Abstract
Objective
To clarify the role of dietary carbohydrate, glycemic index (GI), and glycemic load (GL) in progression from health to coronary heart disease (CHD) by determining disease-nutrient risk relation (RR) values needed for intake ranges within jurisdictions and across the globe.
Methods
We performed a literature search of MEDLINE and EMBASE for prospective cohort studies that used truly valid dietary instruments in heathy adults published from January 1, 2000, to June 5, 2018. Relevant observations were extracted by 2 reviewers independently. We used dose-response meta-analysis accounting for nonindependence of results within studies. Bradford-Hill criteria were used to assess causality.
Results
Eligible studies had a mean follow-up of 11 years (range, 5-19 years), were conducted in North America, Europe, and East Asia, and yielded combined RRs of 1.44 (95% CI, 1.25-1.65) per 65 g/d GL (11 studies) and 1.24 (95% CI, 1.12-1.38) per 10 U GI (10 studies) (glucose scale). The CHD-carbohydrate RR on GI was 1.66 (95% CI, 1.23-2.25) per 98 g/d of carbohydrates per 10 units GI. The 65 g/d GL, 10 U GI, and 98 g/d carbohydrate values corresponded to oral intakes from the 10th to the 90th percentiles within sampled populations. Inconsistencies were minor (I2≤20%), as were small-study effects (P=.61 for GL and P=.26 for GI). Funnel plots were symmetric. Cubic spline dose-response meta-analysis yielded RRs as follows: across the global range for GL (55-290 g/d), 5.5 (95% CI, 3.1-9.8) (I2=0); for GI (47-82 U), 2.71 (95% CI, 1.47-4.40) (I2=0); and for the CHD-carbohydrate dependence on GI (50-80 U), 4.57 (95% CI, 1.86-11.4) (I2=16%). Bradford-Hill criteria indicated that these relations were probably causal.
Conclusion
Strong and probably causal CHD-GL and GI RRs exist within populations. The RRs were remarkably higher across global exposures. The results support the consideration of these markers of carbohydrate food quality in dietary guidelines for general populations.
Trial Registration
PROSPERO Identifier: CRD42013004504
Abbreviations and Acronyms: CHD, coronary heart disease; Corr, correlation coefficients; CVD, cardiovascular disease; DRM, dose-response meta-analysis; EQM, extreme-quantile meta-analysis; GI, glycemic index; GL, glycemic load; HDL, high-density lipoprotein; LCL, lower confidence limit; MI, myocardial infarction; RCT, randomized controlled trial; RR, risk relation
Cardiovascular disease (CVD) is recognized as the number one cause of morbidity and mortality globally, with coronary heart disease (CHD) being the major contributor. This issue is of growing concern in low- and middle-income countries1, 2 in addition to wealthy Western countries, where it has long been recognized that diet and lifestyle contribute to 80% of CHD.3 However, the role of dietary carbohydrate is unclear, it may not even be mentioned in major articles4 or media,2 and it may be concluded to have negligible impact5 even when considering the glycemic index (GI) and glycemic load (GL).6 Meanwhile, Halton et al7 reported a modest increase in incident CHD of 22% from the 10th to the 90th percentile of carbohydrate intake in women nurses in the United States. Dwarfing this finding, they reported a 90% higher risk for CHD from GL, strong enough to suggest a role for this marker in carbohydrate food quality.
Whether the risk relations (RR) between CHD and dietary GL and GI are sufficiently strong (threshold RR >1.20 with a lower bound >1.10) to support risk reduction via nutrition guidance is unclear. Methodological problems in original studies and existing systematic and meta-analytic reviews prevent an understanding of whether this threshold is met. Therefore, we reexamined the available evidence, aiming to avoid pitfalls in the existing literature.
A number of extreme-quantile meta-analyses (EQMs) have supported that a higher risk for CHD arises among women consuming diets of higher GL, by 69% in 5 studies,8 55% in 6 studies,9 and 49% in 5 studies while representing 1 study of high RR twice.10 Each meta-analysis combined mostly the same studies, and they reported CHD-GI RRs approximately half that for GL and that RRs were lower in men than women. Unfortunately, each meta-analysis combined results having different definitions for GL or GI (across tertile or quartile or quintile ranges) and with different ranges per quantile. Also, none of the studies adjusted GL to a common energy intake before analysis. None asked whether the size of these RRs was dependent on the quality of the dietary instrument or selected to use only those studies using truly validated dietary instruments to assess GI and GL intakes. Also not addressed was whether the RRs were independent of macronutrient intakes.
Another EQM with problems similar to those noted previously had additionally undertaken dose-response meta-analyses (DRMs) combining studies on men and women.10 Dose-response meta-analysis helps to avoid combining studies of different definitions of exposure. However, DRM depends on the dietary assessment systems not resulting in overdispersion of the exposure variable, which results in bias toward the null.11 The authors of the EQM10 tabulated the CHD RR to be higher by only 5% per 50-U increment in GL (11 studies), which is hard to reconcile with results from other EQMs.8, 9, 10 Moreover, a graphical presentation in their article shows a pattern of confidence intervals inconsistent with the method of DRM indicated.
Mente et al12 reported evidence supporting a causal link between dietary factors and CHD, indicating a 32% greater risk among persons consuming higher GI and GL diets, a combined value as if the risk was the same from GI and GL. The approach to assessment of causality was that of Bradford-Hill,13 although only the first 4 of the 9 criteria were used, and for GI and GL, none of the criteria were independent of the prospective cohort studies analyzed. Micha et al14 reported some evidence on the CHD-GL RR, although they did address all Bradford-Hill criteria. However, although they reported use of an appropriate DRM, the value for the CHD-GL RR they provided was from an earlier publication,9 which was based on EQM having the problems noted previously.
Usually, relevant studies have assessed the CHD-GL and GI RRs in prospective cohort studies as noted previously. Notably, Jakobsen et al15 adopted a different approach by investigating the CHD-carbohydrate RR, finding this relation was “dependent” on the GI of the carbohydrate, although they did not quantify the “dependence.” Others have also provided estimates of the CHD-carbohydrate RR for dietary carbohydrate of known mean GI.16, 17, 18, 19, 20 To our knowledge, there are no published meta-analyses of these data, which we provide. Also to our knowledge, there is no published quantitative DRM of the CHD-GL and GI RRs across the global (worldwide) range of dietary GL and GI intakes as large as 235 g/d GL and 30 U GI, and just one exists for half these ranges,10 with the attendant problems noted. We aimed to rectify this absence since observations are now available to cover the wider ranges.16, 17, 19, 20, 21, 22, 23, 24 We further explored whether the size of the CHD-GL and GI RRs depends on the absence of study-level adjustments for each specific macronutrient. Finally, in discussion, we address causality.
Methods
Protocol and Guidelines
The study protocol was registered with PROSPERO (CRD42013004504). It was to investigate CHD-GI and GL and type 2 diabetes–GL and GI RRs with control for the validity of the dietary instrument, potential sex differences, and macronutrient adjustments (among others). The present article concerns CHD only.
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses)25 and MOOSE (Meta-analyses of Observational Studies in Epidemiology)26 guidelines for reporting were used.
Literature Searches and Sources
MEDLINE and EMBASE were searched simultaneously using ProQuest LLC via the Royal Society of Medicine, London, United Kingdom. A highly efficient protocol was developed in collaboration with the Royal Society of Medicine LitSearch and adapted to CHD. The search period was January 1, 2000, to June 5, 2018 (Supplemental Figure S1a, available online at http://mcpiqojournal.org). The search included identification of Cochrane studies and was for prospective cohort studies (the most reliable epidemiological design) that investigated the association of CHD (first incidence and deaths) with GI and/or GL and carbohydrate characterized by GI in ostensibly heathy adult populations without selection by age, sex, race/ethnicity, or region.
Inclusion and Exclusion Criteria
For inclusion in our analyses, studies had to meet the following criteria: (1) prospective cohort study, (2) relevant to adult public health, (3) investigated the association between confirmed first nonfatal myocardial infarction (MI) or fatal CHD (collectively, CHD) and GI and/or GL, (4) used at least 2 defined categories of exposure (by quantiles and/or range such as standard deviation of intakes) and (5) included estimates of relative risk (risk ratio, hazard ratio, odds ratios, relative risk) with (6) measures of uncertainty (standard errors or confidence intervals) (7) relative to a referent exposure, (8) used study-level adjustments for major confounding factors, (9) had a follow-up duration of 4 or more years (when there were multiple publications from a study, the longest duration of follow-up was used unless there was a prohibitive reason [eg, insufficient information]), (10) ascertained MI or CHD by clinical record, (11) reported the sex of the participants, and (12) used either food records or a dietary instrument with validation for the investigated population. Validity was accepted by us when the instrumental measure of carbohydrate (or carbohydrate food) intakes correlated (>0.55) with intakes obtained using objective food records (see Exclusions). Adults of any age, sex, race/ethnicity from any region worldwide in a report published at any time during the study paramenters were included without language restriction. External to the registered protocol, we included observations on the CHD-carbohydrate RR when the GI of the carbohydrate was reported.
Studies were excluded if they (1) were not observational, longitudinal, or prospective studies, (2) used self-reported disease ascertainment only, (3) included patients with MI, CHD, or type 2 diabetes at baseline, (4) reported observations other than from fully adjusted models, (5) reported observations including stroke or other CVD, and (6) used invalid dietary instuments (defined as those with energy-adjusted correlation coefficients [Corr] of 0.55 or less for carbohydrate or carbohydrate foods vs objective food records27, 28, 29 unless there was reason to do otherwise).
Data Extraction and Calculation
Data were extracted by 2 reviewers independently (G.L., H.L.), and disparities were resolved by agreement. Data included the following: (1) carbohydrate, GI, and GL by quantiles (means, medians), (2) the reference standard used (glucose or white bread)—to adjust to a common glucose metric (GI glucose = 100, GI white bread = 70), (3) relative risks for first incidence of MI and CHD deaths (risk ratios, hazard ratios, or odds ratios) and their 95% confidence limits by quantiles or by linear relations if not provided by quantiles, (4) energy intake by quantiles and the intake of energy to which GL, GI, and carbohydrate were adjusted, (5) cohort average alcohol intakes, (6) whether RR had received study-level adjustments for intakes of energy, carbohydrate, fat (or fats), protein, fiber, folate, supplemental multivitamins, and alcohol, (7) whether RR had received study-level adjustments for nonnutrient factors—baseline hypertension, hypercholesterolemia, menopausal state and related hormone use, level of education, body mass index, age, physical activity, family history of MI or diabetes, smoking, occupation, income, and marital status, (8) whether studies had excluded CVD other than (in addition to) MI and CHD at baseline, (9) population region, (10) participants’ sex (fraction of men), (11) population race/ethnicity, (12) the number of followed up participants, cases, and person-years of follow-up, (13) follow-up in years, (14) attrition rates during follow-up, (15) whether the dietary instrument was validated and the level of validity, and (16) the method used for ascertainment of MI or CHD. Information not reported directly was calculated when possible or sought from corresponding dietary instrument validation publications (for validity), related publications with dietary details, or correspondence with authors (Supplemental Tables S1, S2, and S3, available online at http://mcpiqojournal.org).
Study Quality
The Newcastle-Ottawa Quality Assessment score for prospective cohort studies was used to generate a score from 0 to 9 “stars” based on criteria for selection of participants, comparability of subcohorts, and study-level outcomes.30 We deducted one star from comparability for those studies using a dietary instrument with an energy-adjusted carbohydrate correlation of 0.55 or less.
Meta-analyses/Synthesis
Analyses used Stata/SE statistical software, version 11.2 (StataCorp) with “mais” (Meta-Analysis in Stata) installation.31 Two-step meta-analysis was used for quantitative DRM for doses within jurisdictions or nationwide exposures. Step 1 used the generalized least squares method for trend estimation of summarized dose-response data of Geenland and Longnecker32 as implemented by Osini et al33 (glst v9.2), which provided individual study dose-response RR values. Step 2 combined the RR values by meta-analysis without covariates (metan v3.03) or with covariates—meta-regression (metareg v6.1) using method of moments and random effects,34 which resolved to fixed effects when results from different studies were consistent (I2=0). Eligible studies providing only extreme quantile RR values or providing dose-response results directly were introduced at step 2.
Global DRM examined the dose response across the global population (sampled worldwide exposures) and used cubic spline (nonlinear) meta-regression analysis placing knots at the 10th, 50th, and 90th percentile of global exposures. For this purpose, the procedure of Geenland and Longnecker32 was used to combine eligible studies in one repeating step (from the second to the last study) using at each repeat the pooling procedure with random effects.33 The repeating steps introduced studies one at a time beginning with the study with the lowest referent exposure to enable prediction from prior studies of the graphical location of the subsequently added study observations. Each study introduced resulted in a third error term equal to the forecast standard error (σfi) for the placement of results from each additional study, the combined square of which (σf2) was additive with heterogeneity (τ2) and the combined within-study variance estimate (σ2). Thus, the usual τ2 + σ2 for random effects became τ2 + σ2 + σf2 in the global analysis (only), in which τ2 was zero for fixed effects. I2 was calculated as 100 · τ2/(τ2 + σ2 + σf2), although it was also calculated as 100 · τ2/(τ2 + σ2) to assess whether inclusion of σf2 made an important difference.
Additional Analyses
Small-study effects were assessed by nonparametric trim-and-fill funnel plots (metatrim in Stata) and by a Galbraith-like regression (Log RRi · Ni on Ni, where RRi was the study-level dose-response RR and Ni was the number of persons followed up).35, 36
Because epidemiological studies have potential to generate precise but spurious results, the possibility of an outlying result (P<.05) was examined by meta-regression using an indicator variable for a suspected study (one for which confidence intervals were not overlapping the combined studies mean RR).
Difference in RR between two subgroups was assessed using meta-regression using an indicator variable for one of the subgroups.
Statistical Tests
The z test was used for combined means, covariates, and outliers, the t test for small-study effects, and the χ2 test for heterogeneity. I2 can be interpreted only approximately.37
Bradford-Hill Rating
All 9 Bradford-Hill criteria38 were used. We limited the ratings to either probable or less than probable for each criterion, with total possible scores of 0 to 9. This procedure involved less subjectivity in decision making (G. Livesey, R. Taylor, H. Livesey, et al, unpublished data, 2018) than 3 categories per criterion.39
Results
Search Outcomes
The search of MEDLINE and EMBASE (including Cochrane studies) for prospective cohort studies on the CHD-GL and GI RRs (Supplemental Figure S1a) identified 176 potentially relevant records without duplicates. After examination of titles and abstracts, 30 were potentially relevant and were retrieved (Supplemental Figure S1a). On examination of the full publications, 16 did not meet the inclusion criteria: an early commentary40; a narrative review on diet and CHD41; a systematic review of randomized controlled trials (RCTs)42; 2 systematic reviews of potentially relevant studies10; 2 cross-sectional studies43, 44; 1 case-control study45; 4 prospective cohort studies examining dietary factors other than GI or GL5, 46, 47, 48; a prospective cohort study on nondietary factors49; a conference report of an otherwise later study report50; a prospective cohort study that focused on the CHD-carbohydrate score RR and reported on relations with GI and GL but without quantitative information on exposures by categories of exposure7; and a conference report of CHD-GI and GL RRs, again with too little quantitative information on GI and GL.51
Among the retrieved publications, 14 included prospective cohort studies reporting on one or more of either the CHD-GL or CHD-GI or the CHD-carbohydrate RRs. Thirteen reported on the CHD-GL RR16, 17, 18, 19, 20, 21, 22, 23, 24, 52, 53, 54, 55 including 19 studies. Twelve reported on the CHD-GI risk RR16, 17, 18, 19, 20, 21, 22, 23, 24, 52, 53, 55 including 17 studies. Six reported on the CHD-carbohydrate RR for carbohydrate with specified GI values15, 16, 17, 18, 19, 20 including 12 studies (Supplemental Figure S1a).
Among the 19 studies on the CHD-GL RR, 7 studies from 4 publications18, 53, 54, 55 were not eligible because they had invalid dietary instruments by our criterion (Supplemental Figure S1b). Similarly, among the 17 studies on the CHD-GI RR, 5 studies from 3 publications18, 53, 55 were not eligible because of invalid dietary instruments by our criterion (Supplemental Figure S1c). Among the 12 studies on the CHD-carbohydrate RR with known GI, 5 studies from 2 publications15, 18 were not eligible because of invalid dietary instruments by our criterion (Supplemental Figure S1d). This left 12, 12, and 7 studies eligible for DRM of the CHD-GL, CHD-GI, and CHD-carbohydrate characterized by GI. These inclusions were provided that results would not prove to be significant outliers (P<.05) (Supplemental Figure S1a-d).
Characteristics of the Study Participants
Eligible studies included only men (6 studies) or only women (6 studies). They included adults with a mean ± SD age at baseline of 51±12 years (range, 26-71 years) and mean ± SD body mass index (calculated as weight in kilograms divided by height in meters squared) of 25±1.0 kg/m2 (range, 23-26 kg/m2). All were ostensibly healthy persons free of prior MI (or CHD) and diabetes at baseline. Participants lived in the United States (1 study), Europe (9 studies), and China (2 studies). Occupations were considered representative of these populations in 8 studies, and 1 study focused on nurses. Urban dwelling was included in 2 studies, and smokers were included in 1. Race/ethnicities were largely European/white (9 studies), European American (1 study), and East Asian (2 studies).
Nutritional Characteristics
Nutritional characteristics were collected for the 12 studies that used valid dietary instruments by our criterion. Population mean ± SD values were as follows: energy intake, 2367±280 kcal/d (range, 1930-2617 kcal/d) (1 kcal = 4.184 kJ) in men (6 studies) and 1792±120 kcal/d (range, 1674-1984 kcal/d) in women (6 studies); alcohol consumption (reported in 11 of the 12 eligible studies), 12.5±7 g/d (range, 2-24 g/d) in men (5 studies) and 6.2±2 g/d (range, 2-9 g/d) in women (6 studies); dietary fiber intake (or cereal fiber in 2 studies) (reported in 10 of 12 eligible studies), 24±3.6 g/d (range, 19-29 g/d) in men (5 studies) and 20±3.6 g/d (range, 15-23 g/d) in women (5 studies); protein intake (reported in 10 of the 12 eligible studies), 69±8 g/2000 kcal (range, 57-79 g/2000 kcal) in men (5 studies) and 79±5 g/2000 kcal (range, 73-86 g/2000 kcal) in women (5 studies); carbohydrate intake (reported in 12 of 12 eligible studies), 236±66 g/2000 kcal (range, 185-366 g/2000 kcal) in men (6 studies) and 271±58 g/2000 kcal (range, 190-369 g/2000 kcal) in women (6 studies); GI (reported in all 12 eligible studies), 62±19 on the glucose scale (range, 55-82) in men (6 studies) and 59±10 (range, 47-82) on same scale in women (6 studies); GL (reported in all 12 eligible studies), 166±63 g/2000 kcal (range, 119-290 g/2000 kcal) in men (6 studies) and 170±59 g/2000 kcal (range, 125-286 g/2000 kcal) in women (6 studies).
The 10th to 90th percentile range of carbohydrate intakes in populations of men and women combined and relevant to the CHD-carbohydrate RR was 98±24 g/d (range 78-144 g/d) (adjusted to 2000 kcal/d) (11 studies). That relevant to the CHD-GL RR was 65±13 g/d (range, 46-83 g/d) (adjusted to 2000 kcal/d) (12 studies), and that relevant to the CHD-GI RR was 10±3.2 (range, 5.7-13.8) (glucose scale) (12 studies).
Study Quality
Newcastle-Ottawa quality scores (from 0-9) for the CHD-GL RRs were 8.1 (range, 7-9) for inlying studies with valid dietary instrument (correlation >0.55), 7 for an outlying study,54 and 7.3 (range, 6-8) for those studies with a correlation of 0.55 or less (Supplemental Figure S2, available online at http://mcpiqojournal.org). Corresponding scores for the CHD-GI RR were 8.1 (range, 7-9), 7.5 (range, 7-8),24, 54 and 7.0 (range, 6-8) (Supplemental Figure S3, available online at http://mcpiqojournal.org). For the CHD-carbohydrate RR, corresponding results were 8.3 (range, 8-9), 7 (range, 7-7), and 7.4 (range, 7-8) (Supplemental Figure S4, available online at http://mcpiqojournal.org).
Study Characteristics
Study characteristics were collected for the 12 studies that used valid dietary instruments by our criterion. Dietary intakes were mostly assessed using food frequency questionnaires (9 studies); 1 study each used a combination of food records and diet history interview, food records, and diet history questionnaire.
Studies using food frequency questionnaires as their dietary instrument were validated using energy-adjusted Pearson (otherwise Spearmen) Corr comparing the dietary instrument values with food records for carbohydrate (9 studies) or for high-carbohydrate foods (1 study). Studies using food records directly (2 studies) were assigned an arbitrary but high value for Corr of 0.8. The mean Corr value for the 12 eligible studies was 0.72 (range, 0.64-0.80). The mean ± SD value was 0.71±0.05 (range, 0.64-0.8) in men (6 studies) and 0.73±0.05 (range, 0.66-0.8) in women (6 studies).
Categories of intakes were presented by tertiles in 1 study, quartiles in 5 studies, and quintiles in 6 studies. The mean ± SD follow-up duration for the 12 studies was 11.4±4.6 years (range, 5-19 years). The median study size was 22,400 persons (range, 646-75,521 persons), and the total number of persons entering the studies was 350,000. The median number of events per study (cases) of first MI and CHD deaths was 614 (range, 114-4379), totaling 10,400 events. Excluded at baseline were MI, CHD, and type 2 diabetes in all 12 studies. All 12 studies ascertained cases from medical records.
The 12 eligible studies made study-level adjustments to relative risks for variance in nutrient intakes: energy (12 studies), alcohol (12 studies), dietary fiber (5 studies, including 1 for cereal fiber alone), protein (9 studies), fat or individual groups of fats (saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids) (10 studies), and carbohydrate for the CHD-GI RRs only (7 studies).
Study-level adjustments made for nonnutritive factors were as follows: smoking (12 studies), body mass index (12 studies), age of participants (12 studies), physical activity (12 studies), history of hypertension (8 studies) and systolic blood pressure (2 studies), level of education (8 studies), hypercholesterolemia (total cholesterol or high-density lipoprotein [HDL] cholesterol) (6 studies), family history of MI or CHD (12 studies), aspirin use (3 studies), income (2 studies), marital status (2 studies), multivitamin use (1 study), and menopausal state and related hormone use in women (3 studies).
Risk of Bias Assessment
All 12 studies were judged to have adequate dietary assessment tools (Corr >0.55), had objective outcome assessment (medical records), had adequate follow-up (>4 years), had been reported with no competing interests, generally had low attrition rates (1%-8% in 9 studies, 20% in 1 study,17 and not reported but seemingly <1% in 2 studies22, 23), and had probable adequate adjustment for confounding (nutrient and nonnutrient factors). From the outset, therefore, there was no appreciable evidence of study-level risk of bias other than what may arise from confounding factors (see Sensitivity of RRs to Study-Level Adjustments section, subsections Macronutrient and Folate Intakes and Nonnutrient Factors) and occurrence of small-study effects (see subsequent results).
Coronary Heart Disease–Glycemic Load RR
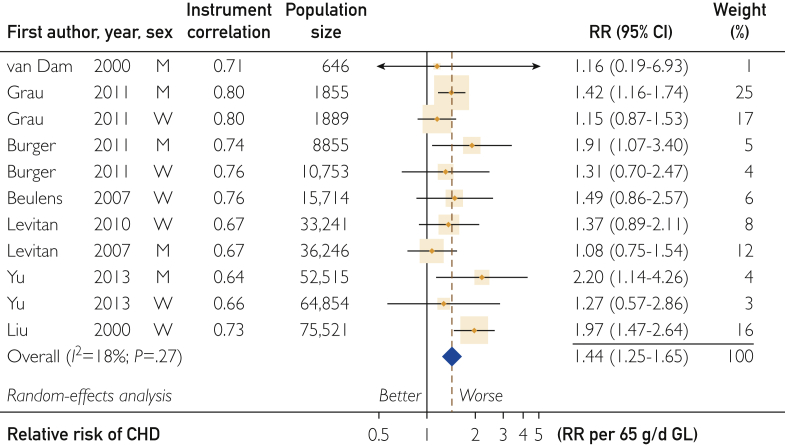
Of 19 studies on the CHD-GL RR, 12 were eligible with dietary instruments having a correlation for carbohydrate greater than 0.55 (Supplemental Figure S1b). Meta-analysis of these valid studies yielded a combined dose-response RR (relative to the lowest dose) that increased in 11 studies by 1.44 (95% CI, 1.25-1.65; P<.001) per 65 g/d GL (adjusted to 2000 kcal/d) with nonsignificant inconsistency among studies (I2=18%; P=.27) (Figure 1). The study of Similä et al52 in men was dropped from the meta-analysis as a significant outlier (P=.005) and remained significantly outlying among the subgroup of men (P=.02).
Figure 1.
Forest plot of the coronary heart disease (CHD)–glycemic load (GL) risk relation (RR). The plot shows for each prospective cohort study the relative risk associated with a higher exposure. Risk relations shown were generated by quantitative dose-response meta-analysis. Box sizes are proportional to the weight contributed by a study to the combined study mean. Horizontal lines span the individual study 95% CIs. Arrowheads indicate truncations. Diamonds represent the combined studies mean RR values and its 95% CI values.
As expected, studies that were less reliable because they used dietary instruments that performed less well (Corr ≤0.55)18, 56, 57, 58 gave a lower mean relative risk of 1.30 (95% CI, 1.13-1.50) (P<.001) per 65 g/d GL (adjusted to 2000 kcal/d) (Table 1).
Table 1.
Sensitivity of the CHD-GL and GI Risk Relations to Dietary Instrument Correlation for Carbohydrate and to Sex of the Study Populationa,b
| Variable | n | Risk relation |
I2 (%) | τc | P value | |
|---|---|---|---|---|---|---|
| Mean (95% CI) | P value | |||||
| Corr ≤0.55 (low or nonvalid studies),d men and women combined | ||||||
| Glycemic loade | 7 | 1.30 (1.13-1.50) | <.001 | 0 | 0 | .66 |
| Glycemic indexf | 5 | 1.18 (1.03-1.34) | .016 | 0 | 0 | .64 |
| Corr >0 .55 (valid studies),c men and women combined | ||||||
| Glycemic loade | 11 | 1.44 (1.25-1.65) | <.001 | 18 | 0.10 | .27 |
| Glycemic indexf | 10 | 1.24 (1.12-1.38) | <.001 | 10 | 0.05 | .35 |
| Men, Corr >0.55d | ||||||
| Glycemic loade | 5 | 1.43 (1.15-1.78) | .001 | 21 | 0.12 | .28 |
| Glycemic indexf | 4 | 1.04 (0.88-1.23) | .62 | 0 | 0 | .96 |
| Women, Corr >0.55d | ||||||
| Glycemic loade | 6 | 1.44 (1.17-1.78) | <.001 | 30 | 0.14 | .21 |
| Glycemic indexf | 6 | 1.35 (1.20-1.52) | <.001 | 0 | 0 | .62 |
CHD = coronary heart disease morbidity (myocardial infarction) and mortality; GI = glycemic index; GL = glycemic load; I2 = inconsistency among study-level risk relation values, ie, the variance among studies expressed as a percentage of the sum of the variance within and among studies; n = number of prospective cohort studies; P = probability values for risk relation, τ, and I2; τ2 = heterogeneity, ie, the variance among studies.
Risk relations obtained by random-effects dose-response meta-analysis.
τ, the square root of τ2, is the standard error among studies and has the same units as risk relation (footnotes e and f).
Corr, dietary instrument correlation for carbohydrate intake (energy adjusted and deattenuated) measured by food frequency questionnaire vs diet records; values ≤0.55 were deemed invalid instruments.
Units: higher risk relation per 65 g/d GL adjusted to 2000 kcal (8400 kJ) of energy intake per day.
Units: higher risk relation per 10 U dietary GI.
The CHD-GL RR was not different in men and women for studies with Corr greater than 0.55 (Table 1). In women-only studies, it was 1.44 (95% CI, 1.17-1.78) (6 studies; P<.001) with nonsignificant inconsistency (I2=30%; P=.21), while in men it was 1.43 (95% CI, 1.15-1.78) (5 studies; P=.001) with nonsignificant inconsistency (I2=21%; P=.28).
Potentially, the low RR in the study of men by Similä et al52 may in part be due to relatively higher alcohol consumption. The population alcohol consumption in studies with a mean of less than 15 g/d was known in 9 studies with Corr greater than 0.55 (from 8 publications).16, 17, 19, 20, 21, 22, 23, 52 Meta-regression using alcohol as a continuous covariate in the low to moderate range of intakes (<15 g/d or approximately 1 drink per day) indicated that at the lowest level of consumption (2 g/d for the present studies), the CHD-GL RR was 1.90 (95% CI, 1.25-2.89; P=.003). By contrast, at a higher level of alcohol consumption (10.9 g/d), this RR was only 1.09 (95% CI, 0.88-1.36; P=.44). Thus, alcohol may significantly attenuate the CHD-GL RR (9 studies; P=.04). However, meta-regression analysis with only 9 studies should be regarded cautiously. Inconsistency was not absent, although it was nonsignificant (I2=27%; P=.20). From this model perspective, the study of Similä et al52 ceased to be significantly outlying (P=.14).
The men and women combined studies RR of 1.44 (95% CI, 1.25-1.65) (11 studies; P<.001) (Figure 1) was sensitive to individual studies. The lowest combined RR of 1.34 (95% CI, 1.18-1.51; P<.001) (I2=0%; P=.06) arose when dropping the study in women by Liu et al,16 and the highest RR of 1.48 (95% CI, 1.29-1.68; I2=4%; P=.40) arose when the study in women by Grau et al24 was dropped.
The Egger test indicated no significant small-study effects (11 studies; P=.61). Trim-and-fill analysis funnel plots indicated symmetry (ie, no trimming or filling to attain this state) (Supplemental Figure S5, available online at http://mcpiqojournal.org).
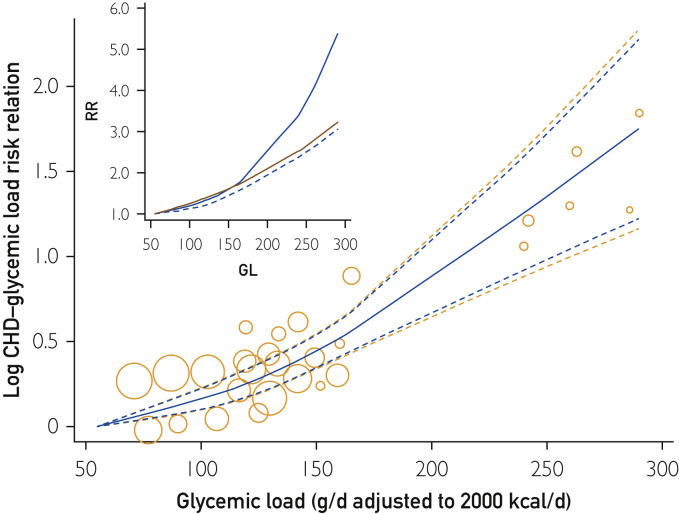
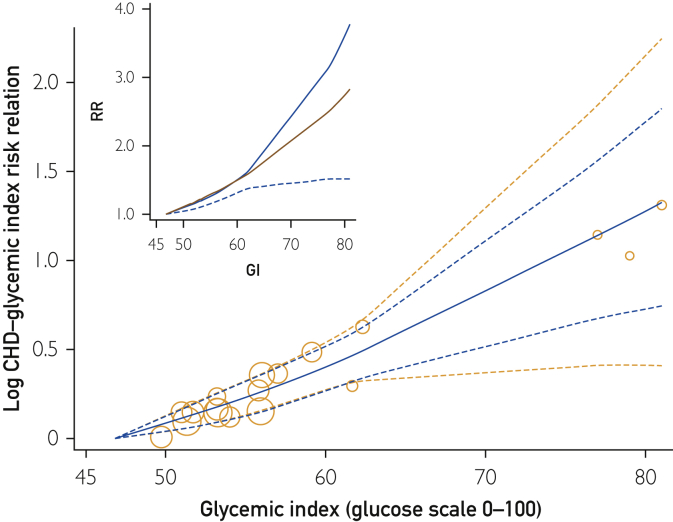
For eligible studies across the globe, GL intakes ranged from 55 to 290 g/d (span 235 g/d), which was more than 3 times wider than the study average range of intakes (65 g/d GL) and reached higher RR values (Figure 2 Inset). The CHD-GL relative risk across the lowest 65 g/d GL range of intakes was 1.32 (1.21-1.45) (Figure 2 and Table 2, row 9). The CHD-GL relative risk across the sampled Western populations range of GL intakes (110 g/d) was 1.78 (1.57-2.02), and across the full range of GL intakes (235 g/d) was 5.5 (3.1-9.8). Heterogeneity and inconsistency among study RR values was absent (I2 and τ = 0 by each method of derivation (see Methods section).
Figure 2.
Glycemic load (GL) and estimates of risk relation (RR) for coronary heart disease (CHD) in men and women combined. Bubbles show results for each cohort from a common referent at 55 g/d GL. Observations were from Beulens et al,17 Burger et al,19 Grau et al,24 Levitan et al,22, 23 Liu et al,16 van Dam et al,21 and Yu et al20 (11 studies from 8 publications). Curves show estimates from a global cubic spline (nonlinear) quantitative dose-response meta-analysis. Bubble areas increase with increasing precision of observation. Blue dashed lines show the 95% confidence limits based on random effects alone. Orange dashed lines show wider 95% CIs based an additional error from forecasting the graphical position of results for each added study. Inset shows the unlogged dose-response curve (blue line), its lower confidence limit (dashed line), and an unlogged log-linear analysis (red line) for comparison.
Table 2.
Summary of the CHD-Carbohydrate, Glycemic Load, and Glycemic Index Risk Relations in Men and Women Combinedab
| Variable | n | Risk relation (95% CI) and unit of measurement | P value | I2 (%) | P value | ||
|---|---|---|---|---|---|---|---|
| CHD-carbohydrate risk relation by GIc | |||||||
| 1 | Over eligible studies,d GI = 50 U | 11b | 1.11 (0.86-1.42) | Per 98 g CHO | .42 | 18 | .28 |
| 2 | Over eligible studies,d GI = 80 U | 5.10 (2.39-10.9) | Per 98 g CHO | <.001 | |||
| CHD-GI risk relation derived from CHD-carbohydrate risk relations per 98 g/d carbohydrate at different glycemic indices (thus avoiding attenuation due to study-level adjustment for carbohydrate intake)e | |||||||
| 3 | Over eligible studiesd | 11 | 1.66 (1.23-2.25) | Per 10 U GI | <.001 | 16 | .30 |
| 4 | Over the 50-80 U GId | 11 | 4.57 (1.86-11.4) | Per 30 U GI | <.001 | 16 | .30 |
| CHD-glycemic index risk relations (not avoiding potential attenuation due to study-level adjustment for carbohydrate intake) | |||||||
| 5 | Over eligible studiesf | 10 | 1.24 (1.12-1.38) | Per 10 U GI | <.001 | 10 | .35 |
| 6 | Lowest range of GI valuesg | 10b | 1.26 (1.15-1.38) | Over 10 U GI | <.001 | 0 | .98 |
| 7 | Full range of GI valuesg | 2.71 (1.47-4.40) | Over 35 U GI | <.001 | |||
| CHD-glycemic load risk relations (avoiding attenuation due to study-level adjustment for carbohydrate) | |||||||
| 8 | Over eligible studiesh | 11 | 1.44 (1.25-1.65) | Per 65 g/d GL | <.001 | 18 | .27 |
| 9 | Lowest range of GL valuesi | 11b | 1.32 (1.21-1.45) | Over 65 g/d GL | <.001 | 0 | .41 |
| 10 | Full range of GL valuesi | 5.5 (3.1-9.8) | Over 235 g/d GL | <.001 | |||
CHD = coronary heart disease; CHO = carbohydrates; DRM = dose-response meta-analysis; GI = glycemic index; GL = glycemic load; I2= inconsistency; n = number of prospective cohort studies; P = probability values for risk relation and τ (and I2); RR = risk relation.
Adjacent rows sharing common values for n, I2 (and its P-value) shared common inputs and common meta-regression models of analysis but differed in the outputs for the relative risk dependent on the question asked related to GL, GI, and CHO and to the level of exposure or range of exposures addressed.
Two-stage quantitative DRM, obtained by centering GI at 50 U and 80 U, respectively, rather than using the noncentered GI as in Figure 7.
Excluding one outlying study (P<.001).
Two-stage quantitative DRM: estimating RR per 98 g/d CHO (adjusted to 2000 kcal) followed by meta-regression (Figure 7).
Two-stage quantitative DRM: estimating RR per 10 U GI followed by meta-analysis without covariates (Figure 3).
One-stage cubic-spline pool-first quantitative DRM (Figure 4).
Two-stage quantitative DRM: estimating RR per 65 g/d GL intake (adjusted to 2000 kcal/d), followed by meta-analysis (Figure 1).
One-stage cubic-spline pool-first quantitative global DRM (Figure 2).
Coronary Heart Disease–Glycemic Index RR
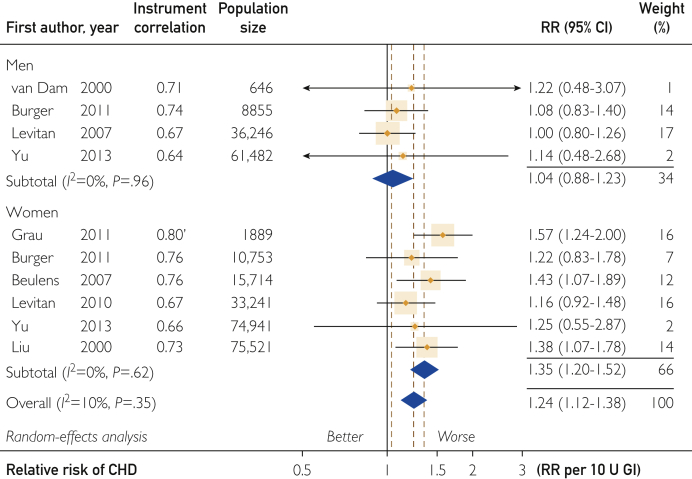
Of 17 studies reporting on the CHD-GI RRs, 12 were eligible with dietary instruments correlation for carbohydrate greater than 0.55 (Supplemental Figure S1c), of which 2 proved to be outliers (see subsequent discussion). Meta-analysis gave a combined studies mean CHD-GI RR of 1.24 (95% CI, 1.12-1.38) (P<.001) per 10 U GI (Figure 3). Inconsistency of results among studies was nonsignificant (10 studies; I2=10%; P=.35). As expected, studies that were less reliable because they used a dietary instrument with Corr of 0.55 or less18, 53, 55 gave a lower RR of 1.18 (95% CI, 1.03-1.34) (P=.02) per 10 U GI (5 studies; I2=0%; P=.64) (Table 1).
Figure 3.
Forest plot of the coronary heart disease (CHD)–glycemic index (GI) risk relation (RR). For explanation of symbols see legend to Figure 1.
The CHD-GI RR was higher in women than in men (4 studies; P=.01) for studies with Corr of greater than .55 (Figure 3 and Table 1). In women-only studies, RR was 1.35 (95% CI, 1.20-1.52) (P<.001) with no inconsistency (6 studies; I2=0%; P=.62). However, in men-only studies, this relation was small and nonsignificant at 1.04 (95% CI, 0.88-1.23) (P=.62) (4 studies; I2=0%; P=.96). This difference should be regarded cautiously because it may not be an inherent sex difference.
Among studies with Corr greater than 0.55, the men-only study of Grau et al24 was a significantly low outlier (P<.001) and therefore not included. Among the remaining studies, the men-only study of Similä et al52 was also a significant outlier (P<.001). Low values of RR for men were therefore not due to these low outlying studies, although they may be related to alcohol consumption.
Meta-regression with population average alcohol intake as a covariate in the low to moderate range of intakes (<15 g/d or approximately 1 drink per day) indicated that at low consumption (2 g/d) the CHD-GI RR was 1.51 (95% CI, 1.17-1.94) (10 studies; P=.001) (I2=10%; P=.35). By contrast, at the higher population average alcohol consumption (10.9 g/d), a CHD-GI RR was not evident because RR was 0.95 (95% CI, 0.86-1.06) (10 studies; P=.38) (I2=10%; P=.35). The study of Similä et al52 remained outlying (P=.02) even with alcohol as a covariate, while the study of women from Grau et al24 became outlyingly high (P=.01).
The men and women combined studies RR without covariates of 1.24 (95% CI, 1.12-1.38) (10 studies; P<.001) (Figure 3) was sensitive to dropping of individual studies. The lowest combined RR of 1.18 (95% CI, 1.07-1.32) (9 studies; P=.002) (I2=0%; P=.70) arose when the study in women from Grau et al24 was dropped, and a highest RR of 1.30 (95% CI, 1.17-1.44) (9 studies; P<.001) (I2=0%; P=.64) arose when the study in men by Levitan et al23 was dropped.
A Galbraith-type plot with Egger test indicated no significant small-study effects (10 studies; P=.26). Trim-and-fill analysis of the funnel plot indicated symmetry (no trimming or filling to attain this state) (Supplemental Figure S6, available online at http://mcpiqojournal.org).
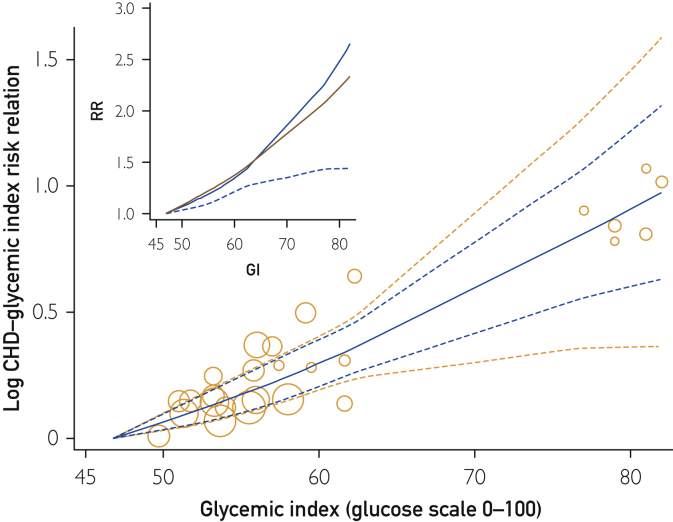
Across the globe, the range of GI values in men and women was 47 to 82 U GI (glucose scale), which covered a range more than 3 times wider than the average range of 10 U GI within study population samples. By global DRM (Figure 4 Inset), the CHD-GI RR over the lowest 10 units range of GI (from 47-57 GI) was 1.26 (95% CI, 1.15-1.38) (Table 2, row 6). Across the sampled Western populations range of GI intakes (lowest 16 U GI), the RR was 1.44 (95% CI, 1.28-1.63), and across sampled global population range of GI intakes (35 U GI), the RR was 2.71 (95% CI, 1.47-4.40). No inconsistency or heterogeneity was evident (I2 and τ = 0), and these relations were significant (P<.001).
Figure 4.
Glycemic index (GI) and estimates of the risk relation (RR) for coronary heart disease (CHD) in men and women. Bubbles show results for each cohort from a common referent at 47 U GI. Observations were from Beulens et al,17 Burger et al,19 Grau et al,24 Levitan et al,22, 23 Liu et al,16 van Dam et al,21 and Yu et al20 (10 studies from 8 publications). For explanation of symbols see legend to Figure 2.
In women (Figure 5 Inset), the global DRM for the CHD-GI RR across the lowest range of 10 U GI (47-57 U GI) was 1.35 (95% CI, 1.21-1.50) per 10 U GI. Across the sampled Western populations range of GI intakes (lowest 16 U GI), the RR was 1.64 (95% CI, 1.38-1.94), and across the sampled global population range of GI intakes (35 U GI), the RR was 3.78 (95% CI, 1.51-9.42). No inconsistency or heterogeneity among observations was evident (I2 and τ = 0), and all 3 RRs were statistically significant (P<.001).
Figure 5.
Glycemic index (GI) and estimates of the risk relation (RR) for coronary heart disease (CHD) in women. Bubbles show results for each cohort from a common referent at 47 U GI. Observations were from Beulens et al,17 Burger et al,19 Grau et al,24 Levitan et al,22 Liu et al,16 and Yu et al20 (6 studies from 6 publications). For explanation of symbols, see legend to Figure 2.
Sensitivity of RRs to Study-Level Adjustments
Macronutrient and Folate Intakes
Where study-level adjustments had been made for intakes of energy, alcohol, fiber, protein, fats, or folate, the CHD-GL RR in men and women combined remained greater than 1.20 with a lower confidence limit (LCL) greater than 1.10. Prudently, no study adjusted for carbohydrate intake (Supplemental Table S4, available online at http://mcpiqojournal.org).
To avoid possible confounding by an apparently low risk in men (Table 1), the CHD-GI RR was examined for women alone. Where study-level adjustments had been made for intakes of the aforementioned factors and carbohydrate or folate, the CHD-GI RR also remained greater than 1.20 with a LCL greater than 1.10 (Supplemental Table S4, available online at http://mcpiqojournal.org).
Nonnutrient Factors
Where study-level adjustments had been made to the CHD-GL and GI RRs for smoking, body mass index, age of participants, physical activity, family history of MI, diabetes status, hypertension, hypercholesterolemia, menopausal state and related hormone use (in women), level of education, and exclusion of other CVD in addition to MI or CHD at baseline, these relations remained greater than 1.20 with an LCL greater than 1.10 (Supplemental Table S4). Aspirin use was associated with variable results. Potentially, it lowered the CHD-GI RR, although nonsignificantly (P=.26), and the RR remained greater than 1.20 for both GL and GI but with an LCL of less than 1.10 for GI (Supplemental Table S5).
Coronary Heart Disease–Carbohydrate RR
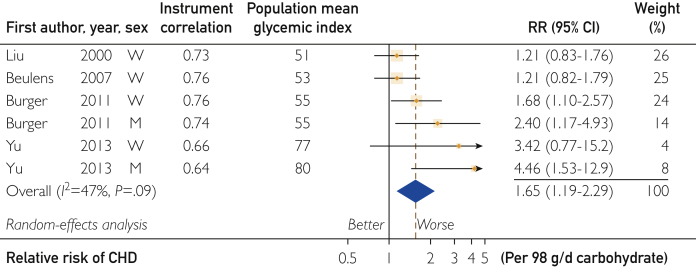
The CHD-carbohydrate RR when using valid dietary instruments (Corr >0.55) was reported in 6 single-sex studies with apparent inconsistency (I2=47%; P=.09) (Figure 6). No study reported on this RR for a GI of less than 50 U on the glucose scale. Carbohydrate with GI of less than 56 U showed no clear association, while at the highest GI, the RR was high at 4.46 (95% CI, 1.53-12.9) per 98 g/d carbohydrate (adjusted to 2000 kcal diet).
Figure 6.
Forest plot of the coronary heart disease (CHD)–carbohydrate risk relation (RR) by population sample mean glycemic index. Observations were from Liu et al,16 Beulens et al,17 Burger et al,19 and Yu et al.20 For explanation of symbols, see legend to Figure 1.
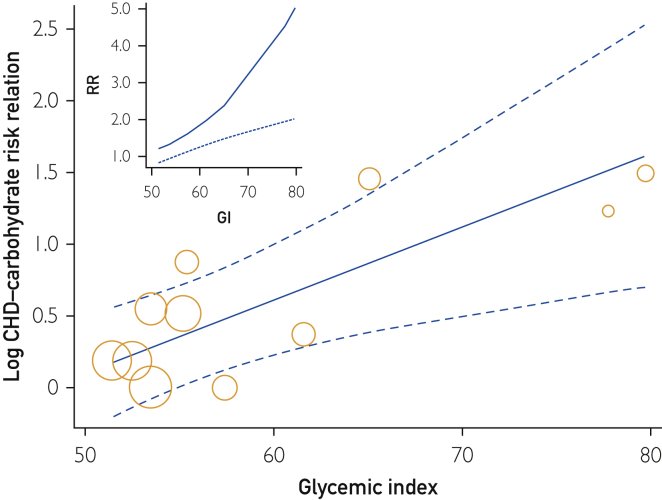
Meta-regression requires more than the 6 studies presented in Figure 6. Therefore, we reintroduced the 3 studies by Jakobsen et al15 and the 2 studies by Sieri et al18 with Corr of 0.55 or less to assess the rate of change in RR with GI (Figure 7). The RR was high at 1.66 (95% CI, 1.23-2.25) per 10 U GI on the glucose scale (P<.001) (Table 2, row 6). Inconsistency was low (I2=16%; P=.30). While the funnel plot for studies in Figure 6 was asymmetrical (Supplemental Figure S7), adjustment for differences in GI (Figure 7) removed 85% of the inconsistency and resulted in a symmetrical funnel plot (Supplemental Figure S8, available online at http://mcpiqojournal.org). Among these studies, the observations from Similä et al52 were excluded as statistically significant outliers (P<.001) both for high and medium GI categories of carbohydrate.
Figure 7.
The log-linear relation between the coronary heart disease (CHD)–carbohydrate risk relation (RR) and the population or cohort average glycemic index. The unlogged slope was 1.66 (1.23-2.25) per 10 units higher GI (P<.001). Observations were from Beulens et al,17 Burger et al,19 Jakobsen et al,15 Liu et al,16 Sieri et al,18 and Yu et al.20 For explanation of symbols, see legend to Figure 2.
Discussion
Risk Relations
To our knowledge no prior meta-analysis on the dependence of the CHD-carbohydrate RR on GI has been undertaken. Carbohydrate with a GI of greater than 50 U on the glucose scale was a strong risk factor for CHD and reached 4.46 (95% CI, 1.53-12.9) (Figure 6) and 5.1 (95% CI, 2.39-10.9) (Figure 7) each per 98 g/d carbohydrate, increasing by 1.66 (95% CI, 1.23-2.25) per 10 U GI. The quantities 98 g/d carbohydrate and 10 U GI each were combined studies mean ranges of intake, from the 10th to the 90th percentile of the study populations. To put this into further context, the ranges of GI values within major food categories (eg, whole-grain, vegetable, fruit) are approximately 60 U for each category.56 This implies that “healthy foods” of extreme high GI for their food category might be a 20 times greater risk to heart health than foods of extreme low GI from the same food category when assuming the increment remains truly log-linear, ie, exp(ln[1.66] · 60/10) = 21. This strong RR on the global scale was supported by high RR values for GL at 5.5 (95% CI, 3.1-9.8) (Figure 2 Inset; Table 2, row 10) and for GI at 2.71 (95% CI, 1.47-4.40) (Figure 4 Inset; Table 2, row 7). It seems worrysome, therefore, that the general public receives little authoritative guidance leading toward the consumption of lower rather than higher GI carbohydrate foods within food groups.
Whether addressing GI or GL of carbohydrate characterized by GI, all 3 associated strongly globally for a nutrient relation with CHD risk. In general, however, harmful RRs greater than 1.20 with a lower 95% CL greater than 1.10 within sample population ranges of intakes have been regarded as sufficient to consider a nutrient for inclusion in nutrition guidance12 (G. Livesey, R. Taylor, H. Livesey, et al, unpublished data, 2018) when sufficiently supported by an assessment such as Bradford-Hill ratings12 (G. Livesey, R. Taylor, H. Livesey, et al, unpublished data, 2018). These within-population RR criteria were clearly met, both for all eligible studies on GL combined (Table 2, row 8) and over the lowest 65 g/d GL range within the global range of GL (Table 2, row 9). These criteria were less clearly met by the CHD-GI RR for all eligible studies (Table 2, row 5) and for the lowest 10 units range of GI within the global range (Table 2, row 6). Avoiding possible attenuation by adjustment for carbohydrate, this RR was possibly stronger (Table 2, RRs in row 3 vs rows 5 or 6).
A strength of our analyses was that eligible studies showed no significant study-level risk of bias (see the Results section, Risk of Bias Assessment). Newcastle-Ottawa study quality scores were high (see Results section, Study Quality), and only studies applying truly valid dietary instruments were used, as first suggested,27 first used in relation to GI and GL,28 and first shown to be a significant determinant of the type 2 diabetes–GL RR.29 A further strength was that we used DRM, which makes use of more of the available observational information than is used by EQM, accounts for different definitions of exposure in respect to the number of quantiles reported, and allows global DRM to be undertaken. In addition, for carbohydrate and GL, which are adjusted within the original studies to different energy intakes, we readjusted to a common energy intake of 2000 kcal/d for each study. Another strength was that DRM as performed obtains efficient estimates of RR by accounting for nonindependence of observations within studies32 and in global DRM additionally took account of the error introduced by the placement of observations graphically. A further strength is that none of the primary relations (Figures 1, 3, and 7 and Table 2, rows 3, 5, and 8) had significant Egger test results for small-study effects (eg, publication bias) or had asymmetric funnel plots (Supplemental Figures S5, S6, and S8, available online at http://mcpiqojournal.org). Further still, all primary RRs had low levels of inconsistency (I2<20%), which for GI and GL were reduced to zero when accounting for curvature in the global dose-response analysis (I2=0%).
A weakness of our study is that residual confounding can never be excluded. Also, weaknesses existed in original reporting of results for eligible studies at the study level. In particular, some studies did not report values of exposure, energy intakes to which exposures were adjusted, number of cases, and number of persons followed up, each by categories of exposure. However, sufficient information was available from related publications, by correspondence with the authors, by calculation from related published data (exposures and energy intakes for exposure adjustments), or approximated (number of persons and cases per quantile) as described study by study (footnotes to Supplemental Tables S1, S2, and S3). For the present purpose, the approximated values contribute negligible error (<3%) to an individual study’s estimated log dose-response RR (see Supplemental Table S1 footnotes d and e).
Bradford-Hill Ratings
Bradford-Hill ratings aim to assess the probability of RRs being causal.38 The ratings fall under 9 headings or viewpoints: strength of association (from meta-analyses when possible), consistency of association, specificity, temporality, biological gradient (dose dependency), plausibility, experimental evidence, analogy, and coherence with the natural history and biology of disease. Ratings increase from 0 to 9 with increasing probability of causality. The ratings provide guidance in the absence of convincing proof from large long-term RCTs or when RCTs might be judged to be unrepresentative of real-world circumstances assessed within prospective cohort studies.
1. Strength of Association
This factor is important for public health when RR is greater than 1.20 and its lower confidence limit is greater than 1.10 from the 10th to 90th percentile of nutrient intake12 (G. Livesey, R. Taylor, H. Livesey, et al, unpublished data, 2018). This criterion was met for the CHD-carbohydrate RR for high GI carbohydrate, the CHD-GL RR, and the CHD-GI RR for men and women combined (Table 2). The last two were examined for the influence of alcohol, finding stronger relations when population average alcohol intake was small (2 g/d), RR then being 1.90 (95% CI, 1.25-2.89) per 65 g/d GL and 1.51 (95% CI, 1.17-1.94) per 10 U GI (see Results section, Coronary Heart Disease–Glycemic Load RR, paragraph 4, and Coronary Heart Disease–Glycemic Index RR, paragraph 4).
2. Consistency of Association
This criterion refers to inconsistency (I2<50%)57 when the lower confidence limit is greater than 1.10 for a harmful relation12 (G. Livesey, R. Taylor, H. Livesey, et al, unpublished data, 2018). National (or local) DRM (Figures 1, 3, and 7) indicated little inconsistency in these relations (I2≤20%), in part attributable to differences in alcohol consumption and/or a sex difference in study-level results and to curvature in the dose responses (when in the latter, I2=0%). The direction of these relations was the same in 100% of eligible studies on GL (Figure 1), 90% of studies on G1 (Figure 3), and 80% of studies on the CHD-carbohydrate on GI RR (Figure 7). Thus, all 3 relations met the consistency criterion for men and women combined.
3. Specificity
Diverse health effects of nutrients are possible (see Coherence section) so that the original definition for this criterion (relating to a single specified disease38) is not possible. To meet this criterion, therefore, the specified association for the disease incidence must be related to the exposure variable hypothesized. Potentially confounding risk factors (see below), both dietary and nondietary, must therefore be adjusted for at the study level or assessed by relevant sensitivity analysis during meta-analyses—finding, RR greater than 1.20 with LCL greater than 1.10 for harmful relations where adjustments were made. This criterion was met for adjustments for energy, fiber, alcohol, protein, and fats or fats and folate for both GI and GL (Supplemental Table S4) and for hypertension, hypercholesterolemia, menopausal state (in women), educational status, and exclusion of other CVD at baseline for both GL and GI (Supplemental Table S5). Other adjustments made at the study level for all eligible studies were smoking, body mass index, age of participants, physical activity, family history of MI, and diabetes status (in addition to exclusion of diabetes at baseline). When these adjustments were made, the CHD-GL and GI RRs were greater than 1.20 with LCL greater than 1.10. An exception was for aspirin consumption (Supplemental Table S5) when the LCL greater than 1.10 criterion was met for neither GL nor GI; however, differences in RR with vs without aspirin use were not statistically significant (P=.78 and P=.16, respectively) (Supplemental Table S5).
4. Temporality
Exposure must precede incidence of disease. This criterion is met by design in all prospective cohort studies (see also Experimental and Analogy sections, which refer to intervention studies).
5. Biological Gradient (Dose Response)
In prospective cohort studies, this criterion is met when the combined studies dose-response RR is statistically significant, as was the case for GL (Figures 1 and 2), GI (Figures 2 and 4), and carbohydrate meta-regressed on GI (Figures 6 and 7).
6. Plausibility
This criterion is met when at least one credible mechanism can explain the association. The GL and GI are major food and dietary markers predictive of a food’s (or diet’s) ability once ingested to both elevate postprandial blood glucose58 and determine longer-term fasting blood glucose and HbA1c concentrations in the nondiabetic and diabetic states.59 Elevated HbA1c and blood glucose concentrations, including postprandial glucose, each are major risk factors for CVD,60, 61 for which CHD is the major contributor.2, 62 This includes elevation of glucose and HbA1c in the normal range in addition to the elevation that occurs in diabetes.60, 61, 63, 64, 65 Further, observations in the general population have shown that HbA1c and blood glucose are better markers of cardiovascular and CHD risk than either HDL cholesterol or total cholesterol66, 67 (see also Experimental section).
7. Experimental
Intervention trials that show either reduction of the specified disease incidence or reduction in markers of disease are needed to meet this criterion. (For trials, see Analogy section.) Several pathogenic pathways from high GL and GI lead to a conclusion that “modern dietary guidelines for patients at risk of CHD should reflect…[the] danger of consuming a HGL [high GL] diet.”68 Further evidence comes from a primary care setting. A study by Unwin et al69, 70 found that lowering the GL of the diet by advice to avoid high GI foods for 13 months in 69 at-risk persons with either prediabetes or diabetes lowered several parameters, including body weight (−9 kg; P<.001), waist circumference (−15 cm; P<.001), HbA1c (−19%; P<.001), total cholesterol (−6%; P<.001), and cholesterol to HDL cholesterol ratio (−9%; P=.001). The study concluded that this dietary approach was a practical alternative to drug therapy (for prediabetes and diabetes, ie, patients at risk for CHD) and had considerable cost savings for general medical practice. Consistently, the present analyses support high GI carbohydrate as a major nutritional risk factor for CHD among general populations.
8. Analogy
Lower GI and GL diets can be achieved using inhibitors of carbohydrate digestion.71 Treatment with acarbose (an α-glucosidase inhibitor) has the same pattern of effect on markers of metabolic disease as does treatment with lower GI or lower GL diets.58 Further, reducing the GI and GL of the diet by use of acarbose has reduced the incidence of any cardiovascular event by 49% (5%-72%) and CHD (as MI) by 91% (28%-99%).71
9. Coherence
To meet this criterion, a disease-exposure association should not conflict with the natural history of disease. Evidence of coherence arises in part from the interventional studies supporting plausibility, experimental, and analogy criteria (see preceding sections). Further evidence comes from the association of one disease with another, each of which is linked to higher blood glucose and insulin concentrations; thus, excess body weight72 and CHD, diabetes, and certain cancers.73, 74 Meta-analysis has revealed that lower GL diets, achieved using lower GI carbohydrate foods, result in a dose-dependent reduction in body weight among persons with varied glycemic control from normal to the diabetic state.59 Further, avoidance of high GI foods to achieve a lower GL diet has proved effective in improving body weight and glycemic and lipidemic parameters over a mean of 13 months in prediabetic and diabetic patients.69, 70 Beneficial effects of low GI and GL have become evident in long-term primary prevention of obesity-associated diseases.75 In longitudinal trials, lower GI and GL due to ingestion of α-glucosidase inhibitors lowers not only the risk of CHD72 but also the risk of diabetes76, 77, 78 and colorectal cancer.79 Likewise, lower GI and GL diets also prospectively associate with a lower risk for type 2 diabetes,28, 29, 80, 81, 82 a disease that increases the risk of subsequent CHD83, 84, 85 and subsequent diagnosis of colon cancer.58
In summary, all 9 of Bradford-Hill’s criteria for probable causality were met in our study. In application of GI and GL to food and dietary guidance, it should be noted that food group–based dietary guidelines would be insufficient because each food group contains foods having a very wide range of GI and GL values56, 86 and that prospective cohort studies have shown that even within beneficial food patterns, such as the Mediterranean diet and the healthy or vegetarian diet in the United Kingdom, there was evidence of added benefits of lower GI and GL.87, 88, 89
Conclusion
Among healthy persons from Europe, North America, and East Asia, strong (RR >1.20, LCL >1.10 within jurisdictions) and probably causal (Bradford-Hill ratings) RRs occur between incident CHD and dietary GI and GL. The CHD-carbohydrate of high GI, the CHD-GL, and the CHD-GI RRs each are markedly greater across the globe than within jurisdictions. The evidence presented supports the use of these markers of carbohydrate food quality in dietary guidelines for general populations.
Acknowledgments
The funding organizations had no role in the design and execution of the study, in the collection, analyses, and interpretation of the data, or in the preparation, review, or approval of the submitted manuscript.
Footnotes
Grant Support: This work was funded by BENEO GmbH.
Potential Competing Interests: Dr Geoffrey Livesey hold shares in Independent Nutrition Logic Ltd, a consultancy, and has received research grants, travel funding, consultant fees, and honoraria, from the American Association for the Advancement of Science, the All-Party Parliamentary Group for Diabetes, the Almond Board of California, BENEO GmbH, Biotechnology and Biological Sciences Research Council, British Nutrition Foundation, Calorie Control Council, Cantox, Colloides Naturel International, Coca-Cola Company, Danisco, Diabetes Nutrition Study Group, Diabetes UK, Elsevier Inc, European Commission, European Polyol Association, EUREKA, Food and Agriculture Organization of the United Nations, Granules India, General Maills Inc, Health Canada, Institute of Food Research, International Carbohydrate Quality Consortium, Institute of Medicine, International Life Sciences Institute, Life Sciences Research Office, Federation of American Societies for Experimental Biology, Kellogg Company, Knights Fitness, Nutrition Society of Australia, Leatherhead Food Research, LighterLife, Matsutani, Inc, Medical Research Council, MSL Group, Porter Novelli, Südzuker, Sugar Nutrition/World Sugar Research Organisation, Tate & Lyle, The Food Group, Weight Watchers, Wiley-Blackwell, and World Health Organization. Ms Helen Livesey holds shares in Independent Nutrition Logic Ltd and has benefitted from the aforementioned organizations.
Supplemental Online Material
Supplemental material can be found online at http://mcpiqojournal.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
References
- 1.Gaziano T.A., Bitton A., Anand S., Abrahams-Gessel S., Murphy A. Growing epidemic of coronary heart disease in low- and middle-income countries. Curr Probl Cardiol. 2010;35(2):72–115. doi: 10.1016/j.cpcardiol.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization Cardiovascular diseases (CVDs) factsheet. World Health Organization website. http://www.who.int/mediacentre/factsheets/fs317/en/ Published May 17, 2017. Accessed May 30, 2018.
- 3.Stampfer M.J., Hu F.B., Manson J.E., Rimm E.B., Willett W.C. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med. 2000;343(1):16–22. doi: 10.1056/NEJM200007063430103. [DOI] [PubMed] [Google Scholar]
- 4.Hajar R. Framingham contribution to cardiovascular disease. Heart Views. 2016;17(2):78–81. doi: 10.4103/1995-705X.185130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dehghan M., Mente A., Zhang X., et al. Prospective Urban Rural Epidemiology (PURE) Study Investigators Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): a prospective cohort study. Lancet. 2017;390(10107):2050–2062. doi: 10.1016/S0140-6736(17)32252-3. [DOI] [PubMed] [Google Scholar]
- 6.Vega-López S., Venn B.J., Slavin J.L. Relevance of the glycemic index and glycemic load for body weight, diabetes, and cardiovascular disease. Nutrients. 2018;10(10):E1361. doi: 10.3390/nu10101361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halton T.L., Willett W.C., Liu S., et al. Low-carbohydrate-diet score and the risk of coronary heart disease in women. N Engl J Med. 2006;355(19):1991–2002. doi: 10.1056/NEJMoa055317. [DOI] [PubMed] [Google Scholar]
- 8.Dong J.Y., Zhang Y.H., Wang P., Qin L.Q. Meta-analysis of dietary glycemic load and glycemic index in relation to risk of coronary heart disease. Am J Cardiol. 2012;109(11):1608–1613. doi: 10.1016/j.amjcard.2012.01.385. [DOI] [PubMed] [Google Scholar]
- 9.Mirrahimi A., de Souza R.J., Chiavaroli L., et al. Associations of glycemic index and load with coronary heart disease events: a systematic review and meta-analysis of prospective cohorts. J Am Heart Assoc. 2012;1(5):e000752. doi: 10.1161/JAHA.112.000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan J., Song Y., Wang Y., Hui R., Zhang W. Dietary glycemic index, glycemic load, and risk of coronary heart disease, stroke, and stroke mortality: a systematic review with meta-analysis. PLoS One. 2012;7(12):e52182. doi: 10.1371/journal.pone.0052182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willett W. 3rd ed. Oxford University Press; New York , NY: 1998. Nutritional Epidemiology. Monographs in Epidemiology and Biostatistics, Vol 40. [Google Scholar]
- 12.Mente A., de Koning L., Shannon H.S., Anand S.S. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med. 2009;169(7):659–669. doi: 10.1001/archinternmed.2009.38. [DOI] [PubMed] [Google Scholar]
- 13.Ledikwe J.H., Blanck H.M., Kettel Khan L., et al. Dietary energy density is associated with energy intake and weight status in US adults. Am J Clin Nutr. 2006;83(6):1362–1368. doi: 10.1093/ajcn/83.6.1362. [DOI] [PubMed] [Google Scholar]
- 14.Micha R., Shulkin M.L., Peñalvo J.L., et al. Etiologic effects and optimal intakes of foods and nutrients for risk of cardiovascular diseases and diabetes: systematic reviews and meta-analyses from the Nutrition and Chronic Diseases Expert Group (NutriCoDE) PLoS One. 2017;12(4):e0175149. doi: 10.1371/journal.pone.0175149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jakobsen M.U., Dethlefsen C., Joensen A.M., et al. Intake of carbohydrates compared with intake of saturated fatty acids and risk of myocardial infarction: importance of the glycemic index. Am J Clin Nutr. 2010;91(6):1764–1768. doi: 10.3945/ajcn.2009.29099. [DOI] [PubMed] [Google Scholar]
- 16.Liu S., Willett W.C., Stampfer M.J., et al. A prospective study of dietary glycemic load, carbohydrate intake, and risk of coronary heart disease in US women. Am J Clin Nutr. 2000;71(6):1455–1461. doi: 10.1093/ajcn/71.6.1455. [DOI] [PubMed] [Google Scholar]
- 17.Beulens J.W., de Bruijne L.M., Stolk R.P., et al. High dietary glycemic load and glycemic index increase risk of cardiovascular disease among middle-aged women: a population-based follow-up study. J Am Coll Cardiol. 2007;50(1):14–21. doi: 10.1016/j.jacc.2007.02.068. [DOI] [PubMed] [Google Scholar]
- 18.Sieri S., Krogh V., Berrino F., et al. Dietary glycemic load and index and risk of coronary heart disease in a large italian cohort: the EPICOR study. Arch Intern Med. 2010;170(7):640–647. doi: 10.1001/archinternmed.2010.15. [DOI] [PubMed] [Google Scholar]
- 19.Burger K.N., Beulens J.W., Boer J.M., Spijkerman A.M., van der A.D. Dietary glycemic load and glycemic index and risk of coronary heart disease and stroke in Dutch men and women: the EPIC-MORGEN study. PLoS One. 2011;6(10):e25955. doi: 10.1371/journal.pone.0025955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu D., Shu X.-O., Li H., et al. Dietary carbohydrates, refined grains, glycemic load, and risk of coronary heart disease in Chinese adults. Am J Epidemiol. 2013;178(10):1542–1549. doi: 10.1093/aje/kwt178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Dam R.M., Visscher A.W., Feskens E.J., Verhoef P., Kromhout D. Dietary glycemic index in relation to metabolic risk factors and incidence of coronary heart disease: the Zutphen Elderly Study. Eur J Clin Nutr. 2000;54(9):726–731. doi: 10.1038/sj.ejcn.1601086. [DOI] [PubMed] [Google Scholar]
- 22.Levitan E.B., Mittleman M.A., Wolk A. Dietary glycaemic index, dietary glycaemic load and incidence of myocardial infarction in women. Br J Nutr. 2010;103(7):1049–1055. doi: 10.1017/S0007114509992674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levitan E.B., Mittleman M.A., Håkansson N., Wolk A. Dietary glycemic index, dietary glycemic load, and cardiovascular disease in middle-aged and older Swedish men. Am J Clin Nutr. 2007;85(6):1521–1526. doi: 10.1093/ajcn/85.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grau K., Tetens I., Bjørnsbo K.S., Heitman B.L. Overall glycaemic index and glycaemic load of habitual diet and risk of heart disease. Public Health Nutr. 2011;14(1):109–118. doi: 10.1017/S136898001000176X. [DOI] [PubMed] [Google Scholar]
- 25.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stroup D.F., Berlin J.A., Morton S.C., et al. Meta-analysis of Observational Studies in Epidemiology (MOOSE) Group Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 27.Brunner E., Stallone D., Juneja M., Bingham S., Marmot M. Dietary assessment in Whitehall II: comparison of 7 d diet diary and food-frequency questionnaire and validity against biomarkers. Br J Nutr. 2001;86(3):405–414. doi: 10.1079/bjn2001414. [DOI] [PubMed] [Google Scholar]
- 28.Barclay A.W., Petocz P., McMillan-Price J., et al. Glycemic index, glycemic load, and chronic disease risk—a meta-analysis of observational studies. Am J Clin Nutr. 2008;87(3):627–637. doi: 10.1093/ajcn/87.3.627. [DOI] [PubMed] [Google Scholar]
- 29.Livesey G., Taylor R., Livesey H., Liu S. Is there a dose-response relation of dietary glycemic load to risk of type 2 diabetes? meta-analysis of prospective cohort studies. Am J Clin Nutr. 2013;97(3):584–596. doi: 10.3945/ajcn.112.041467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wells G.A., Shea B., O'Connell D., et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute website. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Accessed August 28, 2009.
- 31.Sterne J., Newton H.J., Cox N.J., editors. Meta-analysis in Stata: An Updated Collection From the Stata Journal. Stata Press; College Station, TX: 2009. [Google Scholar]
- 32.Greenland S., Longnecker M.P. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135(11):1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 33.Orsini N., Bellocco R., Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata J. 2006;6(1):40–57. [Google Scholar]
- 34.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 35.Galbraith R.F. A note on graphical presentation of estimated odds ratios from several clinical trials. Stat Med. 1988;7(8):889–894. doi: 10.1002/sim.4780070807. [DOI] [PubMed] [Google Scholar]
- 36.Tang J.L., Liu J.L. Misleading funnel plot for detection of bias in meta-analysis. J Clin Epidemiol. 2000;53(5):477–484. doi: 10.1016/s0895-4356(99)00204-8. [DOI] [PubMed] [Google Scholar]
- 37.Hoaglin D.C. Misunderstandings about Q and 'Cochran's Q test' in meta-analysis. Stat Med. 2016;35(4):485–495. doi: 10.1002/sim.6632. [DOI] [PubMed] [Google Scholar]
- 38.Hill A.B. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Health Organization, Food and Agriculture Organization of the United Nations . World Health Organization; Geneva, Switzerland: 2003. Diet, Nutrition and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation. WHO Technical Report Series 916. [Google Scholar]
- 40.Franz M.J. Is there a role for the glycemic index in coronary heart disease prevention or treatment? Curr Atheroscler Rep. 2008;10(6):497–502. doi: 10.1007/s11883-008-0077-0. [DOI] [PubMed] [Google Scholar]
- 41.Hu F.B., Willett W.C. Diet and coronary heart disease: findings from the Nurses' Health Study and Health Professionals' Follow-up Study. J Nutr Health Aging. 2001;5(3):132–138. [PubMed] [Google Scholar]
- 42.Clar C., Al-Khudairy L., Loveman E., et al. Low glycaemic index diets for the prevention of cardiovascular disease. Cochrane Database Syst Rev. 2017;7:CD004467. doi: 10.1002/14651858.CD004467.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Denova-Gutiérrez E., Huitrón-Bravo G., Talavera J.O., et al. Dietary glycemic index, dietary glycemic load, blood lipids, and coronary heart disease. J Nutr Metab. 2010;2010:170680. doi: 10.1155/2010/170680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lofgren I.E., Herron K.L., West K.L., et al. Carbohydrate intake is correlated with biomarkers for coronary heart disease in a population of overweight premenopausal women. J Nutr Biochem. 2005;16(4):245–250. doi: 10.1016/j.jnutbio.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 45.Pierucci P., Misciagna G., Ventura M.T., et al. Diet and myocardial infarction: a nested case-control study in a cohort of elderly subjects in a Mediterranean area of southern Italy. Nutr Metab Cardiovasc Dis. 2012;22(9):727–733. doi: 10.1016/j.numecd.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 46.Eshak E.S., Iso H., Date C., et al. JACC Study Group Rice intake is associated with reduced risk of mortality from cardiovascular disease in Japanese men but not women. J Nutr. 2011;141(4):595–602. doi: 10.3945/jn.110.132167. [DOI] [PubMed] [Google Scholar]
- 47.Sonestedt E., Hellstrand S., Orho-Melander M. Carbohydrate-rich foods and risk of cardiovascular disease in the Malmö diet and cancer cohort [abstract] Eur J Epidemiol. 2013;28(suppl 1):S184. [Google Scholar]
- 48.Al Essa H.B., Cohen R., Adebamowo S.N., et al. Carbohydrate-rich foods and risk of cardiovascular disease in the Malmö diet and cancer cohort. Circulation. 2016;133 [Google Scholar]
- 49.Schulze M.B., Shai I., Manson J.E., et al. Joint role of non-HDL cholesterol and glycated haemoglobin in predicting future coronary heart disease events among women with type 2 diabetes. Diabetologia. 2004;47(12):2129–2136. doi: 10.1007/s00125-004-1593-2. [DOI] [PubMed] [Google Scholar]
- 50.Yu D., Shu X.-O., Li H., et al. High intakes of dietary carbohydrate and rice were associated with increased risk of coronary heart disease in chinese men and women. Circulation. 2013;127(12) [Google Scholar]
- 51.Li S., Hu F.B., Forman J.P., Rimm E.B. Dietary glycemic index and glycemic load and risk of coronary heart disease in a prospective study among US male health professionals. Circulation. 2012;125(10) [Google Scholar]
- 52.Similä M.E., Kontto J.P., Männistö S., Valsta L.M., Virtamo J. Glycaemic index, carbohydrate substitution for fat and risk of CHD in men. Br J Nutr. 2013;110(9):1704–1711. doi: 10.1017/S0007114513000858. [DOI] [PubMed] [Google Scholar]
- 53.Mursu J., Virtanen J.K., Rissanen T.H., et al. Glycemic index, glycemic load, and the risk of acute myocardial infarction in Finnish men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Nutr Metab Cardiovasc Dis. 2011;21(2):144–149. doi: 10.1016/j.numecd.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 54.Turati F., Dilis V., Rossi M., et al. Glycemic load and coronary heart disease in a Mediterranean population: the EPIC Greek cohort study. Nutr Metab Cardiovasc Dis. 2015;25(3):336–342. doi: 10.1016/j.numecd.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 55.Hardy D.S., Hoelscher D.M., Aragaki C., et al. Association of glycemic index and glycemic load with risk of incident coronary heart disease among Whites and African Americans with and without type 2 diabetes: the Atherosclerosis Risk in Communities study. Ann Epidemiol. 2010;20(8):610–616. doi: 10.1016/j.annepidem.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Livesey G. In: Sweeteners and Sugar Alternatives in Food Technology. 2nd ed. O'Donnell K., Kearsley M.W., editors. Wiley-Blackwell; Oxford, US: 2012. Glycaemic responses and toleration; pp. 1–26. [Google Scholar]
- 57.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 58.Augustin L.S., Kendall C.W., Jenkins D.J., et al. Glycemic index, glycemic load and glycemic response: an international scientific consensus summit from the International Carbohydrate Quality Consortium (ICQC) Nutr Metab Cardiovasc Dis. 2015;25(9):795–815. doi: 10.1016/j.numecd.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 59.Livesey G., Taylor R., Hulshof T., Howlett J. Glycemic response and health—a systematic review and meta-analysis: relations between dietary glycemic properties and health outcomes. Am J Clin Nutr. 2008;87(1):258S–268S. doi: 10.1093/ajcn/87.1.258S. [DOI] [PubMed] [Google Scholar]
- 60.Selvin E., Wattanakit K., Steffes M.W., Coresh J., Sharrett A.R. HbA1c and peripheral arterial disease in diabetes: the Atherosclerosis Risk in Communities study. Diabetes Care. 2006;29(4):877–882. doi: 10.2337/diacare.29.04.06.dc05-2018. [DOI] [PubMed] [Google Scholar]
- 61.Khaw K.T., Wareham N., Luben R., et al. Glycated haemoglobin, diabetes, and mortality in men in Norfolk cohort of European Prospective Investigation of Cancer and Nutrition (EPIC-Norfolk) BMJ. 2001;322(7277):15–18. doi: 10.1136/bmj.322.7277.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.World Health Organization Global Health Observatory (GHO) data. World Health Organization website. http://www.who.int/gho/mortality_burden_disease/en/
- 63.Smith S.A. Higher "normal" glycated hemoglobin levels were associated with increased risk for diabetes, CVD, stroke, and mortality in adults. Ann Intern Med. 2010;153(2):JC1–JC13. doi: 10.7326/0003-4819-153-2-201007200-02013. [DOI] [PubMed] [Google Scholar]
- 64.Khaw K.T., Wareham N., Bingham S., Luben R., Welch A., Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European Prospective Investigation into Cancer in Norfolk. Ann Intern Med. 2004;141(6):413–420. doi: 10.7326/0003-4819-141-6-200409210-00006. [DOI] [PubMed] [Google Scholar]
- 65.Pai J.K., Cahill L.E., Hu F.B., Rexrode K.M., Manson J.E., Rimm E.B. Hemoglobin A1c is associated with increased risk of incident coronary heart disease among apparently healthy, nondiabetic men and women. J Am Heart Assoc. 2013;2(2):e000077. doi: 10.1161/JAHA.112.000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Faeh D., Rohrmann S., Braun J. Better risk assessment with glycated hemoglobin instead of cholesterol in CVD risk prediction charts. Eur J Epidemiol. 2013;28(7):551–555. doi: 10.1007/s10654-013-9827-6. [DOI] [PubMed] [Google Scholar]
- 67.Wareham N.J., Pfister R. Diabetes: glycated hemoglobin is a marker of diabetes and CVD risk. Nat Rev Cardiol. 2010;7(7):367–368. doi: 10.1038/nrcardio.2010.84. [DOI] [PubMed] [Google Scholar]
- 68.Mathews M.J., Liebenberg L., Mathews E.H. How do high glycemic load diets influence coronary heart disease? Nutr Metab (Lond) 2015;12:6. doi: 10.1186/s12986-015-0001-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Unwin D.J., Cuthbertson D.J., Feinman R., Sprung V.S. A pilot study to explore the role of a low-carbohydrate intervention to improve GGT levels and HbA1c. Diabetes Pract. 2015;4(3):102–108. [Google Scholar]
- 70.Unwin D., Haslam D., Livesey G. It is the glycaemic response to, not the carbohydrate content of food that matters in diabetes and obesity: the glycaemic index revisited. J Insulin Resistance. 2016;1(1):a8. [Google Scholar]
- 71.Chiasson J.L., Josse R.G., Gomis R., Hanefeld M., Karasik A., Laakso M., STOP-NIDDM Trial Research Group Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003;290(4):486–494. doi: 10.1001/jama.290.4.486. [DOI] [PubMed] [Google Scholar]
- 72.Carey P.E., Stewart M.W., Ashworth L., Taylor R. Effect of insulin therapy on plasma leptin and body weight in patients with type 2 diabetes. Horm Metab Res. 2003;35(6):372–376. doi: 10.1055/s-2003-41360. [DOI] [PubMed] [Google Scholar]
- 73.Hu F.B., Manson J.E., Liu S., et al. Prospective study of adult onset diabetes mellitus (type 2) and risk of colorectal cancer in women. J Natl Cancer Inst. 1999;91(6):542–547. doi: 10.1093/jnci/91.6.542. [DOI] [PubMed] [Google Scholar]
- 74.Guraya S.Y. Association of type 2 diabetes mellitus and the risk of colorectal cancer: a meta-analysis and systematic review. World J Gastroenterol. 2015;21(19):6026–6031. doi: 10.3748/wjg.v21.i19.6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schwingshackl L., Hoffmann G. Long-term effects of low glycemic index/load vs. high glycemic index/load diets on parameters of obesity and obesity-associated risks: a systematic review and meta-analysis. Nutr Metab Cardiovasc Dis. 2013;23(8):699–706. doi: 10.1016/j.numecd.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 76.Chiasson J.L., Josse R.G., Gomis R., Hanefeld M., Karasik A., Laakso M., STOP-NIDDM Trial Research Group Acarbose for the prevention of Type 2 diabetes, hypertension and cardiovascular disease in subjects with impaired glucose tolerance: facts and interpretations concerning the critical analysis of the STOP-NIDDM Trial data. Diabetologia. 2004;47(6):969–975. doi: 10.1007/s00125-004-1409-4. [DOI] [PubMed] [Google Scholar]
- 77.Chiasson J.L., Josse R.G., Gomis R., Hanefeld M., Karasik A., Laakso M., STOP-NIDDM Trail Research Group Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002;359(9323):2072–2077. doi: 10.1016/S0140-6736(02)08905-5. [DOI] [PubMed] [Google Scholar]
- 78.Kawamori R., Tajima N., Iwatmoto Y., Kashiwaqi A., Kashiwagi A., Shimamoto K., Kaku K. Alpha-glucosidase inhibitor for the prevention of type 2 diabetes mellitus: a randomised, double-blind trial in Japanese subjects with impaired glucose tolerance [in Japanese] Nihon Rinsho. 2009;67(9):1821–1825. [PubMed] [Google Scholar]
- 79.Tseng Y.-H., Tsan Y.-T., Chan W.-C., Sheu W.H.-H., Chen P.-C. Use of an α-glucosidase inhibitor and the risk of colorectal cancer in patients with diabetes: a nationwide, population-based cohort study. Diabetes Care. 2015;38(11):2068–2074. doi: 10.2337/dc15-0563. [DOI] [PubMed] [Google Scholar]
- 80.Bhupathiraju S.N., Tobias D.K., Malik V.S., et al. Glycemic index, glycemic load, and risk of type 2 diabetes: results from 3 large US cohorts and an updated meta-analysis. Am J Clin Nutr. 2014;100(1):218–232. doi: 10.3945/ajcn.113.079533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Greenwood D.C., Threapleton D.E., Evans C.E., et al. Glycemic index, glycemic load, carbohydrates, and type 2 diabetes: systematic review and dose-response meta-analysis of prospective studies. Diabetes Care. 2013;36(12):4166–4171. doi: 10.2337/dc13-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dong J.Y., Zhang L., Zhang Y.H., Qin L.Q. Dietary glycaemic index and glycaemic load in relation to the risk of type 2 diabetes: a meta-analysis of prospective cohort studies. Br J Nutr. 2011;106(11):1649–1654. doi: 10.1017/S000711451100540X. [DOI] [PubMed] [Google Scholar]
- 83.Turin T.C., Okamura T., Rumana N., et al. Diabetes and lifetime risk of coronary heart disease. Prim Care Diabetes. 2017;11(5):461–466. doi: 10.1016/j.pcd.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 84.Peters S.A., Huxley R.R., Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia. 2014;57(8):1542–1551. doi: 10.1007/s00125-014-3260-6. [DOI] [PubMed] [Google Scholar]
- 85.Murakami Y., Huxley R.R., Lam T.H., et al. Asia Pacific Cohort Studies Collaboration Diabetes, body mass index and the excess risk of coronary heart disease, ischemic and hemorrhagic stroke in the Asia Pacific Cohort Studies Collaboration. Prev Med. 2012;54(1):38–41. doi: 10.1016/j.ypmed.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 86.Atkinson F.S., Foster-Powell K., Brand-Miller J.C. International tables of glycemic index and glycemic load values: 2008. Diabetes Care. 2008;31(12):2281–2283. doi: 10.2337/dc08-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sluijs I., Beulens J.W., van der Schouw Y.T., et al. InterAct Consortium Dietary glycemic index, glycemic load, and digestible carbohydrate intake are not associated with risk of type 2 diabetes in eight European countries. J Nutr. 2013;143(1):93–99. doi: 10.3945/jn.112.165605. [DOI] [PubMed] [Google Scholar]
- 88.Rossi M., Turati F., Lagiou P., et al. Mediterranean diet and glycaemic load in relation to incidence of type 2 diabetes: results from the Greek cohort of the population-based European Prospective Investigation into Cancer and Nutrition (EPIC) Diabetologia. 2013;56(11):2405–2413. doi: 10.1007/s00125-013-3013-y. [DOI] [PubMed] [Google Scholar]
- 89.Rodríguez-Rejón A.I., Castro-Quezada I., Ruano-Rodríguez C., et al. Effect of a Mediterranean diet intervention on dietary glycemic load and dietary glycemic index: the PREDIMED Study. J Nutr Metab. 2014;2014:985373. doi: 10.1155/2014/985373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.