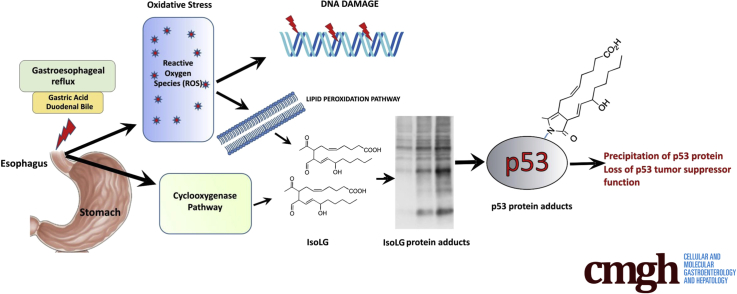
Epidemiologic studies have identified gastroesophageal reflux disease (GERD) as the strongest risk factor for esophageal adenocarcinoma.1 However, it remains not well understood how gastroesophageal reflux facilitates tumor development. In this study, we investigated, for the first time, the role of isolevuglandins (isoLGs), which are formed through free radical and enzymatic cyclooxygenation of polyunsaturated fatty acids.2 Structurally, isoLGs are categorized as lipid-derived γ-ketoaldehydes that are highly reactive with free amines on lysine residues forming LG-lysine lactam protein adducts, protein-protein, and protein-DNA crosslinks.2, 3
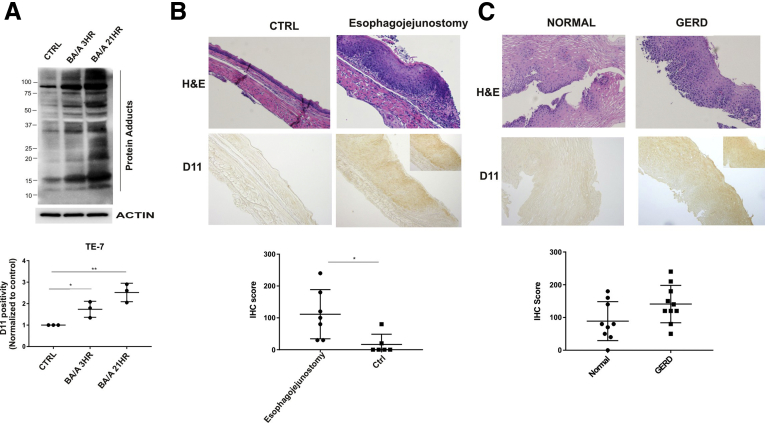
To investigate the reflux-induced cellular alterations, we exposed esophageal cells derived from the normal esophagus (EPC2), Barrett’s esophagus (CP-A), and cancer (TE-7) to acidic growth medium (pH 4.0), supplemented with 100 μM bile salts cocktail (BA/A). The composition, total bile salts concentration and pH, were selected based on previous measurements conducted in patients with GERD.4 IsoLGs were analyzed using D11 single chain antibody. This antibody was generated by screenings of phage-display libraries and tested to specifically recognize isoLG protein adducts independently of protein amino acid sequences.5, 6 We found that treatment of esophageal cells with acidic bile salts led to significant accumulation of isoLG protein adducts compared with untreated control (Figure 1A, Supplementary Figure 1A and B). Notably, multiple proteins were adducted after treatment with BA/A, which was indicated by a strong increase in the intensities of multiple protein bands. Induction of isoLG protein adducts was also observed using immunofluorescence with D11 scFv (Supplementary Figure 2).
Figure 1.
Reflux induces the formation of isoLG protein adducts. (A) Treatment with BA/A leads to significant accumulation of isoLG adducts in TE-7 (n = 3) cells. D11 positivity was arbitrarily set at 1 in control samples. (B) Representative image (×20) of immunohistochemical staining for isoLG protein adducts in the mouse esophagus after induction of reflux by surgery (n = 7). Mice with sham surgery were used as a control (n = 5). Mice with reflux showed significantly higher levels of isoLGs compared with control sham mice (P < .05). Inset shows cells at a ×40 magnification. (C) Representative images (×20) of immunohistochemical staining for isoLG in esophageal biopsies collected from patients with GERD (n = 10) and healthy individuals (n = 9). A trend was found toward increasing formation of isoLG protein adducts in patients with GERD (P = .07). Inset shows cells at a ×40 magnification. Experiments conducted in vitro were evaluated using the Student t test. The Mann-Whitney test was used for data sets generated in vivo. *P < .05, **P < .01. Data are represented as mean ± standard deviation. Ctrl, control; H&E, hematoxylin-eosin; IHC, immunohistochemical; ns = not significant.
Supplementary Figure 1.
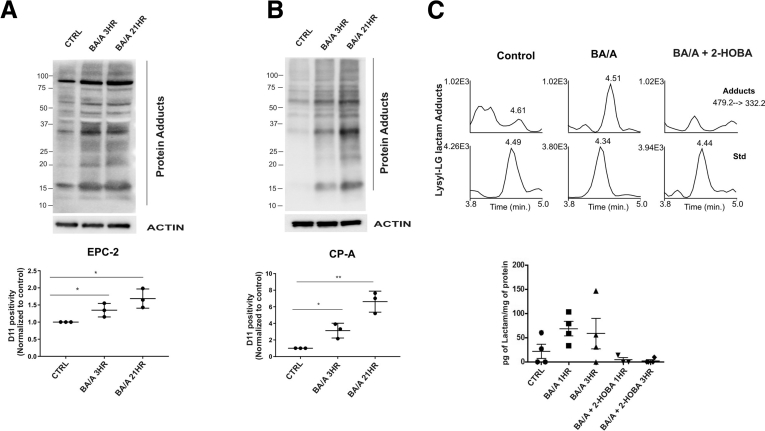
Formation of isoLG protein adducts in vitro. (A) EPC-2 cells were treated with BA/A for 15 minutes and analyzed for isoLG protein adducts with D11 antibody. Treatment with BA/A led to significant accumulation of isoLG adducts in EPC-2 cells. D11 positivity was arbitrarily set at 1 in control samples. (B) The same as A, but CP-A cells are shown. (C) Representative mass spectrometry chromatograms show levels of isoLG-lysine lactam adducts in cellular extracts collected from control (untreated) and BA/A-treated TE-7 cells in the presence or absence of 2-HOBA. Statistical analyses were performed with the Student t test. *P < .05, **P < .01. Data are represented as mean ± standard deviation.
Supplementary Figure 2.
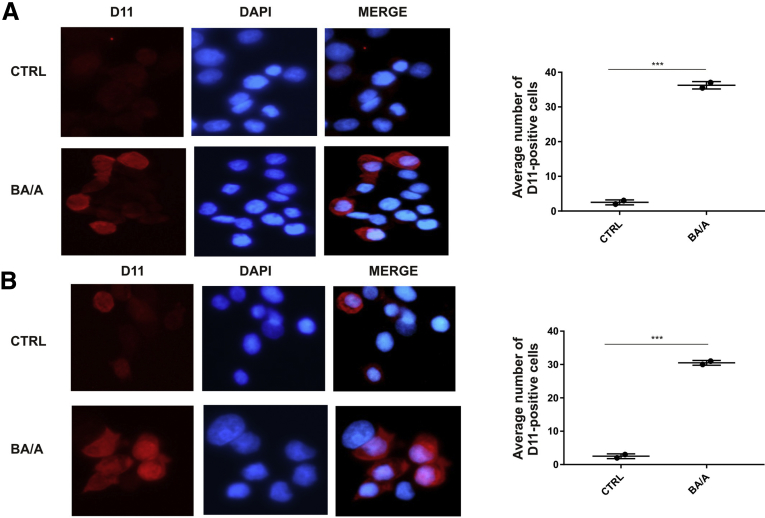
Representative images of isoLG-positive cells. Analyses were performed using immunofluorescence with D11 antibody after treatment with BA/A for 18 hours. Cell nuclei were stained with DAPI. Significant increase in isoLG positivity was found in treated EPC-2 (A) and TE-7 (B) cells. ***P < .001. At least 150 cells were assessed in each experiment. Statistical analyses were performed with Student t test. Data are represented as mean ± standard deviation.
The formation of LG-lysine lactam adducts was further verified by liquid chromatography-electrospray-ionization-tandem mass spectrometry in TE-7 cells. Our quantification analyses were based on specific transitions from the molecular ion at m/z = 479.2 to the specific fragment at m/z = 332.1. The elution of the 2 fragment ions found in BA/A-treated samples was similar to the [13C6] lysine–lactam internal standard. As an additional control we analyzed TE-7 cells treated with BA/A in the presence of the specific isoLG scavenger 2-hydroxybenzylamine (2-HOBA; 50 μM).7 Mass spectrometry analyses found an upregulation of LG-lysine lactam adducts after treatment of esophageal cells with BA/A. Treatment with 2-HOBA inhibited the effect of BA/A, providing further support to our findings (Supplementary Figure 1C).
To investigate the formation of isoLG protein adducts in vivo, we used a mouse model of esophageal reflux injury.8 A section of the mouse jejunum was transected and then anastomosed to the esophagus resulting in increased reflux. Using immunohistochemistry with D11 antibody, the levels of isoLG protein adducts were compared in esophageal specimens collected from animals with reflux and control animals with sham surgery (n = 12). We found significant accumulation of isoLG protein adducts in the esophagus of animals affected by reflux (Figure 1B).
To further investigate isoLG adducts in vivo, we conducted a small-scale study in patients with GERD. Esophageal biopsies collected from 10 GERD patients and 9 healthy individuals were immunostained with D11 antibody and analyzed for isoLG protein adducts. We found a trend toward increasing formation of isoLG protein adducts in patients with GERD (P = .07). An increased staining for the isoLG adducts was observed in 50% (5 out of 10) of patients with GERD (Figure 1C). Normal subjects primarily showed low or undetectable levels of isoLGs. Interestingly, some patients with GERD showed a strong nuclear staining showing that adducts form on nuclear proteins (Figure 1C, inset).
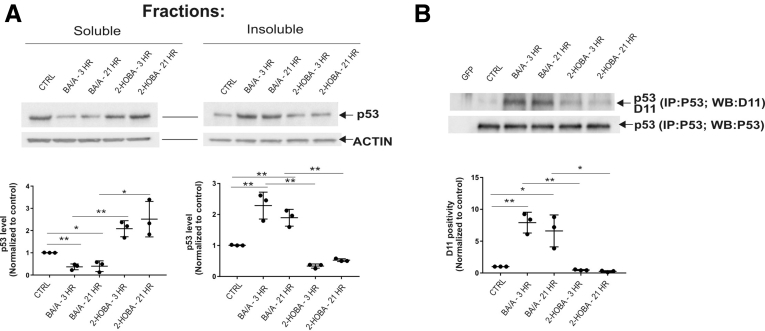
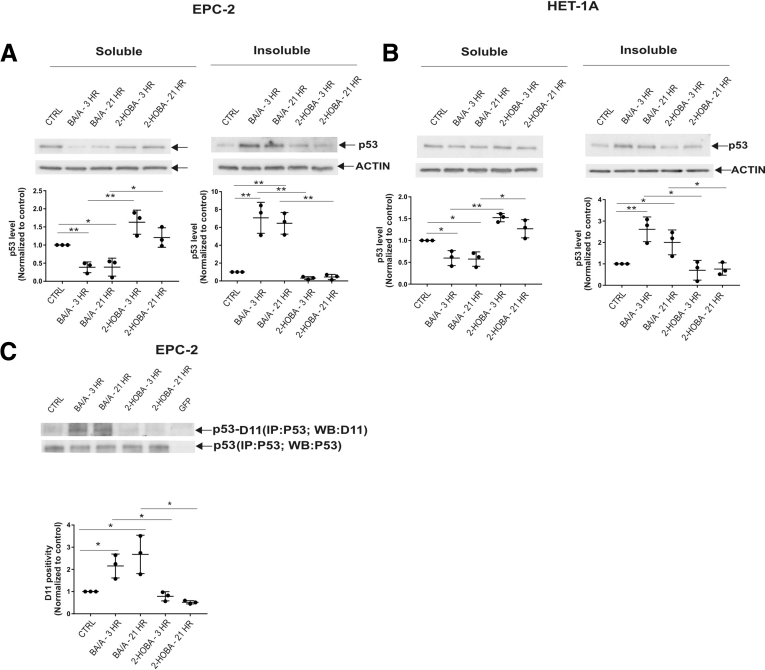
To investigate how induction of isoLGs by reflux affects proteins, we focused our studies on the regulation of p53 protein because it plays a key tumor suppressor role in the esophagus. Given that isoLGs have strong hydrophobic properties and may cause protein aggregation,9 we separately analyzed soluble and insoluble cellular fractions, which were prepared as described in the Supplementary Methods section. Surprisingly, we found that the solubility of the p53 protein significantly decreased after treatment with BA/A, whereas the insoluble cellular fraction was enriched with p53 protein aggregates in all tested cell lines (Figure 2A, Supplementary Figure 3A and B). p53 precipitation was prevented by 2-HOBA, suggesting that isoLGs are responsible for the precipitation of p53 protein (Figure 2A, Supplementary Figure 3A and B).
Figure 2.
Acidic bile salts cause the formation of adducts on p53 protein and its precipitation. (A) p53 protein is precipitated after treatment of CP-A cells with BA/A. 2-HOBA prevents precipitation of p53 protein. (B) Analysis of isoLG protein adducts using D11 scFv after immunoprecipitation of p53 protein with p53-specific antibody (DO1) in CP-A cells. Exposure of esophageal cells to BA/A significantly increases levels of p53 protein adducts. Each analysis is representative of 3 independent experiments and values are expressed as mean ± standard deviation. D11 positivity was arbitrarily set at 1 in control samples. Statistical analyses were performed with the Student t test. *P < .05, **P < .01. Ctrl, control; ns = not significant.
Supplementary Figure 3.
Acidic bile salts cause the formation of adducts on p53 protein and its precipitation. (A) p53 protein is precipitated after treatment of EPC-2 cells with BA/A. 2-HOBA prevents precipitation of p53 protein. (B) The same as A, but HET-1A cells are shown. (C) Analysis of isoLG protein adducts using D11 scFv after immunoprecipitation of p53 protein with p53-specific antibody (DO1) in EPC-2 cells. Exposure of esophageal cells to BA/A significantly increases levels of p53 protein adducts. Each analysis is representative of 3 independent experiments and values are expressed as mean ± standard deviation. D11 positivity was arbitrarily set at 1 in control samples. Statistical analyses were performed with the Student t test. *P < .05, **P < .01.
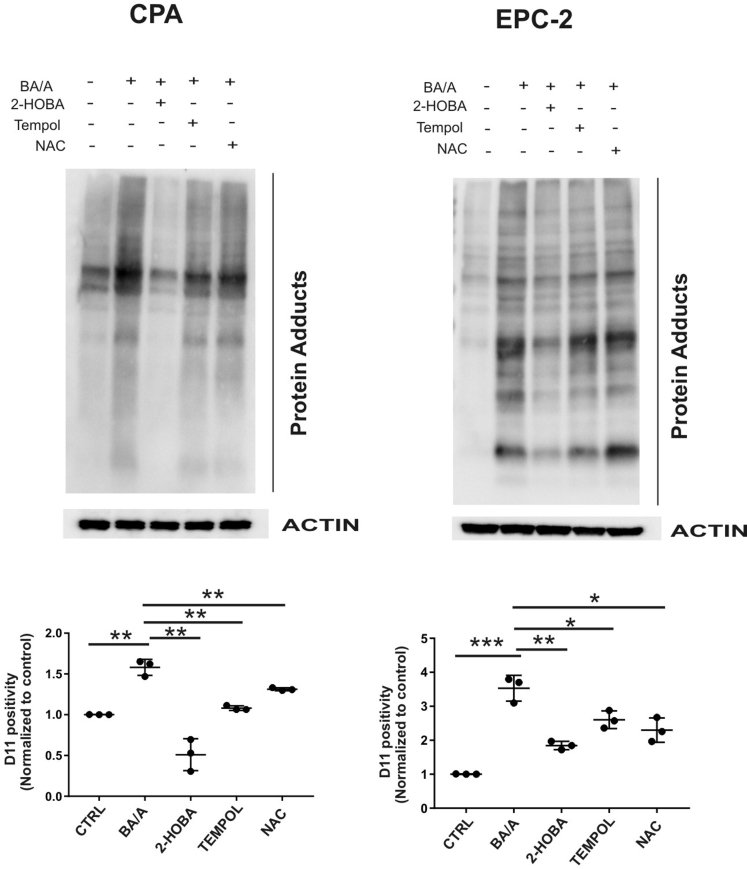
To directly analyze p53 protein adducts, cellular lysates were immunoprecipitated with p53-specific antibody (DO1). The immunoprecipitated p53 protein was then analyzed for the isoLG adducts by Western blotting using D11 scFv. We found that BA/A led to the formation of p53 isoLG protein adducts. Notably, 2-HOBA inhibited the formation of adducts on p53 protein further supporting our findings (Figure 2B, Supplementary Figure 3C). Then, the effect of 2-HOBA was compared with antioxidants (tempol and N-acetylcysteine), which were shown to inhibit the production of reactive oxygen species by acidic bile salts at tested concentrations.10 Although both tested antioxidants had some inhibitory effect, suppression of isoLG protein adducts was significantly stronger by 2-HOBA (Supplementary Figure 4).
Supplementary Figure 4.
Effect of 2-HOBA, Tempol, and NAC on the formation of isoLG protein adducts. EPC-2 and CP-A cells were treated with BA/A alone or in combination with either 2-HOBA or 20 μM of Tempol or 20 μM of NAC. Treated cells were collected and analyzed for the formation of isoLG protein adducts. D11 positivity was arbitrarily set at 1 in control samples. Results from 3 independent experiments are shown. Statistical analyses were performed with the Student t test. *P < .05, **P < .01, ***P < .001. Data are represented as mean ± standard deviation. NAC, N-acetylcysteine.
In summary, this study revealed, for the first time, that gastroesophageal reflux leads to the formation of isoLG and accumulation of isoLG protein adducts causing precipitation and inactivation of p53 tumor suppressor. We also found that isoLG scavenger 2-HOBA efficiently suppresses accumulation of isoLG adducts in the esophagus.
Footnotes
Author contributions Alexander I. Zaika, Sergey I. Dikalov, and Phillip Williams designed research studies. Ravindran Caspa Gokulan, Jamie M. Adcock, Irene Zagol-Ikapitte, and Olivier G. Boutaud conducted experiments. Ravindran Caspa Gokulan, Alexander I. Zaika, and Olivier G. Boutaud analyzed data. Raymond Mernaugh, Kay M. Washington, and Sergey I. Dikalov provided reagents. Ravindran Caspa Gokulan and Alexander I. Zaika wrote the manuscript.
Conflicts of interest The authors disclose no conflicts.
Funding This work was supported by grants from the National Cancer Institute (RO1 206564, R01 138833) and the Department of Veterans Affairs (BX002115). This work was also supported by grants from the National Institute of Health, R21 CA201856 and R21 CA187495. The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the Department of Veterans Affairs, National Institutes of Health, Vanderbilt University, or University of Miami.
Materials and Methods
Cell Lines
Human nontumorous esophageal cell line (HET-1A), human Barrett’s esophageal cell line (CP-A), and human esophageal carcinoma cell line (TE-7) were purchased from American Type Culture Collection. The morphology, karyotyping, and polymerase chain reaction–based techniques were used by American Type Culture Collection to confirm the cell line identity. Human immortalized esophageal epithelial cells, EPC-2, were kindly provided by Dr. Claudia Andl, University of Central Florida. HET-1A cells were cultured in Dulbecco's modified eagle's medium (Life Technologies, Carlsbad, CA) and TE-7 in RPMI (Life Technologies) media. Both Dulbecco's modified eagle's medium and RPMI were supplemented with 10% fetal bovine serum and 100 μg/mL penicillin/streptomycin. EPC-2 and CP-A cells were cultured in keratinocyte SFM media supplemented with 40 μg/mL bovine pituitary extract, 1.0 ng/mL epidermal growth factor (Life Technologies), 5% fetal bovine serum, and 100 μg/mL penicillin/streptomycin. All cells were cultured at 37°C in an atmosphere of 5% CO2.
Treatment With Acidic Bile Salts, Tempol, N-Acetylcysteine, and 2-Hydroxybenzylamine
Cells were treated in acidic Dulbecco's modified eagle's media (pH 4.0), containing bile salts cocktail at a final concentration of 100 μM. The bile salt cocktail was prepared with a combination of glycocholic, taurocholic, glycodeoxycholic, glycochenodeoxycholic, and deoxycholic sodium salts (all reagents were from Sigma-Aldrich, St. Louis, MO) at a concentration of 20 μM each. CP-A, TE-7, and HET-1A cells were treated for 30 minutes, whereas EPC-2 cells were treated for 15 minutes and then the media was replaced. The IsoLG scavenger 2-hydroxybenzylamine (2-HOBA) has been previously characterized.1 Final concentration of 2-HOBA was 50 μM. CP-A and EPC-2 cells were treated with the antioxidants, Tempol (4-hydroxy-2, 2, 6, 6-tetramethylpiperydine-1-oxyl; Enzo Biochem, Farmingdale, NY), and N-acetylcysteine (Sigma-Aldrich), as described previously.2
Antibodies, Cell Fractionation, and Immunoprecipitation
The following antibodies were used: β-actin (Sigma Aldrich), p53 (DO- 1; Millipore, Burlington, MA), and antimouse IgG HRP (Promega, Madison, WI). D-11, an isoLG-lysyl adducts-specific scFv antibody, has been isolated from a phage display recombinant antibody library.3 The resulting scFv antibody displays an E-tag recognized by an anti-E-tag antibody.3 In our study, we used anti-E tag HRP-conjugated secondary antibody from Abcam (Abcam, Cambridge, UK). The binding specificity of D11 scFv was previously characterized.3
After treatment with acidic bile salts, the cell lysates were sonicated and centrifuged at 16,000 × g at 4°C for 20 minutes. The supernatant, which contains the soluble cellular fraction, was carefully collected. The remaining cell pellet, which contains the insoluble cellular fraction, was washed twice, resuspended in phosphate-buffered saline containing protease inhibitor cocktail (Sigma Aldrich), and sonicated. Both soluble and insoluble cellular fractions were analyzed by Western blotting as described previously.4
p53 protein was immunoprecipitated from total cell lysate using p53 (DO-1) antibody and protein G agarose (Roche, Basel, Switzerland). The immunoprecipitated samples were analyzed by Western blotting using D11 antibody. The analyzed membranes were stripped in the Restore Western Blot Stripping Buffer (ThermoFisher Scientific, Waltham, MA) at +50°C for 1 hour, washed, blocked with 5% milk, and analyzed by Western blotting with p53 (DO1) antibody.
Immunofluorescence
TE-7 and EPC-2 cells were grown on chamber slides, treated with BA/A, and cultured in fresh media for 18 hours. After fixation with methanol and acetone mixture at a 1:1 ratio (vol/vol) and blocking with 0.1% Tween 20, the slides were incubated with D11 antibody for 18 hours in a humidified chamber, followed by incubation with rabbit anti-E-tag and goat antirabbit AlexaFluor 594 conjugated antibody (Invitrogen, Carlsbad, CA) for 1 hour. After washing with phosphate-buffered saline, the slides were counterstained with 4, 6-diamidino-2-phenylindole (DAPI; ThermoFisher Scientific) for 1 minute and examined under a fluorescence microscope (Olympus, Pittsburgh, PA). At least 150 cells in 3–5 randomly selected fields were analyzed.
Detection of Isolevuglandin Protein Adducts in Animals and Patients With Gastroesophageal Reflux Disease
Reflux in mice was induced by esophagojejunal anastomosis, as previously described.4 Esophagojejunostomy was performed on 14 129SV mice of 8 weeks old according to the protocol approved by the Vanderbilt University Animal Care and Use Committee. The esophageal and gastroesophageal areas from animals with esophagojejunostomy and sham surgery were harvested 3 weeks after surgery, paraffin-embedded, and analyzed by immunohistochemistry with D11 antibody. The negative control was performed by omitting the primary antibody. Indices of D11 positivity were scored and compared in test and control groups of animals.
Nineteen archival esophageal biopsies collected at Vanderbilt University Medical Center from patients with GERD and healthy individuals were used for analyses. The use of all human pathology specimens for research was approved by the institutional review board. Because only deidentified tissues were included in this retrospective study, the institutional review board waived the requirements for informed consent. Immunohistochemical staining was done with D11-E tag antibody (1 μg/mL) and anti-E tag HRP-conjugated antibody.
Immunohistochemical Analyses
The intensity of staining was graded as 0 (negative), 1 (weak), 2 (moderate), or 3 (strong). The frequency was graded according to the percentage of positive cells. Total scores were calculated by multiplying the intensity score by the percentage of positive cells. The individuals scoring the tissue were blinded.
Detection of Levuglandinyl-Lysine Lactam Using Liquid Chromatography/Electrospray Ionization/Tandem Mass Spectrometry
TE-7 cells were treated with acidic bile salts and collected at the indicated time points. Cells were incubated with indomethacin and pyridoxamine in phosphate-buffered saline, pH 7.4 at 4°C for 30 minutes, homogenized, and centrifugated at 18,000 × g for 10 minutes. Proteins were subjected to complete enzymatic digestion using pronase (1 mg protease per mg of protein) overnight. Samples were then heated at 98°C for 10 minutes, and after cooling, 0.3 μL aminopeptidase M (Calbiochem, EMD Chemicals, Gibbstown, NJ) was added per mg of protein. The digest was incubated at 37°C for 18 hours. The liquid chromatography/electrospray ionization/tandem mass spectrometry of LG adducts was carried out as previously described.5
Statistical Analyses
Statistical analysis was performed using the Student t test and Mann-Whitney test, depending on the data set. Results were shown as mean ± standard deviation and considered significant if P < .05.
Study Approval
All experimental protocols were approved by the Institutional Animal Care and Use Committee and institutional review board of Vanderbilt University Medical Center.
Supplemental Graphical Summary.
References
- 1.Souza R.F. Dig Dis. 2016;34:483–490. doi: 10.1159/000445225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salomon R.G. Antioxid Redox Signal. 2015;22:1703–1718. doi: 10.1089/ars.2014.6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies S.S. FASEB J. 2002;16:715–717. doi: 10.1096/fj.01-0696fje. [DOI] [PubMed] [Google Scholar]
- 4.Dvorak K. Gut. 2007;56:763–771. doi: 10.1136/gut.2006.103697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan H.P. Free Radic Biol Med. 2017;106:62–68. doi: 10.1016/j.freeradbiomed.2017.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirabo A. J Clin Invest. 2014;124:4642–4656. doi: 10.1172/JCI74084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies S.S. Biochemistry. 2006;45:15756–15767. doi: 10.1021/bi061860g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaika E. FASEB J. 2011;25:4406–4414. doi: 10.1096/fj.11-192815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bi W. Chem Res Toxicol. 2016;29:1628–1640. doi: 10.1021/acs.chemrestox.6b00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhardwaj V. Carcinogenesis. 2016;37:1161–1169. doi: 10.1093/carcin/bgw100. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- 1.Davies S.S., Brantley E.J., Voziyan P.A., Amarnath V., Zagol-Ikapitte I., Boutaud O., Hudson B.G., Oates J.A., Roberts L.J., 2nd Pyridoxamine analogues scavenge lipid-derived gamma-ketoaldehydes and protect against H2O2-mediated cytotoxicity. Biochemistry. 2006;45:15756–15767. doi: 10.1021/bi061860g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhardwaj V., Gokulan R.C., Horvat A., Yermalitskaya L., Korolkova O., Washington K.M., El-Rifai W., Dikalov S.I., Zaika A.I. Activation of NADPH oxidases leads to DNA damage in esophageal cells. Sci Rep. 2017;7:9956. doi: 10.1038/s41598-017-09620-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies S.S., Talati M., Wang X., Mernaugh R.L., Amarnath V., Fessel J., Meyrick B.O., Sheller J., Roberts L.J., 2nd Localization of isoketal adducts in vivo using a single-chain antibody. Free Radic Biol Med. 2004;36:1163–1174. doi: 10.1016/j.freeradbiomed.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Zaika E., Wei J., Yin D., Andl C., Moll U., El-Rifai W., Zaika A.I. p73 protein regulates DNA damage repair. FASEB J. 2011;25:4406–4414. doi: 10.1096/fj.11-192815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zagol-Ikapitte I., Masterson T.S., Amarnath V., Montine T.J., Andreasson K.I., Boutaud O., Oates J.A. Prostaglandin H(2)-derived adducts of proteins correlate with Alzheimer's disease severity. J Neurochem. 2005;94:1140–1145. doi: 10.1111/j.1471-4159.2005.03264.x. [DOI] [PubMed] [Google Scholar]