Abstract
Recent investigations have shown that different conditions such as diet, the overuse of antibiotics or the colonization of pathogenic microorganisms can alter the population status of the intestinal microbiota. This modification can produce a change from homeostasis to a condition known as imbalance or dysbiosis; however, the role-played by dysbiosis and the development of inflammatory bowel diseases (IBD) has been poorly understood. It was actually not until a few years ago that studies started to develop regarding the role that dendritic cells (DC) of intestinal mucosa play in the sensing of the gut microbiota population. The latest studies have focused on describing the DC modulation, specifically on tolerance response involving T regulatory cells or on the inflammatory response involving reactive oxygen species and tissue damage. Furthermore, the latest studies have also focused on the protective and restorative effect of the population of the gut microbiota given by probiotic therapy, targeting IBD and other intestinal pathologies. In the present work, the authors propose and summarize a recently studied complex axis of interaction between the population of the gut microbiota, the sensing of the DC and its modulation towards tolerance and inflammation, the development of IBD and the protective and restorative effect of probiotics on other intestinal pathologies.
Keywords: inflammatory bowel disease, dendritic cells, ulcerative colitis, Crohn’s disease, gut microbiota, probiotic
1. Background
Inflammatory bowel disease (IBD) is comprised of a group of pathological entities characterized by inflammation of the small intestine and colon. The two main diseases relative to IBD are ulcerative colitis (UC) and Crohn’s disease (CD) [1] In the population younger than 20 years of age, the incidence of CD amounts to approximately 43 out of 100,000 inhabitants whereas that of UC amounts to 28 out of 100,000. [2]. Given these are chronic diseases there is an incidence increase seen in patients older than 20 years of up to 201 out of 100,000 for CD and 238 out of 100,000 for UC. [2,3]. The highest incidence rates and prevalence of Crohn’s disease and ulcerative colitis are predominantly reported in industrialized countries such as northern Europe, the United Kingdom and North America. These rates have reached a plateau after the steady rise seen in these regions after the end of World War II, while rates continue to rise in low-incidence areas such as southern Europe, Asia and most developing countries [4,5,6]. In the industrialized countries, the incidence rates range from 6.5 to 16.0 cases per 100,000 persons/year, while the prevalence rates range from 26 to 214 patients per 100,000 persons/year [7]. Within these countries, the United States has a prevalence range for UC of 37 to 246 cases per 100,000 persons and an incidence range of 2.2 to 14.3 cases per 100,000 per persons/year. For CD, the prevalence ranges from 26 to 199 cases per 100,000 persons and the incidence ranges from 3.1 to 14. 6 cases per 100,000 persons/year [8]. In Europe, UC has incidence rates range from 1.5 to 20.3 cases per 100,000 person/year, while these rates range from 0.7 to 9.8 cases per 100,000 person-years for CD [4].
One of the developing regions that continues its rise in incidence and prevalence of IBD is Latin America [4]. Still, epidemiologic studies in the countries within Latin America are scarce due to the gradual onset, the lack of universally accepted criteria for diagnosis and the idea that in the past this disease was rare there [7]. Most of these countries do not have an efficient data recording method in order to provide information for epidemiologic studies. However, some have data like Colombia, Peru and Brazil. In Colombia, from 2001 through 2009, 202 cases were diagnosed with IBD, where 80.7% of them had UC and 15.8% had CD [8]. Brazil has made few studies, usually only describing clinical aspects of the patients that arrive at the hospitals in the region with no incidence and prevalence. The most recent data has only proven that CD is more prevalent than UC [7]. In Peru, several studies on UC have been made in hospitals like the Hospital Guillermo Almenara which received 74 cases in 52 years, the Hospital Edgardo Rebagliati which received 43 cases in 2 years and the Hospital Cayetano Heredia which received 27 cases in 7 years. For CD, 17 cases were reported in a period of 20 years in the Hospital Edgardo Rebagliati [8]. Regardless, epidemiologic data is minimum in other countries of Latin America, including Mexico.
2. Microbiota–Dendritic Cell-Mucosal Immune Response–IBD Interaction
The causes leading to the development of IBD are still up to this date uncertain. Regardless, it has been proposed that its origin could be multifactorial, involving the patient’s genetic predisposition, nutrition and eating habits, as well the status of intestinal microbiota and the integrity of the intestinal barrier function [9,10]. The interaction of all these factors has effect on both the intestinal homeostasis and the pathological condition of the uncontrolled immune-mediated inflammatory response present in IBD.
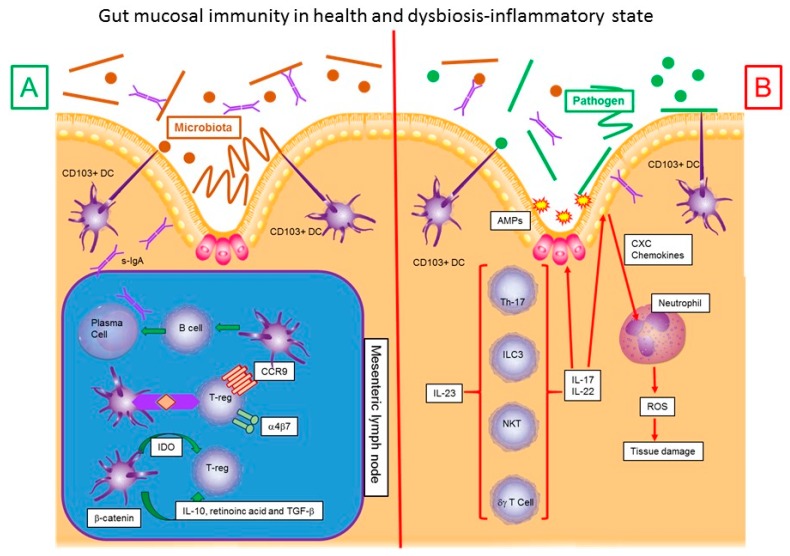
It all in fact originates in the lamina propria (LP) of a healthy intestine, where dendritic cells (DC) sense antigens, which originate from food and bacteria that make up the intestinal microbiota as well as of its metabolite. The count of bacteria and the microbiota is made by the presence of various receptors, made up mainly by different types of toll like receptors (TLR) (Figure 1) [11]. Dendritic cells are surveillance cells that among other tasks, play the indispensable function of distinguishing between self and non-self. They have the capacity to recognize different molecules such as proteins, lipids, carbohydrates and nucleic acids of bacterial, viral, fungal or protozoan origin known as pathogen-associated molecular patterns (PAMPs). To achieve this surveillance task, DCs possess distinct types of receptors among which are: TLR, RIG-I-like receptors (RLR), NOD like receptors (NLR) and C-type lectin receptors (CLR) [12].
Figure 1.
Gut mucosal immunity in health and dysbiosis-inflammatory state schematic representation of the interaction between gut microbiota, dendritic cells and inflammatory response in Gut mucosa in a healthy state (A) and gut mucosa in dysbiotic—inflammatory state (B). Taken from [11]. α4β7: α4β7 Integrin, AMPs: antimicrobial peptides, CCR9: C-C chemokine receptor type 9, CXC: CXC Chemokines family, DC: Dendritic Cell, IDO: Indoleamine-pyrrole 2,3-dioxygenase, IL-10: Interleukin 10, IL-17:Interleukin 17, IL-22: Interleukin 22, IL-23: Interleukin 23, ROS: Reactive oxygen species, s-IgA: Secretory Immunoglobulin A, TGF-β: Transforming growth factor beta, T-reg: Regulatory T cells, Th-17: T-helper cell 17 lineage, NKT: Natural killer T cells.
One of the most important events for keeping intestinal homeostasis is the induction by DCs of anergic and/or regulatory T cells (Tregs), which is crucial for the maintenance of peripheral tolerance in addition to regulating the response by altering the Th1/Th2/Th17 balance. The adequate induction of tolerance by DCs depends on various factors such as the state of maturation, the DC subsets, the exposure to anti-inflammatory, immunosuppressive, environmental or microbial stimuli, among others [13,14,15,16,17]. In relation to the state of maturation of DCs in the induction of tolerance, immature DCs characterized by a low expression of surface major histocompatibility complex class II (MHC II ) and costimulatory molecules, induce suboptimal T-cell priming. Immature DCs promote tolerance in vivo by either deleting antigen-specific T cells or by expanding regulatory T cells [18,19,20,21].
On the other hand, mature DCs promote immunogenic responses [22,23,24], although under some conditions these DCs can be tolerogenic as has been demonstrated with the disruption of E-cadherin-mediated DC-DC interaction that promotes DC maturation and the secretion of high levels of IL-10 that induces the tolerogenic response [25].
The subtype of DC is another factor involved in the induction of tolerance and is influenced by the local environment and state of activation. As example of the former, in the intestine, various factors produced by the epithelial cells and stromal cells such as transforming growth factor beta (TGF-β) [26], Thymic stromal lymphopoietin (TSLP) [27] and retinoic acid (RA) [28] shape the functions of tolerogenic DCs and of the latter, in the resting steady state certain DC subsets have a propensity to induce tolerogenic T cells. In particular, the presence of tolerogenic DC subsets has great relevance at mucosal surfaces where the immune system needs to play a relevant dual role of maintaining tolerance to self-antigens and commensals and mounting strong immune responses to pathogens. Thus, the tolerogenic subsets in the mucosal compartment prevent excessive inflammation and immunity against commensals and food or environmental antigens. As just mentioned, RA is an important compound produced by DCs for the generation of Tregs. The conversion of vitamin A-derived retinol to RA is catalysed by retinaldehyde dehydrogenases (ALDHs), which are crucial enzymes in the induction of Tregs and only DCs that possess them can promote intestinal tolerance and homeostasis. It has been shown that both CD103+CD11b+ and CD103+CD11b− DCs can produce RA and induce Foxp3+ Tregs in vitro [14,29]. Interestingly, the tolerogenic properties of these cells can change under some circumstances as has been shown in colitic mice where CD103+ DCs do not induce Foxp3+ Tregs and instead favour the production of IFN-γ-producing CD4+ T cells [30]. Also, the ratio of DCs:Tcells is important in the induction of tolerance.
Another important factor that influences the tolerogenic properties of DCs is the exposure to microbial products. In many cases this is achieved through the recognition of different microbial ligands by pattern recognition receptors (PRRs) such as TLRs and CLRs that induce Th2 or tolerogenic responses. In relation to TLRs it has been shown that yeast zymosan [31,32,33,34] or Y. pestis virulence factor Lcr [35] signal through TLR2-TLR6 in DCs and induce regulatory T cells. Also, it has been suggested that in pDCs TLR-9 activation induces indole amine 2, 3-Dioxygenase (IDO), which promotes differentiation of Tregs and suppresses T-cell responses [36,37].
As for CLRs, it has been shown that activation of DC-SIGN in DCs by different microbial compounds promotes Tregs-responses [38]. Examples of these are cell surface compounds of Lactobacillus reuteri and L. casei, Lewis antigens on lipopolysaccharide (LPS) from Helicobacter pylori [39] and lpA of L. acidophilus NCFM [40] that bind to dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) and induce IL-10 production and suppress T-cell effector responses. It has also been shown that activation of SIGNR-1 in lamina propria DCs selectively induces IL-10 expression and promotes the induction of Tr1 regulatory cells [41]. All types of galectins, surface, secreted and endogenous, are also important molecules in the promotion of tolerance. In particular, Galectin-1-mediated signals promote tolerance in DCs by inducing the expression of several regulatory molecules like signal transducer and activator of transcription 3 (STAT3), suppressor of cytokine signaling 1 (SOCS1) and histone deacetylase 11 (HDAC11) [42,43].
The interaction of DCs with other cells is another factor that contributes to their tolerogenic profile. As an example, the tolerogenic responses in the intestine are maintained through the concerted action of interleukin 10 (IL-10)-secreting macrophages and DCs and IL-10 is fundamental in the suppression of inflammation such as colitis. Also, the interaction of DCs with non-hematopoietic cells is important for the induction of T-regulatory cells in the intestine. It has been shown that intestinal epithelial cells (IECs) are important in conditioning the intestinal DCs to a tolerogenic state through the secretion of anti-inflammatory mediators such as TGF-β, RA or granulocyte-macrophage colony-stimulating factor (GM-CSF). In addition to ECs, stromal cells also play a critical role in conditioning DCs to a regulatory or tolerogenic state in various organs such as the liver, intestine, gut-associated lymphoid tissues (GALT) and spleen [44,45,46].
As previously mentioned, at mucosal surfaces the immune system has to mount an immune response to microbes and yet be tolerant to commensals. In particular, intestinal commensals play a critical role in shaping DCs functions and promoting tolerance [47,48,49]. The induction of tolerance by commensals can be through the induction of TSLP and TGF-β by IECs or by the promotion of T-regulatory cells. It has been shown that DCs cultured in the presence of IECs and Gram-positive commensal bacteria differentiate into IL-10-producing tolerogenic DCs [50]. Also, in germ-free mice the colonization with the human commensal Bacteroides fragilis induces the development of Foxp3+ T-regulatory cells [51]. The generation of T regs can also be promoted by commensals products as in the case of polysaccharide A (PSA) of B. fragilis that can convert CD4+ T cells into Foxp3+ T-regulatory cells that produce IL-10 during commensal colonization. Contrarily, some commensals can have the capacity to suppress T-regulatory cells and promote Th17 responses. It has been shown that colonization of the small intestine of mice with a segmented filamentous bacterium (SFB) induces the appearance of Th17 cells in the lamina propria [52]. In addition to the commensal bacteria, intestinal helminths can also promote T-regulatory cell differentiation by activating TGF-βR on the antigen-presenting cells [53].
3. Microbiota and Inflammatory Bowel Disease
Concerning the origin of pathologies involving these mechanisms like IBD, the etiology still remains largely unknown. Some studies proclaim that the cause is multifactorial, involving intestinal microbiota, nutrition and the patient’s genetic profile. Recent studies evidence that specifically, the impact on the stability of the population and metabolism balance has a direct relationship in the physiopathology of IBD.
By using ribosomal RNA (rRNA) sequencing, Frank et al. proved in 2007 that the bacterial population in patients with IBD is anomalous. Predominant phyla in the intestinal microbiota of healthy individuals are Firmicutes and Bacteroidetes; however, in patients with IBD there is a decrease of the bacterial population or dysbiosis, a substantial depletion of these phyla and a substitution by phyla Actinobacteria and Proteobacteria (alpha, beta and gamma) [54]. Another study realized in 2012 by Morgan et al. explains that the dysbiosis observed in IBD generates an alteration of metabolism that leads to oxidative stress and perturbed nutrient availability during tissue damage [55]. Gevers et al. in 2014, on the other hand found another cause, demonstrating that antibiotic use amplifies the microbial dysbiosis associated with CD [56].
Another factor associated with dysbiosis is the arrival of pathogenic microorganisms. There are reports indicating that bacteria such as Clostridium difficile, enterotoxigenic Escherichia coli (ETEC) and Salmonella spp may participate in the development of IBD [57]. In a study made by Satokari R. in 2015, rats inhabiting polluted environments with pathogenic bacteria developed IBD, while rats that lived in pathogen-free conditions never developed the disease, suggesting a possible participation of pathogenic microorganism in the physiopathology of this disease [58].
These and other research establish a major participation of the imbalance of the population (dysbiosis) of the intestinal microbiota on IBD physiopathology, antibiotics or infections may induce this dysbiosis. One of the main effects of these previously mentioned conditions is the generation of conditions with high concentrations of reactive oxygen species (ROS) at an intestinal level which will contribute to a more severe inflammatory process at an intestinal level.
Among the causes or triggers of Crohn’s disease, there has been an association between the integrity of the immune system and the patient’s microbiota, this association is of great interest because it starts through the genetic factors of each individual.
Genes that confer a susceptibility to DC have been found, such as the nucleotide-binding oligomerization domain-containing protein 2 (NOD2 gene), whose function is an immune reaction to recognize a peptidoglycan found in the cell of bacterium, both gram positive and negative [59]. Swidsinski et al. found that in patients with mutations in the NOD2 gene there is an increased number of bacteria adhered to the mucosa and a decrease in the transcription of interleukin-10, an anti-inflammatory cytokine [60].
Individuals with mutations in NOD2 and Autophagy-related protein 16-1 (ATG16L1), a gene involved in the process of autophagy that confers susceptibility to CD, present alterations in the strains that make up the microbiota, decreased levels of Faecalibacterium and high levels of Escherichia [61]. Kang et al. found a decrease in diversity within Firmicutes phylum, this decrease has been associated with a temporary instability of the microbiota in both individuals with DC and UC [62].
Enterobacteria are particularly elevated in patients with CD and UC, especially Escherichia coli, which has been isolated from the ileus of individuals with CD through biopsies in several studies [63]. This increase in enterobacteria could indicate a preference for an inflammatory environment such as that of individuals with CD. In fact, a reduction in Escherichia/Shigella levels has been demonstrated in patients with IBD after administration of mesalazine, an anti-inflammatory drug.
A second group of bacteria adhered to the mucosal layer of the colon in patients with CD and UC is Fusobacterium, gram-negative anaerobic bacteria that mainly colonize the oral cavity. Fusobacterium species raised in colorectal cancer biopsies have been found, this is of interest because IBD is one of the most important risk factors for the development of colorectal cancer, suggesting an association between these two diseases [64].
Unlike CD, UC has a less extensive description of the dysbiosis caused by it. In 1988 Tysk et al. found that UC seems to be more related to environmental factors than CD [65]. Spehlmann et al. found in 2008 that monozygotic twins with CD have a concordance of 30% whereas those with UC have one of only 10% [66].
A quantitative and qualitative decrease in the Firmicutes phylum has been shown to be present in both UC and CD. This includes many butyrate producing bacteria such as Faecalobacterium prausnitzii [67]. UC has shown a decrease of the Firmicutes and Bacteroides phylum while having an increase of the Proteobacteria and Actinobacteria phylum [68,69,70].
In a cohort conformed by 127 patients with UC from 2013, Machiels et al. found that UC does not share the microbiota signature of CD. UC patients showed a decrease in numbers of Roseburia hominis and Faecalibacterium prausnitzii relative to the control patients, as well as a decrease in short chain fatty acids (SCFAs) [71]. In 2013, Rajilić-Stojanović et al. found that, even during remission periods of UC, the dysbiosis persists. Thanks to a microarray that detects and quantifies more than 1000 intestinal bacteria, they found a decrease in numbers of the Clostridium cluster IV and the bacteria related to the butyrate and propionate metabolism such as Ruminococcus bromii, Eubacterium rectale, Roseburia sp. and Akermansia sp. They also found an increase in opportunistic bacteria such as Fusobacterium sp., Peptosterptococcus sp., Helicobacter sp., Campilobacter sp. and Clostridium difficile [72].
Dysbiosis consistently shows a decrease in SCFAs such as acetate, propionate and butyrate. These SCFAs are the primary energy source for the epithelial colonic cells [73] and promote the expansion of regulatory T cells in the colon. This decrease in SCFAs has been related to damage to the colonic epithelial cell, causing thinning of this layer, which in turn can cause diarrhoea, colitis and even pouchitis.
4. Inflammation in Inflammatory Bowel Disease
In a physiological way CD103+ DC induce T-reg to express gut-homing markers α4β7 and chemokine receptor type 9 (CCR9), which allow them to localize where the immune response is needed in terms of specific tissues [74,75,76]. This enhanced lymphocyte expression of gut-homing molecule α4β7 results in an alteration in the lymphocytary traffic, which is also observed in IBD [77,78,79].
After pathogenic microorganisms activate macrophages and DC, they produce IL-1b, IL-6, IL12 and IL-23 and thus activate T helper 17 (TH17) cells, γδ T cells, natural killer (NK) cells, natural killer T (NKT) cells and group 3 innate lymphoid cells (ILC3s). When these cells are activated, they will secrete tumour necrosis factor-alpha (TNF-α), interferon-gamma (IFN-γ), IL-17 and IL-22 that will stimulate intestinal epithelium to produce antimicrobial peptides (AMPs). These peptides will help secrete CXC-chemokine that is a chemo-attractant for neutrophils, which will be attracted to a specific location to produce and release ROS [80,81,82]. Out of these participating cells, both UC and CD have been uniquely related to a particular type of NK lymphocytes producing IL-22, a mediator cytokine in the response to bacteria in epithelial cells and regulator of autoimmune response. It has also been widely reported that in UC the response occurs preferentially by Th2 while in CD, Th1 is the most present cell, making the cytokines profile on both diseases vastly different [83,84]. In CD the presence of IL-1a, IL-1b, IL-12, INF-γ is imminent, while in UC IL-13 is the most abundant [85,86]. (See Table 1).
Table 1.
Cytokine’s profile in Crohn’s disease (CD) and ulcerative colitis (UC). IL: Interleukin, TNF: Tumour Necrosis Factor, IFN: Interferon, I: Increased, N: Normal. Information based on: [82,83,84,87].
| Cytokine | CD | UC | Cytokine | CD | UC |
| IL-1b | I | I | IL-5 | N | I |
| IL-6 | I | I | IL-13 | N | I |
| IL-8 | I | I | IL-17 | I | I |
| IL-12 | I | N | IL-21 | I | N |
| IL-18 | I | I | IFN-γ | I | I |
| IL-23 | I | N | IL-4 | N | I |
| IL-27 | I | N | IL-22 | I | I |
| TNF-α | I | I |
With regard to the patient’s genetic predisposition, there is evidence suggesting that individuals presenting mutations in some of the genes involved in the immune response could be more prone to the activation via NFκB for the overproduction of pro-inflammatory cytokines as IL-1b, IL-6, IL-8 and TNF [87].
These mutations have been identified in the genes that encode for the receptor NOD1 (CARD4) which mainly identifies the gram-negative bacteria peptidoglycan, as well as NOD2 (CARD15) which identifies bacteria muramyldipeptide (MDP) both Gram (+) and Gram (−), regulators of TLRs (OCTN, DLG5) and genes related to the autophagy in dendritic cells of the mucosal [88].
Regarding the possible participation of the autoimmune phenomena in IBD, the finding of the auto-antibodies against the proteins of the cytoskeleton, lymphocytes antigens, cardiolipin and pancreatic proteins has been reported both in UC and in CD [89]. Antibodies against glycoproteins of goblet cells, more specific to the intestine, have also been found but have been studied in a more superficial manner [90]. The antibodies that have been found in a more continuous matter in this disease are Anti-Neutrophil Cytoplasmic Antibodies (ANCA) and Anti-Saccharomyces Cerevisiae Antibodies (ASCA) predominantly [83,91].
As established, the role of a stable and healthy microbiota is very important as an inflammation inhibition mechanism and therefore crucially inhibiting IBD’s physiopathology. Clearly, intestinal microbiota exercises an important inhibiting effect of the development of pathogenic microorganisms, which is obtained by means of competitive inhibition, the decrease of permeability at an intestinal level (L. rhamnossus) and chemical inhibition through the secretion of lactic acid (L. acidophilus) and so forth. It has even been reported that the production of short chain fatty acids on by the intestinal microbiota inhibits the production of carcinogenic molecules in the intestine (L. plantarum) [92].
In virtue of the previous, the re-establishment of the population balance of the intestinal microbiota is certainly very important as a corrective measure of the dysbiosis observed in IBD. In fact, based on this theory, probiotics have been proposed as a therapy to avoid IBD all along.
5. Probiotics
The definition of probiotics has been modified and changed over time, but the definition postulated by the World Health Organization (WHO) and accepted by the International Scientific Association for Probiotics and Prebiotics (ISAPP) is the one used today: “living microorganisms that, when administered to a host in a controlled dosage, confers important health benefits.” The knowledge and information about lactic acid fermentation on humans dates back to ancient times, for example, ancient Romans and Greeks used various recipes for fermented milk. In the 20th century, Ilia Miecznikow, a Russian scientist, immunologist and Nobel Prize winner (1907), that worked for the Pasteur Institute in Paris, started a great interest in lactic acid fermentation. Since then, changes on these probiotics have been realized, including changes on their encapsulation resulting in higher resistance, better stability and the formation of a more efficient biofilm [93].
The formation of biofilm, defined by Donlan and Costerton in 2002 as “a sessile community of bacteria characterized by cells that are irreversibly linked to a substrate or interface or among them, are embedded in a matrix of extracellular polymeric substances which have produced and exhibit an altered phenotype regarding the growth speed and the transcription gene,” is a completely substantial process [94]. An effective biofilm gives the colony the capacity to protect itself against external factors, either biotic or antibiotic, to resist changes in pH, temperature and mechanical forces and to remain for longer periods in the binding site. In other words, these resistant biofilms if built in a resistant form, may create a competition with damaging bacteria already living in the host [95,96].
Still, the capacity of formation of a resistant biofilm could be affected by mutations in the genotype of certain bacteria. For example, it has been proven that L. rhamnosus GG decreases its capacity to form biofilms whenever a mutation in the luxS gene is suffered. The make-up of the composition of the media where it is growing has also been seen to be an important factor, since it is subject to temperature changes, can result in an altered chemical make-up, the availability of nutrients can vary vastly.
Aside from the media and mutations, it has been demonstrated that the capacity of bacteria to form biofilms depends on the specific strain producing it and the quantity of bacteria. For example, L. rhamnosus CRL 1332 and L. reuteri CRL 1324 creates a biofilm that is as well-structured but the second uses a higher quantity of extracellular material to create it [97]. Still, this strain of L. reuteri is particularly susceptible to proteases (particularly protein kinase K) since it utilizes proteins more than L. rhamnosus CRL 1332 to produce the biofilm [98].
In order to resist external factors that could destroy the already produced biofilm, probiotics have proved to have the capacity to inhibit its enteric pathogens by the production of lactic acid, hydrogen peroxide and bacteriocins [99]. They also create a competitive exclusion by blocking the adhesion sites of the pathogen, competing for the nutrients and modulating the system will immunize reaching the reduction of the inflammatory response [90]. All these variables complicate the development of a stable colonization. The most extensively used bacteria considering these defence mechanisms are the lactic acid bacteria including the Lactobacillus spp kind, which are gram-positive bacteria and facultative anaerobic [100]. Generally, probiotic products containing specific bacteria strains are developed in different formulations, ranging from chewing gum, fermented milk and capsules. The problem is that these products end up being ineffective due to different mechanisms: (1) Bifidobacterium longum is the only strain that can survive in fermented milk for 2 weeks. (2) The viable bacteria capable of reaching the intestinal tract is limited, because bacteria cannot survive the low pH in the stomach. Anand et al. using Lactobacillus fermentum 2311 in capsules made of hydroxypropyl methylcellulose phthalate (HPMCP), demonstrated that tablet formulation containing HPMCP 55 and sodium alginate showed the property to protect the bacterial strains in acidic environments like the stomach. Also, the storage is an important process to preserve the number of viable cells [101].
In conclusion, the use of probiotics has changed overtime, while new technologies for the alteration and use of probiotics are being developed. At the beginning simple forms of bacteria were used that were contained in dairy products only [102]. After observing these natural forms of probiotics were not as effective, new encapsulating techniques have been developed in order to produce bacteria that could effectively reach its action sites and produce biofilms where needed [103,104,105].
6. Use of Probiotics in Inflammatory Bowel Diseases
Thanks to the capability witnessed of probiotics to modulate the bacterial colonization and prevent the potentially pathogenic bacterial overpopulation, an extensive network of treatments have been used to treat gastrointestinal diseases and reduce the negative effects of antibiotics. The mechanisms of action proposed for the therapeutic effect of probiotics against enterogenic pathogens is its capacity to stabilize the intestinal mucosa, increase secretion and improve intestinal motility. In terms of immunologic action, these also modulate the inflammatory response, increasing immunoglobulin A (IgA), microbicide factors and macrophage activity.
Specifically, for inflammatory diseases, certain lines of lactobacilli and bifidobacteria probiotics have been found to mitigate UC in mice decreasing the production of pro-inflammatory cytokines. In a study made by J. McCarthy with mice knock out (KO) for IL-10, these probiotics mitigated the UC in statistically significant amounts. These results were found to have been caused by the reduction of the secretion of pro-inflammatory cytokines. These include IL-12, transforming growth factor (TGF), INF and TNF, which were observed to decrease in the presence of probiotics such as Bifidobacterium infantis 35624, [106].
Lammers et al. in 2003 demonstrated that stimulation with isolated bacterial DNA of stool in combination with the mixture of VSL#3 probiotics (Bifidobacterium longum, Bifidobacterium breve, Bifidobacterium infantis, Lactobacillus acidophilous, Lactobacillus casei, Lactobacillus delbrueckii subsp. bulgaricus, Lactobacillus plantarum and Streptococcus salivarius) increased the production of IL-1B and IL-10 cytokines. Bacterial DNA on one hand induced a higher production of IL-1B and the production of other pro-inflammatory factors while the treatment with probiotics of VSL #3 mainly resulted in a higher production of IL-10. The production of IL-10 specifically, was seen to produce an improved response in the immune system of the intestinal mucosa, thus mitigating the IBD symptoms [107]. Zaylaa et al. used a mix of 11 Lactobaccillus and Bifidobacterium, where they found 5 strains that had high potential for the management of IBD [108]. It is important to remember something already mentioned, that there are variables both in the host environment and in the probiotic used that complicate the development of a stable colonization.
In a systematic review published by the Mexican consensus about probiotics in gastroenterology, there is enough scientific evidence to prove that probiotics are effective in the prevention of infectious, inflammatory and functional diseases of the digestive system. However, it is necessary to evaluate the strain of the microorganism to be used in each case for its administration [109]. Other therapeutic measures used in the treatment of IBD seeking the reestablishment of intestinal microbiota is the faecal microbiota transplant (FMT). This is a much more direct method in the modification of intestinal microbiota. Although the use and benefits FMT have been controversial there is scientific evidence of its real efficacy, but this will depend of each individual clinical case. Moayyedi et al. in 2015 proved the remission in 24% of patients at the seventh week of the treatment; in their research, they indicate the participation of other factors as part of the success. They mainly refer to the type of donor, the timely treatment regarding the progress of the disease, as well as the immunosuppressant treatment [110].
Most studies of the use of probiotics in the treatment of CD use patients in remission, reporting that it is possible to limit the recurrence of the disease. Historically, the most used probiotics in the treatment of CD have been Lactobacillus sp. such as L. GG and L. johnsonii, however, used by themselves show poor results [111]. The use of Saccharomyces boulardii has shown promise in the prevention of relapse. Guslandi et al. found in 2012 a 6-fold risk of recurrence y patients treated with mesalamine as opposed to those treated with S. boulardii and mesalamine (p = 0.04) [112]. In 1993, Plein et al. found similar results [113]. However, some studies such as the one by Boureille et al. in 2013 found no significant difference between the number of relapses in the S. boulardii and the placebo groups [114].
Steed et al. in 2010 found symptomatic improvements with the use of Bifidobacterium longum with inulin and oligofructose (“Synergy 1”) [115]. Fujimori et al. found in 2007 similar results with the use of B. longum, Lactobacillus casei and plantago ovata [116]. Fedorak et al. in 2014 found that VSL#3 (a mixture of 8 different probiotics: 4 strains of Lactobacillus, 3 strains of Bifidobacterium and Streptococcus thermofilus) decreased the amount of inflammatory citokines in the intestinal mucosal compared to placebo (p < 0.05). However, there was no statistical difference between the number of patients with lesions (both severe and non-severe) [117].
There is a small number of studies about the use of probiotics in IBD and most of them seem to indicate that they are of little to no use. There is, however, a lot of room for research in this area.
Dysbiosis of intestinal microbiota plays an important role in the aetiology and pathogenesis of UC, resulting in increased numbers of proinflammatory citokines. Probiotics have been gaining a lot of attention in the last years due to their different mechanisms of action by which they help to effectively induce and maintain remission in UC patients: restore the function of disturbed mucosal barrier, inhibit competition of potential pathogens, enhance intestinal barrier function, recover intestinal microbiota imbalance and improve local and systemic immunity by decreasing proinflammatory citokines and increasing anti-inflammatory citokines [118].
Even though the data of this topic is reduced, there are recent meta-analyses and clinical trials suggesting that probiotics could be used as adjuvant therapy in some UC patients. Ghouri et al. concluded that probiotics and prebiotics helped to induce and maintain remission of UC, while there was no evidence of benefits in CD [119]. Most trials considered either E. coli Nissle 1917 or the probiotic VSL#3. Kruis et al. concluded that E. coli Nissle 1917 therapy was as effective as mesalamine in maintaining remission of UC [120]. Tsuda et al., used different probiotics, being Enterococcus faecalis, Clostridium butyricum and Bacillus mesentericus showing a reduced disease activity [121]. Although there is a reduced number of large scale and randomized trials on this topic, there is a great potential of probiotics as an alternative for pharmacological therapy in UC patients.
Another mechanism by which probiotics play an important role in the therapy of UC is their ability to recover epithelial barrier integrity, either by down regulating the proinflammatory citokines involved in the pathogenesis and immune cell activation. These limitation of inflammatory signals to the epithelial barrier may help to reduce host-induced epithelium damage. These variety of mechanisms by which probiotics modulate local and systemic immunity will be of interest in the therapy of UC patients [122]. Everard et al. showed that using S. boulardii in antibiotic-treated mice, resulted in a faster return to pre-antibiotic levels of specific bacterial strains, including butyrate producer species [123]. In the context of UC, it may be especially helpful to recover butyrate producers. Al-Sadi et al. showed that increased TNF-α, IFN-γ and IL-23 stimulate the epithelial barrier breakdown and can be modulated with specific probiotic strains [124], including Lactobacillus fermentum, Lactobacillus salivarius, Bifidobacterium lactis and mixtures of Lactobacillus and Bifidobacterium species [125]. Van der Waal et al. used a mixture called Ecologic ® 825 in 2019 and achieved significant lowering of symptoms in UC, bettering the quality of life [126].
Diverse strains of probiotics, including B. breve [127], B. longum [128], L. acidophilus [129], B. longum infantis [130] and Streptococcus thermophilus decrease IL-17 by modulating the citokines that promote Th17 responses, thus there is a downregulation of IL-17 production.
It is important to note that even though there are diverse studies showing the efficacy of probiotics as a treatment for UC, much of them relied on in vitro models rather than in vivo models. Studies have not been consistent and more larger sample sizes and randomized trials are needed to evaluate the diverse mechanisms by which probiotics could be used as a therapy for UC.
7. Conclusions
Although research on probiotics and their effect on gastrointestinal infectious diseases have been realized for several years, the latest studies have focused more on the effects on DCs, as well as their impact on intestinal microbiota population and their relationship with IBD. Specifically, more studies have been recently published focusing on DCs and IBD, since there is now information on T regulator lymphocytes and their involvement in the process known as tolerance, as well as reactive oxygen species and their participation in the inflammatory process and tissue damage. As a result, multiple authors are now further encouraging probiotic therapy for not only infectious diseases but also for these inflammatory diseases, since these have proven to provide an effective homeostatic regulation of the gut microbiota, protection of the intestinal mucosal integrity, a potent anti-inflammatory effect and inhibition of pathogenic microorganism colonization.
Acknowledgments
Authors thank the digital graphic designer Alma Donaji Bravo Vázquez for her support in the design of the image of this article.
Author Contributions
P.A.F.d.C.: Involved in drafting of the manuscript, A.D.O.P.: Involved in drafting of the manuscript, J.R.L.V.: Involved in drafting of the manuscript, J.I.S: Involved in drafting of the manuscript, S.F.N.L.: Involved in drafting of the manuscript, D.A.H.: Involved in drafting of the manuscript, T.B.F.: Involved in drafting of the manuscript, S.G.S.-G.: Contributed substantially to drafting of the manuscript, R.V.-L.: Contributed to the conception and design of this project, general supervision of the research group and gave final approval of this manuscript. All authors read and approve the final manuscript.
Funding
This work is part of the project number 201638-CSNBIAVALR170605171 funded by CICSA, FCS Universidad Anáhuac México, Campus Norte.
Conflicts of Interest
The authors have no competing interests.
References
- 1.Zain K., Belga S., Roifman I., Hirota S., Jijon H., Kaplan G., Ghosh S., Beck P. Inflammatory Bowel Disease Cause-specific Mortality: A Primer for Clinicians. Inflamm. Bowel. Dis. 2014;20:2483–2492. doi: 10.1097/MIB.0000000000000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kappelman M., Rifas-Shiman S., Kleinman K., Ollendorf D., Bousvaros A., Grand R., Finklestein J. The Prevalence and Geographic Distribution of Crohn’s Disease and Ulcerative Colitis in the United States. Clin. Gastroenterol. Hepatol. 2007;5:1424–1429. doi: 10.1016/j.cgh.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Loftus C., Loftus E., Harmsen S., Zinsmeister A., Tremaine W., Melton J., Sandborn W. Update on the Incidence and Prevalence of Crohn’s Disease and Ulcerative Colitis in Olmsted County, Minnesota, 1940–2000. Inflamm. Bowel. Dis. 2007;13:254–261. doi: 10.1002/ibd.20029. [DOI] [PubMed] [Google Scholar]
- 4.Loftus E.V. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 5.Baumgart D.C., Carding S.R. Inflammatory bowel disease: Cause and immunobiology. Lancet. 2007;369:1627–1640. doi: 10.1016/S0140-6736(07)60750-8. [DOI] [PubMed] [Google Scholar]
- 6.Gajendran M., Loganathan P., Catinella A.P., Hashash J.G. A comprehensive review and update on Crohns disease. Dis. Mon. 2018;64:20–57. doi: 10.1016/j.disamonth.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Victoria C.R., Sassak L.Y., Nunes H.R.D.C. Incidence and prevalence rates of inflammatory bowel diseases, in midwestern of São Paulo State, Brazil. Arq. Gastroenterol. 2009;46:20–25. doi: 10.1590/S0004-28032009000100009. [DOI] [PubMed] [Google Scholar]
- 8.Paredes-Mendez J., Otoya G., Mestanza A., Lazo L., Acuña K., Arenas J., Huamán E., Julia F. Caracteristicas epidemiológicas y clínicas de la enfermedad inflamatoria intestinal en un hospital de referencia de Lima-Perú. Rev. Gastroenterol. Perú. 2016;36:209–218. [PubMed] [Google Scholar]
- 9.Kucharzik T., Maaser C., Lügering A., Kagnoff M., Mayer L., Targan S., Domschke W. Recent Understanding of IBD Pathogenesis: Implications for Future Therapies. Inflamm. Bowel Dis. 2006;12:1068–1083. doi: 10.1097/01.mib.0000235827.21778.d5. [DOI] [PubMed] [Google Scholar]
- 10.Kelsen J., Sullivan K. Inflammatory Bowel Disease in Primary Immunodeficiencies. Curr. Allergy Asthma Rep. 2017;17:57. doi: 10.1007/s11882-017-0724-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solano-Gálvez S.G., Tovar-Torres S.M., Tron-Gómez M.S., Weiser-Smeke A.E., Álvarez-Hernández D.A., Franyuti-Kelly G.A., Tapia-Moreno M., Ibarra A., Gutiérrez-Kobeh L., Vázquez-López R. Human Dendritic Cells: Ontogeny and Their Subsets in Health and Disease. Med. Sci. 2018;6:88. doi: 10.3390/medsci6040088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwasaki A., Medzhitov R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015;16:343–353. doi: 10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pulendran B., Tang H., Manicassamy S. Programming dendritic cells to induce TH2 and tolerogenic responses. Nat. Immunol. 2010;11:647–655. doi: 10.1038/ni.1894. [DOI] [PubMed] [Google Scholar]
- 14.Pulendran B. Modulating vaccine responses with dendritic cells and Toll-like receptors. Immunol. Rev. 2004;199:227–250. doi: 10.1111/j.0105-2896.2004.00144.x. [DOI] [PubMed] [Google Scholar]
- 15.Denning T.L., Norris B.A., Medina-Contreras O., Manicassamy S., Geem D., Madan R., Karp C.L., Pulendran B. Functional specializations of intestinal dendritic cell and macrophage subsets that control Th17 and regulatory T cell responses are dependent on the T cell/APC ratio, source of mouse strain, and regional localization. J. Immunol. 2011;187:733–747. doi: 10.4049/jimmunol.1002701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinman R.M., Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 17.Pulendran B., Tang H., Denning T.L. Division of labor, plasticity, and crosstalk between dendritic cell subsets. Curr. Opin. Immunol. 2008;20:61–67. doi: 10.1016/j.coi.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dhodapkar M.V., Steinman R.M., Krasovsky J., Munz C., Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J. Exp. Med. 2001;193:233–238. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jonuleit H., Schmitt E., Schuler G., Knop J., Enk A.H. Induction of interleukin 10-producing, nonproliferating CD4+ T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J. Exp. Med. 2000;192:1213–1222. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahnke K., Qian Y., Knop J., Enk A.H. Induction of CD4+/CD25+ regulatory T cells by targeting of antigens to immature dendritic cells. Blood. 2003;101:4862–4869. doi: 10.1182/blood-2002-10-3229. [DOI] [PubMed] [Google Scholar]
- 21.Hawiger D., Inaba K., Dorsett Y., Guo M., Mahnke K., Rivera M., Ravetch J.V., Steiman R.M., Nussenzweig M.C. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J. Exp. Med. 2001;194:769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steinman R.M., Hawiger D., Nussenzweig M.C. Tolerogenic dendritic cells. Annu. Rev. Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 23.Steinman R.M., Nussenzweig M.C. Avoiding horror autotoxicus: The importance of dendritic cells in peripheral T cell tolerance. Proc. Natl. Acad. Sci. USA. 2002;99:351–358. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maldonado-Lopez R., De Smedt T., Michel P., Godfroid J., Pajak B., Heirman C., Thielemans K., Leo O., Urbain J., Moser M. CD8α+ and CD8α− subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J. Exp. Med. 1999;189:587–592. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang A., Bloom O., Ono S., Cui W., Unternaehrer J., Jiang S., Whitney J.A., Connolly J., Banchereau J., Mellman I. Disruption of E-cadherin-mediated adhesion induces a functionally distinct pathway of dendritic cell maturation. Immunity. 2007;27:610–624. doi: 10.1016/j.immuni.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li M.O., Flavell R.A. TGF-β: A master of all T cell trades. Cell. 2008;134:392–404. doi: 10.1016/j.cell.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y.J., Soumelis V., Watanabe N., Ito T., Wang Y.H., de Waal Malefyt R., Omori M., Zhou B., Ziegler S.F. TSLP: An epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu. Rev. Immunol. 2007;25:193–219. doi: 10.1146/annurev.immunol.25.022106.141718. [DOI] [PubMed] [Google Scholar]
- 28.Manicassamy S., Pulendran B. Retinoic acid-dependent regulation of immune responses by dendritic cells and macrophages. Semin. Immunol. 2009;21:22–27. doi: 10.1016/j.smim.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guilliams K., Crozat S., Henri S., Tamoutounour S., Grenot P., Devilard E., de Bovis B., Alexopoulou L., Dalod M., Malessen B. Skin-draining lymph nodes contain dermis-derived CD103− dendritic cells that constitutively produce retinoic acid and induce Foxp3+ regulatory T cells. Blood. 2010;115:1958–1968. doi: 10.1182/blood-2009-09-245274. [DOI] [PubMed] [Google Scholar]
- 30.Laffont S., Siddiqui K.R., Powrie F. Intestinal inflammation abrogates the tolerogenic properties of MLN CD103+ dendritic cells. Eur J. Immunol. 2010;40:1877–1883. doi: 10.1002/eji.200939957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dillon S., Agrawal S., Banerjee K., Letterio J., Denning T.L., Oswald-Richter K., Kasprowicz D.J., Kellar K., Pare J., van Dyke T., et al. Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J. Clin. Investig. 2006;116:916–928. doi: 10.1172/JCI27203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manicassamy S., Ravindran R., Deng J., Oluoch H., Denning T.L., Kasturi S.P., Rosentahl K.M., Evavold B.D., Pulendran B. Toll-like receptor 2-dependent induction of vitamin A-metabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nat. Med. 2009;15:401–409. doi: 10.1038/nm.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karumuthil-Melethil S., Perez N., Li R., Vasu C. Induction of innate immune response through TLR2 and dectin 1 prevents type 1 diabetes. J. Immunol. 2008;181:8323–8334. doi: 10.4049/jimmunol.181.12.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burton O.T., Zaccone P., Phillips J.M., De la Peña H., Fehérvári Z., Azuma M., Gibbs S., Stockinger B., Cooke A. Roles for TGF-β and programmed cell death 1 ligand 1 in regulatory T cell expansion and diabetes suppression by zymosan in non-obese diabetic mice. J. Immunol. 2010;185:2754–2762. doi: 10.4049/jimmunol.1001365. [DOI] [PubMed] [Google Scholar]
- 35.Depaolo R.W., Tang F., Kim I., Han M., Levin N., Ciletti N., Lin A., Anderson D., Schneewind O., Jabri B. Toll-like receptor 6 drives differentiation of tolerogenic dendritic cells and contributes to LcrV-mediated plague pathogenesis. Cell Host Microbe. 2008;4:350–361. doi: 10.1016/j.chom.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orabona C., Puccetti P., Vacca C., Bicciato S., Luchini A., Fallarino F., Bianchi R., Velardi E., Peruccio K., Velardi A., et al. Toward the identification of a tolerogenic signature in IDO-competent dendritic cells. Blood. 2006;107:2846–2854. doi: 10.1182/blood-2005-10-4077. [DOI] [PubMed] [Google Scholar]
- 37.Moseman E.A., Liang X., Dawson A.J., Panoskaltsis-Mortari A., Krieg A.M., Liu Y.J., Blazar B.R., Chen W. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J. Immunol. 2004;173:4433–4442. doi: 10.4049/jimmunol.173.7.4433. [DOI] [PubMed] [Google Scholar]
- 38.Smits H., Engering A., van der Kleij D., de Jong E., Schipper K., van Capel T., Zaat B., Yazdanbakhsh M., Wierenga E., van Kooyk Y., et al. Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J. Allergy Clin. Immunol. 2005;115:1260–1267. doi: 10.1016/j.jaci.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 39.Bergman M.P., Engering A., Smits H.H., Savan Vliet S.J., Bodegraven A.A., Wirth H.P., Kapsenberg M.L., Vandenbroucke-Grauls C., van Kooyk Y., Appelmelk B.J. Helicobacter pylori modulates the T helper cell 1/T helper cell 2 balance through phase-variable interaction between lipopolysaccharide and DC-SIGN. J. Exp. Med. 2004;200:979–990. doi: 10.1084/jem.20041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Konstantinov S.R., Smidt H., de Vos W.M., Brujins S.C., Singh S.K., Valence F., Molle D., Lortal S., Altermann E., Klaenhammer T.R., et al. S layer protein A of Lactobacillus acidophilus NCFM regulates immature dendritic cell and T cell functions. Proc. Natl. Acad. Sci. USA. 2008;105:19474–19479. doi: 10.1073/pnas.0810305105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou Y., Kawasaki H., Hsu S.C., Lee R.T., Yao X., Plunkett B., Fu J., Yang K., Lee Y.C., Huang S.K. Oral tolerance to food-induced systemic anaphylaxis mediated by the C-type lectin SIGNR1. Nat. Med. 2010;16:1128–1133. doi: 10.1038/nm.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garin M.I., Chu C.C., Golshayan D., Cernuda-Morollon E., Wait R., Lechler R.I. Galectin-1, a key effector of regulation mediated by CD4+CD25+ T cells. Blood. 2007;109:2058–2065. doi: 10.1182/blood-2006-04-016451. [DOI] [PubMed] [Google Scholar]
- 43.Kubach J., Lutter P., Bopp T., Stoll S., Becker C., Huter E., Richter C., Weingarten P., Warger T., Knop J., et al. Human CD4+CD25+ regulatory T cells: Proteome analysis identifies galectin-10 as a novel marker essential for their anergy and suppressive function. Blood. 2007;110:1550–1558. doi: 10.1182/blood-2007-01-069229. [DOI] [PubMed] [Google Scholar]
- 44.Smythies L.E., Sellers M., Clements R.H., Mosteller-Barnum M., Meng G., Benjamin W.H., Orenstein J.M., Smith P.D. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J. Clin. Investig. 2005;115:66–75. doi: 10.1172/JCI200519229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang M., Tang H., Guo Z., An H., Zhu X., Song W., Guo J., Huang X., Chen T., Wang J., et al. Splenic stroma drives mature dendritic cells to differentiate into regulatory dendritic cells. Nat. Immunol. 2004;5:1124–1133. doi: 10.1038/ni1130. [DOI] [PubMed] [Google Scholar]
- 46.Svensson M., Maroof A., Ato M., Kaye P.M. Stromal cells direct local differentiation of regulatory dendritic cells. Immunity. 2004;21:805–816. doi: 10.1016/j.immuni.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 47.Rescigno M., Di Sabatino A. Dendritic cells in intestinal homeostasis and disease. J. Clin. Investig. 2009;119:2441–2450. doi: 10.1172/JCI39134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hill D.A., Artis D. Intestinal bacteria and the regulation of immune cell homeostasis. Annu. Rev. Immunol. 2010;28:623–667. doi: 10.1146/annurev-immunol-030409-101330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grainger J.R., Hall J.A., Bouladoux N., Oldenhove G., Belkaid Y. Microbe-dendritic cell dialog controls regulatory T-cell fate. Immunol. Rev. 2010;234:305–316. doi: 10.1111/j.0105-2896.2009.00880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeuthen L.H., Fink L.N., Frokiaer H. Epithelial cells prime the immune response to an array of gut- derived commensals towards a tolerogenic phenotype through distinct actions of thymic stromal lymphopoietin and transforming growth factor-beta. Immunology. 2008;123:197–208. doi: 10.1111/j.1365-2567.2007.02687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Round J.L., Mazmanian S.K. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. USA. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ivanov I.I., Atarashi K., Manel N., Brodie E.L., Shima T., Karaoz U., Wei D., Goldfarb K.C., Santee C.A., Lynch S.V., Tanoue T., et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grainger J., Smith K., Hewitson J., McSorley H., Harcus Y., Filbey K., Finney C., Greenwood E., Knox D., Wilson M., et al. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-β pathway. J. Exp. Med. 2010;207:2331–2341. doi: 10.1084/jem.20101074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Frank D.N., Amand A.L.S., Feldman R.A., Boedeker E.C., Harpaz N., Pace N.R. Microbiology Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morgan X.C., Tickle T.L., Sokol H., Gevers D., Devaney K.L., Ward D.V., Reyes J.A., Shah S.A., LeLeiko N., Snapper S.B., et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012;13:R79. doi: 10.1186/gb-2012-13-9-r79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gevers D., Kugathasan S., Denson L.A., Vázquez-Baeza Y., Van Treuren W., Ren B., Schwager E., Knights D., Song S.J., Yassour M., et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe. 2014;15:382–392. doi: 10.1016/j.chom.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Salas-Jara J., Ilabaca A., Vega M., García A. Biofilm Forming Lactobacillus: New Challenges for the Development of Probiotics. Microorganisms. 2016;4:35. doi: 10.3390/microorganisms4030035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Satokari R. Contentious host—microbiota relationship in inflammatory bowel disease—can foes become friends again? Scand. J. Gastroenterol. 2015;50:34–42. doi: 10.3109/00365521.2014.966320. [DOI] [PubMed] [Google Scholar]
- 59.Ogura Y., Bonen D.K., Inohara N., Nicolae D., Chen F., Ramos R., Britton H., Moran T., Karaliuskas R., Duerr R., et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 60.Swidsinski A., Ladhoff A., Pernthaler A., Swidsinski S., Loening-Baucke V., Ortner M., Weber J., Hoffma U., Schreiber S., Dietel M., et al. Mucosal flora in inflammatory bowel disease. Gastroenterology. 2002;122:44–54. doi: 10.1053/gast.2002.30294. [DOI] [PubMed] [Google Scholar]
- 61.Noguchi E., Homma Y., Kang X., Netea M., Ma X. A Crohn’s disease–associated NOD2 mutation suppresses transcription of human IL10 by inhibiting activity of the nuclear ribonucleoprotein hnRNP-A1. Nat. Immunol. 2009;10:471–479. doi: 10.1038/ni.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kang S., Denman S.E., Morrison M., Yu Z., Dore J., Leclerc M., McSweeny C. Dysbiosis of fecal microbiota in Crohn’s disease patients as revealed by a custom phylogenetic microarray. Inflamm. Bowel Dis. 2010;16:2034–2042. doi: 10.1002/ibd.21319. [DOI] [PubMed] [Google Scholar]
- 63.Sokol H., Lepage P., Seksik P., Doré J., Marteau P. Temperature gradient gel electrophoresis of fecal 16S rRNA reveals active Escherichia coli in the microbiota of patients with ulcerative colitis. J. Clin. Microbiol. 2006;44:3172–3177. doi: 10.1128/JCM.02600-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kostic A.D., Chun E., Robertson L., Glickman J., Gallini C., Michaud M., Clancy T., Chung D., Locchead P., Hold G. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14:207–215. doi: 10.1016/j.chom.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tysk C., Lindberg E., Jarnerot G., Flodérus-Myrhed B. Ulcerative colitis and Crohn’s disease in an unselected population of monozygotic and dizygotic twins. A study of heritability and the influence of smoking. Gut. 1988;29:990–996. doi: 10.1136/gut.29.7.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spehlmann M.E., Begun A.Z., Burghardt J., Lepage P., Radler A., Schreiber S. Epidemiology of inflammatory bowel disease in a German twin cohort: Results of a nationwide study. Inflamm. Bowel Dis. 2008;14:968–976. doi: 10.1002/ibd.20380. [DOI] [PubMed] [Google Scholar]
- 67.Lopez-Siles M., Duncan S., García-Gil L., Martínez-Medina M. Faecalibacterium prausnitzii: From microbiology to diagnostics and prognostics. ISME J. 2017;11:841–852. doi: 10.1038/ismej.2016.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sjöberg F., Barkman C., Nookaew I., Östman S., Adlerberth I., Saalman R., Wold A. Low-complexity microbiota in the duodenum of children with newly diagnosed ulcerative colitis. PLoS ONE. 2017;12:e0186178. doi: 10.1371/journal.pone.0186178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sokol H., Lay C., Seksik P., Tannock G. Analysis of bacterial bowel communities of IBD patients: What has it revealed? Inflamm. Bowel Dis. 2008;14:858–867. doi: 10.1002/ibd.20392. [DOI] [PubMed] [Google Scholar]
- 70.Lepage P., Hasler R., Spehlmann M.E., Rehman A., Zvibliene A., Begun A., Ott S., Kupcinskas L., Doré J., Raedler A., et al. Twin study indicates loss of interaction between microbiota and mucosa of patients with ulcerative colitis. Gastroenterology. 2011;141:227–236. doi: 10.1053/j.gastro.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 71.Machiels K., Joossens M., Sabino J., De Preter V., Arijs I., Eeckhaut V., Ballet V., Claes K., Van Imerseel F., Verbeke K., et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitziidefines dysbiosis in patients with ulcerative colitis. Gut. 2013;63:1275–1283. doi: 10.1136/gutjnl-2013-304833. [DOI] [PubMed] [Google Scholar]
- 72.Rajilić-Stojanović M., Shanahan F., Guarner F., de Vos W. Phylogenetic Analysis of Dysbiosis in Ulcerative Colitis During Remission. Inflamm. Bowel Dis. 2013;19:481–488. doi: 10.1097/MIB.0b013e31827fec6d. [DOI] [PubMed] [Google Scholar]
- 73.Garcia Vilela E., De Lourdes De Abreu Ferrari M., Oswaldo Da Gama Torres H., Guerra Pinto A., Carolina Carneiro Aguirre A., Paiva Martins F., Marcos Andrade Goulart E., Sales Da Cunha A. Influence of Saccharomyces boulardii on the intestinal permeability of patients with Crohn’s disease in remission. Scand. J. Gastroenterol. 2008;43:842–848. doi: 10.1080/00365520801943354. [DOI] [PubMed] [Google Scholar]
- 74.Orel R., Tina K. Intestinal microbiota, probiotics and prebiotics in inflammatory bowel disease. World J. Gastroenterol. 2014;20:11505. doi: 10.3748/wjg.v20.i33.11505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Johansson-Lindbom B., Svensson M., Wurbel M.A., Malissen B., Márquez G., Agace W. Selective generation of gut tropic T cells in gut-associated lymphoid tissue (GALT): Requirement for GALT dendritic cells and adjuvant. J. Exp. Med. 2003;198:963–969. doi: 10.1084/jem.20031244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mora J.R., Bono M.R., Manjunath N., Weninger W., Cavanagh L., Rosemblatt M., Von Andrian U. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 77.Stagg A.J., Kamm M.A., Knight S.C. Intestinal dendritic cells increase T cell expression of α4β7 integrin. Eur. J. Immunol. 2002;32:1445–1454. doi: 10.1002/1521-4141(200205)32:5<1445::AID-IMMU1445>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 78.Ahiro S., Ohtani H., Suzuki M., Murata M., Ejima C., Oki M., Kinouchi Y., Fukushima K., Sasaki I., Nakamura S., et al. Differential expression of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in ulcerative colitis and Crohn’s disease. Pathol. Int. 2002;52:367–374. doi: 10.1046/j.1440-1827.2002.01365.x. [DOI] [PubMed] [Google Scholar]
- 79.Briskin M., Winsor-Hines D., Shyjan A., Cochran N., Bloom S., Wilson J., McEvoy L., Butcher E., Kassam N., Mackay C., et al. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am. J. Pathol. 1997;151:97–110. [PMC free article] [PubMed] [Google Scholar]
- 80.Hart A.L., Kamm M.A., Knight S.C., Stagg A.J. Prospective evaluation of intestinal homing memory T cells in ulcerative colitis. Inflamm. Bowel Dis. 2004;10:496–503. doi: 10.1097/00054725-200409000-00002. [DOI] [PubMed] [Google Scholar]
- 81.Perez-Lopez A., Behnsen J., Nuccio S.P., Raffatellu M. Mucosal immunity to pathogenic intestinal bacteria. Nat. Rev. Immunol. 2016;16:135–148. doi: 10.1038/nri.2015.17. [DOI] [PubMed] [Google Scholar]
- 82.Mann E.R., Bernardo D., Ng S.C., Rigby R.J., Al-Hassi H.O., Landy J., Peake S.T., Spranger H., English N.R., Thomas L.V., et al. Human gut dendritic cells drive aberrant gut-specific t-cell responses in ulcerative colitis, characterized by increased IL-4 production and loss of IL-22 and IFNγ. Inflamm. Bowel Dis. 2014;20:2299–2307. doi: 10.1097/MIB.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 83.Harbour S., Maynard C., Zindl C., Schoeb T., Weaver C. Th17 cells give rise to Th1 cells that are required for the pathogenesis of colitis. Proc. Natl. Acad. Sci. USA. 2015;112:7061–7066. doi: 10.1073/pnas.1415675112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weaver C., Elson C., Fouser L., Kolls J. The Th17 Pathway and Inflammatory Diseases of the Intestines, Lungs and Skin. Annu. Rev. Pathol. 2013;8:477–512. doi: 10.1146/annurev-pathol-011110-130318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wei W., Feidi C., Zhanju L., Yingzi C. Microbiota-specific Th17 cells: Yin and Yang in regulation of inflammatory bowel disease. Inflamm. Bowel Dis. 2016;22:1473–1482. doi: 10.1097/MIB.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Atreya R., Neurath M. IBD Pathogenesis in 2014, Molecular pathways controlling barrier function in IBD. Nat. Rev. Gastroenterol. Hepatol. 2015;12:67–68. doi: 10.1038/nrgastro.2014.201. [DOI] [PubMed] [Google Scholar]
- 87.Steenholdt C., Coskun M., Buhl S., Bendtzen K., Ainsworth M., Brynskov J., Nielsen O. Circulating Cytokines and Cytokine Receptors in Infliximab Treatment Failure Due to TNF-α Independent Crohn Disease. Medicine. 2016;95:e3417. doi: 10.1097/MD.0000000000003417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shih D., Targan S. Immunopathogenesis of inflammatory bowel disease. World J. Gastroenterol. 2008;14:390–400. doi: 10.3748/wjg.14.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wen Z., Fiocchi C. Inflammatory Bowel Disease: Autoimmune or Immune-mediated Pathogenesis? Clin. Dev. Immunol. 2004;11:195–204. doi: 10.1080/17402520400004201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Aronson R.A., Cook S.L., Roche J.K. Sensitization to epithelial antigens in chronic mucosal inflammatory disease. I. Purification, characterization, and immune reactivity of murine epithelial cell-associated components (ECAC) J. Immunol. 1983;131:2796. [PubMed] [Google Scholar]
- 91.Mitsuyama K., Niwa M., Takedatsu H., Yamasaki H., Kuwaki K., Yoshioka S., Yamauchi R., Fukunaga S., Torimura T. Antibody markers in the diagnosis of inflammatory bowel disease. World J. Gastroenterol. 2015;22:1304–1310. doi: 10.3748/wjg.v22.i3.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Figueroa-González I., Quijano G., Ramírez G., Cruz-Guerrero A. Probiotics and prebiotics—perspectives and challenges. J. Sci. Food Agric. 2011;91:1341–1348. doi: 10.1002/jsfa.4367. [DOI] [PubMed] [Google Scholar]
- 93.Metchnikoff E. Optimistic Studies of the Prolongation of Life. Putman’s Sons; New York, NY, USA: 1998. pp. 161–183. [Google Scholar]
- 94.FAO/WHO . Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food. FAO/WHO; London, UK: 2002. [Google Scholar]
- 95.Costerton J.W., Stewwart P.S., Greenberg E.P. Bacterial biofilms: A common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 96.Post J.C., Stoodley P., Hall-Stoodley L., Ehrlich G.D. The role of biofilms in otolaryngologic infections. Curr. Opin. Otolaryngol. Head Neck Surg. 2004;12:185–190. doi: 10.1097/01.moo.0000124936.46948.6a. [DOI] [PubMed] [Google Scholar]
- 97.Terraf M.C., Juarez M.S., Nader-Macias M.E., Silva C. Screening of biofilm formation by beneficial vaginal lactobacilli and influence of culture media components. J. Appl. Microbiol. 2012;113:1517–1529. doi: 10.1111/j.1365-2672.2012.05429.x. [DOI] [PubMed] [Google Scholar]
- 98.Leccese M., Juárez M., Rault L., Le Loir Y., Even S., Nader-Macías M. Biofilms of vaginal Lactobacillus rhamnosus CRL 1332, Kinetics of formation and matrix characterization. Arch. Microbiol. 2016;198:689–700. doi: 10.1007/s00203-016-1225-5. [DOI] [PubMed] [Google Scholar]
- 99.Sarxelin M., Tynkkynen S., Mattila-sandholm T., Vos W.M. Probiotic and other functional microbes: From markets to mechanisms. Curr. Opin. Microbiol. 2005;16:204–211. doi: 10.1016/j.copbio.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 100.Holzapfel W.H., Haberer P., Geisen R., Björkroth J., Schillinger U. Taxonomy and important features of probiotic microorganisms in food and nutrition. Am. J. Clin. Nutr. 2001;73:365–373. doi: 10.1093/ajcn/73.2.365s. [DOI] [PubMed] [Google Scholar]
- 101.Anand S., Rajagopalan S., Mohan R. Management of liver hydatid cysts—Current perspectives. Med. J. Armed Forces India. 2012;68:304–309. doi: 10.1016/j.mjafi.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 102.De Vos P., Fass M., Spasojevic M., Sikkema J. Encapsulation for preservation of functionality and targeted delivery of bioactive food components. Int. Dairy J. 2010;20:292–302. doi: 10.1016/j.idairyj.2009.11.008. [DOI] [Google Scholar]
- 103.Klayraung S., Viernstein H., Okonogi S. Development of tablet containing probiotics: Effects of formulation and processing parameters on bacterial viability. Int. J. Pharm. 2008;370:54–60. doi: 10.1016/j.ijpharm.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 104.Burgain J., Gaiani C., Linder M., Scher J. Encapsulation of probiotic living cells: From laboratory scale to industrial applications. J. Food Eng. 2011;104:467–493. doi: 10.1016/j.jfoodeng.2010.12.031. [DOI] [Google Scholar]
- 105.Kiew T., Cheow W., Hadinoto K. Importance of biofilm age and growth medium on the viability of probiotics capsules containing Lactobacillus rhamnosus GG biofilm. Food Sci. Technol. 2014;59:956–963. doi: 10.1016/j.lwt.2014.07.053. [DOI] [Google Scholar]
- 106.McCarthy J., O’Mahony L., O’Callaghan L., Sheil B., Vaughan E.E., Fitzsimons N., Fitzgibbon J., O’Sullivan G.C., Kiely B., Collins J.K., et al. Double blind, placebo controlled trial of two probiotic strains in interleukin 10 knockout mice and mechanistic link with cytokine balance. Gut. 2002;52:975–980. doi: 10.1136/gut.52.7.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lammers K., Brigidi P., Vitali B., Gionchetti P., Rizello F., Caramelli E., Matteuzzi D., Campieri M. Immunomodulatory effects of probiotic bacteria DNA: IL-1 and IL-10 response in human peripheral blood mononuclear cells. FEMS Immunol. Med. Microbiol. 2003;38:165–172. doi: 10.1016/S0928-8244(03)00144-5. [DOI] [PubMed] [Google Scholar]
- 108.Zaylaa M., Al Kassaa I., Alard J., Peucelle V., Boutillier D., Desramaut J., Dabboussi F., Pot B., Grangette C. Probiotics in IBD: Combining in vitro and in vivo models for selecting strains with both anti-inflammatory potential as well as a capacity to restore the gut epithelial barrier. J. Funct. Foods. 2018;47:304–315. doi: 10.1016/j.jff.2018.05.029. [DOI] [Google Scholar]
- 109.Valdovinos M.A., Montijo E., Abreu A.T., Heller S., González-Garay A., Bacarreza D., Bielsa-Fernández M., Bojórquez-Ramos M.C., Bosques-Padilla F., Burguete-García A.I., et al. The Mexican consensus on probiotics in gastroenterology. Rev. Gastroenterol. Mex. 2017;82:156–178. doi: 10.1016/j.rgmxen.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 110.Moayyedi P., Surette M.G., Kim P.T., Libertucci J., Wolfe M., Onischi C., Armstrong D., Marshall J.K., Kassam Z., Reinisch W., et al. Fecal Microbiota Transplantation Induces Remission in Patients with Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology. 2017;149:102–109. doi: 10.1053/j.gastro.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 111.Guslandi M. Role of Probiotics in Crohn’s Disease and in Pouchitis. J. Clin. Gastroenterol. 2015;49:S46–S49. doi: 10.1097/MCG.0000000000000351. [DOI] [PubMed] [Google Scholar]
- 112.Guslandi M., Mezzi G., Sorghi M., Testoni P. Saccharomyces boulardii in maintenance treatment of Crohn’s disease. Digest. Dis. Sci. 2000;45:1462–1464. doi: 10.1023/A:1005588911207. [DOI] [PubMed] [Google Scholar]
- 113.Plein K., Hotz J. Therapeutic effects of Saccharomyces boulardii on mild residual symptoms in a stable phase of Crohn’s disease with special respect to chronic diarrhea—a pilot study. Z Gastroenterol. 1993;31:129–134. [PubMed] [Google Scholar]
- 114.Bourreille A., Cadiot G., Le Dreau G., Laharie D., Beaugerie L., Dupas J., Marteau P., Rampai P., Moyse D., Saleh A., et al. Saccharomyces boulardii Does Not Prevent Relapse of Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2013;11:982–987. doi: 10.1016/j.cgh.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 115.Steed H., Macfarlane G., Blackett K., Bahrami B., Reynolds N., Walsh S., Cummings J., Macfarlane S. Clinical trial: The microbiological and immunological effects of synbiotic consumption—A randomized double-blind placebo-controlled study in active Crohn’s disease. Aliment Pharmacol. Ther. 2010;32:872–883. doi: 10.1111/j.1365-2036.2010.04417.x. [DOI] [PubMed] [Google Scholar]
- 116.Fujimori S., Tatsuguchi A., Gudis K., Kishida T., Mitsui K., Ehara A., Kobayashi T., Sekita Y., Seo T., Sakamoto C. High dose probiotic and prebiotic cotherapy for remission induction of active Crohn’s disease. J. Gastroenterol. Hepatol. 2007;22:1199–1204. doi: 10.1111/j.1440-1746.2006.04535.x. [DOI] [PubMed] [Google Scholar]
- 117.Fedorak R., Feagan B., Hotte N., Leddin D., Dieleman L., Petrunia D., Enns R., Bitton A., Chiba N., Paré P., et al. The Probiotic VSL#3 Has Anti-inflammatory Effects and Could Reduce Endoscopic Recurrence After Surgery for Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2015;13:928–935. doi: 10.1016/j.cgh.2014.10.031. [DOI] [PubMed] [Google Scholar]
- 118.Shen Z., Zhu C., Quan Y., Yang Z., Wu S., Luo W., Tan B., Wang X. Relationship between intestinal microbiota and ulcerative colitis: Mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J. Gastroenterol. 2018;24:5–14. doi: 10.3748/wjg.v24.i1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ghouri Y.A., Richards D.M., Rahimi E.F., Krill J.T., Jelinek K.A., DuPont A.W. Systematic review of randomized controlled trials of probiotics, prebiotics, and synbiotics in inflammatory bowel disease. Clin. Exp. Gastroenterol. 2014;7:473–487. doi: 10.2147/CEG.S27530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kruis W., Fric P., Pokrotnieks J., Lukás M., Fixa B., Kascák M., Kamm M., Weismueller J., Beglinger C., Stolte M., et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 2004;53:1617–1623. doi: 10.1136/gut.2003.037747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tsuda Y., Yoshimatsu Y., Aoki H., Nakamura K., Irie M., Fukuda K., Hosoe N., Takada N., Shirai K., Suzuki Y. Clinical effectiveness of probiotics therapy (BIO-THREE) in patients with ulcerative colitis refractory to conventional therapy. Scand J. Gastroenterol. 2007;42:1306–1311. doi: 10.1080/00365520701396091. [DOI] [PubMed] [Google Scholar]
- 122.Hudson L., Anderson S., Corbett A., Lamb T. Gleaning Insights from Fecal Microbiota Transplantation and Probiotic Studies for the Rational Design of Combination Microbial Therapies. Clin. Microbiol. Rev. 2016;30:191–231. doi: 10.1128/CMR.00049-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Everard A., Matamoros S., Geurts L., Delzenne N.M., Cani P.D. Saccharomyces boulardii administration changes gut microbiota and reduces hepatic steatosis, low-grade inflammation, and fat mass in obese and type 2 diabetic db/db mice. mBio. 2014;5:e01011-14. doi: 10.1128/mBio.01011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Al-Sadi R., Boivin M., Ma T. Mechanism of cytokine modulation of epithelial tight junction barrier. Front. Biosci. (Landmark Ed.) 2009;14:2765–2778. doi: 10.2741/3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Roselli M., Finamore A., Nuccitelli S., Carnevali P., Brigidi P., Vitali B., Nobili F., Rami R., Garaguso I., Mengheri E. Prevention of TNBS-induced colitis by different Lactobacillus and Bifidobacterium strains is associ- ated with an expansion of gamma delta T and regulatory T cells of intestinal intraepithelial lymphocytes. Inflamm. Bowel. Dis. 2009;15:1526–1536. doi: 10.1002/ibd.20961. [DOI] [PubMed] [Google Scholar]
- 126.van der Waal M., Flach J., Browne P., Besseling-van der Vaart I., Claassen E., van de Burgwal L. Probiotics for improving quality of life in ulcerative colitis: Exploring the patient perspective. Pharmanutrition. 2019;7:100139. doi: 10.1016/j.phanu.2018.100139. [DOI] [Google Scholar]
- 127.Ghadimi D., Helwig U., Schrezenmeir J., Heller K.J., de Vrese M. Epigenetic imprinting by commensal probiotics inhibits the IL-23/IL-17 axis in an in vitro model of the intestinal mucosal immune system. J. Leukoc Biol. 2012;92:895–911. doi: 10.1189/jlb.0611286. [DOI] [PubMed] [Google Scholar]
- 128.Miyauchi E., Ogita T., Miyamoto J., Kawamoto S., Morita H., Ohno H., Suzuki T., Tanabe S. Bifidobacterium longum alleviates dextran sulfate sodium-induced colitis by suppressing IL-17A response: Involvement of intestinal epithelial costimulatory molecules. PLoS ONE. 2013;8:e79735. doi: 10.1371/journal.pone.0079735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tanabe S., Kinuta Y., Saito Y. Bifidobacterium infantis suppresses proinflammatory interleukin-17 production in murine splenocytes and dextran sodium sulfate-induced intestinal inflammation. Int. J. Mol. Med. 2008;22:181–185. doi: 10.3892/ijmm_00000006. [DOI] [PubMed] [Google Scholar]
- 130.Ogita T., Tanii Y., Morita H., Suzuki T., Tanabe S. Suppression of Th17 response by Streptococcus thermophilus ST28 through induction of IFN-γ. Int. J. Mol. Med. 2011;28:817–822. doi: 10.3892/ijmm.2011.755. [DOI] [PubMed] [Google Scholar]