Abstract
The aerial parts of extensively used ethnomedicinal plant Mikania cordata (Burm. f.) Robinson growing wild in Bangladesh were investigated to isolate and characterize compounds responsible for the bioactivities of the plant. In the present study, a new derivatives of betulinic acid, 16-hydroxy betulinic acid [3β,16-dihydroxy-lup-20(29)-en-28-oic] was isolated and the structure of the compound was determined by NMR spectroscopic means and comparing with available literature data. The isolated compound was then investigated for different pharmacological activities including antibacterial, antifungal, analgesic, anti-inflammatory and antipyretic potential employing different methods. The compound showed potent antibacterial activity with inhibition zone of diameter ranging from 12.0 to 17.5 mm and antifungal activity with mycelial growth inhibition ranging from 37.6 to 54.5%. The MIC values for antibacterial and antifungal activities ranged from 31.5–125 and 250–1000 μg/mL respectively. The compound (50 and 100 mg/kg body weight) showed potent peripheral and central analgesic activity with 55.19% and 41% of writhing inhibition at 90 min after administration of the compound and the highest 55.98%, 79.18% elongation of reaction time, respectively. In anti-inflammatory activity screening, the compound (100 mg/kg b.w.) revealed the highest 77.08% edema inhibition at 4 h after administration of carrageenan. In antipyretic assay, 16-hydroxy betulinic acid displayed a strong antipyretic effect in yeast-induced rats. From the present study it is apparent that 16-hydroxy betulinic acid might play vital role to establish M. cordata as ethnomedicinal plant to treat wound, cuts and fever.
Keywords: Mikania cordata, 16-hydroxy betulinic acid, Antimicrobial, Analgesic, Anti-inflammatory, Antipyretic potential
1. Introduction
Mikania cordata (Burm. f.) Robinson belongs to the family Asteraceae is a climbing herb and distributed in tropical Asia, the Philippines, Papua New Guinea and tropical Africa (Ahmed et al., 2008). Presence of cordate leaves, capitulum inflorescence, whitish flowers, and narrowly oblong cypsela with white pappus distinguishes the species from remaining members of the Asteraceae. In Bangladesh, M. cordata is widely distributed throughout the country and used as an ethnomedicinally important plant in the treatment of cuts and wounds. Leaf decoction is applied for treatment of dysentery, indigestion and gastro-intestinal sore (Ghani, 2003). The plant is also used as leafy vegetable and applied against coughs, eye sores and gastro-intestinal disorders.
Different ethnic communities residing in different districts of Bangladesh use leaves of M. cordata for treating cuts, wounds, fever and pain. The Garo tribal people residing in Netrokona district employ leaves of this species in cuts and wounds (Rahmatullah et al., 2009a). In order to treat gastric pain fried leaves of this species are eaten by the Mandi tribal people in Tangail district (Partha and Hossain, 2007). The Santals tribal people residing in Thakurgaon district use the leaf of M. cordata for the treatment of cuts and wounds, and as remedy of dengue fever (Rahmatullah et al., 2009b).
M. cordata has already been investigated and reported to exhibit different pharmacological activities. Mosaddik and Alam (2000) showed psycho-pharmacological, neuro-pharmacological, antimicrobial and therapeutic properties of M. cordata against pain, inflammation, hyperthermia, ulcer as well as carcinogenesis. The CNS depressant activity with potential antioxidant properties of M. cordata was found (Hasan et al., 2009), while crude ethanolic extract of M. cordata presented analgesic, cytotoxicity, and antibacterial activities (Nayeem et al., 2011). Very recently, chloroform extract of the aerial parts of M. cordata was found very bioactive in nature (Siddiqui et al., 2017). To provide scientific support to traditional and folklore usage of M. cordata in Bangladesh for the treatment of different ailment, in this study, we aimed to explore and identify the chemical compound(s) from M. cordata and evaluate the role of the compounds behind bioactivities of the species. In this view, we subjected chloroform extract for investigation to isolate bioactive compounds and as a consequence, a pentacyclic triterpenoid, 16-hydroxy betulinic acid (16 HBA) was isolated, identified and characterized for the first time from M. cordata. The isolated 16-hydroxy betulinic acid was further studied extensively for its antimicrobial, analgesic, anti-inflammatory and antipyretic activities.
2. Materials and methods
2.1. Plant material
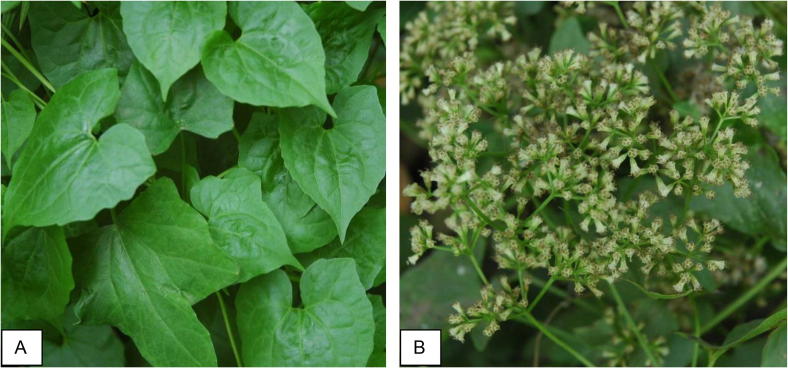
Mikania cordata was collected from Bhabrasur of Muksudpur upazila under Gopalganj district, Bangladesh in 2010. The aerial parts were plucked from the collected specimens. The habit and inflorescence of the plant are shown in Fig. 1. The voucher specimen has been prepared and deposited in Bangladesh National Herbarium for further reference (Voucher No. 37894).
Fig. 1.
Habit and inflorescence of Mikania cordata; A. Habit showing cordate leaves; B. Capitulum inflorescence.
2.2. Drugs and chemicals
Morphine manufactured by Gonoshasthaya Pharmaceuticals Ltd, Dhaka, and Diclofenac sodium manufactured by ACI Pharmaceutical Ltd., Dhaka Bangladesh were used for the experiments. In addition, acetic acid (Merck, Germany) and carrageenan (BDH, UK.) were employed in this study. For other chemicals, only the analytical grade chemicals (Sigma and Merck) were used in the experiments.
2.3. Experimental animal models
Swiss albino mice of 25–30 g and Wister albino rats of 150–200 g were employed in the experiment which were collected from ICDDR, B (International Centre for Diarrhoeal Diseases and Research, Bangladesh). The animals were kept at 25 ± 1 °C with 60–70% humidity and normal day/night cycle (12 h each) in standard polypropylene cages. Standard pellets as basal diet (ICDDR, B formulated) and water ad libitum were provided to the animals for one week. After fasting for overnight, the animals were weighted prior to run the experiment. During carrying out the experiments, the guidelines of the National Institute of Health for the Care and Use of Laboratory Animals (NIH Publication revised in 1996) were strictly followed.
2.4. Preparation of chloroform extract
After air drying, the collected leaves of M. cordata were crushed to make leaf powder. Then 200 g of the powder was subjected to extraction by 1000 ml of chloroform at room temperature for 7 days. After filtration, the solvent was evaporated from the solution with the help of rotary evaporator operated at 50 °C which yielded 7 g of extract.
2.5. Isolation of compound from chloroform extract
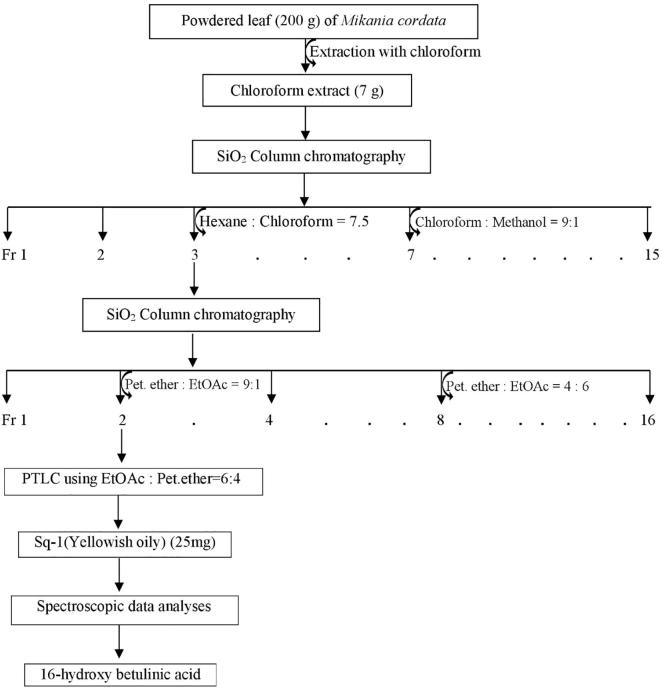
Chloroform extract (7 g) was subjected to silica gel column chromatography (364 g, Kieselgel 60, 230–400 mesh, Merck) and in this case, the mobile phase was started with 100% n-hexane on fraction 1; Hexane-CHCl3 (8.5:1.5) solvent systems on fraction 2; Hexane-CHCl3 (7.5:2.5) on fraction 3; Hexane-CHCl3 (5:5) solvent systems on fraction 4; Hexane-CHCl3 (3:7) solvent systems on fraction 5; CHCl3 (100%) solvent systems on fraction 6; CHCl3-MeOH (9:1) solvent systems on fraction 7; CHCl3-MeOH (8:2) solvent systems on fraction 8; CHCl3-MeOH (7:3) solvent systems on fraction 9–10; CHCl3-MeOH (5:5) solvent systems on fraction 11–12; CHCl3-MeOH (3:7) solvent systems on fraction 13 and MeOH solvent on fraction 14–15. A total of 15 fractions were collected and based on their TLC profiles, some of the fractions were combined with each other. The fraction 3 obtained from first column chromatography was then subjected for further column chromatography using same silica gel for fractionations. The starting solvent system was petroleum ether on fraction 1; Pet.ether-EtOAc (9:1) solvent system on fraction 2; Pet.ether-EtOAc (8:2) solvent system on fraction 3; Pet.ether-EtOAc (7:3) solvent system on fraction 4; Pet.ether-EtOAc (6:4) solvent system on fraction 5; Pet.ether-EtOAc (5:5) solvent system on fraction 6; Pet.ether-EtOAc (4:6) solvent system on fraction 7–8; Pet.ether-EtOAc (3:7) solvent system on fraction 9–10; EtOAc (100%) solvent system on fraction 11; EtOAc-MeOH (9:1) solvent system on fraction 12; EtOAc-MeOH (8:2) solvent system on fraction 13; EtOAc-MeOH (6:4) solvent system on fraction14; EtOAc-MeOH (5:5) solvent system on fraction 15; MeOH (100%) solvent system on fraction 16. Fraction 2 was then subjected to prepare thin layer chromatography using solvent system ethyl acetate: petroleum ether (6:4) as mobile phase on silica gel 60 (mesh 230–400). The compound 16-hydroxy betulinic acid was separated from fraction 2 with the Rf value of 0.66. The compound was characterized as 16-hydroxy betulinic acid on the basis of 1H-NMR spectra recorded at room temperature on a Bruker Avance 300 instrument operating at a frequency of 300 MHz. The schematic diagram for isolation and purification of 16 HBA is shown in Fig. 2.
Fig. 2.
Schematic diagram for isolation and purification of 16-hydroxy betulinic acid.
2.6. Antimicrobial activity
2.6.1. Disc diffusion assay for antibacterial activity
Bacillus subtilis IFO 3232, Sarcina lutea IAM 1671, Escherichia coli IFO 3007, Klebsiella pneumoniae ATTC 10031, Xanthomonas campestris IAM 1671 and Pseudomonas sp. ATCC 13867 were used in this study. For this assay, the method of Murray et al. (1995) was followed. 100 μL of standardized inoculums suspension containing 107 CFU/mL of bacteria was used. Dimethylsulfoxide (DMSO) was used in preparing 16-hydroxy betulinic acid sample by dissolving in it and the concentration of this solution was maintained at 10 mg/mL. Then 10 μL from the solution (containing 100 μg) were soaked on discs (6 mm diameter) made of sterilized Whatman No.1 filter paper and applied on the inoculated LB agar medium. DMSO (used in dissolving samples) was taken as negative control. Kanamycin (30 μg/disc) sourced from Sigma-Aldrich Co. (St. Louis, MO, USA) was employed in the experiment as positive control. All the assays were replicated thrice.
2.6.2. Minimum inhibitory concentration (MIC) against tested bacteria
The MIC values of 16 HBA against the tested bacteria were determined by the method developed by Chandrasekaran and Venkatesalu (2004). At first, the 16 HBA sample was mixed with LB medium in a way that different concentrations ranging from 0 to 1000 μg/mL can easily be obtained. The experiment was done by transferring 20 μL inoculums of bacteria strain (107 CFU/mL) to the tube and the final test volume was 2 mL. For control tube, the test was run using only bacteria strain without samples. The incubation of the culture tubes was carried out at 37 °C over 24 h. The growth of the bacteria was observed by microscopic evaluation. The lowest inhibiting concentration (MIC) was expressed as μg/mL.
2.6.3. Preparation of spore suspension and test samples
The 5–10 days old spore suspension of Rhizoctonia solani AG-1 (IB) KACC 40111, R. solani AG-2 (IV) KACC 40132, Pythium graminicola KACC 40155 Tricoderma harzianum KACC 40791 and Fusarium oxysporum KACC 40052 were homogenized with sterile distilled water to prepare the suspension of 105 spore/ml. 16 HBA was dissolved in DMSO to prepare the stock solution which was further diluted to prepare test samples.
2.6.4. Determination of antifungal activities
Petri dishes (9 cm in diameter) containing 20 ml of potato dextrose agar (PDA) medium were used for assessing antifungal activity using disc diffusion assay (Duru et al., 2003). Sterile Whatman paper discs (6 mm in diameter) impregnated with 100 μL (1000 μg/disc) of 16 HBA were placed at equidistant and near the border of the petri dishes. A disc of fungal inoculum of 6 mm in diameter was placed upside down in the centre of the petri dishes. Then the incubation of the petri dishes at 28 ± 2 °C was continued till the growth of control slates unto the corner of the plates. The plates were used in triplicates for each treatment. Growth inhibition was calculated using the following formula:
2.6.5. Minimum inhibitory concentration (MIC) against tested fungi
Two-fold dilution method (Murray et al., 1995) was used in this assay. Different sequential concentrations of 125, 250, 500 and 1000 μg/ml of 16 HBA were obtained by serial dilution with DMSO and subsequent adding to PDB medium. The minimum concentrations (MIC) which completely inhibit fungal growth were determined by inoculation of 10 μl spore suspension in the test tubes containing specified concentrations of test sample, followed by incubation for 5–7 days at 28 ± 2 °C. The control tubes containing PDB medium were inoculated only with fungal suspension. The MIC values were expressed as μg/mL.
2.7. Analgesic activity study
2.7.1. Analgesic activity against peripheral nociceptive pain
Evaluation of 16 HBA for analgesic activity against peripheral nociceptive pain was performed using the method of Koster et al. (1959). Each group included five randomly selected mice and two different groups were received suspension of 50 and 100 mg/kg b.w. (body weight) of 16 HBA. After 30 min, each treatment group received 3% acetic acid to induce pain and then the writhing count was started from 5 min and continued for 20 min. Diclofenac sodium (40 mg/kg b.w.) was used as standard reference drug, whereas the control group received only 5% Tween 80 in normal saline (10 ml/kg b.w.). The percentage of inhibition was calculated as per following equation:
2.7.2. Analgesic activity against central nociceptive pain
For hot plate experiment, the method of Ojewole (2006) with slight modification was followed. The hot plate temperature was kept at 55 ± 1.0 °C and mice were selected based on their response to hot plate thermal stimulation after 4 s. Suspension of 16 HBA of 50 and 100 mg/kg b.w. and morphine (10 mg/kg) as a positive control were given to mice. The negative control group received only 5% Tween 80 in normal saline (10 ml/kg b.w.). Each group of mice was assayed for reaction time at 30, 60, and 90 min after treatment with test samples or morphine. To avoid tissue impairment a latency period of 30 s was considered as complete analgesia cut off time.
2.8. Carrageenan-induced paw edema assay
The efficacy of 16 HBA in reducing induced inflammation was evaluated by acute inflammation method in rats as designated by Winter et al. (1962). The basal volume (Co) of the right hind paw of each of the five rats of each group was measured by plethysmometer (model 7150, UgoBasile, Italy). After 30 min of treatment with suspension of 16 HBA (50 and 100 mg/kg b.w.) and phenylbutazone (100 mg/kg) as positive control, each of the rats of all groups received 1% carrageenan (0.1 ml) into sub-plantar surface of right hind paw. The control group received only 5% Tween 80 in normal saline (10 ml/kg b.w.). The volume of the right hind paw edema was measured at 0 h, 1 h, 2 h, 3 h and 4 h. Inhibitory activity was calculated by the following formula:
where Ct refers to paw size after a specific time interval in hours after carrageenan injection and Co denotes paw size before carrageenan injection.
2.9. Yeast-induced hyperthermia assay
After recording initial rectal temperature by digital electric thermometer (EXACON, Denmark), the rats were given 20 ml/kg (20%) brewer’s yeast suspension subcutaneously to induce pyrexia (Turner, 1965). The rat groups (n = 5) which exhibited an increase in temperature of 0.3–0.5 °C after 18 h, were further treated with 16 HBA (50 and 100 mg/kg b.w.) and paracetamol (100 mg/kg b.w.) as standard drug, whereas the control group received only 0.3 ml of saline. Then the rectal temperature of the rats was measured at 1 h, 2 h, 3 h and 4 h. The average temperature of every group was compared with mean temperature of the group treated with standard drugs.
2.10. Statistical analysis
All data were analyzed by means of ANOVA test followed by Student’s t-test. Significant levels were determined at P < 0.05 (95% confidence limit).
3. Results and discussion
3.1. Structure elucidation of the compound
Based on bio-assay, the chloroform leaf extract was selected for the purification of compounds. The leaf extract (7 g) was subjected to column chromatography over silica gel (230–400 mesh ASTM, Merck, Germany) to give 15 fractions. The fraction 3 was then further subjected to column chromatography over silica gel to give 16 fractions. Then the compound was obtained from fraction 2 by PTLC using suitable solvent system and was finally characterized as 16-hydroxy betulinic acid by 1H NMR spectroscopic data analysis.
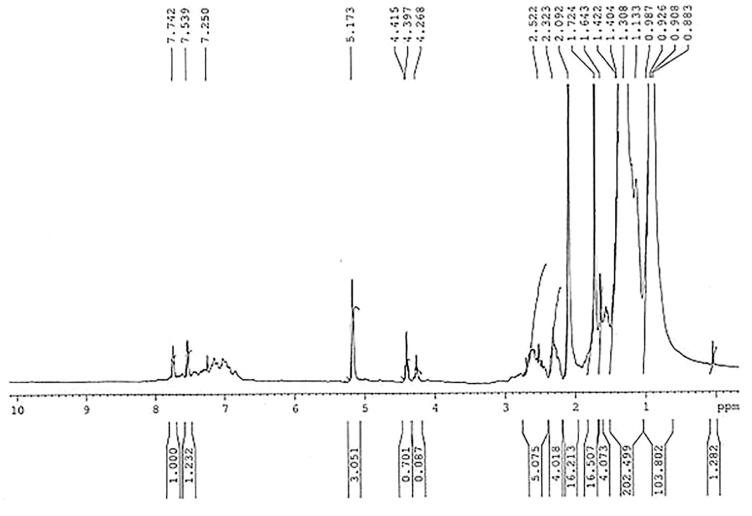
16 HBA was obtained as yellowish oily liquid. It was insoluble in water but highly soluble in chloroform, pyridine and acetic acid. TLC examination showed that it to be a single compound with Rf value of 0.66 in EtOAc: Pet. ether (6:4). It was appeared as a dark spot on TLC plate (silica gel 60 GF254) under UV light at 254 nm and after the development of the chromatoplates in closed iodine chamber, it was appeared as yellow spot. The 1H NMR (CDCl3, 300 MHz) spectrum of the compound (Fig. 3) revealed the presence of a lupine type carbon skeleton. It displayed signals attributable to an exomethylene group at δ 4.39 and 4.41 ppm (1H, each, br.s) which together with an allylic methyl at δ 1.77 that indicated an isopropenyl function. The two olefinic protons H-29 (a) and H-29 (b) resonated at δ 4.39 and δ 4.41 ppm, respectively and their relative assignment could be made due to the vicinity of H-29 (a) to the methyl group at C-30.
Fig. 3.
1H NMR (CDCl3, 300 MHz) spectrum of 16-hydroxy betulinic acid.
The next signal in the proton spectrum at δ 3.5 ppm integrated for two protons which form their chemical shift, and were assigned to H-19 and H-3. The signal of methyl group at C-30 attached to a SP2 centre could be safely identified from its chemical shift of δ 1.77. In addition, the compound showed five singlets at δ 0.83 (3H, Me-25), 1.01 (3H, Me-24), 1.12 (3H, Me-27), 1.13 (3H, Me-26) and 1.21 (3H, Me-23), each integrating for three protons which assigned for five methyl groups in the compound.
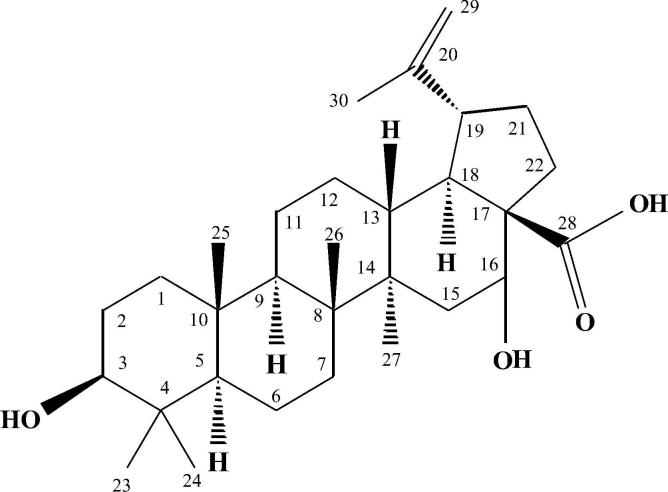
The characteristic signal at δ 5.18 was due to highly deshielding suggested the presence of hydroxyl group at C-16. All other peaks in 1H NMR data of 16 HBA also matching with literature values (Stefan and Dieter, 2009) and are in good agreement with those of 16 HBA. The 1H NMR (CDCl3, 300 MHz) data of 16 HBA acid is shown in Table 1 and its chemical structure is presented in Fig. 4. Betulinic acid and some of its derivatives have been isolated from some other plant species (Yogeeswari and Sriram, 2005). M. cordata has already been reported to contain some chemical compounds e.g. vitamin A, B, and C, mikanin, friedelin, efifriedinol, dilactones, etc. (Ghani, 2003), however, there is no report available so far on isolation of betulinic acids or any of its derivatives from this plant. This is the first report for isolation and purification of a betulinic acid derivative i.e. 16-hydroxyl betulinic acid (a triterpenoid) from the leaves of M. cordata.
Table 1.
1H NMR spectral data of the 16-hydroxy betulinic acid (300 MHz, δ, CDCl3) with reference to Stefan and Dieter (2009).
| Position | δH | Position | δH |
|---|---|---|---|
| 1 | 0.84 (s) | 17 | |
| 2 | 1.24 (m) | 18 | 1.80 (m) |
| 3 | 3.48 (t) | 19 | 3.52 (m) |
| 4 | 20 | ||
| 5 | 0.83 (m) | 21 | |
| 6 | 1.26 (m) | 22 | 1.80 (m) |
| 7 | 23 | 1.21 (s) | |
| 8 | 24 | 1.01 (s) | |
| 9 | 1.28 (t) | 25 | 0.83 (s) |
| 10 | 26 | 1.13 (s) | |
| 11 | 1.18 (m) | 27 | 1.12 (s) |
| 12 | 1.15 (m) | 28 | |
| 13 | 2.46 (m) | 29 | 4.39(br.s)/4.41(br.s) |
| 14 | 30 | 1.77 (s) | |
| 15 | 1.80 (m) | COOH | 7.53 (s) |
| 16 | 5.18 (d) | OH | 7.74 (s) |
Fig. 4.
Structure of 16-hydroxy betulinic acid isolated from Mikania cordata.
3.2. Antimicrobial activity
The results of the assays for antibacterial activities of the 16-hydroxy betulinic acid are shown in Table 2. The 16-hydroxy betulinic acid showed potent efficacy against all six bacteria. For all the six bacteria, the zone of growth inhibition ranged from 12.0 to 17.5 mm. The present study revealed that the efficacy shown by 16-hydroxy betulinic acid is somewhat weaker than that of Kanamycin. The blind control showed no activity against the bacteria. From the minimum inhibitory concentration (MIC) assay, it was observed that the two gram positive bacteria, Sarcina lutea IAM 1671 and Bacillus subtilis IFO 3232 were very much susceptible to 16-hydroxy betulinic acid with lowest MIC 31.5 μg/mL values (Table 2), whereas the gram negative bacteria, Escherichia coli IFO 3007, Klebsiella pneumoniae ATTC 10031, Pseudomonas sp. ATCC 13867 and Xanthomonas campestris IAM 1671 were found somewhat less susceptible to the 16-hydroxy betulinic acid with MIC values (62.5–125) μg/mL. Our findings are in agreement with previous reports where some hydroxy derivatives of betulinic acid were found to have antibacterial activities (Schuhly et al., 1999).
Table 2.
Antibacterial activity and minimum inhibitory concentrations of 16-hydroxy betulinic acid.
| Bacteria | Zone of growth inhibition (in mm)a |
Minimum inhibition concentration (μg/ml) | |
|---|---|---|---|
| 16-hydroxy betulinic acid | Kanb | ||
| Bacillus subtilis IFO 3232 | 16 ± 0.7 | 21.5 ± 0.7 | 31.5 |
| Sarcina lutea IAM 1671 | 17.5 ± 0.4 | 16 ± 1.1 | 31.5 |
| Escherichia coli IFO 3007 | 14 ± 1.3 | 20.5 ± 1.2 | 62.5 |
| Xanthomonas campestris IAM 1671 | 14 ± 0.9 | 24 ± 1.4 | 62.5 |
| Klebsiella pneumoniae ATTC 10,031 | 12 ± 1.7 | 20 ± 0.6 | 125 |
| Pseudomonas sp. ATCC 13,867 | 14.5 ± 0.3 | 20 ± 1.2 | 125 |
Diameter of inhibition zones (mm) of the compound around the discs (6 mm) impregnated with 10 μL/disc corresponding to 100 μg/disc.
Kanamycin (30 μg). Values are given as mean ± S.D of triplicate experiment and considered to be significantly different at P < 0.05.
The 16-hydroxy betulinic acid exhibited a moderate antifungal activity against all the tested fungi except Tricoderma harzianum KACC 40791 and a low inhibition effect was observed against Fusarium oxysporum KACC 41083. At the concentration of 10 μl (1000 μg/mL), the 16-hydroxy betulinic acid showed a potent inhibitory effect on the growth of Rhizoctonia solani AG-1 (IB) KACC 40111 (45.4%), R. solani AG-2 (IV) KACC 40132 (41.9%), Pythium graminicola KACC 40155 (54.5%), and Fusarium oxysporum KACC 40052 (37.6%) as shown in Table 3.
Table 3.
Antifungal activity and minimum inhibitory concentrations of 16-hydroxy betulinic acid.
| Fungal strains | Radial growth inhibition |
Minimum inhibition concentration | |
|---|---|---|---|
| mma | (%)b | (μg/ml) | |
| Rhizoctonia solani AG-1 (IB) KACC 40111 | 2.1 ± 0.4c | 45.4 ± 0.5c | 500 |
| Rhizoctonia solani AG-2-2 (IV) KACC 40132 | 2.7 ± 0.9c | 41.9 ± 0.4c | 500 |
| Pythium graminicola KACC 40155 | 2.5 ± 0.9c | 54.5 ± 0.8c | 250 |
| Tricoderma harzianum KACC 40791 | nd | nd | nd |
| Fusarium oxysporum KACC 40052 | 1.9 ± 0.8c | 37.6 ± 0.9c | 1000 |
Radial growth of fungal pathogens.
Percentage of radial growth inhibition.
Values are given as mean ± S.D. (n = 3), and considered to be significantly different at P < 0.05. nd: not detected of anti-fungal activity.
In the MIC assay, 16-hydroxy betulinic acid was found most effective against P. graminicola (250 μg/mL) as compared to those of R. solani AG-1 and R. solani AG-2 (500 μg/mL), whereas the MIC values were found lowest (1000 μg/ml) in Fusarium oxysporum KACC 40052 (Table 3). Our result is congruent with some other previous studies where betulinic acid and some other derivatives of betulinic acid were reported to have antifungal effects (Shai et al., 2008).
The antimicrobial activities of pentacyclic compounds having lupane skeleton is well reported and the compounds were found to exert their activities by initiating changes in membrane permeability resulting from the leakage of reducing sugars, proteins and reduction of respiratory chain dehydrogenase enzyme’s activity (Sathya Bama et al., 2012). This mechanism can also be attributed to 16-hydroxy betulinic acid for its antimicrobial activity.
3.3. Analgesic activity study
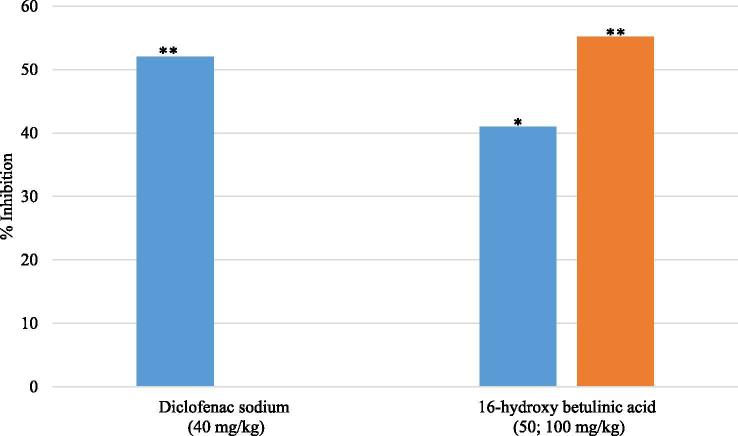
16-hydroxy betulinic acid showed analgesic efficacy against peripheral nociceptive pain by reducing acetic acid induced writhing in mice (Fig. 5). The percentage inhibition of writhing for 16-hydroxy betulinic acid at 50 and 100 mg/kg were 41% and 55.19%, respectively. The percentage inhibitions were determined by comparing the writhing response of animals received test specimen to the animals that received vehicle only. By comparison, 40 mg/kg diclofenac sodium was found to produce highest 52% analgesic effect in this nociception model. In this study, the tested samples exhibited dose dependent analgesia.
Fig. 5.
Effect of the treatment 16-hydroxy betulinic acid (50, 100 mg/kg), and diclofenac sodium as control (40 mg/kg) on the intraperitoneal administration of 10 ml/kg of 3% acetic acid. Each group represents the mean of 5 animals. Values are expressed as mean ± SEM, ∗P < 0.05 and ∗∗P < 0.01 compared with control group.
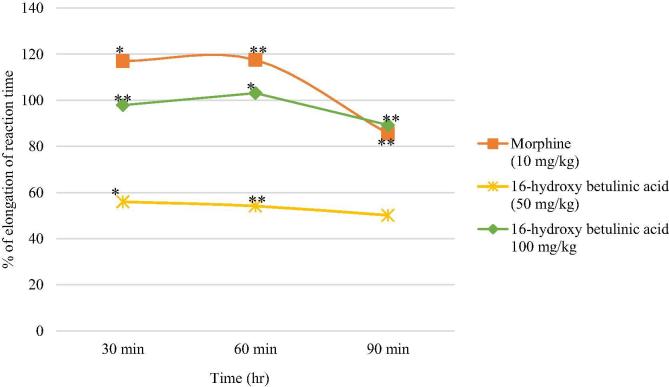
In the hot plate study, the 16-hydroxy betulinic acid (50 and 100 mg/kg) elongated the reaction time at different time points. All these elongation in the reaction time was determined by comparing to the corresponding control groups (Table 4; Fig. 6). Morphine significantly prolonged the reaction time of the animals, e.g. 116.99%, 117.52% and 85.55% relative elongation of reaction time at 30 min, 60 min and 90 min, respectively, confirming centrally mediated activity. The compound 16-hydroxy betulinic acid showed central anti-nociceptive activity in dose dependent manner and the highest analgesia was induced at 100 mg/kg b.w. by elongating the reaction time 97.97%, 103.11%, and 89.29% at 30 min, 60 min and 90 min, respectively.
Table 4.
Effects of 16-hydroxy betulinic acid on hot plate test in mice.
| Treatment | Dose (mg/kg) | Reaction time (s) |
||
|---|---|---|---|---|
| 30 min | 60 min | 90 min | ||
| Control (Vehicle) | – | 6.24 ± 0.61 | 6.28 ± 0.28 | 6.92 ± 0.34 |
| Morphine | 10 | 13.54 ± 0.49* | 13.66 ± 0.39** | 12.84 ± 0.56** |
| 16-hydroxy betulinic acid | 50 | 9.73 ± 0.19** | 9.67 ± 021 | 10.39 ± 0.15** |
| 100 | 12.35 ± 0.12** | 12.75 ± 0.09** | 13.1 ± 0.03** | |
Each value represents the mean (±S.E.M.) of five observations.
∗P < 0.05 and ∗∗P < 0.01 compared with control group.
Fig. 6.
Effect of the treatment with 16-hydroxy betulinic acid (50, 100 mg/kg) and morphine as control (10 mg/kg) on the hot plate analgesic test. Each mouse is served as its own control. Before treatment, the time for hind paw licking or jumping on the heated plate of analgesiometer was determined thrice at 1 h interval and mean of these were taken as the initial reaction time (control latency). Mice in each group were tested 30, 60 and 90 min after drug treatment. Values are expressed as mean ± SEM, ∗P < 0.05 and ∗∗P < 0.01 compared with control group.
The analgesic activity (both peripheral and central) of the test specimens were investigated by applying two different methods. The most suitable and widely used acetic acid induced writhing method was applied to identify the peripheral effects, whereas hot plate method was employed to detect analgesic activity against central nociceptive pain of the test samples. Our findings clearly indicated that 16-hydroxy betulinic acid possessed both peripheral (writhe reduction) and central (thermal reaction time prolongation) analgesic effects.
In the peripheral nociception model, the pain is developed by inducing local inflammatory sensation. Following inflammation, there is biogenesis of prostaglandins from cyclooxygenase pathway and leukotrienes from lipoxygenase pathway (Das et al., 2012). The released prostaglandins, mainly prostacyclin (PGI2), and to a lesser extent, prostaglandin E, are supposed to induce pain and abnormal contraction of mice body that is termed as ‘writhing’. Several lupane triterpenoids found to prevent the biogenesis process of lipooxygenase and/or cyclooxygenase which in turn, affect the formation of PGE2 (Taiwe et al., 2011). Thus, the lupane triterpenoids under investigation (16-hydroxy betulinic acid) might suppress transformation mechanism in the nociceptor which results in reduced or no pain perception. Therefore, to evaluate the actual analgesic activity, another test model e.g. hot plate thermal stimulation in combination with the acetic acid induced writhing model is generally suggested. Likewise, 16-hydroxy betulinic acid was also found to prolong reaction time in hot plate thermal stimulation test. The effect of 16-hydroxy betulinic acid is comparable with that obtained by morphine. Central nervous system depression properties of morphine resulted in reduced behavioral activities. Previous studies revealed that pentacyclic lupane triterpenoids might be able to produce locomotors depressant, muscle relaxant and sedative potential effect which, in turn, leads to the conclusion that those compounds have some central nervous system depression properties (Taiwe et al., 2011) and all those observation can help in explaining central analgesic activity of 16-hydroxy betulinic acid in hot plate analgesic model.
3.4. Carrageenan-induced rat paw edema assay
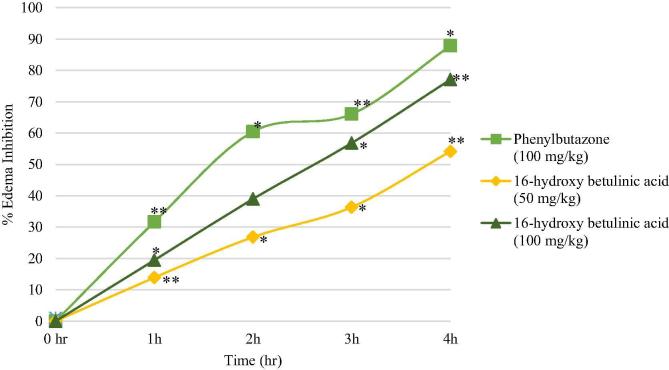
Results of the anti-inflammatory effects of 16-hydroxy betulinic acid are presented in Table 5 and Fig. 7. Dose dependent paw edema inhibition effect was observed and the highest inhibition of paw edema (77.08%) was recorded at 100 mg/kg of 16-hydroxy betulinic acid at 4 h.
Table 5.
Anti-inflammatory activities of 16-hydroxy betulinic acid and phenylbutazone (PBZ) on carrageenan-induced edema in the right hind-limb paw of rats.
| Treatment | Dose (mg/kg) | Time (h) |
Average edema formation | ||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |||
| EV (ml) | EV (ml) | EV (ml) | EV (ml) | EV (ml) | |||
| Control (saline) edema formation (mm) size | – | – | 0.35 ± 0.04 | 0.41 ± 0.05 | 0.44 ± 0.050 | 0.48 ± 0.06 | 0.42 |
| Phenylbutazone | 100 | – | 0.24 ± 0.03 | 0.16 ± 0.05** | 0.15 ± 0.03* | 0.06 ± 0.04** | 0.15 |
| 16-hydroxy betulinic acid | 50 | – | 0.31 ± 0.03 | 0.30 ± 0.01* | 0.28 ± 0.1* | 0.22 ± 0.07** | 0.28 |
| 100 | – | 0.29 ± 0.0* | 0.25 ± 0.04** | 0.19 ± 0.3** | 0.11 ± 0.12** | 0.21 | |
EV: Edema volume. Values represent the mean ± S.E.M. (n = 5), ∗P < 0.05 and ∗∗P < 0.01 compared with control group.
Fig. 7.
Effect of the treatment with 16-hydroxy betulinic acid (50, 100 mg/kg) and Phenylbutazone as control (100 mg/kg) on paw edema induced by intradermal injection of carrageenan (1% 0.1 ml) in rats. Zero (time zero) corresponds to baseline measurement of animals performed 24 h before the test. Each group represents the mean of 5 animals, and the vertical lines indicate the SEM. ∗P< 0.05 and ∗∗P < 0.01 compared with control group.
The anti-inflammatory activity of 16-hydroxy betulinic acid can be explained based on previous studies. IL-10, a cytokine with potent anti-inflammatory properties, inhibits TNF-α release (Fiorentino et al. 1991). It has been reported that the betulinic acid (BA) increase in the levels of serum IL-10 (Costa et al., 2014). Viji et al. (2011) showed that BA inhibits the expression of cyclooxygenase 2 (COX-2) in cultures of human peripheral blood mononuclear cells, leading to a decrease in PGE2 production. The increase in IL-10 induced by betulinic acid might be related to the inhibition of PGE2 production, another inflammatory mediator, since IL-10 is known to inhibit COX-2 expression (Niiro et al., 1995) which play a vital role against inflammation conferred by betulinic acid. However, the fact that betulinic acid modulates IkBα phosphorylation (Viji et al., 2011) suggests that its action on TNF-α inhibition may also be related to alterations in signaling pathways, such as NF-kB activation. Moreover, it has been observed that betulinic acid inhibits the production of inflammatory mediators, such as NO and TNF-α (Costa et al., 2014). All these effects of betulinic acid in suppressing inflammatory mediators and increasing anti-inflammatory cytokines might be applicable for the 16 hydroxy betulinic acid, a derivatives of betulinic acid, to explain its anti-inflammatory effects.
3.5. Yeast-induced hyperthermia assay
The antipyretic results of 16-hydroxy betulinic acid are presented in Table 6. Administration of brewer’s yeast to rats produced a noticeable increase in rectal temperature after 18 h of yeast injection (Table 6). In this study, although 16-hydroxy betulinic acid reduced the rectal temperature right from 1 h onward but the weaker antipyretic effect was observed in comparing to paracetamol (100 mg/kg orally) which significantly reduced the temperature (Table 6).
Table 6.
Antipyretic activities of 16-hydroxy betulinic acid and paracetamol on brewer’s yeast-induced pyrexia in rats.
| Treatment | Dose (mg/kg) | Rectal temperature |
|||||
|---|---|---|---|---|---|---|---|
| Before drug |
After drug |
||||||
| −18 h | 0 h | 1 h | 2 h | 3 h | 4 h | ||
| Control (Saline) | 0.3 ml | 37.22 ± 0.20 | 38.106 ± 0.30 | 38.018 ± 0.28 | 38.00 ± 0.19 | 38.05 ± 0.19 | 38.05 ± 0.20 |
| 16-hydroxy betulinic acid | 50 | 36.88 ± 0.32 | 37.92 ± 0.21 | 37.44 ± 0.15* | 37.04 ± 0.11 | 36.85 ± 0.31** | 36.77 ± 0.17** |
| 100 | 36.84 ± 0.22 | 38.38 ± 0.15* | 38.46 ± 0.12 | 37.62 ± 0.31* | 37.04 ± 0.12** | 36.66 ± 0.19** | |
| Paracetamol | 100 | 36.97 ± 0.28 | 38.19 ± 0.32 | 37.76 ± 0.12** | 37.13 ± 0.19* | 36.98 ± 0.05** | 36.95 ± 0.09** |
Values are mean ± S.E.M. rectal temperature (n = 5), ∗P < 0.05 and ∗∗P < 0.01 compared with control group.
The drugs or medicinal compounds bearing the suppressing effect on biogenesis of prostaglandin generally have antipyretic properties (Vane, 1987). In our study, 16-hydroxy betulinic acid was found to inhibit pyrexia considerably developed by yeast administration. As discussed earlier, 16-hydroxy betulinic acid have impacts on central nervous system and likely to inhibit the synthesis of prostaglandin E2 and thus lessen hyperthermia. In this case, 16-hydroxy betulinic acid might exert some inhibitory effect by its action on COX-3 which decrease the amount prostaglandin E2 in the hypothalamus region of brain (Botting and Ayoub, 2005). There is another possibility in which the tested compounds might have some positive influence to increase the secretion of vasopressin and arginine like antipyretic agents within the body (Chandrasekharan, 2002).
4. Conclusion
The present study confirmed that 16-hydroxy betulinic acid isolated from M. cordata has potent antimicrobial, analgesic, anti-inflammatory and antipyretic properties. In the recent past, betulinic acid and its different derivatives have attracted great research interest in the field of drug discovery. Our findings will not only be a support to bolster the research efforts on betulinic acids to emerge new commercial therapeutic agents from betulinic acid and its derivatives but also attract researchers to study structural and functional relationship of 16 hydroxy betulinic acid. Our results can also be seen as scientific support for the traditional and folklore usage of Mikania cordata in Bangladesh for the treatment of different ailments and provide opportunities to explore this plant as a source of bioactive compounds for biochemical and pharmaceutical industries.
Acknowledgments
Acknowledgements
This work was supported by the Department of Applied Chemistry & Chemical Technology, Islamic University, Kushtia and the Centre for Biomedical Research, University of Dhaka, Bangladesh. The authors are thankful to Prof. Dr. A.S. Shamsur Rouf, Department of Clinical Pharmacy and Pharmacology, University of Dhaka for his cooperation during the course of the study. The authors from KSU extend sincere appreciation to the Deanship of Scientific Research at King Saud University (research group project number RG-195).
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Atiqur Rahman, Email: atiq@acct.iu.ac.bd.
M. Oliur Rahman, Email: oliur.bot@du.ac.bd.
References
- Ahmed Z.U., Begum Z.N.T., Hassan M.A., Khondker M., Kabir S.M.H., Ahmad M., Ahmed A.T.A., Rahman A.K.A., Haque E.U., editors. vol. 6. Asiatic Society of Bangladesh; Dhaka: 2008. (Encyclopedia of Flora and Fauna of Bangladesh). [Google Scholar]
- Botting R., Ayoub S.S. COX-3 and the mechanism of action of paracetamol/ acetaminophen. Prostaglandins Leukot. Essent. Fatty Acids. 2005;72:85–87. doi: 10.1016/j.plefa.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Chandrasekharan N.V. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure and expression. Proc. Nat. Acad. Sci. USA. 2002;99:13926–13931. doi: 10.1073/pnas.162468699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran M., Venkatesalu V. Antibacterial and antifungal activity of Syzygium jambolanum seeds. J Ethnopharmacol. 2004;91:105–108. doi: 10.1016/j.jep.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Costa J.F., Barbosa-Filho J.M., Maia G.L., Guimarães E.T., Meira C.S., Ribeiro-dos-Santos R., de Carvalho L.C., Soares M.B. Potent anti-inflammatory activity of betulinic acid treatment in a model of lethal endotoxemia. Int. Immunopharmacol. 2014;23(2):469–474. doi: 10.1016/j.intimp.2014.09.021. [DOI] [PubMed] [Google Scholar]
- Das S.C., Bhadra S., Roy S., Saha S.K., Islam M.S., Bachar S.C. Analgesic and anti-inflammatory activities of ethanol root extract of Swertia chirata (Gentianaceae) Jordan J. Biol. Sci. 2012;5:31–36. [Google Scholar]
- Duru M.E., Cakir A., Kordali S., Zengin H., Harmandar M., Izumi S., Hirata T. Chemical composition and antifungal properties of essential oils of three Pistacia species. Fitoterapia. 2003;74:170–176. doi: 10.1016/s0367-326x(02)00318-0. [DOI] [PubMed] [Google Scholar]
- Fiorentino D.F., Zlotnik A., Mosmann T.R., Howard M., O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 1991;147:3815–3822. [PubMed] [Google Scholar]
- Ghani A. second. ed. The Asiatic Society of Bangladesh; Dhaka: 2003. Medicinal Plants of Bangladesh; p. 603. [Google Scholar]
- Hasan S.M.R., Jamila M., Majumder M.M., Akter R., Hossain M.M., Mazumder M.E.H., Alam M.A., Jahangir R., Arif M., Rahman S. Analgesic and antioxidant activity of the hydromethanolic extract of Mikania scandens (L.) Willd. leaves. Am. J. Pharmacol. Toxicol. 2009;41:1–7. [Google Scholar]
- Koster R., Anderson M., Beer E.J. Acetic acid for analgesic screening. Fed Proc. 1959;18:412–416. [Google Scholar]
- Mosaddik M.A., Alam K.M. The antiulcerogenic effect of an alkaloidal fraction from Mikania cordata on diclofenac sodium-induced gastrointestinal lesions in rats. J. Pharm. Pharmacol. 2000;52:1157–1162. doi: 10.1211/0022357001774930. [DOI] [PubMed] [Google Scholar]
- Murray P.R., Baron E.J., Pfaller M.A., Tenover F.C., Yolkem R.H. ASM Press; Washington DC: 1995. Manual of Clinical Microbiology. [Google Scholar]
- Nayeem A.A., Khatun A., Rahman M.S., Rahman M. Evaluation of phytochemical and pharmacological properties of Mikania cordata (Asteraceae) leaves. J. Pharmacognosy Phytother. 2011;3(8):118–123. [Google Scholar]
- Niiro H., Otsuka T., Tanabe T., Hara S., Kuga S., Nemoto Y., Tanaka Y., Nakashima H., Kitajima S., Abe M., Niho Y. Inhibition by interleukin-10 of inducible cyclooxygenase expression in lipopolysaccharide-stimulated monocytes: its underlying mechanism in comparison with interleukin-4. Blood. 1995;85:3736–3745. [PubMed] [Google Scholar]
- Ojewole J.A.O. Analgesic, anti-inflammatory and hypoglycaemic effects of ethanol extract of Zingiber officinale (Roscoe) Rhizomes (Zingiberaceae) in mice and rats. Phytother. Res. 2006;20:764–772. doi: 10.1002/ptr.1952. [DOI] [PubMed] [Google Scholar]
- Partha P., Hossain A.B.M.E. Ethnobotanical investigation into the Mandi ethnic community in Bangladesh. Bangladesh J. Plant Taxon. 2007;14(2):129–145. [Google Scholar]
- Rahmatullah M., Mukti I.J., Fahmidul Haque A.K.M., Mollik M.A.H., Parvin K., Jahan R., Chowdhury M.H., Rahman T. An ethnobotanical survey and pharmacological evaluation of medicinal plants used by the Garo tribal community living in Netrakona district, Bangladesh. Adv. Nat. Appl. Sci. 2009;3(3):402–418. [Google Scholar]
- Rahmatullah M., Mollik M.A.H., Azam A.T.M.A., Islam M.R., Chowdhury M.A.M., Jahan R., Chowdhury M.H., Rahman T. Ethnobotanical survey of the Santal tribe residing in Thakurgaon district, Bangladesh. Am.-Euras. J. Sustain. Agric. 2009;3(4):889–898. [Google Scholar]
- Sathya Bama S., Jayasurya Kingsley S., Sankaranarayanan S., Bama P. Antibacterial activity of different phytochemical extracts from the leaves of T. procumbens Linn, Identification and mode of action of the terpenoid compound as antimicrobial. Int. J. Pharm. Pharm. Sci. 2012;4:557–564. [Google Scholar]
- Schuhly W., Heilmann J., Calis I., Sticher O. New triterpenoids with antibacterial activity from Zizyphus joazeiro. Planta Med. 1999;65(8):740–743. doi: 10.1055/s-1999-14054. [DOI] [PubMed] [Google Scholar]
- Shai L.J., McGaw L.J., Aderogba M.A., Mdee L.K., Eloff J.N. Four pentacyclic triterpenoids with antifungal and antibacterial activity from Curtisia dentata (Burm. f) C.A. Sm. Leaves. J. Ethnopharmacol. 2008;119(2):238–244. doi: 10.1016/j.jep.2008.06.036. [DOI] [PubMed] [Google Scholar]
- Siddiqui S.A., Islam R., Jamal A.H.M., Parvin T., Rahman A. Chemical composition and antifungal properties of the essential oil and various extracts of Mikania scandens (L.) Willd. Arabian J. Chem. 2017;10(2):2170–2174. [Google Scholar]
- Stefan, B., Dieter, S., 2009. Classics in Spectroscopy: Isolation and Structure Elucidation of Natural Products. Weily-VCH.
- Taiwe G.S., Bum E.N., Talla E., Dimo T., Weiss N., Sidiki N., Dawe T., Moto F.C.O., Dzeufiet P.D., Waard M.D. Antipyretic and antinociceptive effects of Nauclea latifolia root decoction and possible mechanisms of action. Pharm. Biol. 2011;49:15–25. doi: 10.3109/13880209.2010.492479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner R.A. Academic Press; New York & London: 1965. Screening Method in Pharmacology; p. 268. [Google Scholar]
- Vane J. The evolution of non-steroidal anti-inflammatory drugs and their mechanisms of action. Drugs. 1987;33(suppl. 1):18–27. doi: 10.2165/00003495-198700331-00005. [DOI] [PubMed] [Google Scholar]
- Viji V., Helen A., Luxmi V.R. Betulinic acid inhibits endotoxin-stimulated phosphorylation cascade and pro-inflammatory prostaglandin E2 production in human peripheral blood mononuclear cells. Br. J. Pharmacol. 2011;162:1291–1303. doi: 10.1111/j.1476-5381.2010.01112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter C.A., Risley E.A., Nuss G.W. Carrageenin-induced edema in hind paw of the rat as an assay for anti-inflammatory drugs. Proc. Soc. Exp. Bio. Med. 1962;111:544–547. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- Yogeeswari P., Sriram D. Betulinic acid and its derivatives: A review on their biological properties. Curr. Med. Chem. 2005;12:657–666. doi: 10.2174/0929867053202214. [DOI] [PubMed] [Google Scholar]