Rivero R.M., Shulaev V., Blumwald E., Mittler R. Abiotic and biotic stress combinations. New Phytol. 2014;203:32–43. doi: 10.1111/nph.12797. [DOI] [PubMed] [Google Scholar]
- 10.Pachauri R.K., Allen M.R., Barros V.R., Broome J., Cramer W., Christ R., Church J.A., Clarke L., Dahe Q., Dasgupta P. Climate Change 2014: Synthesis Report. IPCC; Geneva, Switzerland: 2014. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. [Google Scholar]
- 11.Khan A., Ijaz M., Muhammad J., Goheer A., Akbar G., Adnan M. Climate Change Implications for Wheat Crop in Dera Ismail Khan District of Khyber Pakhtunkhwa. Pak. J. Meteorol. 2016;13:17–27. [Google Scholar]
- 12.Kanojia A., Dijkwel P.P. Abiotic Stress Responses are Governed by Reactive Oxygen Species and Age. Annu. Plant Rev. 2018:1–32. doi: 10.1002/9781119312994.apr0611. [DOI] [Google Scholar]
- 13.Lesk C., Rowhani P., Ramankutty N. Influence of extreme weather disasters on global crop production. Nature. 2016;529:84. doi: 10.1038/nature16467. [DOI] [PubMed] [Google Scholar]
- 14.Altieri M.A., Nicholls C.I. The adaptation and mitigation potential of traditional agriculture in a changing climate. Clim. Chang. 2017;140:33–45. doi: 10.1007/s10584-013-0909-y. [DOI] [Google Scholar]
- 15.Richardson K.J., Lewis K.H., Krishnamurthy P.K., Kent C., Wiltshire A.J., Hanlon H.M. Food security outcomes under a changing climate: Impacts of mitigation and adaptaion on vulnerablity to food insecurity. Clim. Chang. 2018;147:327–341. doi: 10.1007/s10584-018-2137-y. [DOI] [Google Scholar]
- 16.Ito R., Vasconcelos H.L., Feeley K.J. Global climate change increases risk of crop yield losses and food insecurity in the tropical Andes. Glob. Chang. Biol. 2018;24:e592–e602. doi: 10.1111/gcb.13959. [DOI] [PubMed] [Google Scholar]
- 17.Rogelj J., Den Elzen M., Höhne N., Fransen T., Fekete H., Winkler H., Schaeffer R., Sha F., Riahi K., Meinshausen M. Paris Agreement climate proposals need a boost to keep warming well below 2 °C. Nature. 2016;534:631. doi: 10.1038/nature18307. [DOI] [PubMed] [Google Scholar]
- 18.FAOSTAT. [(accessed on 2 August 2017)];2017 Available online: http://www.fao.org/faostat/en/#data.
- 19.Reckling M., Döring T.F., Bergkvist G., Chmielewski F., Stoddard F., Watson C., Seddig S., Bachinger J. Grain legume yield instability has increased over 60 years in long-term field experiments as measured by a scale-adjusted coefficient of variation. Asp. Appl. Biol. 2018;138:15–20. [Google Scholar]
- 20.Dhankher O.P., Foyer C.H. Climate resilient crops for improving global food security and safety. Plant Cell Environ. 2018;41:877–884. doi: 10.1111/pce.13207. [DOI] [PubMed] [Google Scholar]
- 21.Kang Y., Khan S., Ma X. Climate change impacts on crop yield, crop water productivity and food security—A review. Prog. Nat. Sci. 2009;19:1665–1674. doi: 10.1016/j.pnsc.2009.08.001. [DOI] [Google Scholar]
- 22.Campbell B.M., Vermeulen S.J., Aggarwal P.K., Corner-Dolloff C., Girvetz E., Loboguerrero A.M., Ramirez-Villegas J., Rosenstock T., Sebastian L., Thornton P.K. Reducing risks to food security from climate change. Glob. Food Sec. 2016;11:34–43. doi: 10.1016/j.gfs.2016.06.002. [DOI] [Google Scholar]
- 23.Thornton P.K., Ericksen P.J., Herrero M., Challinor A.J. Climate variability and vulnerability to climate change: A review. Glob. Chang. Biol. 2014;20:3313–3328. doi: 10.1111/gcb.12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.FAO. IFAD. UNICEF. WEP. WHO . The State of Food Security and Nutrition in the World 2018. FAO; Rome, Italy: Building climate resilience for food security and nutrition. [Google Scholar]
- 25.Emergency Events Database (EM-DAT) [(accessed on 30 January 2019)];2009 Available online: https://www.emdat.be/
- 26.Boyer J.S. Plant productivity and environment. Science. 1982;218:443–448. doi: 10.1126/science.218.4571.443. [DOI] [PubMed] [Google Scholar]
- 27.Van Velthuizen H. Mapping Biophysical Factors That Influence Agricultural Production and Rural Vulnerability. Food & Agriculture Organization; Rome, Italy: 2007. [Google Scholar]
- 28.Tebaldi C., Lobell D. Estimated impacts of emission reductions on wheat and maize crops. Clim. Chang. 2018;146:533–545. doi: 10.1007/s10584-015-1537-5. [DOI] [Google Scholar]
- 29.Bonan G.B., Doney S.C. Climate, ecosystems, and planetary futures: The challenge to predict life in Earth system models. Science. 2018;359:eaam8328. doi: 10.1126/science.aam8328. [DOI] [PubMed] [Google Scholar]
- 30.Olesen J.E., Trnka M., Kersebaum K.C., Skjelvåg A., Seguin B., Peltonen-Sainio P., Rossi F., Kozyra J., Micale F. Impacts and adaptation of European crop production systems to climate change. Eur. J. Agron. 2011;34:96–112. doi: 10.1016/j.eja.2010.11.003. [DOI] [Google Scholar]
- 31.Olesen J.E., Bindi M. Consequences of climate change for European agricultural productivity, land use and policy. Eur. J. Agron. 2002;16:239–262. doi: 10.1016/S1161-0301(02)00004-7. [DOI] [Google Scholar]
- 32.Asseng S., Ewert F., Martre P., Rötter R.P., Lobell D., Cammarano D., Kimball B., Ottman M., Wall G., White J.W. Rising temperatures reduce global wheat production. Nat. Clim. Change. 2015;5:143. doi: 10.1038/nclimate2470. [DOI] [Google Scholar]
- 33.Barnabás B., Jäger K., Fehér A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008;31:11–38. doi: 10.1111/j.1365-3040.2007.01727.x. [DOI] [PubMed] [Google Scholar]
- 34.Griffin J.J., Ranney T.G., Pharr D.M. Heat and drought influence photosynthesis, water relations, and soluble carbohydrates of two ecotypes of redbud (Cercis canadensis) J. Am. Soc. Hortic. Sci. 2004;129:497–502. [Google Scholar]
- 35.Gong M., Chen S.-N., Song Y.-Q., Li Z.-G. Effect of calcium and calmodulin on intrinsic heat tolerance in relation to antioxidant systems in maize seedlings. Funct. Plant Biol. 1997;24:371–379. doi: 10.1071/PP96118. [DOI] [Google Scholar]
- 36.Wang Z., Huang B. Physiological recovery of Kentucky bluegrass from simultaneous drought and heat stress. Crop Sci. 2004;44:1729–1736. doi: 10.2135/cropsci2004.1729. [DOI] [Google Scholar]
- 37.Xu Z.Z., Zhou G.S. Combined effects of water stress and high temperature on photosynthesis, nitrogen metabolism and lipid peroxidation of a perennial grass Leymus chinensis. Planta. 2006;224:1080–1090. doi: 10.1007/s00425-006-0281-5. [DOI] [PubMed] [Google Scholar]
- 38.Winkel T., Renno J.-F., Payne W. Effect of the timing of water deficit on growth, phenology and yield of pearl millet (Pennisetum glaucum (L.) R. Br.) grown in Sahelian conditions. J. Exp. Bot. 1997;48:1001–1009. doi: 10.1093/jxb/48.5.1001. [DOI] [Google Scholar]
- 39.Saini H., Aspinall D. Abnormal sporogenesis in wheat (Triticum aestivum L.) induced by short periods of high temperature. Ann. Bot. 1982;49:835–846. doi: 10.1093/oxfordjournals.aob.a086310. [DOI] [Google Scholar]
- 40.Saini H., Aspinall D. Effect of water deficit on sporogenesis in wheat (Triticum aestivum L.) Ann. Bot. 1981;48:623–633. doi: 10.1093/oxfordjournals.aob.a086170. [DOI] [Google Scholar]
- 41.Sheoran I.S., Saini H.S. Drought-induced male sterility in rice: Changes in carbohydrate levels and enzyme activities associated with the inhibition of starch accumulation in pollen. Sex. Plant Reprod. 1996;9:161–169. doi: 10.1007/BF02221396. [DOI] [Google Scholar]
- 42.Garrity D., O’Toole J. Screening rice for drought resistance at the reproductive phase. Field Crops Res. 1994;39:99–110. doi: 10.1016/0378-4290(94)90012-4. [DOI] [Google Scholar]
- 43.Ruf F., Schroth G., Doffangui K. Climate change, cocoa migrations and deforestation in West Africa: What does the past tell us about the future? Sustain. Sci. 2015;10:101–111. doi: 10.1007/s11625-014-0282-4. [DOI] [Google Scholar]
- 44.Hellin J., Bellon M.R., Hearne S.J. Maize landraces and adaptation to climate change in Mexico. J. Crop Improv. 2014;28:484–501. doi: 10.1080/15427528.2014.921800. [DOI] [Google Scholar]
- 45.Svoboda N., Strer M., Hufnagel J. Rainfed winter wheat cultivation in the North German Plain will be water limited under climate change until 2070. Environ. Sci. Eur. 2015;27:29. doi: 10.1186/s12302-015-0061-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao C., Liu B., Piao S., Wang X., Lobell D.B., Huang Y., Huang M., Yao Y., Bassu S., Ciais P. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA. 2017;114:9326–9331. doi: 10.1073/pnas.1701762114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scheben A., Yuan Y., Edwards D. Advances in genomics for adapting crops to climate change. Curr. Plant Biol. 2016;6:2–10. doi: 10.1016/j.cpb.2016.09.001. [DOI] [Google Scholar]
- 48.Pradhan G.P., Prasad P.V., Fritz A.K., Kirkham M.B., Gill B.S. Effects of drought and high temperature stress on synthetic hexaploid wheat. Funct. Plant Biol. 2012;39:190–198. doi: 10.1071/FP11245. [DOI] [PubMed] [Google Scholar]
- 49.Araus J., Slafer G., Reynolds M., Royo C. Plant breeding and drought in C3 cereals: What should we breed for? Ann. Bot. 2002;89:925–940. doi: 10.1093/aob/mcf049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Oliveira E.D., Bramley H., Siddique K.H., Henty S., Berger J., Palta J.A. Can elevated CO2 combined with high temperature ameliorate the effect of terminal drought in wheat? Funct. Plant Biol. 2013;40:160–171. doi: 10.1071/FP12206. [DOI] [PubMed] [Google Scholar]
- 51.Baroowa B., Gogoi N. Biochemical changes in black gram and green gram genotypes after imposition of drought stress. J. Food Legum. 2014;27:350–353. [Google Scholar]
- 52.Maleki A., Naderi A., Naseri R., Fathi A., Bahamin S., Maleki R. Physiological performance of soybean cultivars under drought stress. Bull. Environ. Pharmacol. Life Sci. 2013;2:38–44. [Google Scholar]
- 53.Schlenker W., Roberts M.J. Nonlinear temperature effects indicate severe damages to US crop yields under climate change. Proc. Natl. Acad. Sci. USA. 2009;106:15594–15598. doi: 10.1073/pnas.0906865106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lobell D.B., Bänziger M., Magorokosho C., Vivek B. Nonlinear heat effects on African maize as evidenced by historical yield trials. Nat. Clim. Chang. 2011;1:42. doi: 10.1038/nclimate1043. [DOI] [Google Scholar]
- 55.Lobell D.B., Field C.B. Global scale climate–crop yield relationships and the impacts of recent warming. Environ. Res. Lett. 2007;2:014002. doi: 10.1088/1748-9326/2/1/014002. [DOI] [Google Scholar]
- 56.Brown L.R. Plan B 3.0: Mobilizing to Save Civilization (Substantially Revised) WW Norton & Company; New York, NY, USA: 2008. [Google Scholar]
- 57.Ray D.K., Gerber J.S., MacDonald G.K., West P.C. Climate variation explains a third of global crop yield variability. Nat. Commun. 2015;6:5989. doi: 10.1038/ncomms6989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Easterling W.E., Aggarwal P.K., Batima P., Brander K.M., Erda L., Howden S.M., Kirilenko A., Morton J., Soussana J.-F., Schmidhuber J. Food, fibre and forest products. Clim. Chang. 2007;273:313. [Google Scholar]
- 59.Kjellstrom E., Nikulin G., Strandberg G., Christensen O.B., Jacob D., Keuler K., Lenderink G., Van Meijgaard E., Schar C., Somot S., et al. European climate change at global mean temperature increases of 1.5 and 2 degrees above pre-industrail conditions as simulated by the EURO-CORDEX regional climate models. Earth. Syst. Dyn. 2018;9:459–478. doi: 10.5194/esd-9-459-2018. [DOI] [Google Scholar]
- 60.Otto I.M., Reckien D., Reyer C.P., Marcus R., Le Masson V., Jones L., Norton A., Serdeczny O. Social vulnerability to climate change: A review of concepts and evidence. Reg. Environ. Chang. 2017;17:1651–1662. doi: 10.1007/s10113-017-1105-9. [DOI] [Google Scholar]
- 61.Eastburn D.M., Degennaro M.M., Delucia E.H., Dermody O., McElrone A.J. Elevated atmospheric carbon dioxide and ozone alter soybean diseases at SoyFACE. Glob. Chang. Biol. 2010;16:320–330. doi: 10.1111/j.1365-2486.2009.01978.x. [DOI] [Google Scholar]
- 62.Kitano H. Systems biology: A brief overview. Science. 2002;295:1662–1664. doi: 10.1126/science.1069492. [DOI] [PubMed] [Google Scholar]
- 63.Intergovernmental Panel on Climate Change (IPCC) Climate Change: Impacts, Adaptation, and Vulnerability. Cambridge University Press; Cambridge, UK: 2007. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. [Google Scholar]
- 64.Schmidhuber J., Tubiello F.N. Global food security under climate change. Proc. Natl. Acad. Sci. USA. 2007;104:19703–19708. doi: 10.1073/pnas.0701976104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Intergovernmental Panel on Climate Change (IPCC) Special Report on Emissions Scenarios. Cambridge University Press; Cambridge, UK: 2000. A Special Report of Working Group III of the Intergovernmental Panel on Climate Change. [Google Scholar]
- 66.Espeland E.K., Kettenring K.M. Strategic plant choices can alleviate climate change impacts: A review. J. Environ. Manag. 2018;222:316–324. doi: 10.1016/j.jenvman.2018.05.042. [DOI] [PubMed] [Google Scholar]
- 67.Pereira A. Plant abiotic stress challenges from the changing environment. Front. Plant Sci. 2016;7:1123. doi: 10.3389/fpls.2016.01123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hatfield J.L., Prueger J.H. Temperature extremes: Effect on plant growth and development. Weather Clim. Extrem. 2015;10:4–10. doi: 10.1016/j.wace.2015.08.001. [DOI] [Google Scholar]
- 69.Barlow K., Christy B., O’leary G., Riffkin P., Nuttall J. Simulating the impact of extreme heat and frost events on wheat crop production: A review. Field Crops Res. 2015;171:109–119. doi: 10.1016/j.fcr.2014.11.010. [DOI] [Google Scholar]
- 70.Salehi-Lisar S.Y., Bakhshayeshan-Agdam H. Drought Stress Tolerance in Plants. Volume 1. Springer; Berlin/Heidelberg, Germany: 2016. Drought stress in plants: Causes, consequences, and tolerance; pp. 1–16. [Google Scholar]
- 71.Zandalinas S.I., Mittler R., Balfagón D., Arbona V., Gómez-Cadenas A. Plant adaptations to the combination of drought and high temperatures. Physiol. Plant. 2018;162:2–12. doi: 10.1111/ppl.12540. [DOI] [PubMed] [Google Scholar]
- 72.Singh P., Basu S., Kumar G. Biochemical, Physiological and Molecular Avenues for Combating Abiotic Stress Tolerance in Plants. Elsevier; Amsterdam, The Netherlands: 2018. Polyamines Metabolism: A Way Ahead for Abiotic Stress Tolerance in Crop Plants; pp. 39–55. [Google Scholar]
- 73.Tack J., Barkley A., Nalley L.L. Effect of warming temperatures on US wheat yields. Proc. Natl. Acad. Sci. USA. 2015 doi: 10.1073/pnas.1415181112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.FAO, FAOSTAT Food Agriculture. Organization. United Nations. [(accessed on 15 October 2017)];2017 Available online: http://www.fao.org/faostat/en/#home.
- 75.Abhinandan K., Skori L., Stanic M., Hickerson N.M., Jamshed M., Samuel M.A. Abiotic Stress Signaling in Wheat—An Inclusive Overview of Hormonal Interactions During Abiotic Stress Responses in Wheat. Front. Plant Sci. 2018;9:734. doi: 10.3389/fpls.2018.00734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Challinor A., Wheeler T., Craufurd P., Ferro C., Stephenson D. Adaptation of crops to climate change through genotypic responses to mean and extreme temperatures. Agric. Ecosyst. Environ. 2007;119:190–204. doi: 10.1016/j.agee.2006.07.009. [DOI] [Google Scholar]
- 77.Zhu J.-K. Abiotic stress signaling and responses in plants. Cell. 2016;167:313–324. doi: 10.1016/j.cell.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dinneny J.R., Long T.A., Wang J.Y., Jung J.W., Mace D., Pointer S., Barron C., Brady S.M., Schiefelbein J., Benfey P.N. Cell identity mediates the response of Arabidopsis roots to abiotic stress. Science. 2008;320:942–945. doi: 10.1126/science.1153795. [DOI] [PubMed] [Google Scholar]
- 79.Carvalho L.C., Amâncio S. Cutting the Gordian Knot of abiotic stress in grapevine: From the test tube to climate change adaptation. Physiol. Plant. 2018 doi: 10.1111/ppl.12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ahmad Z., Anjum S., Waraich E.A., Ayub M.A., Ahmad T., Tariq R.M.S., Ahmad R., Iqbal M.A. Growth, physiology, and biochemical activities of plant responses with foliar potassium application under drought stress—A review. J. Plant Nutr. 2018;41:1734–1743. doi: 10.1080/01904167.2018.1459688. [DOI] [Google Scholar]
- 81.Martinez V., Nieves-Cordones M., Lopez-Delacalle M., Rodenas R., Mestre T.C., Garcia-Sanchez F., Rubio F., Nortes P.A., Mittler R., Rivero R.M. Tolerance to stress combination in tomato plants: New insights in the protective role of melatonin. Molecules. 2018;23:535. doi: 10.3390/molecules23030535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rymaszewski W., Vile D., Bediee A., Dauzat M., Luchaire N., Kamrowska D., Granier C., Hennig J. Stress-related gene expression reflects morphophysiological responses to water deficit. Plant Physiol. 2017;174:1913–1930. doi: 10.1104/pp.17.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu X., Cai K., Zhang G., Zeng F. Metabolite profiling of barley grains subjected to water stress: To Explain the genotypic difference in drought-induced impacts on malting quality. Front. Plant Sci. 2017;8:1547. doi: 10.3389/fpls.2017.01547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vincent D., Ergül A., Bohlman M.C., Tattersall E.A., Tillett R.L., Wheatley M.D., Woolsey R., Quilici D.R., Joets J., Schlauch K. Proteomic analysis reveals differences between Vitis vinifera L. cv. Chardonnay and cv. Cabernet Sauvignon and their responses to water deficit and salinity. J. Exp. Bot. 2007;58:1873–1892. doi: 10.1093/jxb/erm012. [DOI] [PubMed] [Google Scholar]
- 85.Liu J.-X., Howell S.H. Endoplasmic reticulum protein quality control and its relationship to environmental stress responses in plants. Plant Cell. 2010;22:2930–2942. doi: 10.1105/tpc.110.078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Menezes-Silva P.E., Sanglard L.M., Ávila R.T., Morais L.E., Martins S.C., Nobres P., Patreze C.M., Ferreira M.A., Araújo W.L., Fernie A.R. Photosynthetic and metabolic acclimation to repeated drought events play key roles in drought tolerance in coffee. J. Exp. Bot. 2017;68:4309–4322. doi: 10.1093/jxb/erx211. [DOI] [PubMed] [Google Scholar]
- 87.Becklin K.M., Anderson J.T., Gerhart L.M., Wadgymar S.M., Wessinger C.A., Ward J.K. Examining plant physiological responses to climate change through an evolutionary lens. Plant Physiol. 2016;172:635–649. doi: 10.1104/pp.16.00793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.DaMatta F.M., Grandis A., Arenque B.C., Buckeridge M.S. Impacts of climate changes on crop physiology and food quality. Food Res. Int. 2010;43:1814–1823. doi: 10.1016/j.foodres.2009.11.001. [DOI] [Google Scholar]
- 89.Jan S.A., Shinwari Z.K., Rabbani M.A. Morpho-biochemical evaluation of Brassica rapa sub-species for salt tolerance. Genetika. 2016;48:323–338. doi: 10.2298/GENSR1601323J. [DOI] [Google Scholar]
- 90.Tkemaladze G.S., Makhashvili K. Climate changes and photosynthesis. Ann. Agric. Sci. 2016;14:119–126. doi: 10.1016/j.aasci.2016.05.012. [DOI] [Google Scholar]
- 91.Zargar S.M., Gupta N., Nazir M., Mahajan R., Malik F.A., Sofi N.R., Shikari A.B., Salgotra R. Impact of drought on photosynthesis: Molecular perspective. Plant Gene. 2017;11:154–159. doi: 10.1016/j.plgene.2017.04.003. [DOI] [Google Scholar]
- 92.Khan A., Ali M., Siddiqui S.U., Jatoi S.A., Jan S.A., Khan N., Ghafoor A. Effect of Various Temperatures and Duration on Deterioration of Rice Seeds. Science. 2017;36:79–83. [Google Scholar]
- 93.Jan S.A., Bibi N., Shinwari Z.K., Rabbani M.A., Ullah S., Qadir A., Khan N. Impact of salt, drought, heat and frost stresses on morpho-biochemical and physiological properties of Brassica species: An updated review. J. Rural Dev. Agric. 2017;2:1–10. [Google Scholar]
- 94.Nagarajan R., Gill K.S. Evolution of Rubisco activase gene in plants. Plant Mol. Biol. 2018;96:69–87. doi: 10.1007/s11103-017-0680-y. [DOI] [PubMed] [Google Scholar]
- 95.Sage R.F., Way D.A., Kubien D.S. Rubisco, Rubisco activase, and global climate change. J. Exp. Bot. 2008;59:1581–1595. doi: 10.1093/jxb/ern053. [DOI] [PubMed] [Google Scholar]
- 96.Nasim S., Shabbir G., Ilyas M., Cheema N.M., Shah M.K.N. Contemplation of wheat genotypes for enhanced antioxidant enzyme activity. Pak. J. Bot. 2017;49:647–653. [Google Scholar]
- 97.Kurepin L.V., Ivanov A.G., Zaman M., Pharis R.P., Hurry V., Hüner N.P. Photosynthesis: Structures, Mechanisms, and Applications. Springer; Berlin/Heidelberg, Germany: 2017. Interaction of glycine betaine and plant hormones: Protection of the photosynthetic apparatus during abiotic stress; pp. 185–202. [Google Scholar]
- 98.Dong H., Bai L., Chang J., Song C.-P. Chloroplast protein PLGG1 is involved in abscisic acid-regulated lateral root development and stomatal movement in Arabidopsis. Biochem. Biophys. Res. Commun. 2018;495:280–285. doi: 10.1016/j.bbrc.2017.10.113. [DOI] [PubMed] [Google Scholar]
- 99.Kuromori T., Seo M., Shinozaki K. ABA transport and plant water stress responses. Trends Plant Sci. 2018;23:513–522. doi: 10.1016/j.tplants.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 100.Takahashi F., Kuromori T., Sato H., Shinozaki K. Survival Strategies in Extreme Cold and Desiccation. Springer; Berlin/Heidelberg, Germany: 2018. Regulatory Gene Networks in Drought Stress Responses and Resistance in Plants; pp. 189–214. [DOI] [PubMed] [Google Scholar]
- 101.Ma Y., Szostkiewicz I., Korte A., Moes D., Yang Y., Christmann A., Grill E. Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science. 2009;324:1064–1068. doi: 10.1126/science.1172408. [DOI] [PubMed] [Google Scholar]
- 102.Park S.-Y., Fung P., Nishimura N., Jensen D.R., Fujii H., Zhao Y., Lumba S., Santiago J., Rodrigues A., Tsz-Fung F.C. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science. 2009;324:1068–1071. doi: 10.1126/science.1173041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Leung J., Giraudat J. Abscisic acid signal transduction. Annu. Rev. Plant Biol. 1998;49:199–222. doi: 10.1146/annurev.arplant.49.1.199. [DOI] [PubMed] [Google Scholar]
- 104.Yoshida R., Hobo T., Ichimura K., Mizoguchi T., Takahashi F., Aronso J., Ecker J.R., Shinozaki K. ABA-activated SnRK2 protein kinase is required for dehydration stress signaling in Arabidopsis. Plant Cell Physiol. 2002;43:1473–1483. doi: 10.1093/pcp/pcf188. [DOI] [PubMed] [Google Scholar]
- 105.Carswell G., Johnson C., Shillito R., Harms C. O-acetyl-salicylic acid promotes colony formation from protoplasts of an elite maize inbred. Plant Cell Rep. 1989;8:282–284. doi: 10.1007/BF00274130. [DOI] [PubMed] [Google Scholar]
- 106.Eberhard S., Doubrava N., Marfa V., Mohnen D., Southwick A., Darvill A., Albersheim P. Pectic cell wall fragments regulate tobacco thin-cell-layer explant morphogenesis. Plant Cell. 1989;1:747–755. doi: 10.1105/tpc.1.8.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Malamy J., Carr J.P., Klessig D.F., Raskin I. Salicylic acid: A likely endogenous signal in the resistance response of tobacco to viral infection. Science. 1990;250:1002–1004. doi: 10.1126/science.250.4983.1002. [DOI] [PubMed] [Google Scholar]
- 108.Arnao M.B., Hernández-Ruiz J. Melatonin and its relationship to plant hormones. Ann. Bot. 2017;121:195–207. doi: 10.1093/aob/mcx114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Verma V., Ravindran P., Kumar P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016;16:86. doi: 10.1186/s12870-016-0771-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dubois M., Van den Broeck L., Inzé D. The pivotal role of ethylene in plant growth. Trends Plant Sci. 2018;23:313–323. doi: 10.1016/j.tplants.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Klay I., Gouia S., Liu M., Mila I., Khoudi H., Bernadac A., Bouzayen M., Pirrello J. Ethylene Response Factors (ERF) are differentially regulated by different abiotic stress types in tomato plants. Plant Sci. 2018;274:137–145. doi: 10.1016/j.plantsci.2018.05.023. [DOI] [PubMed] [Google Scholar]
- 112.Duku C., Zwart S.J., Hein L. Impacts of climate change on cropping patterns in a tropical, sub-humid watershed. PLoS ONE. 2018;13:e0192642. doi: 10.1371/journal.pone.0192642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Marcinkowski P., Piniewski M. Effect of climate change on sowing and harvest dates of spring barley and maize in Poland. Int. Agrophys. 2018;32:265–271. doi: 10.1515/intag-2017-0015. [DOI] [Google Scholar]
- 114.Teixeira E.I., de Ruiter J., Ausseil A.-G., Daigneault A., Johnstone P., Holmes A., Tait A., Ewert F. Adapting crop rotations to climate change in regional impact modelling assessments. Sci. Total Environ. 2018;616:785–795. doi: 10.1016/j.scitotenv.2017.10.247. [DOI] [PubMed] [Google Scholar]
- 115.Deligios P.A., Chergia A.P., Sanna G., Solinas S., Todde G., Narvarte L., Ledda L. Climate change adaptation and water saving by innovative irrigation management applied on open field globe artichoke. Sci. Total Environ. 2019;649:461–472. doi: 10.1016/j.scitotenv.2018.08.349. [DOI] [PubMed] [Google Scholar]
- 116.Ali A., Erenstein O. Assessing farmer use of climate change adaptation practices and impacts on food security and poverty in Pakistan. Clim. Risk Manag. 2017;16:183–194. doi: 10.1016/j.crm.2016.12.001. [DOI] [Google Scholar]
- 117.Battisti R., Sentelhas P.C., Parker P.S., Nendel C., Gil M.D.S., Farias J.R., Basso C.J. Assessment of crop-management strategies to improve soybean resilience to climate change in Southern Brazil. Crop Pasture Sci. 2018;69:154–162. doi: 10.1071/CP17293. [DOI] [Google Scholar]
- 118.Henderson B., Cacho O., Thornton P., van Wijk M., Herrero M. The economic potential of residue management and fertilizer use to address climate change impacts on mixed smallholder farmers in Burkina Faso. Agric. Syst. 2018;167:195–205. doi: 10.1016/j.agsy.2018.09.012. [DOI] [Google Scholar]
- 119.Blum A. Plant Breeding for Stress Environments: 0. CRC Press; Boca Raton, FL, USA: 2018. [DOI] [Google Scholar]
- 120.Raza A., Mehmood S.S., Ashraf F., Khan R.S.A. Genetic diversity analysis of Brassica species using PCR-based SSR markers. Gesunde Pflanzen. 2018:1–7. doi: 10.1007/s10343-018-0435-y. [DOI] [Google Scholar]
- 121.Raza A., Shaukat H., Ali Q., Habib M. Assessment of RAPD markers to analyse the genetic diversity among sunflower (Helianthus annuus L.) genotypes. Turk. J. Agric. Food Sci. Technol. 2018;6:107–111. doi: 10.24925/turjaf.v6i1.107-111.1710. [DOI] [Google Scholar]
- 122.Lopes M.S., El-Basyoni I., Baenziger P.S., Singh S., Royo C., Ozbek K., Aktas H., Ozer E., Ozdemir F., Manickavelu A. Exploiting genetic diversity from landraces in wheat breeding for adaptation to climate change. J. Exp. Bot. 2015;66:3477–3486. doi: 10.1093/jxb/erv122. [DOI] [PubMed] [Google Scholar]
- 123.Stinchcombe J.R., Hoekstra H.E. Combining population genomics and quantitative genetics: Finding the genes underlying ecologically important traits. Heredity. 2008;100:158. doi: 10.1038/sj.hdy.6800937. [DOI] [PubMed] [Google Scholar]
- 124.Keurentjes J.J., Koornneef M., Vreugdenhil D. Quantitative genetics in the age of omics. Curr. Opin. Plant Biol. 2008;11:123–128. doi: 10.1016/j.pbi.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 125.Bevan M., Waugh R. Applying Plant Genomics to Crop Improvement. BioMed Central; London, UK: 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Des Marais D.L., Hernandez K.M., Juenger T.E. Genotype-by-environment interaction and plasticity: Exploring genomic responses of plants to the abiotic environment. Annu. Rev. Ecol. Evol. Syst. 2013;44:5–29. doi: 10.1146/annurev-ecolsys-110512-135806. [DOI] [Google Scholar]
- 127.Roy S.J., Tucker E.J., Tester M. Genetic analysis of abiotic stress tolerance in crops. Curr. Opin. Plant Biol. 2011;14:232–239. doi: 10.1016/j.pbi.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 128.Kole C., Muthamilarasan M., Henry R., Edwards D., Sharma R., Abberton M., Batley J., Bentley A., Blakeney M., Bryant J. Application of genomics-assisted breeding for generation of climate resilient crops: Progress and prospects. Front. Plant Sci. 2015;6:563. doi: 10.3389/fpls.2015.00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Collins N.C., Tardieu F., Tuberosa R. Quantitative trait loci and crop performance under abiotic stress: Where do we stand? Plant Physiol. 2008;147:469–486. doi: 10.1104/pp.108.118117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wani S.H., Choudhary M., Kumar P., Akram N.A., Surekha C., Ahmad P., Gosal S.S. Biotechnologies of Crop Improvement. Volume 3. Springer; Berlin/Heidelberg, Germany: 2018. Marker-Assisted Breeding for Abiotic Stress Tolerance in Crop Plants; pp. 1–23. [Google Scholar]
- 131.Da Silva Dias J.C. Molecular Approaches to Genetic Diversity. InTech; London, UK: 2015. Biodiversity and Plant Breeding as Tools for Harmony Between Modern Agriculture Production and the Environment. [DOI] [Google Scholar]
- 132.D’Agostino N., Tripodi P. NGS-based genotyping, high-throughput phenotyping and genome-wide association studies laid the foundations for next-generation breeding in horticultural crops. Diversity. 2017;9:38. doi: 10.3390/d9030038. [DOI] [Google Scholar]
- 133.Kearsey M., Farquhar A. QTL analysis in plants; where are we now? Heredity. 1998;80:137. doi: 10.1046/j.1365-2540.1998.00500.x. [DOI] [PubMed] [Google Scholar]
- 134.Yu H., Xie W., Wang J., Xing Y., Xu C., Li X., Xiao J., Zhang Q. Gains in QTL detection using an ultra-high density SNP map based on population sequencing relative to traditional RFLP/SSR markers. PLoS ONE. 2011;6:e17595. doi: 10.1371/journal.pone.0017595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sehgal D., Singh R., Rajpal V.R. Molecular Breeding for Sustainable Crop Improvement. Springer; Berlin/Heidelberg, Germany: 2016. Quantitative trait loci mapping in plants: Concepts and approaches; pp. 31–59. [Google Scholar]
- 136.Araus J.L., Cairns J.E. Field high-throughput phenotyping: The new crop breeding frontier. Trends Plant Sci. 2014;19:52–61. doi: 10.1016/j.tplants.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 137.Pikkuhookana P., Sillanpää M. Combined linkage disequilibrium and linkage mapping: Bayesian multilocus approach. Heredity. 2014;112:351. doi: 10.1038/hdy.2013.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Haley S.D., Johnson J.J., Peairs F.B., Quick J.S., Stromberger J.A., Clayshulte S.R., Butler J.D., Rudolph J.B., Seabourn B.W., Bai G. Registration of ‘Ripper’wheat. J. Plant Regist. 2007;1:1–6. doi: 10.3198/jpr2006.10.0689crc. [DOI] [Google Scholar]
- 139.Badu-Apraku B., Yallou C. Registration of Striga-resistant and drought-tolerant tropical early maize populations TZE-W Pop DT STR C 4 and TZE-Y Pop DT STR C 4. J. Plant Regist. 2009;3:86–90. doi: 10.3198/jpr2008.06.0356crg. [DOI] [Google Scholar]
- 140.Merchuk-Ovnat L., Barak V., Fahima T., Ordon F., Lidzbarsky G.A., Krugman T., Saranga Y. Ancestral QTL alleles from wild emmer wheat improve drought resistance and productivity in modern wheat cultivars. Front. Plant Sci. 2016;7:452. doi: 10.3389/fpls.2016.00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kochevenko A., Jiang Y., Seiler C., Surdonja K., Kollers S., Reif J.C., Korzun V., Graner A. Identification of QTL hot spots for malting quality in two elite breeding lines with distinct tolerance to abiotic stress. BMC Plant Biol. 2018;18:106. doi: 10.1186/s12870-018-1323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dixit S., Singh A., Sandhu N., Bhandari A., Vikram P., Kumar A. Combining drought and submergence tolerance in rice: Marker-assisted breeding and QTL combination effects. Mol. Breed. 2017;37:143. doi: 10.1007/s11032-017-0737-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tahmasebi S., Heidari B., Pakniyat H., McIntyre C.L. Mapping QTLs associated with agronomic and physiological traits under terminal drought and heat stress conditions in wheat (Triticum aestivum L.) Genome. 2016;60:26–45. doi: 10.1139/gen-2016-0017. [DOI] [PubMed] [Google Scholar]
- 144.Manolio T.A. Genomewide association studies and assessment of the risk of disease. N. Engl. J. Med. 2010;363:166–176. doi: 10.1056/NEJMra0905980. [DOI] [PubMed] [Google Scholar]
- 145.Bush W.S., Moore J.H. Genome-wide association studies. PLoS Comput. Biol. 2012;8:e1002822. doi: 10.1371/journal.pcbi.1002822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mousavi-Derazmahalleh M., Bayer P.E., Hane J.K., Babu V., Nguyen H.T., Nelson M.N., Erskine W., Varshney R.K., Papa R., Edwards D. Adapting legume crops to climate change using genomic approaches. Plant Cell Environ. 2018;42:6–19. doi: 10.1111/pce.13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Thoen M.P., Davila Olivas N.H., Kloth K.J., Coolen S., Huang P.P., Aarts M.G., Bac-Molenaar J.A., Bakker J., Bouwmeester H.J., Broekgaarden C. Genetic architecture of plant stress resistance: Multi-trait genome-wide association mapping. New Phytol. 2017;213:1346–1362. doi: 10.1111/nph.14220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wan H., Chen L., Guo J., Li Q., Wen J., Yi B., Ma C., Tu J., Fu T., Shen J. Genome-wide association study reveals the genetic architecture underlying salt tolerance-related traits in rapeseed (Brassica napus L.) Front. Plant Sci. 2017;8:593. doi: 10.3389/fpls.2017.00593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lafarge T., Bueno C., Frouin J., Jacquin L., Courtois B., Ahmadi N. Genome-wide association analysis for heat tolerance at flowering detected a large set of genes involved in adaptation to thermal and other stresses. PLoS ONE. 2017;12:e0171254. doi: 10.1371/journal.pone.0171254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Verslues P.E., Lasky J.R., Juenger T.E., Liu T.-W., Kumar M.N. Genome-wide association mapping combined with reverse genetics identifies new effectors of low water potential-induced proline accumulation in Arabidopsis. Plant Physiol. 2014;164:144–159. doi: 10.1104/pp.113.224014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ashraf M. Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol. Adv. 2009;27:84–93. doi: 10.1016/j.biotechadv.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 152.Qin P., Lin Y., Hu Y., Liu K., Mao S., Li Z., Wang J., Liu Y., Wei Y., Zheng Y. Genome-wide association study of drought-related resistance traits in Aegilops tauschii. Genet. Mol. Biol. 2016;39:398–407. doi: 10.1590/1678-4685-GMB-2015-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kumar V., Singh A., Mithra S.A., Krishnamurthy S., Parida S.K., Jain S., Tiwari K.K., Kumar P., Rao A.R., Sharma S. Genome-wide association mapping of salinity tolerance in rice (Oryza sativa) DNA Res. 2015;22:133–145. doi: 10.1093/dnares/dsu046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chopra R., Burow G., Burke J.J., Gladman N., Xin Z. Genome-wide association analysis of seedling traits in diverse Sorghum germplasm under thermal stress. BMC Plant Biol. 2017;17:12. doi: 10.1186/s12870-016-0966-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chen J., Chopra R., Hayes C., Morris G., Marla S., Burke J., Xin Z., Burow G. Genome-wide association study of developing leaves’ heat tolerance during vegetative growth stages in a sorghum association panel. Plant Genome. 2017;10:1–15. doi: 10.3835/plantgenome2016.09.0091. [DOI] [PubMed] [Google Scholar]
- 156.Kumar S., Muthusamy S.K., Mishra C.N., Gupta V., Venkatesh K. Advanced Molecular Plant Breeding: Meeting the Challenge of Food Security. CRC Press; Boca Raton, FL, USA: 2018. Importance of Genomic Selection in Crop Improvement and Future Prospects; p. 275. [Google Scholar]
- 157.Burgueño J., de los Campos G., Weigel K., Crossa J. Genomic prediction of breeding values when modeling genotype× environment interaction using pedigree and dense molecular markers. Crop Sci. 2012;52:707–719. doi: 10.2135/cropsci2011.06.0299. [DOI] [Google Scholar]
- 158.Jarquín D., Crossa J., Lacaze X., Du Cheyron P., Daucourt J., Lorgeou J., Piraux F., Guerreiro L., Pérez P., Calus M. A reaction norm model for genomic selection using high-dimensional genomic and environmental data. Appl. Genet. 2014;127:595–607. doi: 10.1007/s00122-013-2243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lopez-Cruz M., Crossa J., Bonnett D., Dreisigacker S., Poland J., Jannink J.-L., Singh R.P., Autrique E., de los Campos G. Increased prediction accuracy in wheat breeding trials using a marker × environment interaction genomic selection model. G3: Genes Genom. Genet. 2015;5:569–582. doi: 10.1534/g3.114.016097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cuevas J., Crossa J., Montesinos-López O.A., Burgueño J., Pérez-Rodríguez P., de los Campos G. Bayesian genomic prediction with genotype× environment interaction kernel models. G3: Genes. Genom. Genet. 2017;7:41–53. doi: 10.1534/g3.116.035584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rutkoski J.E., Crain J., Poland J., Sorrells M.E. Genomic Selection for Crop Improvement. Springer; Berlin/Heidelberg, Germany: 2017. Genomic Selection for Small Grain Improvement; pp. 99–130. [Google Scholar]
- 162.Dong H., Wang R., Yuan Y., Anderson J., Pumphrey M., Zhang Z., Chen J. Evaluation of the potential for genomic selection to improve spring wheat resistance to Fusarium head blight in the Pacific Northwest. Front. Plant Sci. 2018;9:911. doi: 10.3389/fpls.2018.00911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Crain J., Mondal S., Rutkoski J., Singh R.P., Poland J. Combining high-throughput phenotyping and genomic information to increase prediction and selection accuracy in wheat breeding. Plant Genome. 2018;11:1–14. doi: 10.3835/plantgenome2017.05.0043. [DOI] [PubMed] [Google Scholar]
- 164.Reynolds M., Tattaris M., Cossani C.M., Ellis M., Yamaguchi-Shinozaki K., Saint Pierre C. Advances in Wheat Genetics: From Genome to Field. Springer; Berlin/Heidelberg, Germany: 2015. Exploring genetic resources to increase adaptation of wheat to climate change; pp. 355–368. [Google Scholar]
- 165.Shah S.H., Ali S., Hussain Z., Jan S.A., Ali G.M. Genetic improvement of tomato (Solanum lycopersicum) with AtDREB1A dene for cold stress tolerance using optimized agrobacterium-mediated transformation system. Int. J. Agric. Biol. 2016;18:471–782. doi: 10.17957/IJAB/15.0107. [DOI] [Google Scholar]
- 166.Nejat N., Mantri N. Plant immune system: Crosstalk between responses to biotic and abiotic stresses the missing link in understanding plant defence. Curr. Issues Mol. Biol. 2017;23:1–16. doi: 10.21775/cimb.023.001. [DOI] [PubMed] [Google Scholar]
- 167.Riechmann J.L., Meyerowitz E.M. The AP2/EREBP family of plant transcription factors. Biol. Chem. 1998;379:633–646. doi: 10.1515/bchm.1998.379.6.633. [DOI] [PubMed] [Google Scholar]
- 168.Licausi F., Giorgi F.M., Zenoni S., Osti F., Pezzotti M., Perata P. Genomic and transcriptomic analysis of the AP2/ERF superfamily in Vitis vinifera. BMC Genom. 2010;11:719. doi: 10.1186/1471-2164-11-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sharoni A.M., Nuruzzaman M., Satoh K., Shimizu T., Kondoh H., Sasaya T., Choi I.-R., Omura T., Kikuchi S. Gene structures, classification and expression models of the AP2/EREBP transcription factor family in rice. Plant Cell Physiol. 2010;52:344–360. doi: 10.1093/pcp/pcq196. [DOI] [PubMed] [Google Scholar]
- 170.Stockinger E.J., Gilmour S.J., Thomashow M.F. Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc. Natl. Acad. Sci. USA. 1997;94:1035–1040. doi: 10.1073/pnas.94.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Agarwal M., Hao Y., Kapoor A., Dong C.-H., Fujii H., Zheng X., Zhu J.-K. A R2R3 type MYB transcription factor is involved in the cold regulation of CBF genes and in acquired freezing tolerance. J. Biol. Chem. 2006;281:37636–37645. doi: 10.1074/jbc.M605895200. [DOI] [PubMed] [Google Scholar]
- 172.Lata C., Prasad M. Role of DREBs in regulation of abiotic stress responses in plants. J. Exp. Bot. 2011;62:4731–4748. doi: 10.1093/jxb/err210. [DOI] [PubMed] [Google Scholar]
- 173.Mizoi J., Shinozaki K., Yamaguchi-Shinozaki K. AP2/ERF family transcription factors in plant abiotic stress responses. Biochim. Biophys. Acta Gene Regul. Mech. 2012;1819:86–96. doi: 10.1016/j.bbagrm.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 174.Liu Q., Kasuga M., Sakuma Y., Abe H., Miura S., Yamaguchi-Shinozaki K., Shinozaki K. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought-and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell. 1998;10:1391–1406. doi: 10.1105/tpc.10.8.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lucas S., Durmaz E., Akpınar B.A., Budak H. The drought response displayed by a DRE-binding protein from Triticum dicoccoides. Plant Physiol. Biochem. 2011;49:346–351. doi: 10.1016/j.plaphy.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 176.Sakuma Y., Liu Q., Dubouzet J.G., Abe H., Shinozaki K., Yamaguchi-Shinozaki K. DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration-and cold-inducible gene expression. Biochem. Biophys. Res. Commun. 2002;290:998–1009. doi: 10.1006/bbrc.2001.6299. [DOI] [PubMed] [Google Scholar]
- 177.Gilmour S.J., Zarka D.G., Stockinger E.J., Salazar M.P., Houghton J.M., Thomashow M.F. Low temperature regulation of the Arabidopsis CBF family of AP2 transcriptional activators as an early step in cold-induced COR gene expression. Plant J. 1998;16:433–442. doi: 10.1046/j.1365-313x.1998.00310.x. [DOI] [PubMed] [Google Scholar]
- 178.Jaglo-Ottosen K.R., Gilmour S.J., Zarka D.G., Schabenberger O., Thomashow M.F. Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science. 1998;280:104–106. doi: 10.1126/science.280.5360.104. [DOI] [PubMed] [Google Scholar]
- 179.Jaglo K.R., Kleff S., Amundsen K.L., Zhang X., Haake V., Zhang J.Z., Deits T., Thomashow M.F. Components of the Arabidopsis C-repeat/dehydration-responsive element binding factor cold-response pathway are conserved inbrassica napus and other plant species. Plant Physiol. 2001;127:910–917. doi: 10.1104/pp.010548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ito Y., Katsura K., Maruyama K., Taji T., Kobayashi M., Seki M., Shinozaki K., Yamaguchi-Shinozaki K. Functional analysis of rice DREB1/CBF-type transcription factors involved in cold-responsive gene expression in transgenic rice. Plant Cell Physiol. 2006;47:141–153. doi: 10.1093/pcp/pci230. [DOI] [PubMed] [Google Scholar]
- 181.Hsieh T.-H., Lee J.-T., Yang P.-T., Chiu L.-H., Charng Y.-Y., Wang Y.-C., Chan M.-T. Heterology expression of the ArabidopsisC-repeat/dehydration response element binding Factor 1 gene confers elevated tolerance to chilling and oxidative stresses in transgenic tomato. Plant Physiol. 2002;129:1086–1094. doi: 10.1104/pp.003442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kasuga M., Miura S., Shinozaki K., Yamaguchi-Shinozaki K. A combination of the Arabidopsis DREB1A gene and stress-inducible rd29A promoter improved drought-and low-temperature stress tolerance in tobacco by gene transfer. Plant Cell Physiol. 2004;45:346–350. doi: 10.1093/pcp/pch037. [DOI] [PubMed] [Google Scholar]
- 183.Dubouzet J.G., Sakuma Y., Ito Y., Kasuga M., Dubouzet E.G., Miura S., Seki M., Shinozaki K., Yamaguchi-Shinozaki K. OsDREB genes in rice, Oryza sativa L., encode transcription activators that function in drought-, high-salt-and cold-responsive gene expression. Plant J. 2003;33:751–763. doi: 10.1046/j.1365-313X.2003.01661.x. [DOI] [PubMed] [Google Scholar]
- 184.Qin F., Sakuma Y., Li J., Liu Q., Li Y.-Q., Shinozaki K., Yamaguchi-Shinozaki K. Cloning and functional analysis of a novel DREB1/CBF transcription factor involved in cold-responsive gene expression in Zea mays L. Plant Cell Physiol. 2004;45:1042–1052. doi: 10.1093/pcp/pch118. [DOI] [PubMed] [Google Scholar]
- 185.Qin F., Kakimoto M., Sakuma Y., Maruyama K., Osakabe Y., Tran L.S.P., Shinozaki K., Yamaguchi-Shinozaki K. Regulation and functional analysis of ZmDREB2A in response to drought and heat stresses in Zea mays L. Plant J. 2007;50:54–69. doi: 10.1111/j.1365-313X.2007.03034.x. [DOI] [PubMed] [Google Scholar]
- 186.Chen M., Wang Q.-Y., Cheng X.-G., Xu Z.-S., Li L.-C., Ye X.-G., Xia L.-Q., Ma Y.-Z. GmDREB2, a soybean DRE-binding transcription factor, conferred drought and high-salt tolerance in transgenic plants. Biochem. Biophys. Res. Commun. 2007;353:299–305. doi: 10.1016/j.bbrc.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 187.Mallikarjuna G., Mallikarjuna K., Reddy M., Kaul T. Expression of OsDREB2A transcription factor confers enhanced dehydration and salt stress tolerance in rice (Oryza sativa L.) Biotechnol. Lett. 2011;33:1689–1697. doi: 10.1007/s10529-011-0620-x. [DOI] [PubMed] [Google Scholar]
- 188.Dietz K.-J., Vogel M.O., Viehhauser A. AP2/EREBP transcription factors are part of gene regulatory networks and integrate metabolic, hormonal and environmental signals in stress acclimation and retrograde signalling. Protoplasma. 2010;245:3–14. doi: 10.1007/s00709-010-0142-8. [DOI] [PubMed] [Google Scholar]
- 189.Hao D., Ohme-Takagi M., Sarai A. Unique mode of GCC box recognition by the DNA-binding domain of ethylene-responsive element-binding factor (ERF domain) in plant. J. Biol. Chem. 1998;273:26857–26861. doi: 10.1074/jbc.273.41.26857. [DOI] [PubMed] [Google Scholar]
- 190.Xu Z.-S., Chen M., Li L.-C., Ma Y.-Z. Functions of the ERF transcription factor family in plants. Botany. 2008;86:969–977. doi: 10.1139/B08-041. [DOI] [Google Scholar]
- 191.Liang H., Lu Y., Liu H., Wang F., Xin Z., Zhang Z. A novel activator-type ERF of Thinopyrum intermedium, TiERF1, positively regulates defence responses. J. Exp. Bot. 2008;59:3111–3120. doi: 10.1093/jxb/ern165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhu X., Qi L., Liu X., Cai S., Xu H., Huang R., Li J., Wei X., Zhang Z. The wheat ethylene response factor transcription factor pathogen-induced ERF1 mediates host responses to both the necrotrophic pathogen Rhizoctonia cerealis and freezing stresses. Plant Physiol. 2014;164:1499–1514. doi: 10.1104/pp.113.229575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhang G., Chen M., Li L., Xu Z., Chen X., Guo J., Ma Y. Overexpression of the soybean GmERF3 gene, an AP2/ERF type transcription factor for increased tolerances to salt, drought, and diseases in transgenic tobacco. J. Exp. Bot. 2009;60:3781–3796. doi: 10.1093/jxb/erp214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Baldoni E., Genga A., Cominelli E. Plant MYB transcription factors: Their role in drought response mechanisms. Int. J. Mol. Sci. 2015;16:15811–15851. doi: 10.3390/ijms160715811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Li C., Ng C.K.-Y., Fan L.-M. MYB transcription factors, active players in abiotic stress signaling. Environ. Exp. Bot. 2015;114:80–91. doi: 10.1016/j.envexpbot.2014.06.014. [DOI] [Google Scholar]
- 196.Ambawat S., Sharma P., Yadav N.R., Yadav R.C. MYB transcription factor genes as regulators for plant responses: An overview. Physiol. Mol. Biol. Plants. 2013;19:307–321. doi: 10.1007/s12298-013-0179-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Cominelli E., Galbiati M., Vavasseur A., Conti L., Sala T., Vuylsteke M., Leonhardt N., Dellaporta S.L., Tonelli C. A guard-cell-specific MYB transcription factor regulates stomatal movements and plant drought tolerance. Curr. Biol. 2005;15:1196–1200. doi: 10.1016/j.cub.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 198.Liang Y.-K., Dubos C., Dodd I.C., Holroyd G.H., Hetherington A.M., Campbell M.M. AtMYB61, an R2R3-MYB transcription factor controlling stomatal aperture in Arabidopsis thaliana. Curr. Biol. 2005;15:1201–1206. doi: 10.1016/j.cub.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 199.Jung C., Seo J.S., Han S.W., Koo Y.J., Kim C.H., Song S.I., Nahm B.H., Do Choi Y., Cheong J.-J. Overexpression of AtMYB44 enhances stomatal closure to confer abiotic stress tolerance in transgenic Arabidopsis. Plant Physiol. 2008;146:623–635. doi: 10.1104/pp.107.110981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Seo P.J., Xiang F., Qiao M., Park J.-Y., Lee Y.N., Kim S.-G., Lee Y.-H., Park W.J., Park C.-M. The MYB96 transcription factor mediates abscisic acid signaling during drought stress response in Arabidopsis. Plant Physiol. 2009;151:275–289. doi: 10.1104/pp.109.144220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Seo P.J., Lee S.B., Suh M.C., Park M.-J., Go Y.S., Park C.-M. The MYB96 transcription factor regulates cuticular wax biosynthesis under drought conditions in Arabidopsis. Plant Cell. 2011 doi: 10.1105/tpc.111.083485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Yang A., Dai X., Zhang W.-H. A R2R3-type MYB gene, OsMYB2, is involved in salt, cold, and dehydration tolerance in rice. J. Exp. Bot. 2012;63:2541–2556. doi: 10.1093/jxb/err431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Liao Y., Zou H.-F., Wang H.-W., Zhang W.-K., Ma B., Zhang J.-S., Chen S.-Y. Soybean GmMYB76, GmMYB92, and GmMYB177 genes confer stress tolerance in transgenic Arabidopsis plants. Cell Res. 2008;18:1047. doi: 10.1038/cr.2008.280. [DOI] [PubMed] [Google Scholar]
- 204.Cao Z.-H., Zhang S.-Z., Wang R.-K., Zhang R.-F., Hao Y.-J. Genome wide analysis of the apple MYB transcription factor family allows the identification of MdoMYB121 gene confering abiotic stress tolerance in plants. PLoS ONE. 2013;8:e69955. doi: 10.1371/journal.pone.0069955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Chen Y., Cao Y., Wang L., Li L., Yang J., Zou M. Identification of MYB transcription factor genes and their expression during abiotic stresses in maize. Biol. Plant. 2017;62:1–9. doi: 10.1007/s10535-017-0756-1. [DOI] [Google Scholar]
- 206.Wang R.K., Cao Z.H., Hao Y.J. Overexpression of a R2R3 MYB gene MdSIMYB1 increases tolerance to multiple stresses in transgenic tobacco and apples. Physiol. Plant. 2014;150:76–87. doi: 10.1111/ppl.12069. [DOI] [PubMed] [Google Scholar]
- 207.Zhang Z., Liu X., Wang X., Zhou M., Zhou X., Ye X., Wei X. An R2R3 MYB transcription factor in wheat, Ta PIMP 1, mediates host resistance to Bipolaris sorokiniana and drought stresses through regulation of defense-and stress-related genes. New Phytol. 2012;196:1155–1170. doi: 10.1111/j.1469-8137.2012.04353.x. [DOI] [PubMed] [Google Scholar]
- 208.Liu H., Zhou X., Dong N., Liu X., Zhang H., Zhang Z. Expression of a wheat MYB gene in transgenic tobacco enhances resistance to Ralstonia solanacearum, and to drought and salt stresses. Funct. Integr. Genom. 2011;11:431–443. doi: 10.1007/s10142-011-0228-1. [DOI] [PubMed] [Google Scholar]
- 209.Muthamilarasan M., Bonthala V.S., Khandelwal R., Jaishankar J., Shweta S., Nawaz K., Prasad M. Global analysis of WRKY transcription factor superfamily in Setaria identifies potential candidates involved in abiotic stress signaling. Front. Plant Sci. 2015;6:910. doi: 10.3389/fpls.2015.00910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Phukan U.J., Jeena G.S., Shukla R.K. WRKY transcription factors: Molecular regulation and stress responses in plants. Front. Plant Sci. 2016;7:760. doi: 10.3389/fpls.2016.00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wu X., Shiroto Y., Kishitani S., Ito Y., Toriyama K. Enhanced heat and drought tolerance in transgenic rice seedlings overexpressing OsWRKY11 under the control of HSP101 promoter. Plant Cell Rep. 2009;28:21–30. doi: 10.1007/s00299-008-0614-x. [DOI] [PubMed] [Google Scholar]
- 212.Zhou Q.Y., Tian A.G., Zou H.F., Xie Z.M., Lei G., Huang J., Wang C.M., Wang H.W., Zhang J.S., Chen S.Y. Soybean WRKY-type transcription factor genes, GmWRKY13, GmWRKY21, and GmWRKY54, confer differential tolerance to abiotic stresses in transgenic Arabidopsis plants. Plant Biotechnol. J. 2008;6:486–503. doi: 10.1111/j.1467-7652.2008.00336.x. [DOI] [PubMed] [Google Scholar]
- 213.Niu C.F., Wei W., Zhou Q.Y., Tian A.G., Hao Y.J., Zhang W.K., Ma B., Lin Q., Zhang Z.B., Zhang J.S. Wheat WRKY genes TaWRKY2 and TaWRKY19 regulate abiotic stress tolerance in transgenic Arabidopsis plants. Plant Cell Environ. 2012;35:1156–1170. doi: 10.1111/j.1365-3040.2012.02480.x. [DOI] [PubMed] [Google Scholar]
- 214.He G.-H., Xu J.-Y., Wang Y.-X., Liu J.-M., Li P.-S., Chen M., Ma Y.-Z., Xu Z.-S. Drought-responsive WRKY transcription factor genes TaWRKY1 and TaWRKY33 from wheat confer drought and/or heat resistance in Arabidopsis. BMC Plant Biol. 2016;16:116. doi: 10.1186/s12870-016-0806-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Li H., Gao Y., Xu H., Dai Y., Deng D., Chen J. ZmWRKY33, a WRKY maize transcription factor conferring enhanced salt stress tolerances in Arabidopsis. Plant Growth Regul. 2013;70:207–216. doi: 10.1007/s10725-013-9792-9. [DOI] [Google Scholar]
- 216.Fan Q., Song A., Jiang J., Zhang T., Sun H., Wang Y., Chen S., Chen F. CmWRKY1 enhances the dehydration tolerance of chrysanthemum through the regulation of ABA-associated genes. PLoS ONE. 2016;11:e0150572. doi: 10.1371/journal.pone.0150572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Nuruzzaman M., Sharoni A.M., Kikuchi S. Roles of NAC transcription factors in the regulation of biotic and abiotic stress responses in plants. Front. Microbiol. 2013;4:248. doi: 10.3389/fmicb.2013.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Banerjee A., Roychoudhury A. WRKY proteins: Signaling and regulation of expression during abiotic stress responses. Sci. World J. 2015;2015:1–17. doi: 10.1155/2015/807560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Nuruzzaman M., Manimekalai R., Sharoni A.M., Satoh K., Kondoh H., Ooka H., Kikuchi S. Genome-wide analysis of NAC transcription factor family in rice. Gene. 2010;465:30–44. doi: 10.1016/j.gene.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 220.Shiriga K., Sharma R., Kumar K., Yadav S.K., Hossain F., Thirunavukkarasu N. Genome-wide identification and expression pattern of drought-responsive members of the NAC family in maize. Meta Gene. 2014;2:407–417. doi: 10.1016/j.mgene.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Le D.T., Nishiyama R., Watanabe Y., Mochida K., Yamaguchi-Shinozaki K., Shinozaki K., Tran L.-S.P. Genome-wide survey and expression analysis of the plant-specific NAC transcription factor family in soybean during development and dehydration stress. DNA Res. 2011;18:263–276. doi: 10.1093/dnares/dsr015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Jiang Y., Deyholos M.K. Comprehensive transcriptional profiling of NaCl-stressed Arabidopsis roots reveals novel classes of responsive genes. BMC Plant Biol. 2006;6:25. doi: 10.1186/1471-2229-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Fang Y., You J., Xie K., Xie W., Xiong L. Systematic sequence analysis and identification of tissue-specific or stress-responsive genes of NAC transcription factor family in rice. Mol. Genet. Genom. 2008;280:547–563. doi: 10.1007/s00438-008-0386-6. [DOI] [PubMed] [Google Scholar]
- 224.Lu M., Zhang D.-F., Shi Y.-S., Song Y.-C., Wang T.-Y., Li Y. Expression of SbSNAC1, a NAC transcription factor from sorghum, confers drought tolerance to transgenic Arabidopsis. Plant Cell Tissue Organ. Cult. 2013;115:443–455. doi: 10.1007/s11240-013-0375-2. [DOI] [Google Scholar]
- 225.Zheng X., Chen B., Lu G., Han B. Overexpression of a NAC transcription factor enhances rice drought and salt tolerance. Biochem. Biophys. Res. Commun. 2009;379:985–989. doi: 10.1016/j.bbrc.2008.12.163. [DOI] [PubMed] [Google Scholar]
- 226.Hu H., Dai M., Yao J., Xiao B., Li X., Zhang Q., Xiong L. Overexpressing a NAM, ATAF, and CUC (NAC) transcription factor enhances drought resistance and salt tolerance in rice. Proc. Natl. Acad. Sci. USA. 2006;103:12987–12992. doi: 10.1073/pnas.0604882103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Liu W., Yuan J.S., Stewart C.N., Jr. Advanced genetic tools for plant biotechnology. Nat. Rev. Genet. 2013;14:781. doi: 10.1038/nrg3583. [DOI] [PubMed] [Google Scholar]
- 228.Abdelrahman M., El-Sayed M., Sato S., Hirakawa H., Ito S.-I., Tanaka K., Mine Y., Sugiyama N., Suzuki M., Yamauchi N. RNA-sequencing-based transcriptome and biochemical analyses of steroidal saponin pathway in a complete set of Allium fistulosum—A. cepa monosomic addition lines. PLoS ONE. 2017;12:e0181784. doi: 10.1371/journal.pone.0181784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Flint-Garcia S.A. Genetics and consequences of crop domestication. J. Agric. Food Chem. 2013;61:8267–8276. doi: 10.1021/jf305511d. [DOI] [PubMed] [Google Scholar]
- 230.Abdelrahman M., Jogaiah S., Burritt D.J., Tran L.S.P. Legume genetic resources and transcriptome dynamics under abiotic stress conditions. Plant Cell Environ. 2018;41:1972–1983. doi: 10.1111/pce.13123. [DOI] [PubMed] [Google Scholar]
- 231.Taranto F., Nicolia A., Pavan S., De Vita P., D’Agostino N. Biotechnological and digital revolution for climate-smart plant breeding. Agronomy. 2018;8:277. doi: 10.3390/agronomy8120277. [DOI] [Google Scholar]
- 232.Kamburova V.S., Nikitina E.V., Shermatov S.E., Buriev Z.T., Kumpatla S.P., Emani C., Abdurakhmonov I.Y. Genome editing in plants: An overview of tools and applications. Int. J. Agron. 2017;2017:1–15. doi: 10.1155/2017/7315351. [DOI] [Google Scholar]
- 233.Zhu C., Bortesi L., Baysal C., Twyman R.M., Fischer R., Capell T., Schillberg S., Christou P. Characteristics of genome editing mutations in cereal crops. Trends Plant Sci. 2017;22:38–52. doi: 10.1016/j.tplants.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 234.Abdelrahman M., Al-Sadi A.M., Pour-Aboughadareh A., Burritt D.J., Tran L.-S.P. Genome editing using CRISPR/Cas9–targeted mutagenesis: An opportunity for yield improvements of crop plants grown under environmental stresses. Plant Physiol. Biochem. 2018;131:31–36. doi: 10.1016/j.plaphy.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 235.Hussain B., Lucas S.J., Budak H. CRISPR/Cas9 in plants: At play in the genome and at work for crop improvement. Brief. Funct. Genom. 2018;17:319–328. doi: 10.1093/bfgp/ely016. [DOI] [PubMed] [Google Scholar]
- 236.Lino C.A., Harper J.C., Carney J.P., Timlin J.A. Delivering CRISPR: A review of the challenges and approaches. Drug Deliv. 2018;25:1234–1257. doi: 10.1080/10717544.2018.1474964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Larochelle S. Genomics: CRISPR–Cas Goes RNA. Nat. Methods. 2018;15:312. doi: 10.1038/nmeth.4681. [DOI] [Google Scholar]
- 238.Jaganathan D., Ramasamy K., Sellamuthu G., Jayabalan S., Venkataraman G. CRISPR for crop improvement: An update review. Front. Plant Sci. 2018;9:985. doi: 10.3389/fpls.2018.00985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Haque E., Taniguchi H., Hassan M.M., Bhowmik P., Karim M.R., Śmiech M., Zhao K., Rahman M., Islam T. Application of CRISPR/Cas9 Genome Editing Technology for the Improvement of Crops Cultivated in Tropical Climates: Recent Progress, Prospects, and Challenges. Front. Plant Sci. 2018;9:617. doi: 10.3389/fpls.2018.00617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Khurshid H., Jan S.A., Shinwari Z.K., Jamal M., Shah S.H. An Era of CRISPR/Cas9 Mediated Plant Genome Editing. Curr. Issues Mol. Biol. 2017;26:47–54. doi: 10.21775/cimb.026.047. [DOI] [PubMed] [Google Scholar]
- 241.Kim D., Alptekin B., Budak H. CRISPR/Cas9 genome editing in wheat. Funct. Integr. Genom. 2018;18:31–41. doi: 10.1007/s10142-017-0572-x. [DOI] [PubMed] [Google Scholar]
- 242.Ou W., Mao X., Huang C., Tie W., Yan Y., Ding Z., Wu C., Xia Z., Wang W., Zhou S. Genome-wide identification and expression analysis of the KUP family under abiotic stress in cassava (Manihot esculenta Crantz) Front. Physiol. 2018;9:17. doi: 10.3389/fphys.2018.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Ye J., Yang H., Shi H., Wei Y., Tie W., Ding Z., Yan Y., Luo Y., Xia Z., Wang W. The MAPKKK gene family in cassava: Genome-wide identification and expression analysis against drought stress. Sci. Rep. 2017;7:14939. doi: 10.1038/s41598-017-13988-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Xu R., Yang Y., Qin R., Li H., Qiu C., Li L., Wei P., Yang J. Rapid improvement of grain weight via highly efficient CRISPR/Cas9-mediated multiplex genome editing in rice. J. Genet. Genom. 2016;43:529–532. doi: 10.1016/j.jgg.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 245.Wang W., Pan Q., He F., Akhunova A., Chao S., Trick H., Akhunov E. Transgenerational CRISPR-Cas9 activity facilitates multiplex gene editing in allopolyploid wheat. CRISPR J. 2018;1:65–74. doi: 10.1089/crispr.2017.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Sánchez-León S., Gil-Humanes J., Ozuna C.V., Giménez M.J., Sousa C., Voytas D.F., Barro F. Low-gluten, nontransgenic wheat engineered with CRISPR/Cas9. Plant Biotechnol. J. 2018;16:902–910. doi: 10.1111/pbi.12837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Wang L., Chen L., Li R., Zhao R., Yang M., Sheng J., Shen L. Reduced drought tolerance by CRISPR/Cas9-mediated SlMAPK3 mutagenesis in tomato plants. J. Agric. Food Chem. 2017;65:8674–8682. doi: 10.1021/acs.jafc.7b02745. [DOI] [PubMed] [Google Scholar]
- 248.Chen Klap E.Y., Bolger A.M., Arazi T., Gupta S.K., Shabtai S., Usadel B., Salts Y., Barg R. Tomato facultative parthenocarpy results from SlAGAMOUS-LIKE 6 loss of function. Plant Biotechnol. J. 2017;15:634. doi: 10.1111/pbi.12662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Shen C., Que Z., Xia Y., Tang N., Li D., He R., Cao M. Knock out of the annexin gene OsAnn3 via CRISPR/Cas9-mediated genome editing decreased cold tolerance in rice. J. Plant Biol. 2017;60:539–547. doi: 10.1007/s12374-016-0400-1. [DOI] [Google Scholar]
- 250.Shimatani Z., Kashojiya S., Takayama M., Terada R., Arazoe T., Ishii H., Teramura H., Yamamoto T., Komatsu H., Miura K. Targeted base editing in rice and tomato using a CRISPR-Cas9 cytidine deaminase fusion. Nat. Biotechnol. 2017;35:441. doi: 10.1038/nbt.3833. [DOI] [PubMed] [Google Scholar]