Abstract
Most ribbon worms (phylum: Nemertea) are found in marine environments, where they act as predators and scavengers. They are characterized by an eversible proboscis that is used to hunt for prey and thick mucus covering their skin. Both proboscis and epidermal mucus mediate toxicity to predators and preys. Research into the chemical nature of the substances that render toxicity has not been extensive, but it has nevertheless led to the identification of several compounds of potential medicinal use or for application in biotechnology. This review provides a complete account of the current status of research into nemertean toxins.
Keywords: Anabaseine, cytotoxin, DMXBA, nemertea, nemertide, parborlysin, ribbon worm, tetrodotoxin
1. Introduction
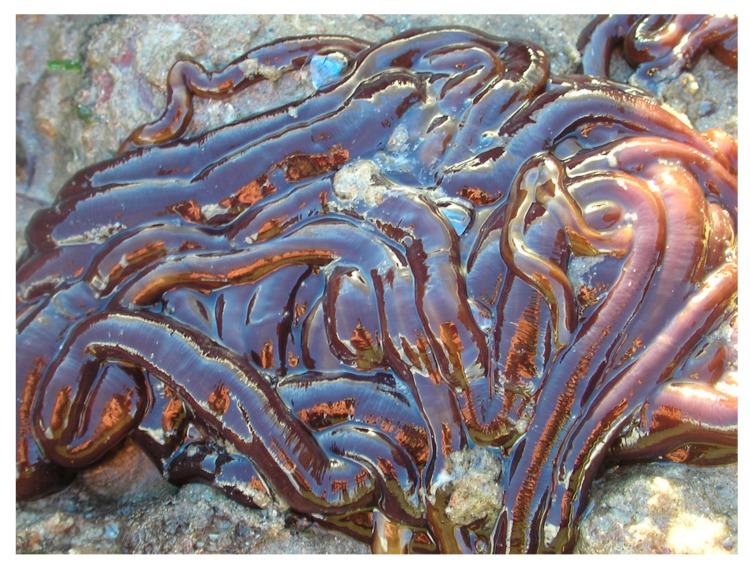
Approximately 1300 species currently comprise the phylum of nemerteans, or ribbon worms (also known as nemertini or rhyncocoeles) [1,2]. Most species are found in marine environments, but 13 terrestrial [3] and 22 freshwater [4] species are described. They are poorly known to the general public and the body of research into nemertean biology and ecology is limited. However, the phylum includes some remarkable species: Parborlasia corrugatus, which is the major scavenger on the sea floor in Antarctica, and Lineus longissimus, Figure 1, which is known as the longest animal on earth, reaching lengths of 50 m. The eversible proboscis of nemerteans can be armed with a stylet. Certain nemertean species are known to contain remarkably potent toxins: pyridine alkaloids, tetrodotoxin (TTX), and cytolytic or neurotoxic peptides. In the current review, we show the plethora of pharmacologically active compounds that have been discovered in nemerteans.
Figure 1.
Lineus longissimus, the world’s longest animal? Note the characteristic mucus covering the whole body. Photography © Sion Roberts (https://bit.ly/2tlzRAI). Used with permission.
W.R. Kem, the main pioneer in the area of nemertean chemistry, has previously given detailed accounts [5,6,7,8], but new methodologies and new discoveries prompt a comprehensive update. This review aims to detail the present knowledge on the topic of nemertean toxins with regard to the chemistry, mechanisms of action, and biological functions. Throughout, the term toxin is used for any pharmacologically active compound in this paper. We avoid the division into poisons, venoms, or toxungens [9], because delivery mechanisms as well as the potential storage and sources of production of nemertean toxic compounds are only known in parts. In addition, nemerteans are both preys [10] and predators [11], and knowledge is mostly insufficient in determining whether a particular toxin is used for defense or for hunting, or potentially both. The terms nemerteans, nemertean worms, and ribbon worms are used interchangeably.
2. Taxonomy and Phylogeny
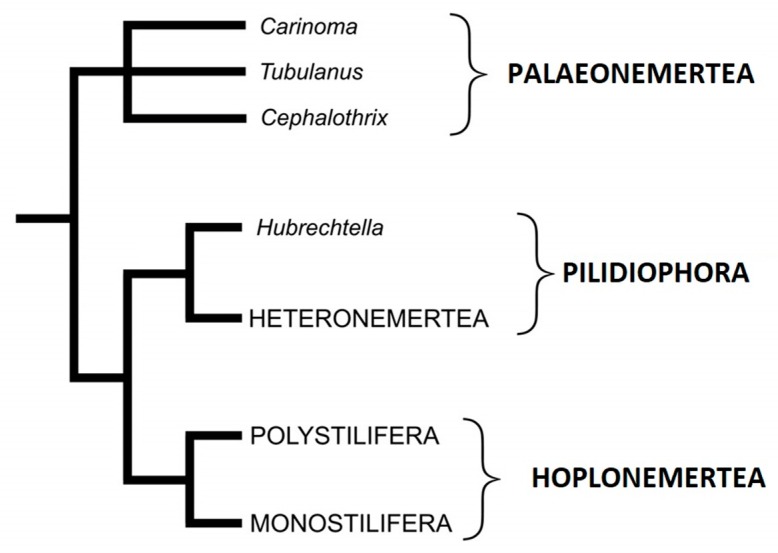
The classification of nemerteans has been in constant flux, both at the intra-phylum level and with respect to the position of the phylum among the metazoans. Relations between higher taxa are not steadily positioned within a phylogenetic framework and some taxonomic groups within Nemertea are clearly not monophyletic. Traditionally, the two suborders, Anopla and Enopla, have been used following Johnston’s (1837) [12] grouping that is based primarily on the absence or presence of the stylet apparatus in the proboscis. Recently, these suborders were dismissed [13] and instead three classes (natural groups) are maintained from the compiled evidence of the last 15 years: Palaeonemertea, Pilidiophora, and Hoplonemertea. The main morphological features that are used for further classification are muscle layers in the body wall, armament of proboscis (Hoplonemertea), and placement of mouth opening.
Until 2007, 1275 species in 285 genera were counted [2]. This is most certainly an underestimation of the actual number, and genetic evidence (see for example [14]) shows that the sibling and cryptic species are more common than previously recognized.
Phylogenetic analyses support that nemerteans are affiliated to protostome coelomates in Lophotrochozoa. Recent studies support the hypothesis that phoronids (horseshoe worms) are their closest relatives within this group [15]. Intra-phylum phylogeny molecular studies, although based on different markers and non-overlapping taxa, have agreed at some fundamental points: monophyly of Hoplonemertea, paraphyly of Anopla [16]. The proposed modified taxonomic structure is hence presented in Figure 2.
Figure 2.
Schematic phylogenetic tree over the Nemertea phylum with names as proposed by Strand et al. [13]. Current intraphylum theories suggest relatively closer relationship between Pilidiophora and Hoplonemertea with Palaeonemertea outside this branch. Italics refer to genera.
3. Anatomy
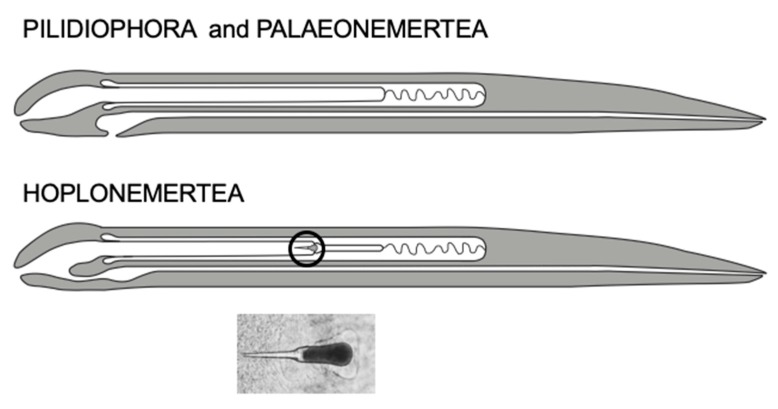
Nemerteans are unsegmented animals with an eversible proboscis and the capability of extreme contraction/elongation as distinctive features. Many species are brightly coloured with different patterns of pigmentation. Different species range in size from microscopic, such as Carcinonemertes sp., which only reaches 2 mm in length, to 50 m, as reported for Lineus longissimus. The most prominent synapomorphic anatomical feature of nemerteans is the eversible proboscis that lies within a special coelom called the rhynchocoel. A well-developed nervous system renders the ability to detect and catch prey with precision. The proboscis is used to catch prey (e.g., molluscs, crustaceans, worms) and the toxin(s) are is considered to be concentrated to the anterior part of the proboscis [17]. The hoplonemerteans carry a stylet [18], Figure 3, that is attached at its outermost tip, and in association a sac assumed to contain toxins [19]. This armed proboscis can puncture the prey and quickly immobilize it. The nemertean can then feed upon the prey.
Figure 3.
Differences between the Pilidiophora and Hoplonemertea anatomies. Top: The mouth opening of Pilidiophora and Palaeonemertea is separated from the proboscis, whereas a common opening is the case for Hoplonemerteans. Bottom: The hoplonemertean proboscis (in circle) contains a stylet (or several in the case of Polystilifera) by which a prey epithelium can be punctured. Photography of a stylet from Amphiporus lactifloreus.
Nemerteans that do not belong to the hoplonemerteans generally lack the stylet but they can still be very effective predators. Certain cells form papillous structures on the proboscis [20,21], releasing glandulous secretions that may contain both toxins and glue-like substances that help to hold the prey until death/immobilization occurs [22]. Tissue dissolving capacities seem to have been exhibited by some of these substances. Among these structures, the so-called pseudocnidae have been subjected to detailed study [23,24], but more work remains in order to ascertain their role in toxin production and delivery.
Most species secrete epidermal mucus that covers the whole body. It facilitates the “gliding” movement of the animals. Many species respond to tactile stimuli with enhanced mucus production. This mucus is not well chemically explored, although some studies have displayed the presence of both cytolytic toxins and neurotoxins [8,25,26]. The mucoid neurotoxins could be functional in chemical defense or pheromonal/odour-driven activities (chemical signalling), but there is little empirical evidence in the literature.
4. Early Descriptions of Ribbon Worms and Their Toxins
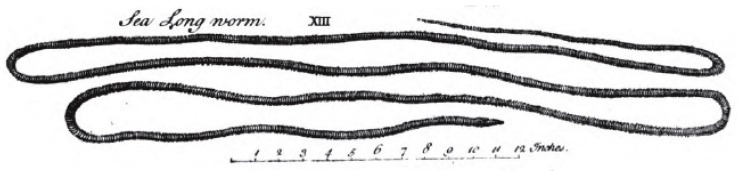
The perhaps earliest description of a ribbon worm in the literature is also the first indication of its toxicity. The Swedish ecclesiastic Olaus Magnus described in his Historia de Gentibus Septentrionalibus from 1555 [27] how a (worm is entirely harmless, unless touched by a human hand. In that case, the fingers will swell when the animal comes into contact with the skin of the hand). The credibility of the source may be somewhat hampered by the fact that it also contains descriptions of giants, sea monsters, and unicorns, but the species discussed has nevertheless been interpreted as Lineus longissimus [28], Figure 1. An early illustration of a ribbon worm (possibly L. longissimus) is seen in William Borlase’s Natural History of Cornwall [29], Figure 4. It was denoted a “Sea long worm” and then categorized as belonging to the “less perfect kind of sea-animals”, but no mention was made of any toxicity.
Figure 4.
The “Sea long worm”, an early depiction of what is likely Lineus sp. Reproduced from [29], 1758, Oxford.
In 1900, Wilson described the toxicity of the mucus as “it will be found so intensely arid as to parch the whole mouth, and the taste remains for a long time” after having placed a drop of mucus from the heteronemertean Cerebratulus lacteus on the tongue [30]. Reisinger [31] described the attack of Prostoma graecense and then suggested that the paralyzing poison originated from the epithelium of the posterior proboscis. The first systematic investigations of the toxic matter were carried out in the 1930’s by Bacq [32,33]. An account was given by Kem [5], which, in brief, is described here: aqueous extracts of whole hoplonemerteans (Amphiporus lactifloreus and Drepanophorus crassus) were shown to exhibit a nicotine-like effect on frog smooth muscles. The A. lactifloreus extract was still active after boiling under acid and alkaline conditions both, and the active compound could be extracted into chloroform under basic conditions. Bacq named this substance “amphiporine” and then concluded that it was an alkaloid similar to nicotine, Figure 5. The nicotine-like effect appeared to be absent in heteronemerteans, but extracts of those were shown to induce repetitive spiking in an isolated crab nerve preparation. As this effect appeared to be general for nemerteans, it was assumed that the effect came from another type of toxin, which was named “nemertine”. In 1939, King [34] reported a series of attempts to purify the amphiporine fraction, but was unable to decipher the identity of this toxin, and since then it appears that no further studies were reported regarding the nature of nemertean toxins until the early 1970’s.
Figure 5.
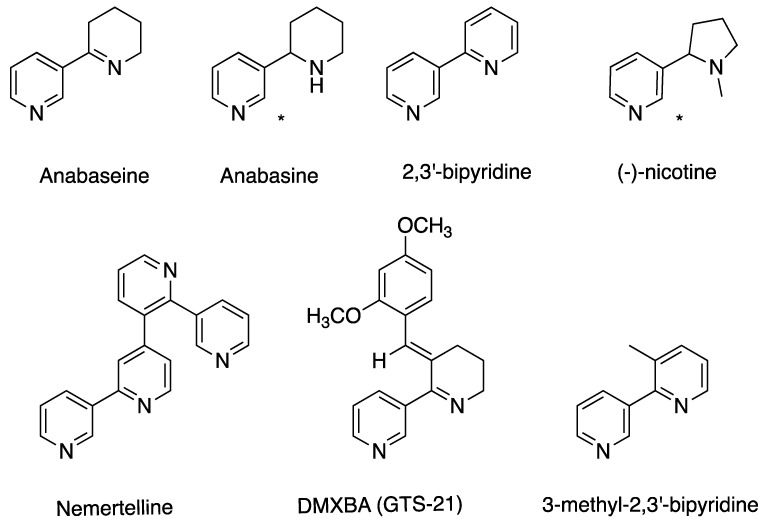
Structures of nemertean pyridine alkaloids, nicotine, and the derivative 3-(2,4-dimethoxybenzylidene)-anabaseine (DMXBA).
5. Ribbon Worm Toxins
The discovery of ribbon worm toxins on a molecular level can be attributed to a few research groups, with each focusing on their specific types of target compounds. Kem and co-workers pioneered pyridine alkaloids. TTX and analogs have been the focus of research groups from Hiroshima and Tokyo, and also lately Vladivostok. Peptide toxins have been explored by the groups of Kem and Blumenthal. In recent years, the interest in this field has been reinvigorated by the development of techniques, such as peptidomics and next-generation sequencing. In addition to the known toxins, gene sequences of other peptides and proteins that are related to toxins known from other organisms have been identified. These sequences represent a fourth category, albeit the presence of the actual molecules have not yet been confirmed. This review follows this subdivision.
5.1. Pyridine Alkaloids
During the summer of 1967, W.R. Kem collected some 10,000 specimens of Paranemertes peregrina along the coast of San Juan Island, aiming to uncover the identity of Bacq’s amphiporine [33]. The nemerteans were homogenized, centrifuged, and then subsequently extracted with chloroform. The effects of resultant extracts were studied in bioassays of crabs (Hemigrapsus nudus) and crayfish (Cambarus virilis). A toxic fraction was obtained and then subjected to mass spectrometry (MS) and NMR analyses, identifying the active principle as anabaseine [35], Figure 5. This was followed by an extensive investigation of the localization of anabaseine in P. peregrina, and its occurrence in other nemertean species [17]. The concentration of anabaseine was found to be the highest by far in the proboscis, where it is concentrated to the anterior and median regions. High concentrations were also found in the peripheral part of the body wall. In addition to P. peregrina, anabaseine was present in the stylet-carrying Amphiporus lactifloreus and Tetrastemma worki, whereas it was not present in any of the other species that were studied. Kem et al. identified two major alkaloids in A. angulatus, neurotoxic 2,3′-bipyridyl and a tetrapyridyl, they named nemertelline because of its similarity with the tobacco alkaloid nicotelline [36], Figure 5. Later, the structure of nemertelline was revised by Cruskie et al. [37]).
In another study, the content of pyridine alkaloids in 19 nemertean species was assayed [38]. The analysis was complicated by limited sample availability of some species and long storage times, resulting in the possible degradation of toxic constituents. Nevertheless, it was shown that anabasine (not to be confused with anabaseine), Figure 4, was present in two of the species, Amphiporus angulatus and Zygonemertes virescens [38]. Moreover, several other unidentified pyridines appeared to be widespread among the species that were analyzed. A later study showed the presence of 15 different alkaloids in a basic chloroform soluble fraction derived from A. angulatus [39]. However, the details of the analysis and the identities of the alkaloids were not described. One of the compounds found in A. angulatus, 3-methyl-2,3′bipyridyl, was later identified and synthesized [40].
The pyridyl compounds that were identified were all shown to be active against invertebrates in the μM range, Table 1. A series of bioassays were carried out for the whole range of compounds and synthetic derivatives: crayfish paralysis, feeding behaviour of spiny lobsters, electrophysiological recordings of chemoreceptor neurons, and patch clamp recordings of crayfish gastric mill chloride channels. The results suggested that crayfish paralysis was due to the effect of pyridyl toxins on nicotinic cholinergic receptors in the crustacean central nervous system.
Table 1.
Biological activities of nemertean pyridyl alkaloids (data from [41]).
| Compound | Barnacle Larvae Settlement Inhibition IC50 (μM) |
Barnacle Larvae Median Lethal Concentration IL50 (μM) | Crayfish Acute Paralytic Dose PD50 (μg) |
|---|---|---|---|
| 2,3′-bipyridyl | 4.1 (3.2–5.3) a | 1.9 (1.0–4.3) | 0.88 (0.71–1.1) |
| Anabaseine | 1.2 (0.91–1.7) | 2 | 3.6 (3.1–4.1) |
| Nemertelline | 3.2 (1.8–6.0) | - | >120 |
| Anabasine | 3.0 (1.5–4.9) | - | 3.9 (3.4–4.5) |
a Parentheses indicate standard deviations.
The results also indicated the partial deterrence of spiny lobsters from A. angulatus toxins. Cleaning off the mucus from the nemertean led them to be consumed. In addition, nemertelline (inactive in the other assays), together with anabaseine and 2,3′-bipyridyl, stimulated a stomatogastric muscle nicotinic receptor calcium channel of crayfish. Permanent cation derivatives of 2,3′-bipyridyl maintained activity on this receptor, a result that is in contrast to the case for CNS activity, supposedly due to the permanent charge hindering blood-brain passage. These results, taken altogether, suggested that the various pyridine compounds may have different activities and that their combined presence brings on a multimodal defense [39].
A later study showed that 2,3′-bipyridyl, anabaseine, nemertelline, and anabasine all inhibited barnacle larvae settlement. Analysis of effects from a series of analogues to the natural pyridyl alkaloids suggested that both of the nitrogen atoms of 2,3′-bipyridyl are important for action, as are their relative positions. Moreover, the protonation of these nitrogens inhibited the antifouling activity [41]. This work generated a patent covering application of these and related compounds as anti-fouling materials [42]. To our knowledge, these compounds have not been developed further for this purpose.
In the 1970’s, Kem observed that the toxicity of anabaseine was not exclusive to crayfish, and that it was equally potent as nicotine when injected into mice [36]. This prompted studies of its potential pharmacological activities. Meyer et al. demonstrated that anabaseine stimulated acetylcholine release from the rat brain cortex [43], and anabaseine activity was subsequently studied in comparison to a series of alkaloids, including nicotine and anabasine to vertebrate nicotinergic receptors [44]. It was shown that anabaseine selectively stimulates nicotinic receptors, in particular, α-7 and neuromuscular type receptors.
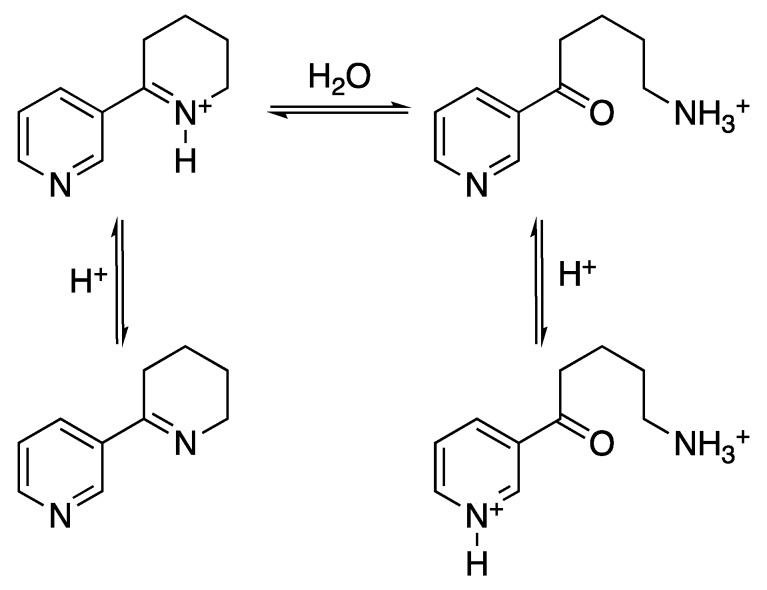
The comparative analyses provided an understanding of some structural aspects of anabaseine recognition. Moreover, a systematic study of the anabaseine solution equilibrium demonstrated how its molecular structure shifts with pH. At neutral pH, it exists in an equilibrium with an almost equal amount of protonated cyclic structure and the corresponding hydrolyzed product and minor amounts of the non-protonated cyclic species, the abundance of which increases with pH [45], Figure 6.
Figure 6.
Anabaseine equilibrium states. Lower pH shifts the equilibrium towards the hydrolyzed product, whereas the cyclic anabaseine is favoured by higher pH. Adapted from [45].
Taken together, this provided rationale in understanding the effects of a series of cinnamylidene and benzylidene derivative of anabasine, among them 3-(2,4-dimethoxybenzylidene)-anabaseine or DMXBA [46], also known as GTS-21 [47]. It is the anabaseine derivative that has come closest to medicinal application. DMXBA shows long-term potentiation via the selective binding to CNS α-7 nicotinic acetylcholine receptors [47]. This sparked interest by the similarity to effects of nicotine on memory-related behaviours, specifically Alzheimer’s disease [48]. DMBXA showed promising results in eyeblink classical conditioning (EBCC), a rabbit model to parallel aging in humans [49]. It was also shown to improve memory-related behaviour in Sprague–Dawley rats [50]. DMXBA in Alzheimer’s has been reviewed by Zawieja et al. [51], and Kem et al. reviewed studies on the use of anabaseine and DMXBA against memory loss and schizophrenia [46]. A comprehensive paper by Rangel and Falkenberg discussed the recent status of clinical trials with regard to compounds of marine origin and their derivatives in the pharmaceutical pipeline [52]. As of January 2019, eight studies regarding DMXBA are listed in ClinicalTrials.gov, but no outcome is reported.
An overview of nemertean species (and their place of origin), which have been analyzed with respect to anabaseine related compounds, is presented in Table 2.
Table 2.
Overview of nemertean species analyzed for anabaseine related compounds.
| Species | Origin | Toxin | Sample | Extraction and Analysis | Source |
|---|---|---|---|---|---|
| Class Hoplonemertea | Order Monostilifera | ||||
| Amphiporus angulatus | NH + ME shores, USA | Anabaseine | Whole body | TLC - alkal CHCl3 extr., DMAB deriv. | [5,17] |
| Amphiporus angulatus | NH + ME shores, USA | Anabaseine | Whole body | Chromatogr - alkal CHCl3 extr | [36] |
| Amphiporus angulatus | NH + ME shores, USA | Nemertelline | Whole body | Chromatogr - alkal CHCl3 extr | [36] |
| Amphiporus angulatus | NH + ME shores, USA | 2,3′-bipyridyl | Whole body | Chromatogr - alkal CHCl3 extr | [36] |
| Amphiporus angulatus | NH + ME shores, USA | 3-methyl-2,3′-bipyridyl | Whole body | Chromatogr - alkal CHCl3 extr | [36,40] |
| Amphiporus angulatus | Not stated | Bipyridyl toxins | Live animal | Not stated | [53] |
| Amphiporus angulatus | Eastport, MA, USA. | 2,3′-bipyridyl, nemertelline + 4 unidentified | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Amphiporus bimaculatus | San Juan Island, Washington, USA. | Unidentified pyridyl | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Amphiporus cruentatus | Woods hole, MA, USA. | 5 unidentified | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Amphiporus formidabilis | San Juan Island, Washington, USA. | 3 unidentified | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Amphiporus lactifloreus | Not stated | Low anabaseine activity | Whole body | TLC - alkal CHCl3 extr., DMAB deriv. | [17] |
| Amphiporus lactifloreus | Not stated | Low anabaseine activity | Whole body | TLC - alkal CHCl3 extr., DMAB deriv. | [5] |
| Amphiporus lactifloreus | Bangor, Wales, UK | Anabasine | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Amphiporus ochraceus | Not stated | None found | Whole body | TLC - alkal CHCl3 extr., DMAB deriv. | [5,17] |
| Amphiporus ochraceus | Woods hole, MA, USA | 5 unidentified | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Argonemertes dendyi | Tomales bay, CA, USA | 1 unidentified | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Carcinonemertes errans | Bodega bay, CA, USA | None found | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Emplectonema gracile | San Juan Island, WA, USA | Possibly 1 unidentified | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Emplectonema gracile | San Juan Island, WA, USA | 1 unidentified | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Emplectonema neesi | Bangor, Wales, UK | None found | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Fasciculonemertes arenicola | Los Molles, Chile | 5 unidentified | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Geonemertes pelaensis | Miami, FL, USA | 1 unidentified | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Malacobdella grossa | Not stated | None found | Whole body | TLC - alkal CHCl3 extr - DMAB deriv | [5,17] |
| Nipponnemertes pulchra a | Helsingør, Dk | 1 unidentified | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Paranemertes peregrina | San Juan Island, WA, USA | Anabaseine | Whole body | Al2O3 chrom-alkal CHCl3 extr. | [35] |
| Paranemertes peregrina | San Juan Island, WA, USA | Anabaseine | Body w.o. proboscis | MS + UV - DMAB deriv | [5,17] |
| Paranemertes peregrina | San Juan Island, WA, USA | Anabaseine | Anterior proboscis | MS + UV - DMAB deriv | [5,17] |
| Paranemertes peregrina | San Juan Island, WA, USA | Anabaseine | Median proboscis | MS + UV - DMAB deriv | [5,17] |
| Paranemertes peregrina | San Juan Island, WA, USA | Anabaseine | Posterior proboscis | MS + UV - DMAB deriv | [5,17] |
| Paranemertes peregrina | San Juan Island, WA, USA | Anabaseine | Periph part body wall | MS + UV - DMAB deriv | [17] |
| Paranemertes peregrina | San Juan Island, WA, USA | Anabaseine | Periph part body wall | MS + UV - DMAB deriv | [5] |
| Paranemertes peregrina | San Juan Island, WA, USA | Low anabaseine | Body core tissues | MS + UV - DMAB deriv | [17] |
| Paranemertes peregrina | San Juan Island, WA, USA | Low anabaseine | Body core tissues | MS + UV - DMAB deriv | [5] |
| Paranemertes peregrina | San Juan Island, WA, USA | Anabaseine | Whole body | TLC - alkal CHCl3 extr - DMAB deriv | [5,17] |
| Paranemertes peregrina | Bodega Bay, CA, USA | 2 unidentified | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Prosadenoporus californiensis b | Tomales Bay, CA, USA | 2 unidentified | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Prostoma graecense c | Not stated | Possibly anabaseine | Whole body | TLC - alkal CHCl3 extr - DMAB deriv | [5,17] |
| Tetrastemma candidum | Woods hole, CA, USA | 1 unidentified | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Tetrastemma reticulatum | San Juan Island, WA, USA | 1 unidentified | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Tetrastemma worki | Not stated | Anabaseine | Whole body | TLC - alkal CHCl3 extr - DMAB deriv | [5,17] |
| Zygonemertes virescens | Not stated | None found | Whole body | TLC - alkal CHCl3 extr - DMAB deriv | [5] |
| Zygonemertes virescens | Woods hole, MA, USA | Anabasine | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Class Palaeonemertea | |||||
| Carinoma sp. | Not stated | None found | Whole body | TLC - alkal CHCl3 extr., DMAB deriv. | [5] |
| Carinoma tremaphoros d | Woods hole, MA, USA. | Not reported | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Cephalothrix spiralis d,e | Not stated | None found | Whole body | TLC - alkal CHCl3 extr., DMAB deriv. | [5,17] |
| Class Pilidiophora | Order Heteronemertea | ||||
| Cerebratulus lacteus | Woods hole, MA, USA. | None found | Whole body | TLC - alkal CHCl3 extr., DMAB deriv. | [5,17] |
| Cerebratulus lacteus | Boston, MA, USA | None found | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Lineus ruber | Not stated | Anabaseine/Nemertine Below detection level |
Whole body | TLC - alkal CHCl3 extr., DMAB deriv. | [17] |
| Lineus ruber | NH + ME shores, USA | Anabaseine/Nemertine Below detection level |
Whole body | TLC - alkal CHCl3 extr., DMAB deriv. | [5] |
| Lineus sanguineus f | Not stated | Anabaseine/Nemertine Below detection level |
Whole body | TLC - alkal CHCl3 extr., DMAB deriv. | [5,17] |
| Lineus viridis | Not stated | Anabaseine/Nemertine | Whole body | TLC - alkal CHCl3 extr., DMAB deriv. | [17] |
| Lineus viridis | NH + ME shores, USA | Anabaseine/Nemertine | Whole body | TLC - alkal CHCl3 extr., DMAB deriv. | [5] |
| Lineus viridis | Woods hole, MA, USA | None found | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
| Micrura leidyi | Not stated | Anabaseine/Nemertine | Whole body | TLC - alkal CHCl3 extr - DMAB deriv | [17] |
| Micrura leidyi | Woods hole, MA, USA | None found | Whole body Frozen | TLC - alkal CHCl3 extr | [38] |
| Micrura leidyi | Not stated | Anabaseine/Nemertine Below detection level | Whole body | TLC - alkal CHCl3 extr - DMAB deriv | [5] |
| Parvicirrus dubius g | Not stated | Anabaseine/Nemertine Below detection level | Whole body | TLC - alkal CHCl3 extr - DMAB deriv | [5] |
| Siphonenteron bicolour h | Not stated | Anabaseine/Nemertine Below detection level | Whole body | TLC - alkal CHCl3 extr - DMAB deriv | [5] |
| Siphonenteron bicolour h | Woods hole, MA, USA | None found | Whole body Frozen | TLC - alkal/acid CHCl3 extr | [38] |
Abbreviations: Alkal—alkaline; Chrom—chromatography; DMAB deriv—p-dimethyl aminobenzylidene deriative; Extr—extraction; MS—mass spectrometry; TLC—thin layer chromatography; UV—ultraviolet spectroscopy. a In source referred to as Nipponemertes pulcher (synonymous). b In source referred to as Pantinonemertes californiensis (synonymous). c In source referred to as Prostoma rubrum (synonymous). d Used as negative control. e In source referred to as Procephalothrix spiralis (synonymous). f In source referred to as Lineus socialis (synonymous). g In source referred to as Lineus dubius (synonymous). h In source referred to as Lineus bicolor (synonymous). Species are denoted according to WoRMS, World Register of Marine Species as of 19-02-11 [54].
5.2. Tetrodotoxin (TTX)
TTX, as in Figure 7, is well known as the toxin in puffer fish [55], but it has been found in several other genera, including salamanders, frogs, octopi, flatworms, and crustaceans [56]. It acts via binding to the extracellular pore opening site 1 (i.e. the P-loop between domain V and VI) in voltage-gated sodium channels (VGSC). Thereby, it blocks Na+ conduction and causes its strong paralytic effect [57]. TTX has been a key tool for the characterization of ion channels and for fundamental studies in neurology as a selective blocker [58], and it is still widely used as a pharmacological tool. In addition, it is the subject of several clinical trials for its potential use in pain relief [59,60]. The biosynthesis of TTX is not clear, but bacterial and/or symbiotic routes have been suggested [61]. However, there are no methods to produce TTX in sustained cultures [62] and TTX production is not feasible for commercial synthesis [56]. TTX production therefore relies on purification from pufferfish; 1–2 g of TTX is considered to be a good yield from 100 kg of puffer fish ovaries [63].
Figure 7.
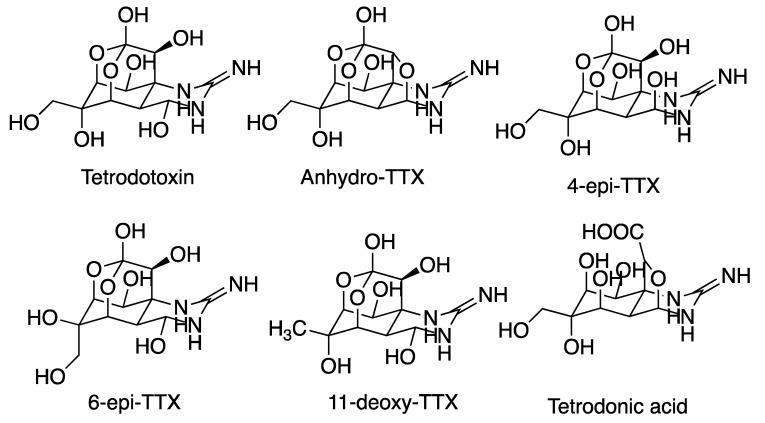
Tetrodotoxin (TTX) and a selection of active analogs identified in nemertans.
Miyazawa et al. were the first to report the occurrence of TTX in ribbon worms [64], namely in Lineus fuscoviridis and Tubulanus punctatus that were collected from intertidal zones in the south of Japan. TLC showed the presence of TTX, which was confirmed by HPLC, GC-MS, and lethal effects on mice measured in mouse units (MU). One (1) MU corresponds to the death of one mouse within 30 min. Out of 56 sampled individuals (both species), 32 were found to contain toxin in the range 10–500 MU/g live nemertean.
The same group showed that Cephalotrix linearis was about tenfold more active [65]. Toxicity was again ascribed to TTX, and the compounds were suggested to be localized to the proboscis, as this exhibited about twice the lethal potency (MU/g) as the rest of the body. The potency of mucus was about ¼ of that of the proboscis. In retrospect, other compounds may have contributed to these results. A concurrent study suggested that one unknown such toxin, called “tetrodonic acid-like substance” was a likely precursor to TTX, although no structures were characterized [66]. A few years later, Asawaka et al. conducted surveillance work by the Hiroshima Bay oyster farms for sources to paralytic shellfish poisoning (PSP). They found that “himomushi” (Cephalotrix sp.), clinging on to oyster shells, showed paralytic toxicity that could be attributed to TTX and its derivatives [67], as identified by HPLC and GC-MS. Although the toxic content substantially varied among the worms that were collected, the potency was noteworthy, the most active sample exhibiting 14,734 MU/g. This prompted an extensive investigation into the toxic nature of the himomushi toxin. LC-MS and NMR evidence conclusively demonstrated that the observed activity was due to TTX [68].
A more recent paper detailed toxicological surveillance work carried out between 1998–2005 at several Japanese sites. Out of 764 specimens of Cephalothrix simula that were collected, approximately 80% exhibited strong toxicity (≥1000 MU/g), which was ascribed to TTX and the derivatives 4-epi-TTX and 4,9-anhydro-TTX [69]. This suggests that TTX is a common constituent of C. simula, at least in these waters. In addition, HPLC and GC data indicated that TTX was also present in other species, e.g., Lineus torquatus, L. alborostratus, and Nipponemertes punctatula.
TTX does not appear to be synthesized by the nemerteans themselves, so how does this toxin emerge in these worms? The possibility of bioaccumulation, as has been observed e.g., in Fugu from eating TTX-containing flatworms [70], is one possibility. However, a common hypothesis suggests that the presence of TTX is due to production from commensal bacteria. Vibrio alginolyticus bacteria was provisionally identified in the intestinal contents as a plausible source of TTX. Previous work by Carroll et al. [71,72] had elaborated on the idea that a symbiotic relationship between Vibrio bacteria and nemerteans might be the origin of TTX. V. alginolyticus was isolated from the epidermal mucus and whole body extracts of a selection of nemertean species that were collected outside of North Wales. The extracts and bacteria cultures that were grown in the presence of the nemertea samples were then subjected to ultraviolet UV spectroscopy, from which it was concluded that TTX was present in several of these samples. At a closer look, this data is inconclusive at best. Neither did HPLC analyses, where the samples were compared to puffer fish TTX controls, provide solid evidence for the presence of TTX, because the retention time of the “TTX” differed between the samples.
Nevertheless, it inspired an idea to use V. alginolyticus cultures that were grown with mucus from L. longissimus as a continuous production system for TTX [73]. Although toxicity was evident, as assayed using Carcinus maenas (green crab), an in-depth analysis of this system was unable to identify any traces of TTX. Moreover, the toxic action was found to originate from the mucus, and it was unrelated to the presence of V. alginolyticus [25]. This work highlights the difficulty of conclusively demonstrating the presence of the complex TTX molecule, and it is not the first time that doubts have been raised. In two papers, [74,75], Matsumura challenged claims of TTX production in Vibrio sp. cultures. Two standard methods were employed: HPLC-UV and GC-MS. High “TTX” peaks were found by both techniques, but these peaks were also found in the polypeptone and yeast extracts that were used for cultivation. It is obvious that the sole use of HPLC-UV may generate false TTX positives. In addition, the common GC-MS method is based on harsh alkali hydrolysis, leading to a C9 base compound, which is common to a group of related compounds.
The need for caution can further be stressed as judged by a study by Salvitti et al. [76]. They reported the analysis of 102 bacterial strains that were isolated from the marine slug Pleurobrancheaea maculata and the marine flatworm Stylochoplana sp., whereby both have previously been shown to contain TTX [77], without finding any evidence for TTX in these cultures [76]. A literature survey in that paper of 25 reports on bacterial TTX production showed that they relied on indirect evidence in all cases but one, in which HPLC-MS data was included. However, there is also support for the hypothesis that bacteria produce TTX with or in conjunction with nemerteans. Beleneva et al. isolated bacterial strains from Cephalothrix simula and then identified TTX producing cultures using polyclonal rabbit TTX-antibodies, Alexa 488 secondary antibodies, and confocal microscopy [78]. A positive result was found for Bacillus sp. 1839. Transmission-electron microscopy was then used to localize antibody binding, both to immature forespores and to mature spores of the bacteria [79]. Lately, the screening of total bacterial cultures that were isolated from a number of nemertea species indicated TTX-positive cells originating from Hubrechtiella juliae and Lineus alborostratus [80]. A previous study had used anti-TTX monoclonal antibodies to identify TTX at several epithelial and intestinal sites in Cephalotrix sp. [81], and recently Lineus alborostratus was subjected to a similar analysis, generating detailed pictures, which indicated that TTX was primarily located to the cutis layer, and a hypothesis for its intracellular delivery was also proposed [82]. A 2018 study by Vlasenko et al. [83] provided HPLC-MS/MS evidence regarding the presence of seven different TTX analogues in extracts from Cephalotrix simula and three in Kulikovia manchenkoi. However, the presence of TTX itself was not observed. In another study, Kwon et al. combined MALDI-MS with cytotoxicity assays on HPLC fractions from Yininemertes pratensis, which were collected in the Han River estuary in South Korea. Several masses corresponding to known TTX derivatives were found, as was cytotoxicity in certain fractions. However, correlation between the two was poor, again raising the question of the origin of activity [84].
To conclude, the evidence that TTX is present in a range of nemertean species is accumulating, especially for Cephalotrix sp. that were collected in Japanese/Russian waters, but many observations rely on the selectivity of TTX antibodies. It is not clear to the authors of this review that the risk of cross reactions can be completely excluded. Nearly all of the examples where nemertean worms have been shown unequivocally to contain TTX originate from the Sea of Japan or its vicinity, although a recent report of Cephalothrix simula caught in Cornwall, UK, demonstrated TTX and TTX derivative content analyzed by HPLC-MS/MS [85]. Although originating from the Pacific, this species appears to be establishing itself in Europe [86], which is why this first observation in the UK was not totally unexpected.
The apparent geographical concentration of TTX bearing nemerteans could of course be a function of limited search efforts elsewhere, but it does raise questions regarding the general role of TTX versus other known toxins, such as peptides, anabaseine, and other pyridyl compounds. An overview of nemertean species that were analyzed with respect to TTX related compounds is presented in Table 3.
Table 3.
Nemertean species analyzed for TTX content.
| Species | Origin | Toxin/-s | Sample | Extraction and Analysis | Ref |
|---|---|---|---|---|---|
| Class Hoplonemertea | Order Monostilifera | ||||
| Amphiporus lactifloreus | Llandudno, Wales, UK, or Rhosneigr, Wales, UK | TTX-like cpds | Acidic whole body extract | UV spectroscopy and HPLC | [72] |
| Amphiporus sp. | Akkeshi Bay, Hokkaido, Jpn | TTX + analogs | Acidic whole body extract | Defatted, charcoal purif, HPLC and GC-MS-C9 base | [69] |
| Nipponnemertes bimaculata a | Peter the Great Bay, Rus/Jpn | Very low TTX | Acidic MeOH extract | HPLC-MS/MS | [83] |
| Malacobdella japonica | Akkeshi Bay, Hokkaido, Jpn | TTX + analogs Anhydro-TTX |
Acidic whole body extract | Defatted, charcoal purif, HPLC, GC-MS-C9 base | [69] |
| Malacobdella grossa | Peter the Great Bay, Rus/Jpn | Antimicrobial activity. No TTX. | Bacteria - whole body homogenate | Confocal laser microscopy after TTX antibody labeling | [80] |
| Nemertellina yamaokai | Akkeshi Bay, Hokkaido, Jpn | None found | Acidic whole body extract | Defatted, charcoal purif, HPLC, GC-MS-C9 base | [69] |
| Nipponnemertes punctatula | Otsuchi Bay, Iwate, Jpn | TTX, epi-, anhydro-TTX | Acidic whole body extract | Defatted, charcoal purif, HPLC, GC-MS-C9 base | [69] |
| Paranemertes sp. | Peter the Great Bay, Rus/Jpn | None found | Acidic methanol extract | HPLC-MS/MS | [83] |
| Quasitetrastemma nigrifrons b | Akkeshi Bay, Hokkaido, Jpn | None found | Acidic whole body extract | Defatted, charcoal purif, HPLC, GC-MS-C9 base | [69] |
| Quasitetrastemma stimpsoni c | Akkeshi Bay, Hokkaido, Jpn | (TTX and analogues) Not analyzed |
Acidic whole body extract | Defatted, charcoal purif, HPLC, GC-MS-C9 base | [69] |
| Quasitetrastemma stimpsoni c | Peter the Great Bay, Rus/Jpn | Bact. cultures Antimicrob activity. TTX. | Bacteria from whole body homogenate | Confocal laser microscopy after TTX antibody labeling | [80] |
| Quasitetrastemma stimpsoni | Peter the Great Bay, Rus/Jpn | Very low TTX | Acidic methanol extract | HPLC-MS/MS | [83] |
| Class Palaeonemertea | |||||
| Cephalothrix linearis | Shimoda, Shizuoka, Jpn | TDA-like, TTX, anhydro-, epi-TTX | Proboscis, body | SEC, TLC, el.phoresis, HPLC, GC-C9 base | [65] |
| Cephalothrix linearis | Shimoda, Shizuoka, Jpn | TDA-like | Handling stimulus secretion | SEC, TLC, el.phoresis, HPLC, GC-C9 base | [65,66] |
| Cephalothrix rufifrons | Llandudno, Wales, UK, or Rhosneigr, Wales, UK | TTX-like cpds | Acidic whole body extract | UV spectroscopy and HPLC | [72] |
| Cephalotrix rufifrons d | Cornwall, UK | None in extract, but TTX in bacteria isolate | Acidic whole body extract and bacteria isolates | HPLC-MS/MS of extract and isolates | [85] |
| Cephalothrix simula | Hiroshima Bay, Jpn | TTX, epi-, anhydro-TTX | Acidic whole body extract | Defatted, charcoal purif, HPLC and GC-MS-C9 base | [69] |
| Cephalothrix simula | Akkeshi Bay, Hokkaido, Jpn | TTX, epi-, anhydro-TTX | Acidic whole body extract | Defatted, charcoal purif, HPLC and GC-MS-C9 base | [69] |
| Cephalothrix simula | Otsuchi Bay, Iwate, Jpn | TTX, epi-, anhydro-TTX | Acidic whole body extract | Defatted, charcoal purif, HPLC and GC-MS- C9 base | [69] |
| Cephalothrix simula e | Peter the Great Bay, Rus/Jpn | TTX-Bacillus sp. | Bacteria isolates | Immunovisualization | [78,79] |
| Cephalothrix simula | Peter the Great Bay, Rus/Jpn | 7 TTX derivatives | Acidic MeOH extract | HPLC-MS/MS | [83] |
| Cephalotrix simula | Cornwall, UK | TTX and derivatives | Acidic whole body extract and bacteria isolates | HPLC-MS/MS of extract and isolates | [85] |
| Cephalothrix sp. | Hiroshima Bay, Jpn | TTX, epi-, anhydro-TTX | Acidic whole body extract | Defatted, SEC, IEC. TLC, HPLC, GCMS-C9 base | [67] |
| Cephalothrix sp. | Hiroshima Bay, Jpn | TTX, epi-, anhydro-TTX | Whole body, frozen | Activated charcoal, SEC, IEC, cryst from acidic CH3OH soln, GCMS-C9 base, NMR + MS | [68] |
| Cephalothrix sp. | Hiroshima Bay, Jpn | TTX | Whole body, cross-section | Anti-TTX antibodies | [81] |
| Tubulanus annulatus | Cornwall, UK | None found | Acidic whole body extract and bacteria isolates | HPLC-MS/MS of extract and isolates | [85] |
| Tubulanus polymorphus f | Not stated | TTX | Not stated | Immunostaining | [87] |
| Tubulanus punctatus | Seto Inland sea Hiroshima, Jpn | Anhydro-TTX | Whole body | Defatted, charcoal purif, HPLC, GC-MS-C9 base | [64] |
| Tubulanus punctatus | Peter the Great Bay, Rus/Jpn | Very low TTX | Acidic MeOH extract | HPLC-MS/MS | [83] |
| Class Pilidiophora | Order Heteronemertea | ||||
| Cerebratulus marginatus | Peter the Great Bay, Rus/Jpn | None found | Acidic MeOH extract | HPLC-MS/MS | [83] |
| Dushia atra f | Not stated | TTX | Not stated | Immunostaining | [87,88] |
| Kulikovia alborostrata | Peter the Great Bay, Rus/Jpn | Very low TTX | Acidic MeOH extract | HPLC-MS/MS | [83] |
| Kulikovia manchenkoi | Peter the Great Bay, Rus/Jpn | TTX + 3 deriv | Acidic MeOH extract | HPLC-MS/MS | [83] |
| Lineus alborostratus | Akkeshi Bay, Hokkaido, Jpn | TTX, anhydro-, epi-TTX | Acidic whole body extract | Defatted, charcoal purif, HPLC, GC-MS-C9 base | [69] |
| Lineus alborostratus | Peter the Great Bay, Rus/Jpn | TTX | Whole body | Immunostaining | [82] |
| Lineus alborostratus | Peter the Great Bay, Rus/Jpn | Bact cultured for TTX. Antimicrob activity. | Bacteria from whole body homogenate | Confocal laser microscopy after TTX antibody labeling | [80] |
| Lineus bilineatus | Akkeshi Bay, Hokkaido, Jpn | None found | Acidic whole body extract | Defatted, charcoal purif, HPLC, GC-MS-C9 base | [69] |
| Lineus fuscoviridis | Seto Inland sea, Hiroshima, Jpn | TTX, anhydro-TTX | Whole body | Defatted, SEC, IEC. TLC, HPLC., GC-MS-C9 base | [64] |
| Lineus longissimus | Llandudno, Wales, UK, or Rhosneigr, Wales, UK | TTX-like cpds | Acidic whole body extract and mucus | UV spectroscopy and HPLC | [72] |
| Lineus longissimus | Koster Fiord, Swe, and Millport, Scot, UK | <5 kDa cpd | Mucus + Vibrio cultures | Various purification methods | [25] |
| Lineus ruber | Llandudno, Wales, UK, or Rhosneigr, Wales, UK | TTX-like cpds | Acidic whole body extract | UV spectroscopy and HPLC | [72] |
| Lineus sanguineus g | Llandudno, Wales, UK, or Rhosneigr, Wales, UK | TTX-like cpds | Acidic whole body extract | UV spectroscopy and HPLC | [72] |
| Lineus torquatus | Akkeshi Bay, Hokkaido, Jpn | TTX, anhydro-, epi-TTX | Acidic whole body extract | Defatted, charcoal purif, HPLC, GC-MS-C9 base | [69] |
| Lineus viridis | Llandudno, Wales, UK, or Rhosneigr, Wales, UK | TTX-like cpds | Acidic whole body extract | UV spectroscopy and HPLC | [72] |
| Micrura akkeshiensis | Akkeshi Bay, Hokkaido, Jpn | None found | Acidic whole body extract | Defatted, charcoal purif, HPLC, GC-MS-C9 base | [69] |
| Micrura bella | Peter the Great Bay, Rus/Jpn | None found | Acidic MeOH extract | HPLC-MS/MS | [83] |
| Micrura verrilli f | Not stated | TTX | Not stated | Immunostaining | [87,88] |
| Nipponomicrura uchidai | Peter the Great Bay, Rus/Jpn | None found | Acidic methanol extract | HPLC-MS/MS | [83] |
| Riseriellus occultus | Llandudno, Wales, UK, or Rhosneigr, Wales, UK | TTX-like cpds | Acidic whole body extract | UV spectroscopy and HPLC | [72] |
| Yininemertes pratensis | Haengjunaru, Han river Estuary, South Korea | TTX + analogs, derivatives. Tox cpd mass 291.1 | EtOH extract | Dry homogenate lysis in pure EtOH, hydrophobic HPLC, MALDI-TOF | [84] |
| Class Pilidiophora | Genus Hubrechtella | ||||
| Hubrechtella juliae | Peter the Great Bay, Rus/Jpn | Bact cultured for TTX. Antimicrob activity. | Bacteria from whole body homogenate | Confocal laser microscopy after TTX antibody labeling | [80] |
Abbreviations: Cpd—compound; Cryst—crystallization; Deriv—derivatization/derivative; El.phoresis—electrophoresis; EtOH; ethanol; GC-MS-C9 base—gas chromatography—mass spectrometry of TTX C9 base derivative; IEC—ion-exchange chromatography; MeOH—methanol; Purif —purification; SEC—size exclusion chromatography (gel filtration); TDA—tetrodonic acid. a In source referred to as Collarenemertes bimaculata. b In source referred to as Tetrastemma nigrifrons (synonymous). c In source referred to as Tetrastemma stimpsoni (synonymous). d In source referred to as Cephalothrix rubifrons. e Host organism for bacteria claimed to contain TTX. f Conference abstract only. g In source referred to as Ramphogordius sanguineus (synonymous). Species are denoted according to WoRMS, World Register of Marine Species as of 19-02-11 [54].
5.3. Peptide Toxins
5.3.1. Cerebratulus Toxins
B-neurotoxins
Some advances into the nature of Bacq’s nemertine were reported already in the early 1970’s [5,17]. This will be touched upon in Section 5.3.3. However, to shed further light on the identity and the character of nemertine, Kem turned to Cerebratulus lacteus, extracting mucus from 160 specimens, which were then subjected to several rounds of purification [89]. Two fractions that were obtained by size exclusion chromatography stood out with regard to their toxic activity, the first containing several compounds of of approximately 11 kDa. These were designated Cerebratulus A-toxins. The A-toxin fraction was toxic to mice as well as crayfish, but its toxicity appeared to be more gradual and paralysis was less severe than in the case of a second fraction, containing smaller proteins, designated Cerebratulus B-toxins. The B-toxins were highly toxic to crayfish, leading to convulsions, persistent paralysis, and death, but they affected neither cockroach nor mice. Several steps of CM-cellulose gradient purification led to the identification of four main proteins: B-I to B-IV. It was hypothesized that the four proteins are homologous, with molecular weights of approximately 5.4 kD (B-I) and 5.9 kD (B-II to IV), containing six (B-I) or eight (B-II to IV) cysteines. Although B-II appeared to be the most toxic compound, when assayed against Procambarus clarkii, more focus was placed on B-IV, on account of availability (B-I: 7.0 mg; B-II: 6.0 mg; B-III: 3.9 mg; B-IV: 101.0 mg). The full primary sequence of B-IV was resolved the same year by Blumenthal and Kem [90] (later revised, [91]). Subsequently, the primary sequence of B-II was reported, demonstrating a high degree of homology between the two peptides (41 out of 55 amino acids were identical); both contained a hydroxyproline at position 10 and four disulfides [91]. In parallel, the structural features of relevance for B-IV activity were identified. It was shown that the nitration of tyrosine-9 almost completely abolished the toxic activity on P. clarkii without inducing any major disruptions to the secondary structure [92], and via reaction with 2-hydroxy-5-nitrobenzyl bromide (HNB), it was shown that Trp-30 was similarly crucial for activity, whereas Trp-5 was apparently not [93]. Reduction of the disulfides generated a structure that was devoid of P. clarkii activity, whereas renaturation restored activity [94].
In attempts to identify the target receptor, Toth and Blumenthal used sucrose gradient centrifugation to purify membrane fractions of lobster (Homarus americus) nervous tissue [95]. The axonal vesicles that were obtained were found to bind B-IV with a single class of binding sites at a Kd of 5–20 nM, and a binding capacity of 6–9 pmol/mg membrane protein. The pharmacological activity of B-IV is consistent with the effects on ion channels in the nerve membrane, and from competition experiments with other toxins and studies of Na+ flux, it was suggested that action was most likely due to effects on voltage-gated Na+ channels or K+ channels. In a follow-up study, a pull-down strategy was attempted, where B-IV was conjugated with 125I-azidosalicylic acid (ASA), followed by the incubation of this derivative with lobster axonal and muscular vesicles, respectively. The derivative was thereby linked photochemically to the receptor. After subsequent SDS-electrophoresis, major bands could be identified for both preparations, which, after correction (B-IV, 125I-ASA masses subtracted), gave the molecular masses 40 and 38 kDa, matching those of ß1 and ß2 subunits of mammalian Na-channels. Accordingly, the authors tentatively suggested that binding occurred at these components of the Na+ channel in lobster nerves [96].
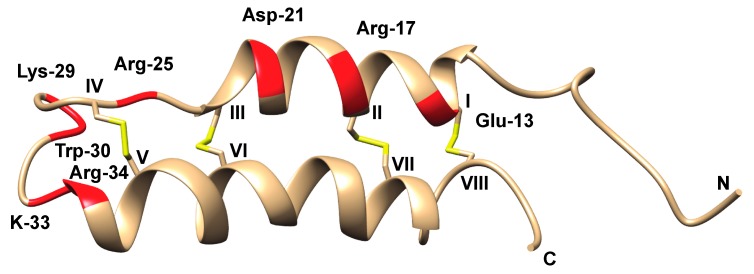
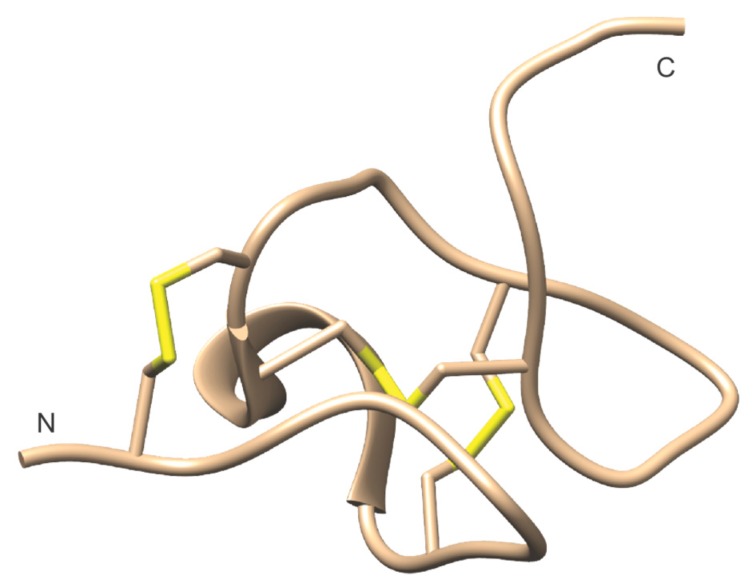
Detailed investigations of the three-dimensional (3D) structure of B-IV have been carried out. Using circular dichroism (CD) and Raman spectroscopy, Kem et al. showed that B-IV had a high α-helix content (49–78%), but no detectable ß-sheets [97]. This character was subsequently confirmed by 1H-NMR, showing the presence of two α-helices, incorporating residues 13–26 and 33–49, and a helix-like structure was also seen in the five C-terminal residues, whereas the N-terminus appeared to be unordered [98]. In 1997, Barnham et al. demonstrated the well-defined helical hairpin structure that was obtained in solution via 600 MHz 1H-NMR, Figure 8 [99].
Figure 8.
B-IV NMR structure (RCSB id: 1VIB) [99] with residues important for activity marked in red, Cys residues in roman numerals, and disulfide bonds in yellow.
Recombinant production was first reported by Howell and Blumenthal in 1989 [100]. A synthetic B-IV gene was introduced via a plasmid in E. coli and produced as a fusion protein either with ß-galactosidase or the gene 9 protein of bacteriophage T7, which was subsequently cleaved off. The peptide folded as natural B-IV, differing only by virtue of an extra methionine at the N-terminus and the replacement of Hyp-10 for Pro, with specific toxicity of 35-40% to that of the naturally occurring form. The extra codon for Met was deleted in the following study [101], and the resultant peptide was shown to be fully active. Using the recombinant approach, a series of mutants of the Met-B-IV was produced, where N-terminal alanines (3 and 8) were replaced by serines or glycines. Interestingly, the double-Ser mutant was about two-fold more active than its parental form, whereas the double-Gly mutant was slightly less active. No effects on folding were observed from these mutations, as analyzed by CD. In two follow-up papers [102,103], a series of recombinant mutants were compared. Table 4 summarizes the outcomes of these mutations, as well as those from earlier studies.
Table 4.
Summary of neurotoxin B-IV mutant analyses.
| Residue | AA | Modification | Effect | Source |
|---|---|---|---|---|
| 1 | Ala | Extra Met-1 | 35-40% of native | [100] |
| 3 | Ala | Ser (+8 Ser) | x2 activity | [101] |
| Gly (+8 Gly) | Slightly less active | [101] | ||
| 5 | Trp | HNB a w Trp 30, 1 eq. | Slightly less active | [92] |
| HNB w Trp 30, 2 eq. | Inactive | [93] | ||
| 8 | Ala | Ser (+8 Ser) | x2 activity | [101] |
| Gly (+8 Gly) | Slightly less active | [101] | ||
| 9 | Tyr | Nitration | Inactive | [92] |
| 10 | Hyp | Pro | Active | [101] |
| 12 | Cys | Reduction (all Cys) | Inactive | [94] |
| 13 | Glu | Gly | Active | [102] |
| Ala | Active | [102] | ||
| Gln | Active | [102] | ||
| 16 | Cys | Reduction (all Cys) | Inactive | [94] |
| 17 | Arg | Gln | Inactive | [102] |
| Ala | Inactive | [102] | ||
| Lys | Inactive | [102] | ||
| 18 | Lys | Gln | Active | [103] |
| Gln + Gln 19 | Slightly less active | [103] | ||
| 19 | Lys | Gln + Gln 18 | Slightly less active | [103] |
| 21 | Asp | Ala | Active | [102] |
| Asn | Active | [102] | ||
| Pro | 75% helix reduction. 10-fold reduction in activity | [102] | ||
| 22 | Leu | Asp | Active | [103] |
| 23 | Cys | Reduction (all Cys) | Inactive | [94] |
| 25 | Arg | Gln | 400-fold reduction | [102] |
| Lys | Slightly less active | [102] | ||
| 26 | Cys | Reduction (all Cys) | Inactive | [94] |
| 29 | Lys | Asn | Active | [103] |
| 30 | Trp | HNB | Inactive | [93] |
| Ser | 40-fold reduction | [103] | ||
| Tyr | Active | [103] | ||
| Phe | Active | [103] | ||
| HNB with Trp 5, 1 eq. | Slightly less active | [93] | ||
| HNB with Trp 5, 2 eq. | Inactive | [93] | ||
| 33 | Lys | Asn | Active | [103] |
| 34 | Arg | Gln | 20-fold reduction. Structure destabilized. | [102] |
| Ala | 80-fold reduction | [102] | ||
| 37 | Cys | Reduction (all Cys) | Inactive | [94] |
| 41 | Cys | Reduction (all Cys) | Inactive | [94] |
| 48 | Cys | Reduction (all Cys) | Inactive | [94] |
| 52 | Cys | Reduction (all Cys) | Inactive | [94] |
| 53 | Lys | Truncation | Active | [102] |
| 54 | Lys | Truncation | Active | [102] |
| 55 | Glu | Truncation | Active | [102] |
a HNB: 2-hydroxy-5-nitrobenzyl bromide.
The extensive study of the B-IV neurotoxin by Blumenthal and co-workers has provided a detailed understanding of structural features that are crucial for activity. Arg-17 is a key amino acid for activity, lost upon replacement by either Gln, Ala, or Lys. Likewise, when Arg-25 is replaced by Gln, activity is lost, whereas replacement by Lys does not alter activity. At position 30, Trp can be replaced by other aromatic amino acids without the loss of activity, whereas replacement by Ser leads to a 40-fold reduction. A number of other positively charged amino acids are of importance for activity, albeit less so than those mentioned above [102], Figure 8.
Cerebratulus A-Toxins
The larger (11 kDa) Cerebratulus A-toxins have also been rigorously studied, and progress has been thoroughly reviewed [26]. Briefly, initial experiments [89] were carried out on a size exclusion chromatography (SEC) fraction rather than on purified toxin, but already at this stage, it was clear that the A-toxins exhibited a cell-lytic mode of action. In a following study, partial (A-I) and full purification (A-II to A-IV) was achieved, and the peptides were subjected to both structure and activity studies [104]. The N-termini of the latter three were sequenced by Edman degradation, showing chemical homology (seven positions of 12 were identical) and amino acid analysis revealed very high Lys content, and the presence of three (A-II, A-III) or four (A-IV) disulfides. In one study, A-IV was shown to inhibit phospholipid-sensitive Ca2+-dependent protein kinases [105], but, beyond this, most of the subsequent effort has focused on A-III: its full primary sequence was determined [106], as was its disulfide connectivity [107]. A high α-helical content was suggested from these studies, and Dumont and Blumenthal studied this peptide using CD [108], indicating that A-III contained 37% α-helix and 14% ß-sheet structure. An enzymatic cleavage product (1–86) exhibited four-fold lower cytolytic effect as compared to the native peptide, suggesting that the proposed helical sequence (63–95 was suggested to consist of an amphipathic helix) of the C-terminal is important for the lytic activity. This activity was known already from the first paper while using purified toxin [104], where a number of assays were carried out, the most striking result of which being the hemolytic activity on human erythrocytes (A-III: HC50 0.3 μmol/L). LD50 measurements of A-II–A-IV were performed on crayfish and fiddler crab (values ranging between 0.1–0.5 mg/kg) and mouse (1.5–2.8 mg/kg). In addition, paralytic and hemolytic activity studies on extracts from different body parts indicated that the A-toxins are mainly present in the nemertean integument. In another study, the cardiac effects of A-III toxin were investigated on canine cardiac Purkinje fibers [109]. Brief exposure of sublytic concentrations led to reversible membrane depolarization, but the addition ofCa2+ was found to reduce this effect. Higher concentrations (>2 mg/L) led to irreversible cell damage.
As for the underlying explanation for cell lysis, a number of studies were carried out. A-III-liposome experiments indicated that the N-terminal end plays an important role for the insertion of the peptide into the membrane [110]. This was followed through with release studies on DOPC-containing liposomes showing an A-III concentration dependent release of small molecular markers [111]. It was shown that tetrameric concanavalin-A leaked out from the liposome following the addition of A-III, indicating that the lesion produced exceeded 90 Å, corresponding to the rotational diameter of Con-A. Blumenthal and co-workers investigated the mechanism of A-III hemolysis in a series of papers [112,113,114,115], which showed that A-III forms tetramers at the membrane, a formation that is promoted by oleic acid binding at specific sites of the peptides. High concentrations of mono- and divalent ions were seen to inhibit hemolysis, by analogy to the Posner and Kem study [109], proposedly due to the inhibition of A-III self-association following membrane binding.
5.3.2. Parborlasia Toxins
In 1991, Heine et al. reported a multi-faceted investigation on Parborlasia corrugatus, a top predator and scavenger in the Antarctic fauna [116]. They demonstrated cytotoxicity of whole body extracts (aqueous, 3%), which proved to be lethal to spermatozoa of the Antarctic sea urchin Sterechinus neumayeri. Moreover, feeding experiments demonstrated significant deterrence both for the Antarctic cod (Dissosticus mawsoni) and for the benthic fish Trematomus bernacchi. The identity or type of toxins involved was not investigated further, although a later review stated that the worm harboured a “potent toxic neuropeptide” [117], which is an early hint of ongoing work where the freeze-dried mucus from P. corrugatus was also analyzed by chemical means. This work [118] involved the size exclusion of resuspended lyophilized mucus, followed by HPLC fractionation on a hydroxyapatite column. Hemolytic fractions were further chromagraphed on C18 and C4 using MeCN/H2O gradients. One fraction displayed hemolytic activity towards erythrocytes, and it was subject to electrophoretic analysis, and subsequent MALDI-TOF analysis revealed the presence of two ions, one at 10,324 Da and a less prominent at 10,097 Da. N-terminal Edman sequencing provided a partial N-terminal sequence, which also suggested the presence of isotoxins, but the authors were unable to separate these compounds. Sequence alignment of the first 25 amino acids of the protein, named parborlysin, to the C. lacteaus A-III cytolysin demonstrated 70% homology. The pI, 9.1, also pointed to a close relationship between these proteins. However, some differences were evident; (i) erythrocyte lysis via Cerebratulus A-toxins is Ca2+ sensitive, but parborlysin was adversely affected by Ca2+; (ii) the identities and concentrations of the added membrane lipids required the inhibition of A-III and paborlysin lysis, respectively, varied substantially. Twelve years later, a full length parborlysin sequence was reported by Butala et al. [119]. Furthermore, by PCR amplification and sequencing, six additional parborlysins were identified, whereby all but one contained six cysteines, suggesting three disulfides in the structure. The peptides were all in the range 9400–10,100 Da, with a calculated pI in the interval 10–10.4. A putative structure was modelled ab initio from the sequence, suggesting a globular peptide with secondary structure that solely consists of alpha-helices. Parborlysin sequences were also cloned into expression vectors that were introduced into E. coli and then subsequently expressed. The process was complicated by the inherent toxicity of parborlysin, and although it was shown that the construct had indeed been produced in one case, no hemolytic activity could be shown. This suggested that the protein was not properly folded, or that the N-terminal peptide requires a free N-terminal end for activity, which was blocked by a His-tag.
5.3.3. Lineus Toxins
In a 1971 paper, Kem reported the screening of nemertea species for anabaseine activity [17]. Among the 14 species thatwere investigated, three stood out—Lineus ruber, L. sanguineus, and L. viridis, exhibiting crayfish paralytic activity that was far beyond what could be explained by anabaseine content. The Lineus toxin was described as a polypeptide neurotoxin, corresponding to Bacq’s “nemertine” [32,33]. Size exclusion experiments on these polypeptides indicated a molecular mass of approximately 3500 Da [5]. For L. ruber mucus extracts, it was shown that at least two separate polypeptide toxins were present. These were shown to cause spike activity in isolated lobster (Homarus americanus) preparations, whereas no effect was evident in a frog assay or in the hemolysis of human erythrocytes. The lack of material hampered further efforts into the nature and identity of these peptides.
More recently, Lineus nemerteans were explored as a production system for TTX [73]. No TTX could be identified, although the mucus was shown to be highly toxic to crustaceans [25]. Instead, the size exclusion of mucus from L. longissimus and subsequent UPLC-MS analysis led to the discovery of one peptide (named nemertide ß-1) closely resembling neurotoxin B-IV, and importantly, two peptides of lower mass (3308 and 3360, respectively) [120]. Although the relationship is not conclusive, these α-nemertides, as they were called, bear resemblance both in the size and toxicity to Bacq’s nemertine, as well as the Lineus peptides that were discovered by Kem. MALDI imaging, which was carried out on cross-sections of the nemertean, showed that the presence of all three peptides was focused to its epithelial/mucus layer. For further evaluation, initial focus was placed on the m/z 3308 peptide, designated nemertide α-1. The α-1 fraction was highly toxic when injected into Carcinus maenas, and the peptide was subsequently sequenced, synthesized, and its solution structure dwas etermined by 2D-NMR, Figure 9. α-1 has 31 residues containing two hydroxyprolines, and three disulfides forming an inhibitor cysteine knot motif in the peptide core. Sequence loops between cysteines are surface-exposed, and whereas the N-terminus is stabilized by a disulfide (Cys2–Cys16), the C-terminus appears to be flexible. The only segment of the regular secondary structure is a short α-helix in the loop between Cys-9 and Cys-15, and a series of tight turns. Three positions (Phe-8, Trp-22, Phe-24) make up a hydrophobic patch. The α-2 peptide differs only by a Val substitution of Phe-8.
Figure 9.
NMR structure of nemertide alpha-1 (RCSB id: 6ENA) [120].
α-1 was shown to be extremely potent. Injections into green crabs (Carcinus maenas) led to paralysis and death in sub nmol/kg doses, and comparable results were given by an in vivo assay on orange-spotted cockroach (Blaptica dubia). Voltage-clamp experiments using heterologously expressed voltage-gated sodium channels (VGSC) showed an EC50 value that was in the low nanomolar range for the German cockroach (Blattella germanica), and the activity was found to be selective for invertebrates (activity was also demonstrated on Drosophila melanogaster and Varroa destructor), as the corresponding experiment on mammalian VGSCs demonstrated an EC50 in the micromolar range, with certain subtype specificities. The B. germanica experiments also generated the hypothesis that α-1 acts via binding to the so-called site three, thereby inhibiting the inactivation of this channel [121], leading to constitutive activity. Some additional preliminary activity data [122], using an Artemia salina bioassay, [123] indicates that the nemertides α-2-6 exhibit similar toxicities to α-1 (EC50 ranges: 0.5–5 μM).
An overview of peptide toxins that were detected and characterized in nemerteans is displayed in Table 5.
Table 5.
Overview of peptide toxins found in nemerteans.
| ID | Toxin | Species | Geographic Origin | Function | Ref |
|---|---|---|---|---|---|
| Pore-forming/hemolytic | |||||
| - | Cytotoxin A-I a | Cerebratulus lacteus | Woods hole, MA, USA | Pore-forming; hemolytic | [5,104] |
| - | Cytotoxin A-II a | Cerebratulus lacteus | Woods hole, MA, USA | Pore-forming; hemolytic | [5,104] |
| P01527 | Cytotoxin A-III a | Cerebratulus lacteus | Woods hole, MA, USA | Pore-forming; hemolytic | [5,104] |
| - | Cytotoxin A-IV a | Cerebratulus lacteus | Woods hole, MA, USA | Pore-forming; hemolytic | [104] |
| 6ENA b | Nemertide α-1 | Lineus longissimus | Koster Fiord, Swe | VGSC activator, paralytic | [120] |
| - | Nemertide α-2 | Lineus longissimus | Koster Fiord, Swe | Likely VGSC activator, paralytic | [120] |
| - | Nemertide β-1 | Lineus longissimus | Koster Fiord, Swe | Neurotoxin B-IV homolog. Likely paralytic. | [120] |
| - | ”Nemertine” | Lineus ruber | Possible nemertide | [5] | |
| - | ”Nemertine” | Tenuilineus bicolor f | Not stated | Possible nemertide | [5] |
| - | ”Nemertine” | Lineus viridis | Not stated | Possible nemertide | [5] |
| - | <5 kDa component | Lineus longissimus | Koster Fiord, Swe, and Millport Scot, UK | Paralytic. Likely α-nemertide | [25] |
| - | Neurotoxin B-I c | Cerebratulus lacteus | Long Island Sound, NY, USA | Likely paralytic | [89] |
| P01526 | Neurotoxin B-II c | Cerebratulus lacteus | Long Island Sound, NY, USA | Paralytic | [89] |
| P01526 | Neurotoxin B-II | Cerebratulus lacteus | Woods hole, MA, USA | Paralytic | [104] |
| - | Neurotoxin B-III c | Cerebratulus lacteus | Long Island Sound, NY, USA | Likely paralytic | [89] |
| P01525 | Neurotoxin B-IV | Cerebratulus lacteus | Woods hole, MA, USA | Paralytic | [104] |
ID is short for UniProtKB entry (uniprot.org). a In source referred to as Cerebratulus toxin A (I-IV). b PDB code. c In source referred to as Cerebratulus toxin B (I-IV). Species are denoted according to WoRMS, World Register of Marine Species as of 19-02-11 [54].
5.3.4. Other Peptide and Protein Toxins
The impact of molecular biology is only beginning to take its effect on the progress in nemertea toxin research. A milestone was the recent publication of the first full genome of a ribbon worm, the heteronemertean Notospermus geniculatus [15]. Prior to this, a limited number of papers have made use of genomic methods to discover new peptide toxins, notably in the aforementioned work by Butala et al. [119], leading to the discovery of additional parborlysin sequences. Whelan et al. analysed the transcriptomes from nine nemertean species (the heteronemerteans Cerebratulus marginatus, Lineus lacteus, Lineus longissimus, Lineus ruber, the hoplonemerteans Malacobdella grossa, Paranemertes peregrine, and the palaeonemerteans Tubulanus polymorphus, Cephalothrix hongkongiensis, and Cephalothrix linearis), finding an abundance of sequences that were identical or similar to those of known toxins [124]. Cytotoxin A-III was found in the four heteronemerteans, but the most prominent sequence was that of plancitoxin-1, which was present in all species. Plancitoxin-1, a DNase II hepatotoxin that is found in the crown-of-thorns starfish (Acanthaster planci) [125], was suggested to be secreted with the mucus to improve the effect of other toxin peptides, rather than acting on its own. Sequences that were related to the SNTX/VTX protein family (either of neoverrucotoxin, verrucotoxin, or stonustoxin), were also found in all of the sequences. These proteins have cytolytic properties, but their functional roles in nemerteans remain unclear. Interestingly, two Shk toxin-like sequences were found, a pseudechetoxin-like venom protein (M. grossa, P. peregrina) and that of a Cys-rich venom protein ENH1 (C. linearis). Shk toxins have been shown to act as potassium channel blockers, thus attracting interest as pharmaceutical targets, e.g., against auto-immune reactions [126]. SE-cephalotoxin genes were found in six species (all but M. grossa, P. peregrine, and T. polymorphus). SE-cephalotoxin appears to have a role in predation, having been found in the salivary glands of the cuttlefish (Sepia esculenta), although its biochemical role is unclear [127]. Similar distribution was found for sequences related to the haemolytic echotoxin-2. Two species (C. hongkongiensis and C. marginatus) exhibited natterin-4-related sequences, containing an aerolysin segment. This domain has been linked to edema and nociception [128]. In addition, two species (C. hongkongiensis and C. linearis) contained MACPF (membrane attack complex/perforin) toxin genes (PsTX 60B and ATX-60-A), which are known to have a variety of roles but most commonly as components in native immunity [129]. In the heteronemertean Notospermus geniculatus genome [15], 63 putative toxin genes were annotated. Fifteen of these were shared with other lophotrochozoans without reported toxicity and they are not further discussed here, but 26 sequences were found to be differentially expressed in eggs and tissues. In many cases, variants of toxin sequences that were found in Whelan’s study were also found in the N. geniculatus genome, such as cytotoxin A-III, plancitoxin-1, a number of SNTX/VTX protein or Shk-protein related sequences, and natterin sequences. Moreover, several neurotoxin-related sequences that were not previously found in nemerteans, such as delta-actitoxin-Amc1a, Mu-theraphotoxin-Hhn2a 4, perivitellin-2, and turripeptide Gsg9.2 were found, and a series of sequences involved in various aspects of blood coagulation. A handful of putative toxin sequences for which the function is presently poorly known was also found.
The proteins themselves were not identified in these studies, and little can be said regarding the relevance and actual roles of these proteins for nemerteans. However, it indicates that there is much yet to discover. This is further stressed by the aforementioned recent study by Jacobsson et al. [120], who performed analyses of 17 transcriptomes, some of which are previously reported [119,124,130]. This analysis revealed seven novel α-nemertides, eight novel β-nemertide sequences (neurotoxin B-analogs), and 29 parborlysin related sequences.
Although limitations do exist in terms of finding completely new toxin families while using data mining alone, the above examples demonstrate how molecular biology techniques without doubt will accelerate the toxin discovery rate within the nemertean phylum. Putative toxin gene sequences that are found in nemertean genomes and transcriptomes are summarized in Table 6.
Table 6.
Overview of putative toxin gene sequences found in nemerteans.
| ID | Toxin Gene Homolog | Species | Geographic Origin | Proposed Function | Ref |
|---|---|---|---|---|---|
| Pore-forming/hemolytic | |||||
| P01527 | Cytotoxin A-III | Cerebratulus marginatus | San Juan Island, WA, USA [130] |
Pore-forming; hemolytic | [124] |
| P01527 | Cytotoxin A-III | Lineus lacteus a | Banyuls, Fréjus, Fra [131] | Pore-forming; hemolytic | [124] |
| P01527 | Cytotoxin A-III | Lineus longissimus | Erdeven + Roscoff, Fra [131] | Pore-forming; hemolytic | [124] |
| P01527 | Cytotoxin A-III | Lineus ruber | Roscoff, Wimereux, Fra [131] | Pore-forming; hemolytic | [124] |
| P01527 | Cytotoxin A-III | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Pore-forming; hemolytic | [15] |
| Q54316 | Hemolysin B | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Hemolytic | [15] |
| P54176 | Hemolysin-3 | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Hemolytic | [15] |
| A0A0N7HUN6 | Parborlysin-4 | Parborlasia corrugatus | Adelaide Island, Antarctica | Likely pore-forming, hemolytic | [119] |
| A0A0P0CC97 | Parborlysin-5 | Parborlasia corrugatus | Adelaide Island, Antarctica | Likely pore-forming, hemolytic | [119] |
| A0A0P0BUQ6 | Parborlysin-6 | Parborlasia corrugatus | Adelaide Island, Antarctica | Liekely pore-forming, hemolytic | [119] |
| A0A0P0CHY3 | Parborlysin-7 | Parborlasia corrugatus | Adelaide Island, Antarctica | Likely pore-forming, hemolytic | [119] |
| - | Parborlysin/cytotoxin homolog 1 (Locus_9778) | Cerebratulus marginatus | San Juan Island, WA, USA [124,130,132] | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 2 ( Locus_9778) | Cerebratulus marginatus | San Juan Island, WA, USA [124,130,132] | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 3 (Locus_40830) | Hubrechtella ijimai | Hamanko, Honshu, Jpn [132] | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 4 (comp17199) | Lineus lacteus a | Banyuls, Fréjus, Fra [131] | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 5 (comp55821) | Lineus lacteus a | Banyuls, Fréjus, Fra [131] | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 6 (Contig1463) | Lineus lacteus a | Banyuls, Fréjus, Fra [131] | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 7 (Comp16298) | Lineus lacteus a | Banyuls, Fréjus, Fra [131] | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 8 (Comp9226) | Lineus lacteus a | Banyuls, Fréjus, Fra [131] | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 9 (contig46055) | Lineus lacteus a | Banyuls, Fréjus, Fra [131] | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 10 (Comp45258) | Lineus longissimus | Koster Fiord, Swe | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 11 (comp48) | Lineus longissimus | Koster Fiord, Swe | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 12 (comp21702) | Lineus longissimus | Koster Fiord, Swe | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 13 (contig49129) | Lineus longissimus | Koster Fiord, Swe | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 14 (comp17823/17-134) | Lineus longissimus | Koster Fiord, Swe | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 15 (contig31748) | Lineus longissimus | Koster Fiord, Swe | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 16 (comp17823/17-147) | Lineus longissimus | Koster Fiord, Swe | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 17 (comp17823/17-145) | Lineus longissimus | Koster Fiord, Swe | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 17 (contig56815) | Lineus longissimus | Koster Fiord, Swe | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 18 (comp52392) | Lineus ruber | Roscoff, Wimereux, Fra [131] | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 19 (contig63996) | Lineus ruber | Roscoff, Wimereux, Fra [131] | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 20 (contig3234) | Lineus ruber | Roscoff, Wimereux, Fra [131] | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 21 (comp40150/seq2) | Lineus ruber | Roscoff, Wimereux, Fra [131] | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 22 (comp40150/seq2) | Lineus ruber | Roscoff, Wimereux, Fra [131] | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 23 (contig21527) | Lineus sanguineus | Not stated [131] | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 24 (contig61445) | Lineus sanguineus | Not stated [131] | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 25 (contig2073) | Ramphogordius pseudolacteus b | Not stated [131] | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 26 (contig6541) | Ramphogordius pseudolacteus b | Not stated [131] | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 27 (Locus_8475) | Riseriellus occultus | Liverpool, UK [132] | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 28 (Locus_39410) | Riseriellus occultus | Liverpool, UK [132] | Likely pore-forming | [120] |
| - | Parborlysin/cytotoxin homolog 29 (Locus_13571) | Riseriellus occultus | Liverpool, UK [132] | Likely pore-forming | [120] |
| Q76CA2 | Echotoxin-2 | Cephalothrix hongkongiensis | Akkeshi, Hokkaido, Jpn [130] | Pore forming, hemolytic | [124] |
| Q76CA2 | Echotoxin-2 | Cephalothrix linearis | Not stated | Pore forming, hemolytic | [124] |
| Q76CA2 | Echotoxin-2 | Cerebratulus marginatus | San Juan Island, WA, USA [130] |
Pore forming, hemolytic | [124] |
| Q76CA2 | Echotoxin-2 | Lineus ruber | Roscoff, Wimereux, Fra [131] | Pore forming, hemolytic | [124] |
| Q76CA2 | Echotoxin-2 | Tubulanus polymorphus | San Juan Island, WA, USA | Pore forming, hemolytic | [124] |
| Q66SO3 | Galactose-specific lectin nattectin | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Ca2+-dependent hemagglutination activity | [15] |
| A0ZSK3 | Neoverrucotoxin subunit alpha | Cerebratulus marginatus | San Juan Island, WA, USA [130] |
SNTX/VTX toxin; hemolytic activity | [124] |
| A0ZSK3 | Neoverrucotoxin subunit alpha | Paranemertes peregrina | San Juan Island, WA, USA | SNTX/VTX toxin; hemolytic activity | [124] |
| A0ZSK4 | Neoverrucotoxin subunit beta c | Lineus longissimus | Erdeven + Roscoff, Fra [131] | SNTX/VTX toxin; hemolytic activity | [124] |
| A0ZSK4 | Neoverrucotoxin subunit beta | Malacobdella grossa | Rhode Island, USA | SNTX/VTX toxin; hemolytic activity | [124] |
| Q98989 | Stonustoxin | Lineus lacteus a | Banyuls, Fréjus, Fra [131] | SNTX/VTX toxin; pore-forming, hemolytic | [124] |
| Q98989 | Stonustoxin | Lineus ruber | Roscoff, Wimereux, Fra [131] | SNTX/VTX toxin; pore-forming, hemolytic | [124] |
| Q91453 | Stonustoxin subunit beta | Tubulanus polymorphus | San Juan Island, WA, USA | SNTX/VTX toxin; pore-forming, hemolytic | [124] |
| P58912 | Toxin PsTX-60B | Cephalothrix hongkongiensis | Akkeshi, Hokkaido, Jpn [130] | MACPF toxin domain; hemolytic | [124] |
| P58912 | Toxin PsTX-60B | Cephalothrix linearis | Not stated | MACPF toxin domain; hemolytic | [124] |
| Q76DT2 | Toxin AvTX-60A | Cephalothrix linearis | Not stated | MACPF toxin domain; hemolytic | [124] |
| Neurotoxins/Acting on ion-channels | |||||
| Q92035 | Acetylcholinesterase | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Synaptic cleft hydrolysis of acetylcholine | [15] |
| P05486 | Conophysin-conopressin | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Targets vasopressin-oxytocin related receptors | [15] |
| 6ENA d | Nemertide α-1 | Lineus lacteus a | Banyuls, Fréjus, Fra [131] | VGSC activator, paralytic | [120] |
| 6ENA d | Nemertide α-1 | Lineus longissimus | Koster Fiord, Swe | VGSC activator, paralytic | [120] |
| 6ENA d | Nemertide α-1 | Lineus ruber | Roscoff, Wimereux, Fra [131] | VGSC activator, paralytic | [120] |
| - | Nemertide α-2 | Lineus longissimus | Koster Fiord, Swe | Likely VGSC activator, paralytic | [120] |
| - | Nemertide α-2 | Lineus ruber | Roscoff, Wimereux, Fra [131] | Likely VGSC activator, paralytic | [120] |
| - | Nemertide α-3 | Lineus lacteus a | Banyuls, Fréjus, Fra [131] | Likely VGSC activator, paralytic | [120] |
| - | Nemertide α-3 | Ramphogordius pseudolacteus b | Not stated [131] | Likely VGSC activator, paralytic | [120] |
| - | Nemertide α-4 | Lineus sanguineus | Not stated [131] | Likely VGSC activator, paralytic | [120] |
| - | Nemertide α-5 | Ramphogordius pseudolacteus b | Not stated [131] | Likely VGSC activator, paralytic | [120] |
| - | Nemertide α-6 | Lineus sanguineus | Not stated [131] | Likely VGSC activator, paralytic | [120] |
| - | Nemertide α-7 | Lineus ruber | Roscoff, Wimereux, Fra [131] | Likely VGSC activator, paralytic | [120] |
| - | Nemertide α-8 | Riseriellus occultus | Liverpool, UK [132] | Likely VGSC activator, paralytic; incomplete sequence | [120] |
| - | Nemertide β-1 | Lineus longissimus | Koster Fiord, Swe | Neurotoxin B-IV homolog, likely paralytic | [120] |
| - | Nemertide β-1 | Lineus ruber | Roscoff, Wimereux, Fra [131] | Neurotoxin B-IV homolog, likely paralytic | [120] |
| - | Nemertide β-2 | Lineus longissimus | Koster Fiord, Swe | Neurotoxin B-IV homolog, likely paralytic | [120] |
| - | Nemertide β-2 | Lineus ruber | Roscoff, Wimereux, Fra [131] | Neurotoxin B-IV homolog, likely paralytic | [120] |
| - | Nemertide β-3 | Lineus longissimus | Koster Fiord, Swe | Neurotoxin B-IV homolog, likely paralytic | [120] |
| - | Nemertide β-4 | Lineus lacteus a | Banyuls, Fréjus, Fra [131] | Neurotoxin B-IV homolog, likely paralytic | [120] |
| - | Nemertide β-5 | Ramphogordius pseudolacteus b | Not stated [131] | Neurotoxin B-IV homolog, likely paralytic | [120] |
| - | Nemertide β-6 | Ramphogordius pseudolacteus b | Not stated [131] | Neurotoxin B-IV homolog, likely paralytic | [120] |
| - | Nemertide β-7 | Lineus ruber | Roscoff, Wimereux, Fra [131] | Neurotoxin B-IV homolog, likely paralytic | [120] |
| - | Nemertide β-8 | Lineus sanguineus | Not stated [131] | Neurotoxin B-IV homolog, likely paralytic | [120] |
| - | Nemertide β-9 | Lineus sanguineus | Not stated [131] | Neurotoxin B-IV homolog, likely paralytic | [120] |
| Q09GJ9 | Cysteine-rich venom protein | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Ca2+-channel impairment; paralytic | [15] |
| Q3SB03 | Cysteine-rich venom protein pseudechetoxin-like | Malacobdella grossa | Rhode Island, USA | Possible Shk-toxin | [124] |
| Q3SB03 | Cysteine-rich venom protein pseudechetoxin-like | Paranemertes peregrina | San Juan Island, WA, USA | Possible Shk-toxin | [124] |
| Q3SB05 | Cys-rich venom protein pseudechetoxin-like | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Possible Shk-toxin | [15] |
| P69929 | Delta-actitoxin-Amc1a | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | May inhibit VGSC | [15] |
| D2Y1Y2 | Mu-theraphotoxin-Hhn2a 4 | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Blocks TTX-sensitive VGSC | [15] |
| P0C8G6 | Perivitellin-2 67 kDa subunit | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Neurotoxin present in eggs, acts on enterocytes | [15] |
| P0DKT2 | Turripeptide Gsg9.2 | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Ion-channel inhibitor | [15] |
| Coagulation | |||||
| Q7T3S7 | Acidic phospholipase A2 EC-I | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Inhibits platelet aggregation | [15] |
| Q3C2C1 | Phospholipase A2 AP-PLA2-II | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Capillary permeability-increasing and hemorrhagic activities | [15] |
| Q0ZZJ6 | A. superbus venom factor 1 | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Complement-activating | [15] |
| P0CH47 | Probable phospholipase A1 magnifin | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Causes platelet aggreagation, phospholipase activity | [15] |
| E0Y418 | Serine protease VLSP-1 | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | May act in prey hemostasis system | [15] |
| Q6X5S5 | Snaclec 27 | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Interferes with platelet aggregation | [15] |
| Q9DEA1 | Snaclec agkicetin-C subunit beta | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Antithrombotic action | [15] |
| P0C929 | Snaclec bothroinsularin subunit alpha | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Inhibits platelet aggregation | [15] |
| I7ICN3 | Snaclec bothroinsularin subunit beta | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Integrin antagonist | [15] |
| Q38L02 | Snaclec dabocetin subunit alpha | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Inhibits platelet aggregation | [15] |
| Q58L91 | Venom prothrombin activator oscutarin-C non-catalytic subunit | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Prothrombin activator; attacks hemostatic system | [15] |
| J3SBP3 | Venom phosphodiesterase 2 | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Nuclease activity and platelet aggregator | [15] |
| Other proposed activities | |||||
| A0FKN6 | Astacin-like metalloprotease toxin 1 | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Degrades fibronectin, fibrinogen and gelatin in vitro | [15] |
| P0DN10 | U-actitoxin-Avd3i | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Serine protease inhibitor of kallikreins | [15] |
| P81382 | L-amino acid oxidase | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Oxidative deamination of hydrophobic and aromatic residues | [15] |
| B2DCR8 | SE-cephalotoxin | Cephalothrix linearis | Not stated | Toxic function unknown | [124] |
| B2DCR8 | SE-cephalotoxin | Cerebratulus marginatus | San Juan Island, WA, USA [130] |
Toxic function unknown | [124] |
| B2DCR8 | SE-cephalotoxin | Lineus lacteus a | Banyuls, Fréjus, Fra [131] | Toxic function unknown | [124] |
| B2DCR8 | SE-cephalotoxin | Lineus longissimus | Erdeven + Roscoff, Fra [131] | Toxic function unknown | [124] |
| B2DCR8 | SE-cephalotoxin | Lineus ruber | Roscoff, Wimereux, Fra [131] | Toxic function unknown | [124] |
| P0A968 | Cold shock-like protein CspD | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Inhibitor of DNA replication | [15] |
| P26831 | Hyaluronoglycosaminidase | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Likely to act on connective tissue | [15] |
| P00984 | Kunitz-type serine protease inhibitor dendrotoxin E | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Inhibitor of trypsin and chymotrypsin | [15] |
| Q9KS12 | Multifunctional-autoprocessing repeats-in-toxin | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Destroys actin cytoskeleton | [15] |
| Q66S25 | Natterin-1 | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Edema and nociception | [15] |
| Q66S13 | Natterin-4 | Lineus lacteus a | Banyuls, Fréjus, Fra [131] | Edema and nociception | [124] |
| Q66S13 | Natterin-4 | Tubulanus polymorphus | San Juan Island, WA, USA | Edema and nociception | [124] |
| Q66S13 | Natterin-4 | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Edema and nociception | [15] |
| - | Natterin-4 homolog (Cm6) | Cerebratulus marginatus | San Juan Island, WA, USA [130] |
Inferred from reciprocal BLAST, likely edema and nociception | [124] |
| K7Z9Q9 | Nematocyst expressed protein 6 | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Metalloendopeptidase activity | [15] |
| A4USB4 | Phospholipase D LiSicTox-AlphaVI | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Hydrolysis of sphingomyelin; causes dermonecrosis and inflammation | [15] |
| Q1W694 | Phosopholipase D LiSicTox-betaID1 | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Hydrolysis of sphingomyelin; causes dermonecrosis and inflammation | [15] |
| Q75WF2 | Plancitoxin-1 | Cephalothrix hongkongiensis | Akkeshi, Hokkaido, Jpn [130] | Hepatotoxin | [124] |
| Q75WF2 | Plancitoxin-1 | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Hepatotoxin | [15] |
| Q75WF2 | Plancitoxin-1 | Cephalothrix linearis | Not stated | Hepatotoxin | [124] |
| Q75WF2 | Plancitoxin-1 | Cerebratulus marginatus | San Juan Island, WA, USA [130] |
Hepatotoxin | [124] |
| Q75WF2 | Plancitoxin-1 | Lineus lacteus a | Banyuls, Fréjus, Fra [131] | Hepatotoxin | [124] |
| Q75WF2 | Plancitoxin-1 | Lineus longissimus | Erdeven + Roscoff, Fra [131] | Hepatotoxin | [124] |
| Q75WF2 | Plancitoxin-1 | Lineus ruber | Roscoff, Bretagne, Wimereux, France [131] | Hepatotoxin | [124] |
| Q75WF2 | Plancitoxin-1 | Malacobdella grossa | Rhode Island, USA | Hepatotoxin | [124] |
| Q75WF2 | Plancitoxin-1 | Paranemertes peregrina | San Juan Island, WA, USA | Hepatotoxin | [124] |
| Q75WF2 | Plancitoxin-1 | Tubulanus polymorphus | San Juan Island, WA, USA | Hepatotoxin | [124] |
| C1IC50 | Protease inhibitor-1 | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Serine protease inhibitor | [15] |
| A8YPR9 | Snake venom metalloprotease inhibitor 02A10 | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Metalloproteinase inhibitor during glandular storage | [15] |
| P86548 | Soybean toxin 17 kDa chain | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Involved in plant defense | [15] |
| B5U2W0 | Venom serine protease Bi-VSP | Notospermus geniculatus | Ushimado Mar Inst Okayama Univ, Jpn | Serine protease, induces melanization in target insects | [15] |
ID is short for UniProtKB entry (uniprot.org). The proposed functions listed are the result of automatic annotation, extracted from uniprot.org entries, or for sequences lacking uniprot entry, inferred from activities of known homologs by the authors of this review. a In source also referred to as Ramphogordius lacteus (synonymous). b In source referred to as Lineus pseudolacteus (synonymous). c In source assigned as subunit alpha. d PDB code. Species are denoted according to WoRMS, World Register of Marine Species as of 19-02-11 [54].
6. The Ecological Roles of Nemertean Toxins
6.1. Nemerteans as Preys
On a large scale, nemerteans are rarely eaten by others. McDermott et al. [10] studied gut content in fish and birds, and generally found low percentages of nemerteans. Seemingly, some flat fish do eat nemerteans within Cerebratulus spp. Although some Cerebratulus species have been shown to have toxins, their effect on flat fish GI tract has not been studied, to the best of our knowledge. In addition, some species can be used as bait to catch fish, e.g., Cerebratulus lacteus [133], Malacobdella spp., and Ototyphlonemertes brevis [134], as well as Polybrachiyorhynchus dayi [135]. Conversely, other nemertean species (e.g., Tubulanus annulatus) are directly rejected should they be swallowed by fish (e.g., Cottidae spp., personal observation, Malin Strand). The puffer fish Takifugu niphobles has been shown to digest Cephalothrix simula [86]. It was suggested in this study that TTX from the nemertean might be accumulated in the fish (both are known to contain TTX). The puffer fish showed no appetite for Baseodiscus curtus, and in the same study, a goby fish rejected both nemertean species. A few echinoderms that digest some nemerteans seem ti ve unaffected; the small ophiuroid Ophiocomina nigra can swallow several specimens of Lineus spp. (viridis/ruber) and Ramphogordius sanguineus a day, and the same is true for the asteroid Marthasterias glacialis (personal observation, Malin Strand). Nemerteans also eat each other to some extent; for instance, Lineus sanguineus has been observed to ingest Amphiporus lactifloreus [136]. There is nothing in the literature that rejects the hypotheses that toxin production helps in avoiding becoming prey, although very few studies actually pinpoint specific responses between toxin production of a certain kind and predator defence. Moles et al. concluded that lipophilic extracts of the Antarctic species Parborlasia corrugatus displayed significant feeding deterrence [137] when presented to the common seastar Odontaster validus.
6.2. Nemerteans as Predators and Scavengers
Nemerteans are carnivorous and they feed on both dead and alive prey [134]. Some are scavenger species that eat through tissue suction, but current knowledge is that many nemerteans are voracious predators that actively hunt living preys [11]. The armed hoplonemerteans can locate and stab a prey with the stylet that is associated with toxins that act as both paralytic and tissue dissolving [138]. The prey is immobilized within seconds and the nemertean can then safely ingest the prey innards or the whole prey. Many hoplonemerteans seemingly prefer arthropods as prey, although there are observations on attacks of molluscs and other worm groups. The concentration of toxins to the proboscis of hoplonemerteans, as confirmed in the case of pyridine alkaloids [17], points to their role in predation.
Heteronemerteans, which lack the stylet, appear to be equally well-developed active predators. The proboscis is still useful to effectively immobilize or slow the locomotion of its polychaete prey, as demonstrated in the case of Lineus sangineus [139]. Brief contact between the prey and the everted proboscis is seemingly enough to affect the prey’s mobility. The proboscis of L. sanguineus is equipped with secretory cells with rod-shaped structures. These may serve to grip and possibly puncture prey, allowing for toxin entry (although the toxin used has not been characterized). Another example is Cerebratulus lacteus, which was studied in a soft-shell clam (Mya arenaria)-community where surface exploration was directly associated with the presence of prey [140]. The attack does not even necessarily involve proboscis evertion; the prey (in this case, a fairly slow moving bivalve) can simply be located and consumed by swallowing. Similarly, the predatory behaviour of Riseriellus occultus both in field observations and laboratory experiments was elegantly documented by Beckers et al. [141]. The nemertean was demonstrated to sneak into the soft tissue of a Patella gastropod and start eating without provoking any discernable counter reaction. In the case of Gibbula umbilicalis, the flat top shell, the attack was followed in close detail. The nemertean first attacked the snail foot, which then retracted into the mantle cavity, with the proboscis clinging onto it. The proboscis was thereby positioned next to the gills. Shortly thereafter, froth bubbles, which were interpreted as a protective reaction, emerged. A little while later, the foot retractor relaxed, suggesting a function of a toxin, and the nemertean moved in towards the apex of the shell, from where it digested the snail.
A number of organisms have been found to co-exist in or on nemerteans in a symbiontic relationship, including Haplosporidia and Microsporidia protists and Conoidasida [142]. Whether these have a role for predation is unclear, however for TTX containing bacterial symbionts, this might be the case [62].
6.3. Commensal Nemerteans
Approximately 40 species, most of which hoplonemerteans within Malacobdellidae, Carcinonemertidae, Tetrastemmatidae, and Emplectonematidae, are considered to be commensal species [143], but whether the nemertean is mutualistic or parasitic is often unclear. Six species within Malacobdellidae have been found in the mantle cavities of bivalves, and studies on Malacobdella grossa [144] suggested that it does not harm its host, supporting the classification of Malacobdellidae as endocommensals. In contrast, some Carcinonemertes species are ectocommensal specialized egg predators of decapod crustaceans [145], using the stylet to pierce egg coats to access yolk. This is not exclusive for Carcinonemertes, and egg predation has been suggested to sometimes emerge as outbreaks of epidemic levels, causing heavy crustacean brood mortality [146].
The benefit of toxin production in commensal nemerteans is not obvious, but toxins can potentially provide the host with protection from other predators. No functional studies that are dedicated to toxins of commensal nemerteans appear to exist, but toxin presence has nevertheless been indicated in a few studies. The Whelan transcriptome study [124] demonstrated the presence of a number of putative toxin sequences in Malacobdella grossa, such as neoverrucotin subunit beta, a pseudechetoxin-like cysteine-rich venom protein, and plancitoxin-1. M. grossa has been the subject of study for toxin content in some other studies: the trancriptome analysis by Jacobsson et al. [120] did not indicated the presence of either alpha- or beta-nemertide and neither parborlysin sequences. Neither was Kem et al. able to find pyridine alkaloids in M. grossa [5,17], and TTX also appears to be absent [80]. Apparently, the most commonly known nemertean toxins are absent or present in very low amounts in these nemerteans. However, Asakawa et al. [69] indicated the possible presence of 4,9-anhydro-TTX in Malacobdella japonica. Moreover, Kem screened a number of nemertean species for pyridine alkaloids. Carcinonemertes errans was not found to contain pyridine alkaloids, whereas the presence of “unidentified” compounds of pyridine type was indicated in Tetrastemma candidum and Tetrastemma reticulatum [38]. This observation is perhaps significant in light of the observation that Tetrastemma worki contains anabaseine [5,17].
7. Conclusions and Future Outlook
The aim of this review was to provide a complete account of nemertean toxins that are known to date. Currently, three main groups of toxins can be distinguished: pyridine alkaloids, TTX and derivatives thereof, and peptides.
Pyridine alkaloids have mainly been found in hoplonemerteans, but the discovery efforts are scarce. The perhaps most extensive study on this topic was published in 1988 [38]. Around 15 related compounds were left uncharacterized in this study, suggesting that there is yet more to be found. Technical improvements over 30 years allow for the characterization of significantly smaller amounts, so time has come for further studies of nemertean pyridine alkaloids. In contrast, TTX and related compounds in nemerteans is an area of active research. Most likely, TTX is not produced by nemerteans themselves, and the role of the compound in nemerteans is unclear. Although most studies demonstrating the presence of TTX in nemerteans analyzed specimens from the Sea of Japan or Peter the Great Bay, it is noteworthy that TTX carrying nemerteans recently emerged in European waters [85]. Studies to understand the mechanisms of this “TTX migration” would be of great value. A large number of putative peptide and protein toxin genes have been accounted for in this review. However, at the peptide level, merely three main families of peptide toxins have been confirmed: neurotoxins of about 3 kDa; neurotoxins of about 6 kDa; and, cytolytic peptides in the size range 8–10 kDa. The exceptional toxicity of the neurotoxins suggests that they may find practical use, and we predict that this will undoubtedly attract more research in the area. As the arsenal of nemertean peptide toxins is growing, a comment on nomenclature is warranted. We have introduced the term nemertide [120] and propose that this could be employed as a general term for nemertean peptide toxins; Greek prefixes are then used to indicate families. Thus, the α-nemertides is used for the family of 3 kDa cystine knot neurotoxins, and we argue that the ß-nemertides (6 kDa), in effect, can be viewed as a part of the same family as the neurotoxins BI to BIV. Similarly, the parborlysins likely belong to the same family as cytotoxins AI to AIV.
The development of marine natural products chemistry can be illustrated by the number of compounds isolated/discovered each year (one in 1963 vs. 1277 in 2016) [147,148], with the last 15 years seeing an average increase of approximately 50 compounds per annum. From this body of work, seven out of 1453 FDA-approved drugs as of 2013 were of marine origin [149]. Current progress is substantial, and today an abundance of pharmacologically active compounds are being evaluated for potential medicinal use [150,151,152].
Only a minute number of those natural products originate from the nemertea phylum. However, as shown in this review, it is clear that nemerteans represent a promising source of bioactive compounds, given that less than 5% of the world’s ribbon worm species have been analyzed for toxin content to any extent. Moreover, in no single case can it be stated that a complete analysis, which takes into account both macromolecular toxins and small molecules, has been carried out. There is also a geographical limitation to the efforts. Sampling has been focused to a limited number of sites and, at this point, it appears that no nemertean species have been sampled in Africa nor India, and only one from Oceania and a few examples from South America have ever been investigated in this context, Figure 10. Only one terrestrial species has been investigated, and no fresh water species. We are only beginning to uncover the chemistry of nemertean worms.
Figure 10.
Schematic illustration of sampling sites for nemerteans analyzed with regard to toxin content reported in scientific publications. Each dot represents any unique species investigated in one report (if the same sample was interpreted as being used in several studies, it is still one dot). Blue dots correspond to pyridine alkaloids, yellow dots: TTX, and red dots: peptide toxins.
Hence, what lies in the future of nemertean toxins? Whereas TTX has been an important tool in the characterization of sodium channels and offers promise as a drug lead [153], it is doubtful that nemerteans will be a source for large-scale production of TTX, unless of course enzymes that are involved in its biosynthesis could be identified. In the case of pyridine alkaloids, DMXBA has been extensively investigated, and its medicinal potential is still under investigation [52]. As for peptide toxins, the use of the α-nemertides in pesticide applications indeed appears to be feasible [122]. It is difficult to predict the outcome of nemertean toxins, but it is clear that they provide an intriguing addition to the field of toxin research. Taking into consideration that the present status has been achieved through a research volume of little more than 100 published scientific articles, ample opportunities for discovery should lie ahead.
Acknowledgments
The authors wish to express their gratitude to Dr Luke Robertson and Mr Quentin Laborde for valuable input on this manuscript.
Funding
Funding from the Swedish National Science Foundation (Vetenskapsrådet grants no 214-3327 and 2018-005403), FORMAS (grant no 2018-00613) and the Crafoord foundation (grant no 20160810) are gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Key Contribution
This review covers all research published by the end of 2018, as far as known by these authors, with content directly relating to nemertean toxins.
References
- 1.Gibson R. Nemertean genera and species of the world: an annotated checklist of original names and description citations, synonyms, current taxonomic status, habitats and recorded zoogeographic distribution. J. Nat. Hist. 1995;29:271–561. doi: 10.1080/00222939500770161. [DOI] [Google Scholar]
- 2.Kajihara H., Chernyshev A.V., Sun S.C., Sundberg P., Crandall F.B. Checklist of nemertean genera and species published between 1995 and 2007. Spec. Div. 2008;13:145–174. doi: 10.12782/specdiv.13.245. [DOI] [Google Scholar]
- 3.Moore J., Gibson R., Jones H.D. Terrestrial nemerteans thirty years on. Hydrobiologia. 2001;456:1–6. doi: 10.1023/A:1013052728257. [DOI] [Google Scholar]
- 4.Sundberg P., Gibson R. Global diversity of nemerteans (Nemertea) in freshwater. Hydrobiologia. 2008;595:61–66. doi: 10.1007/s10750-007-9004-6. [DOI] [Google Scholar]
- 5.Kem W.R. Biochemistry of nemertine toxins. In: Martin D., Padilla G., editors. Marine Pharmacognosy. Action of Marine Biotoxins at the Cellular Level. Academic Press; New York, NY, USA: pp. 38–85. [Google Scholar]
- 6.Kem W.R. Structure and activity of Nemertine Toxins. Am. Zool. 1985;25:99–111. doi: 10.1093/icb/25.1.99. [DOI] [Google Scholar]
- 7.Kem W.R. Chapter 57—Worm Venom Peptides. In: Kastin A.J., editor. Handbook of Biologically Active Peptides. Academic Press; Burlington, VT, USA: 2006. pp. 397–401. [Google Scholar]
- 8.Kem W.R. Nemertine Toxins. In: Massaro E.J., editor. Handbook of Neurotoxicology: Volume I. Humana Press; Totowa, NJ, USA: 2002. pp. 573–593. [Google Scholar]
- 9.Nelsen D.R., Nisani Z., Cooper A.M., Fox G.A., Gren E.C., Corbit A.G., Hayes W.K. Poisons, toxungens, and venoms: redefining and classifying toxic biological secretions and the organisms that employ them. Biol. Rev. 2014;89:450–465. doi: 10.1111/brv.12062. [DOI] [PubMed] [Google Scholar]
- 10.McDermott J.J. Status of the Nemertea as prey in marine ecosystems. Hydrobiologia. 2001;456:7–20. doi: 10.1023/A:1013001729166. [DOI] [Google Scholar]
- 11.Thiel M., Kruse I. Status of the Nemertea as predators in marine ecosystems. Hydrobiologia. 2001;456:21–32. doi: 10.1023/A:1013005814145. [DOI] [Google Scholar]
- 12.Johnston G. Miscellanea Zoologica. A description of some planarian worms. Mag. Zool. Bot. 1837;1:529–538. [Google Scholar]
- 13.Strand M., Norenburg J., Alfaya J.E., Fernández-Álvarez F.Á., Andersson H.S., Andrade S.C., Bartolomeus T., Beckers P., Bigatti G., Cherneva I., et al. Nemertean taxonomy-implementing changes in the higher ranks, dismissing Anopla and Enopla. Zool. Scr. 2019;48:118–119. doi: 10.1111/zsc.12317. [DOI] [Google Scholar]
- 14.Krämer D., Schmidt C., Podsiadlowski L., Beckers P., Horn L., Döhren J. Unravelling the Lineus ruber/viridis species complex (Nemertea, Heteronemertea) Zool. Scr. 2017;46:111–126. doi: 10.1111/zsc.12185. [DOI] [Google Scholar]
- 15.Luo Y.-J., Kanda M., Koyanagi R., Hisata K., Akiyama T., Sakamoto H., Sakamoto T., Satoh N. Nemertean and phoronid genomes reveal lophotrochozoan evolution and the origin of bilaterian heads. Nature Ecol. Evol. 2018;2:141. doi: 10.1038/s41559-017-0389-y. [DOI] [PubMed] [Google Scholar]
- 16.Andrade S., Strand M., Schwartz M., Chen H., Kajihara H., von Döhren J., Sun S., Junoy J., Thiel M., Norenburg J.L. Disentangling ribbon worm relationships: multi-locus analysis supports traditional classification of the phylum Nemertea. Cladistics. 2012;28:141–159. doi: 10.1111/j.1096-0031.2011.00376.x. [DOI] [PubMed] [Google Scholar]
- 17.Kem W.R. A study of the occurrence of anabaseine in Paranemertes and other nemertines. Toxicon. 1971;9:23–32. doi: 10.1016/0041-0101(71)90040-7. [DOI] [PubMed] [Google Scholar]
- 18.Stricker S.A., Cloney R.A. The stylet apparatus of the nemertean Paranemertes peregrina: Its ultrastructure and role in prey capture. Zoomorphology. 1981;97:205–223. doi: 10.1007/BF00310277. [DOI] [Google Scholar]
- 19.Stricker S.A., Cloney R.A. The ultrastructure of venom-producing cells in Paranemertes peregrina (Nemertea, Hoplonemertea) J. Morphol. 1983;177:89–107. doi: 10.1002/jmor.1051770108. [DOI] [PubMed] [Google Scholar]
- 20.Montalvo S., Junoy J., Roldán C., García-Corrales P. Ultrastructural study of sensory cells of the proboscidial glandular epithelium of Riseriellus occultus (Nemertea, Heteronemertea) J. Morphol. 1996;229:83–96. doi: 10.1002/(SICI)1097-4687(199607)229:1<83::AID-JMOR5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 21.Montalvo S., Roldan C., Junoy J., Garcia-Corrales P. Ultrastructural study of two glandular systems in the proboscidial glandular epithelium of Riseriellus occultus (Nemertea, Heteronemertea) Zoomorphology. 1998;117:247–257. doi: 10.1007/s004350050049. [DOI] [PubMed] [Google Scholar]
- 22.Junoy J., Montalvo S., Roldán C., García-Corrales P. Ultrastructural study of the bacillary, granular and mucoid proboscidial gland cells of Riseriellus occultus (Nemertini, Heteronemertini) Acta Zool. 2000;81:235–242. doi: 10.1046/j.1463-6395.2000.00053.x. [DOI] [Google Scholar]
- 23.Turbeville J.M. Ultrastructure of the pseudocnidae of the palaeonemerteans Cephalothrix cf. rufifrons and Carinomella lactea and an assessment of their phylogenetic utility. J. Nat. Hist. 2006;40:967–979. doi: 10.1080/00222930600834006. [DOI] [Google Scholar]
- 24.Magarlamov T.Y., Chernyshev A.V., Turbeville J.M. Pseudocnidae of archinemerteans (nemertea, palaeonemertea) and their implications for nemertean systematics. J. Morphol. 2018;279:1–11. doi: 10.1002/jmor.20881. [DOI] [PubMed] [Google Scholar]
- 25.Strand M., Hedström M., Seth H., McEvoy E.G., Jacobsson E., Goransson U., Andersson H.S., Sundberg P. The bacterial (Vibrio alginolyticus) production of tetrodotoxin in the ribbon worm Lineus longissimus—Just a false positive? Mar. Drugs. 2016;14:63. doi: 10.3390/md14040063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kem W.R. Structure and membrane actions of a marine worm protein cytolysin, Cerebratulus toxin A-III. Toxicology. 1994;87:189–203. doi: 10.1016/0300-483X(94)90251-8. [DOI] [PubMed] [Google Scholar]
- 27.Magnus O. 1555. Historia de gentibus septentrionalibus. Rome. Translated into Swedish. 1909;1925 [Google Scholar]
- 28.Cedhagen T., Sundberg P. A previously unrecognized report of a nemertean in the literature. Arch. Nat. Hist. 1986;13:7–8. doi: 10.3366/anh.1986.13.1.7. [DOI] [Google Scholar]
- 29.Borlase W. The Natural History of Cornwall. Coastal; Wilmington, NC, USA: 1758. [Google Scholar]
- 30.Wilson C.B. The Habits and Early Development of Cerebratulus lacteus (Verrill) J. and A. Churchill Ltd.; London, UK: 1900. A contribution to physiological morphology. [Google Scholar]
- 31.Reisinger E. Nemertini. Biol. Tiere Deutchl. 1926;1:1–24. [Google Scholar]
- 32.Bacq Z.M. Les poisons des némertiens. Bull. Cl. Sci., Acad. Roy. Belg. 1936;22:1072–1079. [Google Scholar]
- 33.Bacq Z.M. L’“amphiporine” et la “nemertine”, poisons des vers némertiens. Arch. Int. Physiol. 1937;44:109–124. doi: 10.3109/13813453709145202. [DOI] [Google Scholar]
- 34.King H. Amphiporine, an active base from the marine worm Amphiporus lactifloreus. J. Chem. Soc. Lond. 1939:1365. [Google Scholar]
- 35.Kem W.R., Abbott B.C., Coates R.M. Isolation and structure of a hoplonemertine toxin. Toxicon. 1971;9:15–22. doi: 10.1016/0041-0101(71)90039-0. [DOI] [PubMed] [Google Scholar]
- 36.Kem W.R., Scott K.N., Duncan J.H. Hoplonemertine worms—A new source of pyridine neurotoxins. Experientia. 1976;32:684–686. doi: 10.1007/BF01919831. [DOI] [PubMed] [Google Scholar]
- 37.Cruskie M.P.J., Zoltewicz J.A., Abboud K.A. Revised structure and convergent synthesis of nemertelline, the neurotoxic quaterpyridine isolated from the hoplonemertine sea worm. J. Org. Chem. 1995;60:7491–7495. doi: 10.1021/jo00128a021. [DOI] [Google Scholar]
- 38.Kem W.R. Pyridine alkaloid distribution in the hoplonemertines. Hydrobiologia. 1988;156:145–151. doi: 10.1007/BF00027988. [DOI] [Google Scholar]
- 39.Kem W.R., Soti F. Amphiporus alkaloid multiplicity implies functional diversity: initial studies on crustacean pyridyl receptors. Hydrobiologia. 2001;456:221–231. doi: 10.1023/A:1013063726807. [DOI] [Google Scholar]
- 40.Kem W.R., Rocca J., Garraffo H.M., Spande T.F., Daly J.W., Soti F. Synthesis and spectroscopic copmarison of the eight methyl-2,3′-bipyridyls and identification of a hoplonemertine alkaloid as 3-methyl-2,3′-bipyridyl. Heterocycles. 2009;79:1025–1041. doi: 10.3987/COM-08-S(D)80. [DOI] [Google Scholar]
- 41.Kem W.R., Soti F., Rittschof D. Inhibition of barnacle larval settlement and crustacean toxicity of some hoplonemertine pyridyl alkaloids. Biomol. Eng. 2003;20:355–361. doi: 10.1016/S1389-0344(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 42.Kem W.R., Soti F., Rittschof D. Materials and Methods for Inhibiting Fouling of Surfaces Exposed to Aquatic Environments. US730717B2. U.S. Patent. 2006
- 43.Meyer E.M., W Arendash G., Judkins J.H., Ying L., Wade C., Ken W.R. Effects of nucleus basalis lesions on the muscarinic and nicotinic modulation of [3H] acetylcholine release in the rat cerebral cortex. J. Neurochem. 1987;49:1758–1762. doi: 10.1111/j.1471-4159.1987.tb02433.x. [DOI] [PubMed] [Google Scholar]
- 44.Kem W.R., Mahnir V.M., Papke R.L., Lingle C.J. Anabaseine is a potent agonist on muscle and neuronal alpha-bungarotoxin-sensitive nicotinic receptors. J. Pharmacol. Exp. Ther. 1997;283:979–992. [PubMed] [Google Scholar]
- 45.Zoltewicz J.A., Bloom L.B., Kem W.R. Quantitative determination of the ring-chain hydrolysis equilibrium constant for anabaseine and related tobacco alkaloids. J. Org. Chem. 1989;54:4462–4468. doi: 10.1021/jo00279a042. [DOI] [Google Scholar]
- 46.Kem W., Soti F., Wildeboer K., LeFrancois S., MacDougall K., Wei D.-Q., Chou K.-C., Arias H.R. The nemertine toxin anabaseine and its derivative DMXBA (GTS-21): Chemical and pharmacological properties. Mar. Drugs. 2006;4:255–273. doi: 10.3390/md403255. [DOI] [Google Scholar]
- 47.Hunter B.E., Christopher M., Papke R.L., Kem W.R., Meyer E.M. A novel nicotinic agonist facilitates induction of long-term potentiation in the rat hippocampus. Neurosci. Lett. 1994;168:130–134. doi: 10.1016/0304-3940(94)90433-2. [DOI] [PubMed] [Google Scholar]
- 48.Sahakian B., Jones G., Levy R., Gray J., Warburton D. The effects of nicotine on attention, information processing, and short-term memory in patients with dementia of the Alzheimer type. Br. J. Psychiat. 1989;154:797–800. doi: 10.1192/bjp.154.6.797. [DOI] [PubMed] [Google Scholar]
- 49.Woodruff-Pak D.S., Li Y.T., Kem W.R. A nicotinic agonist (GTS-21), eyeblink classical conditioning, and nicotinic receptor binding in rabbit brain. Brain Res. 1994;645:309–317. doi: 10.1016/0006-8993(94)91665-9. [DOI] [PubMed] [Google Scholar]
- 50.Meyer E.M., Tay E.T., Papke R.L., Meyers C., Huang G.-L., de Fiebre C.M. 3-[2,4-Dimethoxybenzylidene] anabaseine (DMXB) selectively activates rat α7 receptors and improves memory-related behaviors in a mecamylamine-sensitive manner. Brain Res. 1997;768:49–56. doi: 10.1016/S0006-8993(97)00536-2. [DOI] [PubMed] [Google Scholar]
- 51.Zawieja P., Kornprobst J.M., Métais P. 3-(2,4-Dimethoxybenzylidene)-anabaseine: A promising candidate drug for Alzheimer’s disease? Geriatr. Gerontol. Int. 2012;12:365–371. doi: 10.1111/j.1447-0594.2011.00827.x. [DOI] [PubMed] [Google Scholar]
- 52.Rangel M., Falkenberg M. An overview of the marine natural products in clinical trials and on the market. J. Coast. Life Med. 2015;3:421–428. [Google Scholar]
- 53.Kline J.K. Behavioral Responses of the Spiny Lobster (Panulirus argus) to Live Amphiporous Angulatus and Its Bipyridyl Toxins. [(accessed on 20 December 2012)];1986 Biology Senior Project Report, Kalamazoo College. Available online: http://hdl.handle.net/10920/23073.
- 54.Horton T., Kroh A., Bailly N., Boury-Esnault N., Brandão S.N., Costello M.J., Gofas S., Hernandez F., Mees J., Paulay G., et al. World Register of Marine Species (WoRMS). WoRMS Editorial Board. [(accessed on 20 December 2012)];2017 Available online: http://www.marinespecies.org.
- 55.Hwang D.F., Noguchi T. Tetrodotoxin Poisoning. In: Steve L.T., editor. Advances in Food and Nutrition Research. Vol. 52. Academic Press; Amsterdam, The Netherlands: 2007. pp. 141–236. [DOI] [PubMed] [Google Scholar]
- 56.Bane V., Lehane M., Dikshit M., O’Riordan A., Furey A. Tetrodotoxin: Chemistry, toxicity, source, distribution and detection. Toxins. 2014;6:693–755. doi: 10.3390/toxins6020693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stevens M., Peigneur S., Tytgat J. Neurotoxins and their binding areas on voltage-gated sodium channels. Front. Pharmacol. 2011;2:71. doi: 10.3389/fphar.2011.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blankenship J. Tetrodotoxin: from poison to powerful tool. Perspect. Biol. Med. 1976;19:509–526. doi: 10.1353/pbm.1976.0071. [DOI] [PubMed] [Google Scholar]
- 59.Nieto F.R., Cobos E.J., Tejada M.Á., Sánchez-Fernández C., González-Cano R., Cendán C.M. Tetrodotoxin (TTX) as a therapeutic agent for pain. Mar. Drugs. 2012;10:281–305. doi: 10.3390/md10020281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.National Institute of Health U.S. National Library of Medicine. [(accessed on 10 January 2019)]; Available online: https://clinicaltrials.gov/
- 61.Chau R., Kalaitzis J.A., Neilan B.A. On the origins and biosynthesis of tetrodotoxin. Aquat. Toxicol. 2011;104:61–72. doi: 10.1016/j.aquatox.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 62.Magarlamov T.Y., Melnikova D.I., Chernyshev A.V. Tetrodotoxin-producing bacteria: Detection, distribution and migration of the toxin in aquatic systems. Toxins. 2017;9:166. doi: 10.3390/toxins9050166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou M., Shum F.H.K. Method of Extracting Tetrodotoxin. US6552191B1. U.S. Patent. 22 April 2003
- 64.Miyazawa K., Higashiyama M., Ito K., Noguchi T., Arakawa O., Shida Y., Hashimoto K. Tetrodotoxin in two species of ribbon worm (Nemertini), Lineus fuscoviridis and Tubulanus punctatus. Toxicon. 1988;26:867–874. doi: 10.1016/0041-0101(88)90327-3. [DOI] [PubMed] [Google Scholar]
- 65.Ali A.E., Arakawa O., Noguchi T., Miyazawa K., Shida Y., Hashimoto K. Tetrodotoxin and related substances in a ribbon worm Cephalothrix linearis (Nemertean) Toxicon. 1990;28:1083–1093. doi: 10.1016/0041-0101(90)90147-Y. [DOI] [PubMed] [Google Scholar]
- 66.Noguchi T., Ali A.E., Arakawa O., Miyazawa K., Kanoh S., Shida Y., Nishio S., Hashimoto K. Tetrodonic acid-like substance; A possible precursor of tetrodotoxin. Toxicon. 1991;29:845–855. doi: 10.1016/0041-0101(91)90221-C. [DOI] [PubMed] [Google Scholar]
- 67.Asakawa M., Toyoshima T., Shida Y., Noguchi T., Miyazawa K. Paralytic toxins in a ribbon worm Cephalothrix species (Nemertean) adherent to cultured oysters in Hiroshima Bay, Hiroshima Prefecture, Japan. Toxicon. 2000;38:763–773. doi: 10.1016/S0041-0101(99)00172-5. [DOI] [PubMed] [Google Scholar]
- 68.Asakawa M., Toyoshima T., Ito K., Bessho K., Yamaguchi C., Tsunetsugu S., Shida Y., Kajihara H., Mawatari S.F., Noguchi T., et al. Paralytic toxicity in the ribbon worm Cephalothrix species (Nemertea) in Hiroshima Bay, Hiroshima Prefecture, Japan and the isolation of tetrodotoxin as a main component of its toxins. Toxicon. 2003;41:747–753. doi: 10.1016/S0041-0101(03)00009-6. [DOI] [PubMed] [Google Scholar]
- 69.Asakawa M., Ito K., Kajihara H. Highly toxic ribbon worm Cephalothrix simula containing tetrodotoxin in Hiroshima Bay, Hiroshima Prefecture, Japan. Toxins. 2013;5:376–395. doi: 10.3390/toxins5020376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ueda H., Itoi S., Sugita H. TTX-bearing planocerid flatworm (Platyhelminthes: Acotylea) in the Ryukyu Islands, Japan. Mar. Drugs. 2018;16:37. doi: 10.3390/md16010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McEvoy E.G., Rogers A., Gibson R. Preliminary investigation of Vibrio alginolyticus-like bacteria associated with marine nemerteans. Hydrobiologia. 1998;365:287–291. doi: 10.1023/A:1003174320123. [DOI] [Google Scholar]
- 72.Carroll S., McEvoy E.G., Gibson R. The production of tetrodotoxin-like substances by nemertean worms in conjunction with bacteria. J. Exp. Mar. Biol. Ecol. 2003;288:51–63. doi: 10.1016/S0022-0981(02)00595-6. [DOI] [Google Scholar]
- 73.Strand M., Sundberg P. Sustainable Method for Synthesizing Tetrodotoxin (TTX) US20110081690A1. U.S. Patent. 2011
- 74.Matsumura K. Reexamination of tetrodotoxin production by bacteria. Appl. Environ. Microbiol. 1995;61:3468–3470. doi: 10.1128/aem.61.9.3468-3470.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Matsumura K. No ability to produce tetrodotoxin in bacteria. Appl. Environ. Microbiol. 2001;67:2393–2394. doi: 10.1128/AEM.8.3.2393-2394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Salvitti L., Wood S., McNabb P., Cary S. No evidence for a culturable bacterial tetrodotoxin producer in Pleurobranchaea maculata (Gastropoda: Pleurobranchidae) and Stylochoplana sp. (Platyhelminthes: Polycladida) Toxins. 2015;7:255–273. doi: 10.3390/toxins7020255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Salvitti L., Wood S.A., Taylor D.I., McNabb P., Cary S.C. First identification of tetrodotoxin (TTX) in the flatworm Stylochoplana sp.; a source of TTX for the sea slug Pleurobranchaea maculata. Toxicon. 2015;95:23–29. doi: 10.1016/j.toxicon.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 78.Beleneva I., Magarlamov T.Y., Kukhlevsky A. Characterization, identification, and screening for tetrodotoxin production by bacteria associated with the ribbon worm (Nemertea) Cephalotrix simula (Ivata, 1952) Microbiology. 2014;83:220–226. doi: 10.1134/S0026261714030059. [DOI] [PubMed] [Google Scholar]
- 79.Magarlamov T.Y., Beleneva I.A., Chernyshev A.V., Kuhlevsky A.D. Tetrodotoxin-producing Bacillus sp. from the ribbon worm (Nemertea) Cephalothrix simula (Iwata, 1952) Toxicon. 2014;85:46–51. doi: 10.1016/j.toxicon.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 80.Melnikova D.I., Beleneva I.A., Tyunin A.P., Magarlamov T.Y. The taxonomic composition, characteristics, and neurotoxic activities of ribbon worm-associated bacteria from the Sea of Japan. Russ. J. Mar. Biol. 2017;43:383–391. doi: 10.1134/S1063074017050066. [DOI] [Google Scholar]
- 81.Tanu M.B., Mahmud Y., Arakawa O., Takatani T., Kajihara H., Kawatsu K., Hamano Y., Asakawa M., Miyazawa K., Noguchi T. Immunoenzymatic visualization of tetrodotoxin (TTX) in Cephalothrix species (Nemertea: Anopla: Palaeonemertea: Cephalotrichidae) and Planocera reticulata (Platyhelminthes: Turbellaria: Polycladida: Planoceridae) Toxicon. 2004;44:515–520. doi: 10.1016/j.toxicon.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 82.Magarlamov T.Y., Shokur O.A., Chernyshev A.V. Distribution of tetrodotoxin in the ribbon worm Lineus alborostratus (Takakura, 1898)(Nemertea): Immunoelectron and immunofluorescence studies. Toxicon. 2016;112:29–34. doi: 10.1016/j.toxicon.2016.01.060. [DOI] [PubMed] [Google Scholar]
- 83.Vlasenko A., Velansky P., Chernyshev A., Kuznetsov V., Magarlamov T.Y. Tetrodotoxin and its analogues profile in nemertean species from the Sea of Japan. Toxicon. 2018;156:48–51. doi: 10.1016/j.toxicon.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 84.Kwon Y.S., Min S.K., Yeon S.J., Hwang J.H., Hong J., Shin H.S. Assessment of neuronal cell-based cytotoxicity of neurotoxins from an estuarine nemertean in the Han River Estuary. J. Microbiol. Biotechnol. 2017;27:725–730. doi: 10.4014/jmb.1611.11027. [DOI] [PubMed] [Google Scholar]
- 85.Turner A., Fenwick D., Powell A., Dhanji-Rapkova M., Ford C., Hatfield R., Santos A., Martinez-Urtaza J., Bean T., Baker-Austin C. New Invasive Nemertean Species (Cephalothrix Simula) in England with high levels of tetrodotoxin and a microbiome linked to toxin metabolism. Mar. Drugs. 2018;16:452. doi: 10.3390/md16110452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kajihara H., Sun S.C., Chernyshev A.V., Chen H.X., Ito K., Asakawa M., Maslakova S.A., Norenburg J.L., Strand M., Sundberg P., et al. Taxonomic identity of a tetrodotoxin-accumulating ribbon-worm Cephalothrix simula (Nemertea: Palaeonemertea): A species artificially introduced from the Pacific to Europe. Zool. Sci. 2013;30:985–997. doi: 10.2108/zsj.30.985. [DOI] [PubMed] [Google Scholar]
- 87.Lousalet M., Campbell M.E., Schwartz M.L. Microdistribution of tetrodotoxin in three species of nemerteans. Abstract; Proceedings of the 7th International Conference on Nemertean Biology; Santa Barbara, CA, USA. 29 June–3 July 2009. [Google Scholar]
- 88.Campbell M.E., Schwartz M.L. Immunohistological Visualization of Tetrodotoxin in Micrura Verrili and Dushia Atra (Phylum Nemertea) [(accessed on 20 December 2012)];2008 Available online: https://apps.cur.org/ncur2018/archive/Display_NCUR.aspx?id=10545.
- 89.Kem W.R. Purification and characterization of a new family of polypeptide neurotoxins from the heteronemertine Cerebratulus lacteus (Leidy) J. Biol. Chem. 1976;251:4184–4192. [PubMed] [Google Scholar]
- 90.Blumenthal K.M., Kem W.R. Structure and action of heteronemertine polypeptide toxins. Primary structure of Cerebratulus lacteus toxin B-IV. J. Biol. Chem. 1976;251:6025–6029. [PubMed] [Google Scholar]
- 91.Blumenthal K.M., Keim P.S., Heinrikson R.L., Kem W.R. Structure and action of heteronemertine polypeptide toxins. Amino acid sequence of Cerebratulus lacteus toxin B-II and revised structure of toxin B-IV. J. Biol. Chem. 1981;256:9063–9067. [PubMed] [Google Scholar]
- 92.Blumenthal K.M., Kem W.R. Structure and action of heteronemertine polypeptide toxins: Inactivation of Cerebratulus lacteus toxin B-IV by tyrosine nitration. Arch. Biochem. Biophys. 1980;203:816–821. doi: 10.1016/0003-9861(80)90243-X. [DOI] [PubMed] [Google Scholar]
- 93.Blumenthal K.M. Structure and action of heteronemertine polypeptide toxins: inactivation of Cerebratulus lacteus toxin B-IV concomitant with tryptophan alkylation. Arch. Biochem. Biophys. 1980;203:822–826. doi: 10.1016/0003-9861(80)90244-1. [DOI] [PubMed] [Google Scholar]
- 94.Blumenthal K.M. Renaturation of neurotoxin B-IV from the heteronemertine Cerebratulus lacteus. Toxicon. 1986;24:63–69. doi: 10.1016/0041-0101(86)90166-2. [DOI] [PubMed] [Google Scholar]
- 95.Toth G.P., Blumenthal K.M. Structure and action of heteronemertine polypeptide toxins: Binding of Cerebratulus lacteus toxin B-IV to axon membrane vesicles. Biochim. Biophys. Acta. 1983;732:160–169. doi: 10.1016/0005-2736(83)90199-2. [DOI] [PubMed] [Google Scholar]
- 96.Lieberman D.L., Blumenthal K.M. Structure and action of heteronemertine polypeptide toxins. Specific cross-linking of Cerebratulus lacteus toxin B-IV to lobster axon membrane vesicles. Biochim. Biophys. Acta. 1986;855:41–48. doi: 10.1016/0005-2736(86)90186-0. [DOI] [PubMed] [Google Scholar]
- 97.Kem W.R., Tu C.-K., Williams R.W., Toumadje A., Johnson W.C. Circular dichroism and laser Raman spectroscopic analysis of the secondary structure of Cerebratulus lacteus toxin B-IV. J. Protein Chem. 1990;9:433–443. doi: 10.1007/BF01024619. [DOI] [PubMed] [Google Scholar]
- 98.Hansen P.E., Kem W.R., Bieber A.L., Norton R.S. 1H-NMR study of neurotoxin B-IV from the marine worm Cerebratulus lacteus. Solution properties, sequence-specific resonance assignments, secondary structure and global fold. Eu. J. Biochem. 1992;210:231–240. doi: 10.1111/j.1432-1033.1992.tb17413.x. [DOI] [PubMed] [Google Scholar]
- 99.Barnham K.J., Dyke T.R., Kem W.R., Norton R.S. Structure of neurotoxin B-IV from the marine worm Cerebratulus lacteus: a helical hairpin cross-linked by disulphide bonding. J. Mol. Biol. 1997;268:886–902. doi: 10.1006/jmbi.1997.0980. [DOI] [PubMed] [Google Scholar]
- 100.Howell M.L., Blumenthal K.M. Cloning and expression of a synthetic gene for Cerebratulus lacteus neurotoxin B-IV. J. Biol. Chem. 1989;264:15268–15273. [PubMed] [Google Scholar]
- 101.Howell M.L., Blumenthal K.M. Mutagenesis of Cerebratulus lacteus neurotoxin B-IV identifies NH2-terminal sequences important for biological activity. J. Biol. Chem. 1991;266:12884–12888. [PubMed] [Google Scholar]
- 102.Wen P.H., Blumenthal K.M. Role of electrostatic interactions in defining the potency of neurotoxin B-IV from Cerebratulus lacteus. J. Biol. Chem. 1996;271:29752–29758. doi: 10.1074/jbc.271.47.29752. [DOI] [PubMed] [Google Scholar]
- 103.Wen P.H., Blumenthal K.M. Structure and function of Cerebratulus lacteus neurotoxin B-IV: tryptophan-30 is critical for function while lysines-18, -19, -29, and -33 are not required. Biochemistry. 1997;36:13435–13440. doi: 10.1021/bi970957n. [DOI] [PubMed] [Google Scholar]
- 104.Kem W.R., Blumenthal K.M. Purification and characterization of the cytotoxic Cerebratulus A toxins. J. Biol. Chem. 1978;253:5752–5757. [PubMed] [Google Scholar]
- 105.Kuo J., Raynor R.L., Mazzei G.J., Schatzman R.C., Turner R.S., Kem W.R. Cobra polypeptide cytotoxin I and marine worm polypeptide cytotoxin A-IV are potent and selective inhibitors of phospholipid-sensitive Ca2+-dependent protein kinase. FEBS Lett. 1983;153:183–186. doi: 10.1016/0014-5793(83)80144-6. [DOI] [PubMed] [Google Scholar]
- 106.Blumenthal K.M., Kem W.R. Structure and action of heteronemertine polypeptide toxins. Primary structure of Cerebratulus lacteus toxin A-III. J. Biol. Chem. 1980;255:8266–8272. [PubMed] [Google Scholar]
- 107.Blumenthal K.M. Structure and action of heteronemertine polypeptide toxins. Disulfide bonds of Cerebratulus lacteus toxin A-III. J. Biol. Chem. 1980;255:8273–8274. [PubMed] [Google Scholar]
- 108.Dumont J.A., Blumenthal K.M. Structure and action of heteronemertine polypeptide toxins: importance of amphipathic helix for activity of Cerebratulus lacteus toxin A-III. Arch. Biochem. Biophys. 1985;236:167–175. doi: 10.1016/0003-9861(85)90616-2. [DOI] [PubMed] [Google Scholar]
- 109.Posner P., Kem W.R. Cardiac effects of toxin A-III from the heteronemertine worm Cerebratulus lacteus (Leidy) Toxicon. 1978;16:343–349. doi: 10.1016/0041-0101(78)90154-X. [DOI] [PubMed] [Google Scholar]
- 110.Blumenthal K.M. Structure and action of heteronemertine polypeptide toxins. Membrane penetration by Cerebratulus lacteus toxin A-III. Biochemistry. 1982;21:4229–4233. doi: 10.1021/bi00261a007. [DOI] [PubMed] [Google Scholar]
- 111.Blumenthal K.M. Release of liposomal markers by Cerebratulus toxin A-III. Biochem. Biophys. Res. Commun. 1984;121:14–18. doi: 10.1016/0006-291X(84)90681-8. [DOI] [PubMed] [Google Scholar]
- 112.Blumenthal K.M. Binding of Cerebratulus cytolysin A-III to human erythrocyte membranes. Biochim. Biophys. Acta. 1985;812:127–132. doi: 10.1016/0005-2736(85)90529-2. [DOI] [PubMed] [Google Scholar]
- 113.Liu J.W., Blumenthal K.M. Membrane damage by Cerebratulus lacteus cytolysin A-III. Effects of monovalent and divalent cations on A-III hemolytic activity. Biochim. Biophys. Acta. 1988;937:153–160. doi: 10.1016/0005-2736(88)90237-4. [DOI] [PubMed] [Google Scholar]
- 114.Liu J.W., Blumenthal K.M. Functional interaction between Cerebratulus lacteus cytolysin A-III and phospholipase A2. Implications for the mechanism of cytolysis. J. Biol. Chem. 1988;263:6619–6624. [PubMed] [Google Scholar]
- 115.Liu J., Blumenthal K.M. Identification of oleic acid binding sites in cytolysin A-III from the heteronemertine Cerebratulus lacteus. Toxicon. 1991;29:13–20. doi: 10.1016/0041-0101(91)90035-P. [DOI] [PubMed] [Google Scholar]
- 116.Heine J., McClintock J., Slattery M., Weston J. Energetic composition, biomass, and chemical defense in the common Antarctic nemertean Parborlasia corrugatus (McIntosh) J. Exp. Mar. Biol. Ecol. 1991;153:15–25. doi: 10.1016/S0022-0981(05)80003-6. [DOI] [Google Scholar]
- 117.McClintock J.B., Baker B.J. A review of the chemical ecology of Antarctic marine invertebrates. Am. Zool. 1997;37:329–342. doi: 10.1093/icb/37.4.329. [DOI] [Google Scholar]
- 118.Berne S., Sepcic K., Krizaj I., Kem W.R., McClintock J.B., Turk T. Isolation and characterisation of a cytolytic protein from mucus secretions of the Antarctic heteronemertine Parborlasia corrugatus. Toxicon. 2003;41:483–491. doi: 10.1016/S0041-0101(02)00386-0. [DOI] [PubMed] [Google Scholar]
- 119.Butala M., Sega D., Tomc B., Podlesek Z., Kem W.R., Kupper F.C., Turk T. Recombinant expression and predicted structure of parborlysin, a cytolytic protein from the Antarctic heteronemertine Parborlasia corrugatus. Toxicon. 2015;108:32–37. doi: 10.1016/j.toxicon.2015.09.044. [DOI] [PubMed] [Google Scholar]
- 120.Jacobsson E., Andersson H.S., Strand M., Peigneur S., Eriksson C., Lodén H., Shariatgorji M., Andren P.E., Lebbe E., Rosengren K.J., Tytgat J., Göransson U. Peptide ion channel toxins from the bootlace worm, the longest animal on Earth. Sci. Rep. 2018;8:4596. doi: 10.1038/s41598-018-22305-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rogers J.C., Qu Y., Tanada T.N., Scheuer T., Catterall W.A. Molecular determinants of high affinity binding of α-scorpion toxin and sea anemone toxin in the S3-S4 extracellular loop in domain IV of the Na+ channel α subunit. J. Biol. Chem. 1996;271:15950–15962. doi: 10.1074/jbc.271.27.15950. [DOI] [PubMed] [Google Scholar]
- 122.Göransson U., Rosengren K.J., Jacobsson E., Andersson H.S., Strand M. Nemertea-Derived Bioactive Compounds. World Patent. WO/2018/004433. 2018
- 123.Solis P.N., Wright C.W., Anderson M.M., Gupta M.P., Phillipson J.D. A microwell cytotoxicity assay using Artemia salina (brine shrimp) Planta Med. 1993;59:250–252. doi: 10.1055/s-2006-959661. [DOI] [PubMed] [Google Scholar]
- 124.Whelan N.V., Kocot K.M., Santos S.R., Halanych K.M. Nemertean toxin genes revealed through transcriptome sequencing. Genome Biol. Evol. 2014;6:3314–3325. doi: 10.1093/gbe/evu258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shiomi K., Midorikawa S., Ishida M., Nagashima Y., Nagai H. Plancitoxins, lethal factors from the crown-of-thorns starfish Acanthaster planci, are deoxyribonucleases II. Toxicon. 2004;44:499–506. doi: 10.1016/j.toxicon.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 126.Harvey A.L. Toxins and drug discovery. Toxicon. 2014;92:193–200. doi: 10.1016/j.toxicon.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 127.Ueda A., Nagai H., Ishida M., Nagashima Y., Shiomi K. Purification and molecular cloning of SE-cephalotoxin, a novel proteinaceous toxin from the posterior salivary gland of cuttlefish Sepia esculenta. Toxicon. 2008;52:574–581. doi: 10.1016/j.toxicon.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 128.Xue Z., Liu X., Pang Y., Yu T., Xiao R., Jin M., Han Y., Su P., Wang J., Lv L. Characterization, phylogenetic analysis and cDNA cloning of natterin-like gene from the blood of lamprey, Lampetra japonica. Immunol. Lett. 2012;148:1–10. doi: 10.1016/j.imlet.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 129.Rosado C.J., Kondos S., Bull T.E., Kuiper M.J., Law R.H., Buckle A.M., Voskoboinik I., Bird P.I., Trapani J.A., Whisstock J.C. The MACPF/CDC family of pore-forming toxins. Cell. Microbiol. 2008;10:1765–1774. doi: 10.1111/j.1462-5822.2008.01191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Riesgo A., Andrade S.C., Sharma P.P., Novo M., Perez-Porro A.R., Vahtera V., Gonzalez V.L., Kawauchi G.Y., Giribet G. Comparative description of ten transcriptomes of newly sequenced invertebrates and efficiency estimation of genomic sampling in non-model taxa. Front. Zool. 2012;9:33. doi: 10.1186/1742-9994-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Romiguier J., Gayral P., Ballenghien M., Bernard A., Cahais V., Chenuil A., Chiari Y., Dernat R., Duret L., Faivre N., et al. Comparative population genomics in animals uncovers the determinants of genetic diversity. Nature. 2014;515:261–263. doi: 10.1038/nature13685. [DOI] [PubMed] [Google Scholar]
- 132.Andrade S.C., Montenegro H., Strand M., Schwartz M.L., Kajihara H., Norenburg J.L., Turbeville J.M., Sundberg P., Giribet G. A transcriptomic approach to ribbon worm systematics (nemertea): resolving the Pilidiophora problem. Mol. Biol. Evol. 2014;31:3206–3215. doi: 10.1093/molbev/msu253. [DOI] [PubMed] [Google Scholar]
- 133.MacKenzie C.L., Jr. History of the fisheries of Raritan Bay, New York and New Jersey. Mar. Fish. Rev. 1990;52:1–45. [Google Scholar]
- 134.McDermott J.J., Roe P. Food, feeding behavior and feeding ecology of nemerteans. Am. Zool. 1985:113–125. doi: 10.1093/icb/25.1.113. [DOI] [Google Scholar]
- 135.Napier V., Turpie J., Clark B. Value and management of the subsistence fishery at Knysna Estuary, South Africa. Afr. J. Mar. Sci. 2009;31:297–310. doi: 10.2989/AJMS.2009.31.3.3.991. [DOI] [Google Scholar]
- 136.Jennings J., Gibson R. Observations on the nutrition of seven species of Rhynchocoelan worms. Biol. Bull. 1969;136:405–433. doi: 10.2307/1539685. [DOI] [Google Scholar]
- 137.Moles J., Núñez-Pons L., Taboada S., Figuerola B., Cristobo J., Avila C. Anti-predatory chemical defences in Antarctic benthic fauna. Mar. Biol. 2015;162:1813–1821. doi: 10.1007/s00227-015-2714-9. [DOI] [Google Scholar]
- 138.Kruse I., Buhs F. Preying at the edge of the sea: the nemertine Tetrastemma melanocephalum and its amphipod prey on high intertidal sandflats. Hydrobiologia. 2000;426:43–55. doi: 10.1023/A:1003955523468. [DOI] [Google Scholar]
- 139.Caplins S.A., Turbeville J.M. The occurrence of Ramphogordius sanguineus (Nemertea, Heteronemertea) in the intertidal zone of the Atlantic coast of Virginia and new observations on its feeding behavior. Banisteria. 2011;38:65–70. [Google Scholar]
- 140.Bourque D., Miron G., Landry T. Predator prey relationship between the nemertean Cerebratulus lacteus and the soft-shell clam, Mya arenaria: surface-exploration activity and qualitative observations on feeding behaviour. Can. J. Zool. 2002;80:1204–1211. doi: 10.1139/z02-095. [DOI] [Google Scholar]
- 141.Beckers P., Bartolomaeus T., von Döhren J. Observations and experiments on the biology and life history of Riseriellus occultus (Heteronemertea: Lineidae) Zool. Sci. 2015;32:531–546. doi: 10.2108/zs140270. [DOI] [PubMed] [Google Scholar]
- 142.McDermott J.J. Nemerteans as hosts for symbionts: a review. J. Nat. Hist. 2006;40:1007–1020. doi: 10.1080/00222930600834121. [DOI] [Google Scholar]
- 143.Jensen K., Sadeghian P.S. Nemerteans (ribbon worms) In: Rohde K., editor. Marine parasitology. CSIRO publishing; Clayton, Australia: 2015. [Google Scholar]
- 144.Gibson R. Studies on the biology of the entocommensal rhynchocoelan Malacobdella grossa. J. Mar. Biol. Assoc. UK. 1968;48:637–656. doi: 10.1017/S0025315400019202. [DOI] [Google Scholar]
- 145.Roe P. Laboratory studies of feeding and mating in species of Carcinonemertes (Nemertea: Hoplonemertea) Biol. Bull. 1984;167:426–436. doi: 10.2307/1541287. [DOI] [PubMed] [Google Scholar]
- 146.Kuris A.M., Wickham D.E. Effect of nemertean egg predators on crustaceans. Bul. Mar. Sci. 1987;41:151–164. [Google Scholar]
- 147.Blunt J.W., Copp B.R., Keyzers R.A., Munro M.H., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2015;32:116–211. doi: 10.1039/C4NP00144C. [DOI] [PubMed] [Google Scholar]
- 148.Blunt J.W., Carroll A.R., Copp B.R., Davis R.A., Keyzers R.A., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2018;35:8–53. doi: 10.1039/C7NP00052A. [DOI] [PubMed] [Google Scholar]
- 149.Kinch M.S., Haynesworth A., Kinch S.L., Hoyer D. An overview of FDA-approved new molecular entities: 1827–2013. Drug Discov. Today. 2014;19:1033–1039. doi: 10.1016/j.drudis.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 150.Molinski T.F., Dalisay D.S., Lievens S.L., Saludes J.P. Drug development from marine natural products. Nat. Rev. Drug Discov. 2009;8:69–85. doi: 10.1038/nrd2487. [DOI] [PubMed] [Google Scholar]
- 151.Jaspars M., De Pascale D., Andersen J.H., Reyes F., Crawford A.D., Ianora A. The marine biodiscovery pipeline and ocean medicines of tomorrow. J. Mar. Biol. Assoc. UK. 2016;96:151–158. doi: 10.1017/S0025315415002106. [DOI] [Google Scholar]
- 152.Malve H. Exploring the ocean for new drug developments: Marine pharmacology. J. Pharm. Bioall. Sci. 2016;8:83–91. doi: 10.4103/0975-7406.171700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Moczydlowski E.G. The molecular mystique of tetrodotoxin. Toxicon. 2013;63:165–183. doi: 10.1016/j.toxicon.2012.11.026. [DOI] [PubMed] [Google Scholar]