Abstract
Objective
To describe rates of low-dose computed tomography (LDCT) and all chest computerized tomography (CT) before and after Centers for Medicare and Medicaid Services (CMS) initiated reimbursement and requirements for screening and to describe factors associated with receipt of LDCT.
Patients and Methods
Retrospective cross-sectional study of Medicare enrollees aged 55 to 77 in Parts A and B Medicare without HMO enrollment in a 20% national sample (n=3,887,430 in 2010, 4,200,875 in 2015, and 4,145,542 in 2016). The outcomes were receipt of LDCT and any chest CT from January 1, 2010, to December 31, 2016. Other measures included enrollee demographic characteristics and diagnoses, including diagnoses of tobacco use.
Results
The number of enrollees aged 55 to 77 with LDCT rose throughout 2015 and early 2016, and then plateaued. In 2016, 0.44% of enrollees, and 2.21% of those with a tobacco-use diagnosis, underwent LDCT screening. There were increases in the rate of any chest CT (LDCT or diagnostic) between January 1, 2010 and December 31, 2016, and most of this was accounted for by LDCTs.
Conclusions
Two years after CMS approval for lung cancer screening reimbursement, less than 5% of the Medicare population eligible for screening received LDCT. More work is required to identify and modify the barriers for LDCT screening.
Abbreviations and Acronyms: CMS, Center for Medicare and Medicaid Services; COPD, chronic obstructive pulmonary disease; CPT, current procedural terminology; HMO, Health Maintenance Organization; ICD-9, International Classification of Diseases Ninth Revision; ICD-10, International Classification of Diseases Tenth Revision; LDCT, low dose computed tomography; NHIS, National Health Interview Study; USPSTF, United States Preventive Services Task Force
Lung cancer is the leading cause of cancer-related mortality in the United States, with an estimated 154,050 attributable deaths in 2018.1 The 5-year survival rate for lung cancer is only 18% owing to the fact that a majority of lung cancers are diagnosed late in the disease when cure is no longer possible.2 In early 2015, the Centers for Medicare and Medicaid Services (CMS) initiated reimbursement for low-dose computerized tomography (LDCT) screening for lung cancer in centers that meet eligibility and screening requirements after a shared decision-making visit.3 This was based on a large trial published in 2011, demonstrating lower mortality from lung cancer and from all causes using annual LDCT screening and subsequent United States Preventive Services Task Force (USPSTF) recommendation in 2013.4, 5 In 2018, a Dutch-Belgian trial showed even larger reductions in lung cancer mortality with screening LDCT.6
As it is the only procedure known to reduce lung cancer mortality, implementation of LDCT and outcomes are the focus of ongoing research. Two recent publications examined use of lung cancer screening in 2010 and 2015, before and after publication of the USPSTF guidelines, using the National Health Interview Study (NHIS). The NHIS surveyed respondents who had undergone computerized tomography (CT) scans and asked whether the CT was performed specifically to check for lung cancer.7, 8 One study found no significant change over the 5-year period, and the other, using somewhat different methodology, reported a 50% increase.7, 8 Both studies reported that less than 4% of eligible respondents received LDCT screening; however, results were based on respondents’ ability to recall and differentiate whether they received LDCT imaging for lung cancer screening or whether CT imaging was for another purpose. It is unclear if this is an over- or underestimation of LDCT screening. A large California community health care system found a 7.3% increase in LDCT orders—not necessarily completed screenings—from 2010 to 2016 of eligible patients.9
We hypothesize that lung cancer screening is underused. To ascertain use of lung cancer screening use in the United States, we used national Medicare data from January 1, 2010 and December 31, 2016 to examine use of LDCT screening before and after CMS initiated reimbursement and requirements for screening. In addition, we describe factors associated with receipt of LDCT.
Methods
Source of Data
This was a retrospective cross-sectional study using enrollment and claims data for a 20% national sample of Medicare beneficiaries between January 1, 2010 and December 31, 2016. More than 98% of adults in the United States, aged 65 years and older, are enrolled in Medicare, which serves more than 45 million beneficiaries. CMS selected a random sample of 20% Medicare beneficiaries based on the last 2 digits of their Medicare claim account numbers, and this sample has been shown to be representative of the whole cohort.10 This included Medicare beneficiary summary files, Medicare Provider Analysis and Review files, Outpatient Standard Analytic Files, and Medicare Carrier files. The University of Texas Medical Branch Institutional Review Board approved the research and waived informed consent.
Cohorts
We developed separate cohorts for each calendar year: 2010 (n=3,887,430), 2015 (n=4,200,875), and 2016 (n=4,145,542). Each cohort included beneficiaries aged 55 to 77 years old on January 1 of that year, with complete Medicare Part A (hospital insurance) and Part B (outpatient and preventative services) coverage and no health maintenance organization (HMO) enrollment in that year. HMO enrollees (including Medicare Advantage) were excluded, as there may be incomplete data if enrollees are referred to external plan providers. In 2016, this cohort represented 50.4% of all Medicare enrollees (33.9% of enrollees age 55 to 64 and 54.3% of enrollees age 65 to 77). For analyses that included comorbidity measures, we further restricted the cohorts to those with complete Parts A and B and no HMO coverage in the study year and in the previous year (n=3,412,011 for 2010; 3,673,046 for 2015; and 3,568,760 for 2016). This allowed for the assessment of comorbidities in the year prior to receipt of LDCT. In the 2016 analyses that included estimated life expectancy, we further restricted the cohorts to those aged older than 65 (n=3,071,441). The steps for the selection of cohorts are outlined in the Figure.
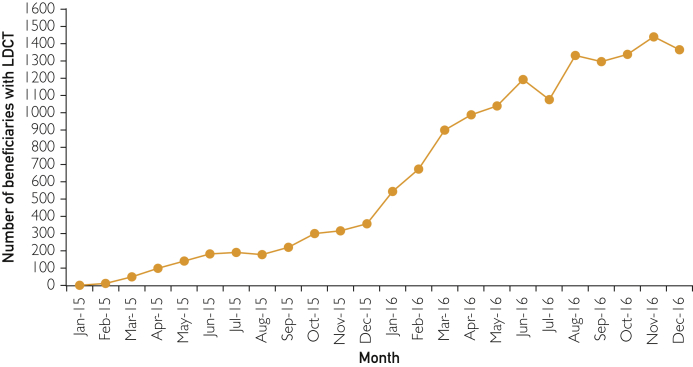
Figure.
Number of Medicare enrollees with claims for LDCT lung cancer screening, by month, in 2015 and 2016. The number of beneficiaries with LDCT equals all enrollees with complete Parts A&B, no HMOs in 2015 or 2016, and no chest CTs in the 12 months prior to the LDCT examination date.
Beneficiary and Regional Characteristics
Medicare enrollment files provided information on beneficiary age, sex, race and ethnicity, and reason for original entitlement (age 65, disability or end-stage renal disease). We used Medicaid enrollment file as a proxy for low income. Chronic obstructive pulmonary disease (COPD) or emphysema were identified by International Classification of Diseases, Ninth Revision (ICD-9) codes 490, 491, 492, 496 or International Classification of Diseases, Tenth Revision (ICD-10) codes J410, J411, J430, J431, J432, J438 J439, J440, J441, or J449 associated with inpatient or outpatient billing claims in the previous 12 months. Elixhauser comorbidity measures with COPD or emphysema excluded were generated from all claims in the 12 months before each study year and categorized according to number of comorbidities (0, 1, 2, 3, 4+).11 We estimated the rate of previous or current smokers for each year, defined by the code V15.82 (history of tobacco use), or ICD-9 codes 305.1 (tobacco-use disorder), or 989.84 (toxic effect of tobacco). Life expectancy of the enrollees who were older than 65 and who underwent LDCT was estimated using an algorithm based on a person’s age and the presence or absence of any of the comorbidities included in the Elixhauser comorbidity measure.12, 13 The C-statistics for the models predicting 10-year mortality were 0.77 for men and 0.80 for women.12
Outcomes
Outcomes were receipt of low dose CT (CPT G0297 or S8032); any chest CT (CPT 71250, 71260, 71270, G0297, or S8032; or ICD-9 874.1, 874.2, or ICD-10 BW2400Z, BW240ZZ, BW2410Z, BW241ZZ, BW24Y0Z, BW24YZZ, BW24ZZZ, BW2500Z, BW250ZZ, BW2510Z, BW251ZZ, BW25Y0Z, BW25YZZ, BW25ZZZ).
Statistical Analyses
We calculated the number and proportion of Medicare beneficiaries with a charge for LDCT or any chest CT in 2010, 2015, and 2016. The number of LDCT charges prior to 2015 was 0 because there was no code for that service before 2015. For 2016, we calculated the proportions of enrollees stratified by patient characteristics. We also estimated the odds of undergoing LDCT in 2016 using logistic regression. Because of the size of the cohorts, the 95% confidence intervals for estimates were small, and small differences were statistically significant. Our focus was more on clinically meaningful differences. The numbers and proportions of beneficiaries receiving LDCT or any chest CT were also calculated for each month from January 2015 to December 2016. We then analyzed the time trends in LDCT using Joinpoint analysis (SAS Analytics, Cary, NC), with a nonlinear model, to identify change points and 95% confidence intervals and also slopes between the change points. Statistical significance for the Joinpoint analysis was at P<0.0001.
Results
Table 1 presents the characteristics of the Medicare population studied for 2015. The enrollees younger than age 65 (12.8%) all had either disability or end-stage renal disease as the reason for Medicare eligibility. More than half the cohort had ≤1 comorbidity, and only 5.63% had diagnoses for COPD or emphysema. Approximately 17.3% of enrollees had diagnoses of current or previous tobacco use. There were small changes in these characteristics in the 2010 and 2016 cohorts (not displayed).
Table 1.
Characteristics of Medicare Enrollees in the 20% National Sample in 2015a
| Number of enrollees (%) | |
|---|---|
| All | 3,673,046 (100%) |
| Age | |
| 55-59 | 225,987 (6.15%) |
| 60-64 | 243,474 (6.63%) |
| 65-69 | 1,349,285 (36.73%) |
| 70-74 | 1,256,280 (34.20%) |
| 75-77 | 598,019 (16.28%) |
| Race/Ethnicity | |
| White | 2,969,632 (80.85%) |
| Black | 330,699 (9.00%) |
| Hispanic | 197,013 (5.36%) |
| Others | 175,701 (4.78%) |
| Gender | |
| Female | 1,972,535 (53.70%) |
| Male | 1,700,510 (46.30%) |
| Medicaid eligible | |
| Yes | 525,068 (14.30%) |
| No | 3,147,977 (85.70%) |
| Initial eligibility by disability or age | |
| Yes | 879,516 (23.95%) |
| No | 2,793,529 (76.05%) |
| Previous COPD or emphysema? | |
| Yes | 206,916 (5.63%) |
| No | 3,466,129 (94.37%) |
| Any tobacco diagnoses? | |
| Yes | 638,836 (17.34%) |
| No | 3,036,209 (82.66%) |
| Number of comorbiditiesb | |
| 0 | 1,308,685 (35.63%) |
| 1 | 795,487 (21.66%) |
| 2 | 637,809 (17.36%) |
| 3 | 380,835 (10.37%) |
| 4+ | 550,229 (14.98%) |
COPD = chronic obstructive pulmonary disease
All enrollees had both Part A and B Medicare and were not enrolled in HMOs in 2014 and 2015.
Excluding COPD or emphysema
The percentage of Medicare enrollees who had charges for LDCT lung cancer screening and for those with smoking diagnoses is shown in Table 2. There were no specific Medicare billing codes for LDCT before 2015, so the rate for 2010 is given as 0%. By 2016, the rate of LDCT in the overall sample was 0.44% (confidence interval [CI] 0.43-0.45), and 2.21% (CI 2.18-2.25) among those with tobacco diagnoses. The 2016 LDCT rate for persons aged 65 to 77 was 0.41% (data not shown). Over the January 1, 2010 and December 31, 2016 period, the absolute increase of any chest CT (including LDCT) was 0.94% among all enrollees and 1.44% among those with smoking diagnoses.
Table 2.
Percent of Enrollees With Claims Data for LDCT and All CTs in 2010, 2015, 2016, for all Medicare Enrollees Aged 55 to 77 and for Enrollees With Smoking Diagnoses
| Year | LDCT |
Any chest CT |
||
|---|---|---|---|---|
| All enrollees (95% CI) | Those with smoking diagnostic codes (95% CI) | All enrollees (95% CI) | Those with smoking diagnostic codes (95% CI) | |
| 2010 | 0% | 0% | 7.38% (7.35%-7.41%) | 20.38% (20.24%-20.52%) |
| 2015 | 0.09% (0.08%-0.09%) | 0.49% (0.47%-0.51%) | 7.59% (7.56%-7.61%) | 20.61% (20.51%-20.71%) |
| 2016 | 0.44% (0.43%-0.45%) | 2.21% (2.18%-2.25%) | 8.32% (8.29%-8.35%) | 21.82% (21.73%-21.91%) |
Denominator is all enrollees with complete Part A&B in 2010, 2015, or 2016 and not enrolled in HMOs in that year.
CI = confidence interval; CT = computed tomography; LDCT = low-dose computed tomography
The number of Medicare enrollees in the 20% sample, aged 55 to 77, who underwent LDCT in each month in 2015 and 2016 is shown in the Figure. There was a significant (P<.001) increase in slope December of 2015 (95% CI, November 2015, January 2016) and leveling of the slope in April of 2016 (95% CI, March, May). After CMS approval for LDCT screening, the slope throughout 2015 shows an increase of 54.8 LDCTs in enrollees per month. After December 2015, the slope showed sharper increase of 220 per month and in April less of an increase of 35 per month. By inspection, there were no further increases in number of LDCTs starting in August of 2016, at about 1800 per month.
The characteristics of those who underwent LDCT screening in 2016, the first full year with Medicare reimbursement, is presented in Table 3. Those patients younger than 65 years of age account for 13.1% of beneficiaries but for one fifth of all LDCTs in 2016. More than 98% had diagnoses of current or previous tobacco use. More than half (55.0%) had diagnoses of COPD or emphysema. Among those aged 65 to 77 who had LDCTs, 75.9% had estimated life expectancies of greater than10 years at the time of screening, whereas 19.8% had life expectancies of more than 5 to less than 10 years, and 4.4% had life expectancies of less than 5 years. Older beneficiaries and those with increased number of comorbidities were less likely to receive LDCT.
Table 3.
Characteristics of Medicare Enrollees with LDCT Claims in 2016
| Number of enrollees | Number of patients with LDCT (Column %) | Percent screened (95% CI) | Adjusted OR | |
|---|---|---|---|---|
| Alla | 4,145,542 | 18,244 (100%) | 0.44% (0.43%-0.45%) | |
| Agea | ||||
| <65 | 542,258 | 3,627 (19.88%) | 0.67% (0.64%-0.69%) | Reference |
| 65-68 | 1,399,626 | 6,726 (36.87%) | 0.48% (0.46%-0.50%) | 0.85 (0.81-0.90) |
| 69-71 | 853,554 | 3,876 (21.25%) | 0.45% (0.43%-0.47%) | 0.75 (0.71-0.80) |
| 72-74 | 749,155 | 2,771 (15.19%) | 0.37% (0.35%-0.39%) | 0.59 (0.55-0.63) |
| 75-77 | 600,949 | 1,244 (6.82%) | 0.21% (0.19%-0.22%) | 0.32 (0.30-0.35) |
| Race/Ethnicitya | ||||
| Black | 376,719 | 1,115 (6.11%) | 0.30% (0.27%-0.32%) | 0.59 (0.55-0.63) |
| Hispanic | 237,536 | 429 (2.35%) | 0.18% (0.16%-0.20%) | 0.41 (0.36-0.46) |
| Others | 231,951 | 675 (3.70%) | 0.29% (0.26%-0.32%) | 0.66 (0.60-0.72) |
| White | 3,299,336 | 16,025 (87.84%) | 0.49% (0.47%-0.50%) | Reference |
| Gendera | ||||
| Female | 2,223,040 | 8,894 (48.75%) | 0.40% (0.39%-0.41%) | 0.82 (0.79-0.85) |
| Male | 1,922,502 | 9,350 (51.25%) | 0.49% (0.47%-0.50%) | Reference |
| Medicaid eligiblea | ||||
| No | 3,524,948 | 14,810 (81.18%) | 0.42% (0.41%-0.43%) | 0.98 (0.93-1.02) |
| Yes | 620,594 | 3,434 (18.82%) | 0.55% (0.53%-0.58%) | Reference |
| Comorbidity, excluding COPDb | ||||
| 0 | 1,450,318 | 4144 (26.39%) | 0.29% (0.28%-0.30%) | 1.57 (1.48-1.66) |
| 1 | 756,769 | 4098 (26.10%) | 0.54% (0.52%-0.56%) | 1.95 (1.84-2.06) |
| 2 | 558,787 | 3155 (20.09%) | 0.56% (0.54%-0.59%) | 1.86 (1.75-1.98) |
| 3 | 328,754 | 1882 (11.99%) | 0.57% (0.54%-0.60%) | 1.57 (1.46-1.68) |
| 4+ | 492,131 | 2423 (15.43%) | 0.49% (0.47%-0.52%) | Reference |
| Previous COPD/emphysemab | ||||
| No | 3,253,661 | 7061 (44.97%) | 0.22% (0.10%-0.33%) | 0.18 (0.17-0.19) |
| Yes | 333,098 | 8641 (55.03%) | 2.59% (2.25%-2.93%) | Reference |
| Tobacco diagnosisb | ||||
| Yes | 972,462 | 15,522 (98.85%) | 1.60% (1.57%-1.63%) | |
| No | 2,614,298 | 180 (1.15%) | 0.007% (0.005%-0.008%) | |
| Life expectancyc | ||||
| ≥10 years | 2,488,382 | 9293 (75.88%) | 0.37% (0.36%-0.39%) | |
| >5 and <10 years | 444,958 | 2,419 (19.75%) | 0.54% (0.52%-0.57%) | |
| ≤5 years | 138,101 | 535 (4.37%) | 0.39% (0.35%-0.42%) |
CI = confidence interval; COPD = chronic obstructive pulmonary disease; LDCT = low-dose computed tomography; OR = odds ratio
Denominator is all enrollees have complete Parts A&B with no HMOs in 2016.
Denominator is all enrollees have complete Parts A&B, with no HMOs in 2015 and 2016.
Denominator is all enrollees aged 66+ and have complete Parts A&B, with no HMOs in 2015 and 2016. Life expectancy is estimated only for those older than age 65 because the algorithm was derived from those older than age 65.
Discussion
We found use of LDCT in the first 2 years of lung cancer screening coverage in the Medicare population is low. We compared our results to 2 estimates of eligible Medicare beneficiaries. One study estimates that 12.5% of Medicare beneficiaries are eligible for lung cancer screening.14 We found the rate of LDCT screening in 2016 was 0.44% or 91,220 Medicare enrollees aged 55 to 77 with Parts A and B Medicare not enrolled in HMOs. If we postulate that the LDCT rate for the 49.6% of Medicare enrollees in an HMO or without Part B is also 0.44%, the estimated total Medicare population age 55 to 77 undergoing screening in 2016 numbered approximately 181,000 beneficiaries. This is less than 4% of another study, which estimates 4.9 million Medicare beneficiaries are eligible for LDCT screening, although this estimate included persons aged 78 to 80 who are not included in CMS guidelines,15 After subtracting 78 to 80 year olds, our findings suggest that less than 5% of Medicare enrollees estimated to meet CMS eligibility criteria underwent LDCT screening in 2016.
Using, NHIS data, Huo et al reported a 2.1% overall rate of LDCT in those age 40 and older and estimated that approximately 880,000 persons aged 55 to 74 underwent LDCT screening in 2015.8 Jemal and Fedewa also used NHIS but restricted their analyses to those who met the age and smoking criteria for LDCT screening.7 They estimated that 262,700 of 6,800,000 persons aged 55 to 80 who met criteria for screening underwent LDCT in 2015. Authors of both studies noted that LDCT rates were lower than expected. Our estimates of LDCT use are even lower than those reported from NHIS. LDCT rates from NHIS depend on accuracy of recall and the respondent’s ability to distinguish among different imaging tests. The fact that 1.2% of never smokers aged 40 and older reported a 2015 LDCT in the NHIS supports the possibility of false positive self-reports.8 Other estimates of LDCT use are also based on surveys of selected centers and have also reported modest rates.16, 17, 18
We cannot compare our results directly with those from the NHIS for several reasons. The NHIS sample was constructed to represent all community-dwelling people, whereas the Medicare cohort included those in institutions and excluded those without Part A and B Medicare and those enrolled in HMOs. In addition, the Medicare population below age 65 is limited to those with disabilities or end-stage renal disease. Finally, NHIS data provide information on quantity and years of smoking, which allows for better estimations of eligibility for LDCT screening, whereas the Medicare data are limited to whether the enrollee had current or previous exposure to tobacco.
Concern has been raised that many older patients undergoing cancer screening tests may not live long enough to benefit, leading to overtreatment.19 Approximately 24% of the Medicare enrollees undergoing LDCT had projected life expectancies of less than 10 years, with 4.4% less than 5 years. Such patients would encounter similar harm from further diagnostic tests and treatments but with less time to experience the benefits of those treatments.
There many possible contributing factors to the low rates of LDCT screening including lack of awareness among primary care providers regarding evidence, recommendations, patient eligibility criteria, or physician “buy-in.” The number of screening-eligible smokers decreased from 8.4 million in 2010 to 6.8 million in 2015.7 Trends in documentation of smoking history to determine lung cancer screening eligibility has shown improvement, but even after efforts to improve documentation, remains less than 50%.9, 20, 21, 22 Among Medicare recipients, prevalence of diagnostic codes for current and former smokers was approximately 55% of national survey estimates by the Behavioral Risk Factor Surveillance System, suggesting that this population has even poorer attention to tobacco documentation and cessation counseling.23, 24 The requirement for a separate shared decision-making visit may be a barrier.25, 26, 27, 28 Also, clinicians may be focusing on those at highest risk among their patients who meet CMS screening criteria.29, 30 The high false positive rate and potential for negative lung biopsies and thoracotomies may discourage some patients and their physicians.26 Concern about potential adverse effects of LDCT screening prompted CMS to issue numerous requirements, including specific criteria for both radiologists and LDCT facilities, and participation in a national registry of patients receiving LDCT.3 These may be slowing dissemination. Also, low rates of screening may simply be related to slow uptake during the first 2 years after CMS approval.
Limitations
As noted above, the results from enrollees aged 65 to 77 with fee-for-service Part A and B Medicare are not generalizable to those in Medicare HMOs, and the results from those age 55 to 64 are from those with Medicare because of disability or end-stage renal disease. It is possible that some LDCTs received by Medicare enrollees were coded as other chest CTs. However, the monthly rates of charges for LDCT appeared to stabilize by August of 2016, suggesting that early issues with use of correct billing codes has been resolved.
We were unable to assess eligibility directly for LDCT using Medicare data. Specifically, we cannot identify amount and timing of tobacco exposure, lack of symptoms, willingness of a patient to pursue treatment if cancer is found, or results of shared decision-making discussion. Smoking history, a key component in determining eligibility, is poorly documented for current or past tobacco use, with a 7% sensitivity but more than 98% specificity.31 As a result, some LDCT recipients may not have met CMS criteria for lung cancer screening. Although we assessed whether patients had tobacco diagnoses in their Medicare charges, such diagnoses may not be accurate and do not indicate either type of amount or period of tobacco use.31, 32
Conclusions
These results suggest a low uptake of LDCT screening for lung cancer in the initial 2 years after CMS-approved reimbursement for the Medicare population, with no evidence from late 2016 that the rate is continuing to rise. Further work is required to improve the implementation of LDCT for lung cancer screening.
Footnotes
Grant Support: The study was supported by the Cancer Prevention and Treatment Institute of Texas (RP160674) and the National Institutes of Health (K05 CA134923, P30 AG024832, and UL1TR001439).
Potential Competing Interests: The authors report no competing interests.
Supplemental Online Material
Supplemental material can be found online at http://mcpiqojournal.org/. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.SEER Cancer Statistics Review, 1975-2015. Based on November 2017 SEER data submission, posted to the SEER web site, April 2018. https://seer.cancer.gov/csr/1975_2015/ Available at: Accessed May 1, 2018.
- 3.Decision memo for screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439N) https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274 Accessed April 26, 2018.
- 4.National Lung Screening Trial Research Team. Aberle D.R., Adams A.M., et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moyer V.A., LeFevre M.L., Siu A.L., et al. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330–338. doi: 10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]
- 6.IASLC 19th World Conference on Lung Cancer (WCLC) NELSON study shows CT screening for nodule volume management reduces lung cancer mortality by 26% in men [press release] ECancer news; September. 2018;25 [Google Scholar]
- 7.Jemal A., Fedewa S.A. Lung cancer screening with low-dose computed tomography in the United States: 2010 to 2015. JAMA Oncol. 2017;3(9):1278–1281. doi: 10.1001/jamaoncol.2016.6416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huo J., Shen C., Volk R.J., Shih Y.T. Use of CT and chest radiography for lung cancer screening before and after publication of screening guidelines: intended and unintended uptake. JAMA Intern Med. 2017;177(3):439–441. doi: 10.1001/jamainternmed.2016.9016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J., Chung S., Wei E.K., Luft H.S. New recommendation and coverage of low-dose computed tomography for lung cancer screening: uptake has increased but is still low. BMC Health Serv Res. 2018;18(1):525. doi: 10.1186/s12913-018-3338-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medicare data file descriptions. www.resdac.umb.edu/Medicare/file_descritions.asp Accessed January 24, 2013.
- 11.Elixhauser A., Steiner C., Harris D.R., Coffey R.M. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Tan A., Kuo Y.F., Goodwin J.S. Predicting life expectancy for community-dwelling older adults from Medicare claims data. Am J Epidemiol. 2013;178(6):974–983. doi: 10.1093/aje/kwt054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodwin J.S., Sheffield K., Li S., Tan A. Receipt of cancer screening is a predictor of life expectancy. J Gen Intern Med. 2016;31(11):1308–1314. doi: 10.1007/s11606-016-3787-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roth J.A., Sullivan S.D., Goulart B.H.L., Ravelo A., Sanderson J.C., Ramsey S.D. Projected clinical, resource use, and fiscal impacts of implementing low-dose computed tomography lung cancer screening in Medicare. J Oncol Pract. 2015;11(4):267–272. doi: 10.1200/JOP.2014.002600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pyenson B.S., Henschke C.I., Yankelevitz D.F., Yip R., Dec E. Offering lung cancer screening to high-risk Medicare beneficiaries saves lives and is cost-effective: an actuarial analysis. Am Health Drug Benefits. 2014;7(5):272–282. [PMC free article] [PubMed] [Google Scholar]
- 16.Boiselle P.M., Chiles C., Ravenel J.G., White C.S. Computed tomographic screening for lung cancer trends at leading academic medical centers from 2013 to 2015. JAMA Oncol. 2016;2(5):682–684. doi: 10.1001/jamaoncol.2015.6419. [DOI] [PubMed] [Google Scholar]
- 17.Henderson L.M., Jones L.M., Marsh M.W., Benefield T., Rivera M.P., Molina P.L. Lung cancer screening practices in North Carolina CT facilities. J Am Coll Radiol. 2017;14(2):166–170. doi: 10.1016/j.jacr.2016.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeliadt S., Birkby G., Eberth J.M., et al. Early implementation of lung cancer screening across federally qualified health centers in the US. Am J Respir Crit Care Med. 2017;195:A5193. [Google Scholar]
- 19.Walter L.C., Covinsky K.E. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285(21):2750–2756. doi: 10.1001/jama.285.21.2750. [DOI] [PubMed] [Google Scholar]
- 20.Ferketich A.K., Khan Y., Wewers M.E. Are physicians asking about tobacco use and assisting with cessation? Results from the 2001-2004 national ambulatory medical care survey (NAMCS) Prev Med. 2006;43(6):472–476. doi: 10.1016/j.ypmed.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Linder J.A., Rigotti N.A., Schneider L.I., Kelley J.H., Brawarsky P., Haas J.S. An electronic health record-based intervention to improve tobacco treatment in primary care: a cluster-randomized controlled trial. Arch Intern Med. 2009;169(8):781–787. doi: 10.1001/archinternmed.2009.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyle R.G., Solberg L.I., Fiore M.C. Electronic medical records to increase the clinical treatment of tobacco dependence: a systematic review. Am J Prev Med. 2010;39(6 suppl 1):S77–82. doi: 10.1016/j.amepre.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 23.Shadel W.G., Elliott M.N., Haas A.C., et al. Clinician advice to quit smoking among seniors. Prev Med. 2015;70:83–89. doi: 10.1016/j.ypmed.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jamal A., King B.A., Neff L.J., Whitmill J., Babb S.D., Graffunder C.M. Current cigarette smoking among adults: United States, 2005-2015. MMWR Morb Mortal Wkly Rep. 2016;65(44):1205–1211. doi: 10.15585/mmwr.mm6544a2. [DOI] [PubMed] [Google Scholar]
- 25.Mazzone P.J., Tenenbaum A., Seeley M., et al. Impact of a lung cancer screening counseling and shared decision-making visit. Chest. 2017;151(3):572–578. doi: 10.1016/j.chest.2016.10.027. [DOI] [PubMed] [Google Scholar]
- 26.Ersek J.L., Eberth J.M., McDonnell K.K., et al. Knowledge of, attitudes toward, and use of low-dose computed tomography for lung cancer screening among family physicians. Cancer. 2016;122(15):2324–2331. doi: 10.1002/cncr.29944. [DOI] [PubMed] [Google Scholar]
- 27.Carter-Harris L., Gould M.K. Multilevel barriers to the successful implementation of lung cancer screening: why does it have to be so hard? Ann Am Thorac Soc. 2017;14(8):1261–1265. doi: 10.1513/AnnalsATS.201703-204PS. [DOI] [PubMed] [Google Scholar]
- 28.Lewis J.A., Petty W.J., Tooze J.A., et al. Low-dose CT lung cancer screening practices and attitudes among primary care providers at an academic medical center. Cancer Epidemiol Biomarkers Prev. 2015;24(4):664–670. doi: 10.1158/1055-9965.EPI-14-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pinsky P.F., Church T.R., Izmirlian G., Kramer B.S. The National Lung Screening Trial: results stratified by demographics, smoking history, and lung cancer histology. Cancer. 2013;119(22):3976–3983. doi: 10.1002/cncr.28326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gould M.K. Who should be screened for lung cancer? And who gets to decide? JAMA. 2016;315(21):2279–2281. doi: 10.1001/jama.2016.5986. [DOI] [PubMed] [Google Scholar]
- 31.Kokotailo R.A., Hill M.D. Coding of stroke and stroke risk factors using international classification of diseases, revisions 9 and 10. Stroke. 2005;36(8):1776–1781. doi: 10.1161/01.STR.0000174293.17959.a1. [DOI] [PubMed] [Google Scholar]
- 32.Wiley L.K., Shah A., Xu H., Bush W.S. ICD-9 tobacco use codes are effective identifiers of smoking status. J Am Med Inform Assoc. 2013;20(4):652–658. doi: 10.1136/amiajnl-2012-001557. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.