Abstract
Background:
The Sentinel Distributed Database (SDD) is a large database of patient-level administrative healthcare records, primarily derived from insurance claims and electronic health records, and is sponsored by the U.S. Food and Drug Administration for medical product safety evaluations. Acute myocardial infarction (AMI) is a common study endpoint for drug safety studies that rely on health records from the SDD and other administrative databases.
Purpose:
In this chart validation study, we report on the positive predictive value (PPV) of inpatient ICD-9-CM AMI administrative diagnosis codes (410.x1 and 410.x0) in the SDD.
Methods:
As part of an assessment of thromboembolic adverse event risk following treatment with intravenous immune globulin (IVIG), charts were obtained for 103 potential post-IVIG AMI cases. Charts were abstracted by trained nurses and physician-adjudicated based on pre-specified diagnostic criteria.
Results:
AMI status could be determined for 89 potential cases. The PPVs for the inpatient AMI diagnoses recorded in the SDD were 75% overall (95% CI: 65–84%), 93% (95% CI: 78–99%) for principal-position diagnoses, 88% (95% CI: 72–97%) for secondary diagnoses, and 38% (95% CI: 20–59%) for position-unspecified diagnoses (e.g., diagnoses originating from separate physician claims associated with an inpatient stay). Of the confirmed AMI cases, demand ischemia was the suspected etiology more often for those coded in secondary or unspecified positions (72% and 40%, respectively) than for principal-position AMI diagnoses (21%).
Conclusions:
PPVs for principal and secondary AMI diagnoses were high and similar to estimates from prior chart validation studies. Position-unspecified diagnosis codes were less likely to represent true AMI cases.
Keywords: diagnosis, medical records, myocardial infarction, pharmacoepidemiology, predictive value of tests, validation studies
Introduction
In this paper we report on the positive predictive values (PPVs) of inpatient diagnosis codes for acute myocardial infarction (AMI) within the Sentinel Distributed Database (SDD). The SDD is a database of longitudinal, patient-level medical and prescription data obtained from a number of participating Data Partners (i.e., large insurers and integrated care delivery systems) from across the U.S. The SDD and Sentinel program are sponsored by the U.S. Food and Drug Administration (FDA) for active safety surveillance of marketed medical products. As of August 2015, the SDD had 351 million person-years of longitudinal patient-level data for 193 million health plan members from 2000–2015.1
AMI is a common endpoint in drug safety studies based on administrative healthcare records. Prior U.S. and non-U.S. validation studies of AMI diagnoses recorded in databases of insurance claims, hospital discharge abstracts, and electronic health records (EHRs) have generally found that principal diagnoses of AMI from inpatient encounters have a high positive predictive value (PPV), typically in the range of 75–95%,2–15 and a sensitivity of 60–90%.3,5,15,16 The PPV associated with secondary inpatient diagnoses of AMI, which has been evaluated in fewer studies, has been found to be modestly lower.7,15,16Since not all AMI cases are identified with algorithms based on the principal diagnosis field only, a common study design dilemma is whether to additionally use other types of AMI diagnoses (e.g., secondary inpatient diagnoses, emergency department diagnoses) to identify AMIs. While the high PPV of principal inpatient diagnoses for AMI was confirmed by a prior validation study conducted within the SDD,11 to date the validity of non-principal inpatient AMI diagnoses has not been assessed in the SDD.
To provide researchers and other stakeholders with data on the validity of non-principal AMI diagnoses within the SDD, we report on the results of a chart validation study of potential AMI cases identified as part of a safety assessment of intravenous immune globulin (IGIV) products.
METHODS
Data sources and study population
The administrative healthcare records and patient medical charts used to identify and validate potential AMI cases came from 13 SDD Data Partners who participated in the protocol-based Sentinel assessment of Thromboembolic Events Following Immunoglobulin Administration.17 Potential cases from the years 2006–2012 were selected for chart review if an inpatient AMI diagnosis code was recorded in the SDD up to one month following a non-specific (i.e., polyvalent) IGIV treatment episode. A complete description of the criteria used to select potential cases can be found in the Appendix. Additional details concerning the design and objectives of the parent study have been described previously.17
IGIV is used in the treatment of primary and secondary immunoglobulin deficiencies, and a variety of inflammatory and autoimmune disorders (e.g., chronic demyelinating polyneuropathy and immune thrombocytopenic purpura).18 In Table 1 we provide descriptive information on the patients included in this chart validation study, including their possible indications for IGIV use and major cardiovascular risk factors. These health conditions were defined as previously described in the protocol for the parent study.19
Table 1.
Baseline characteristics of 103 potential acute myocardial infarction (AMI) cases identified from the Sentinel Distributed Database for whom chart retrieval was completed.
| Characteristic | N (%) |
|---|---|
| Demographics | |
| Age | |
| • 0–19 years | 3 (3%) |
| • 20–39 years | 6 (6%) |
| • 40–59 years | 28 (27%) |
| • 60–79 years | 51 (50%) |
| • 80+ years | 15 (15%) |
| Sex | |
| • Female | 49 (48%) |
| • Male | 54 (52%) |
| Possible indication for IGIV use* | |
| Autoimmune/inflammatory condition | 68 (66%) |
| Immune deficiency | 39 (38%) |
| Infection | 16 (16%) |
| Bone marrow or hematopoietic stem cell transplant | 8 (8%) |
| Other indication | 26 (25%) |
| Major cardiovascular risk factors* | |
| Myocardial infarction | 23 (22%) |
| Angina | 40 (39%) |
| Atrial fibrillation or flutter | 15 (15%) |
| Ischemic stroke | 6 (6%) |
| Peripheral vascular disease | 18 (17%) |
| Hypertension, uncomplicated | 58 (56%) |
| Hypertension, complicated (i.e., with end-organ damage) | 20 (19%) |
| Diabetes mellitus | 27 (26%) |
| Data Partner type | |
| Insurer/claims-based | 83 (81%) |
| Integrated healthcare delivery system | 20 (19%) |
Possible indications and cardiovascular risk factors were assessed using diagnoses and procedures recorded in administrative data during the 183 days prior to the patient’s IGIV treatment date. The indication indicator variables are not mutually exclusive, so indication percentages may sum to greater than 100%.
The data presented in this paper were collected as part of a public health surveillance activity conducted under the auspices of the FDA Sentinel Initiative. For this reason, the collection and analysis of these data did not qualify as human subjects research under the Common Rule and were not subject to institutional review board (IRB) review.20–22
Case identification and chart retrieval
The endpoint definition used to identify potential AMI cases included any International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis code of 410.x1 or 410.x0 that originated from an inpatient hospital encounter. Within the Sentinel Common Data Model (SCDM), diagnosis codes associated with inpatient encounters are categorized as principal, secondary, or “unable to classify” (i.e., position unspecified). These classifications reflect standard coding practices and the addition of a third category to accommodate heterogeneity across Sentinel Data Partners in how encounters and coding positions are defined. Under Uniform Hospital Discharge Data Set (UHDDS) guidelines used by U.S. hospitals and insurers,23 inpatient diagnoses are coded as follows:
Principal diagnosis: the condition established after study to be chiefly responsible for occasioning the admission of the patient to the hospital
Secondary diagnosis: a condition also present on admission, that developed during the hospital stay, or that influenced the care of the patient or length of stay
In the SDD, there are also position-unspecified diagnoses that cannot be classified as principal or secondary. These diagnosis codes may represent diagnoses originating from non-facility claims associated with an inpatient stay, e.g., a physician services claim submitted separately from the facility claim. Codes of this type generally come from claims-based Data Partners.
Eligible post-IVIG inpatient encounters with an AMI diagnosis code listed in any position (principal, secondary or unspecified) were selected for review. For each potential AMI case meeting study eligibility criteria, Sentinel Data Partners were asked to retrieve a medical chart corresponding to the encounter during which the AMI diagnosis was recorded. In this validation report, we restricted the denominator for our PPV calculations to the sub-sample of potential cases for which we received a chart that was sufficiently complete to determine whether an AMI occurred (Figure 1).
Figure 1.
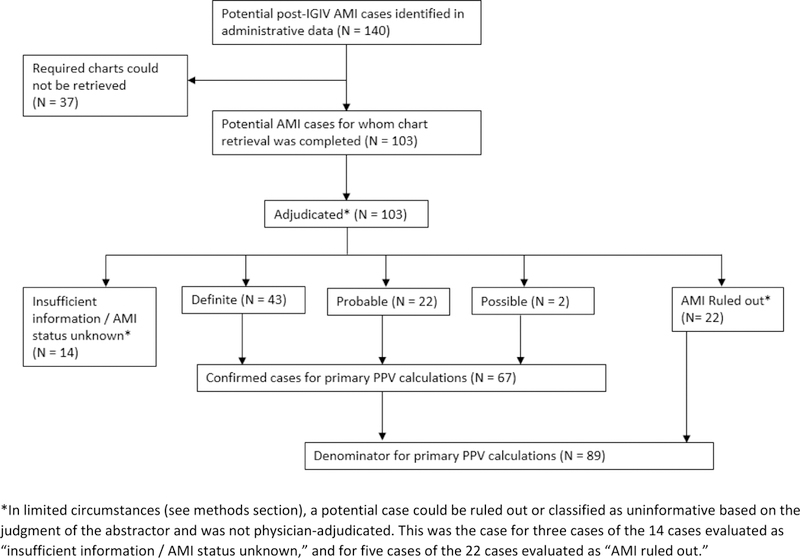
Disposition of potential acute myocardial infarction (AMI) cases identified in the SDD.
Chart abstraction
A trained nurse abstractor (L.P., K.P., A.N., or E.R) reviewed the medical chart(s) associated with the index AMI hospital encounter. The abstractors recorded information concerning symptom onset, relevant clinician notes, results from diagnostic testing including electrocardiograms, echocardiography, cardiac biomarkers, and cardiac catheterization reports, and other factors relevant for the IGIV safety assessment.
Case adjudication
Completed abstraction forms (and the original medical charts if needed) were reviewed by single a physician adjudicator with relevant clinical expertise (J.G.R., J.O.E., R.K. or S.G.). Based on the documentation available in the charts, potential cases were adjudicated as a definite, probable, or possible AMI, no AMI, or status unknown / insufficient information. Adjudication criteria, detailed in the Appendix, were adapted from the third universal definition of myocardial infarction developed under the auspices of the European Society of Cardiology, the American College of Cardiology Foundation, the American Heart Association, and the World Health Federation.24 In addition to the third universal definitions of definite and probable AMIs, we added the category of possible AMI to account for cases where parts of a patient’s medical chart were unavailable or illegible. If a case could not be counted as a definite or probable AMI or ruled out based on the information available in the chart, a case was classified as a possible AMI if there was a documented physician diagnosis of AMI; otherwise the patient was classified as AMI status unknown / insufficient information. The adjudication form, which was adapted from the Women’s Health Initiative AMI adjudication form,12 includes a full description of the adjudication criteria (see Appendix).
For a small number of potential cases, the chart(s) received contained no recorded diagnosis of an acute AMI, no indication that an acute AMI was considered as part of a differential diagnosis, no diagnostic testing, and no symptoms suggestive of a possible AMI. These cases were flagged by the abstractors and not reviewed by the physician adjudicators due to resource constraints. For these cases, if the chart(s) received included the discharge summary for the index AMI hospital encounter, the potential case was considered to have been miscoded and classified as no AMI. Otherwise the case was classified as having an unknown status due to chart incompleteness.
Positive predictive value (PPV) calculation
We calculated the PPV of the AMI diagnosis codes identified in the administrative data by dividing the number of confirmed AMI cases (definite, probable or possible) by the total number of cases for whom a sufficiently complete chart was obtained for the index AMI hospitalization. Potential cases adjudicated as unknown AMI status due to insufficient information were removed from the denominator for the PPV calculation (Figure 1). Exact binomial 95% confidence intervals (Clopper-Pearson) were calculated for the PPV estimates to quantify their precision.
RESULTS
One hundred forty potential post-IGIV AMI cases were identified in the SDD; required charts could be obtained for 103 (74%; see Figure 1). Common reasons that charts were unavailable included an inability to map the encounter record in the SDD to patient and provider identifiers required for chart requests, an inability to locate the medical chart corresponding to the requested encounter, and refusal by the healthcare provider. (See Appendix Table A1 for a complete list of reasons that charts were unobtainable.) Of the 103 cases for which charts were available, 83 were from claims-based Data Partners, and 20 from integrated care delivery systems. The median age of the patients was 65 years; 48% were female. Based on administrative diagnoses recorded during the six months prior, these patients had a high burden of pre-existing atherosclerotic cardiovascular disease (22% with prior AMI diagnosis; 39% with angina) and other major cardiovascular risk factors (75% with hypertension; 26% with diabetes). Additional descriptive information on these patients is provided in Table 1.
Outcome status could be determined for 89 potential AMI cases, of which 67 were confirmed by physician adjudicators (43 definite, 22 probable and 2 possible AMIs; see Figure 1). The PPVs for the inpatient AMI diagnoses recorded in the administrative data were 75% overall (67/89, 95% CI: 65–84%), 93% (28/30, 95% CI: 78–99%) for principal-position diagnoses, 88% (29/33, 95% CI: 72–97%) for secondary diagnoses, and 38% (10/26, 95% CI: 20–59%) for position-unspecified diagnoses. PPVs were higher for 410.x1 diagnosis codes (which denote an “initial episode of care” for AMI) than for 410.x0 codes (“episode of care unspecified”). Detailed PPV estimates stratified by coding position, ICD-9-CM diagnosis code, Data Partner type, and prior AMI diagnosis are provided in Table 2.
Table 2.
Positive predictive values (PPVs)* associated with inpatient administrative diagnosis codes for acute myocardial infarction (AMI) by position.
| PPVs for all potential AMI cases (N = 89) | PPVs for principal position AMI diagnoses (N = 30) | PPVs for secondary AMI diagnoses (N = 33) | PPVs for position-unspecified** AMI diagnoses (N = 26) | |
|---|---|---|---|---|
| All AMI codes | 75% (67/89, 95% CI: 65–84%) | 93% (28/30, 95% CI: 78–99%) | 88% (29/33, 95% CI: 72–97%) | 38% (10/26, 95% CI: 20–59%) |
| By diagnosis code recorded in administrative data | ||||
| 410.x0 | 33% (5/15, 95% CI: 12–62%) | 100% (1/1, 95% CI: 3–100%) | 33% (1/3, 95% CI: 1–91%) | 27% (3/11, 95% CI: 6–61%) |
| 410.x1 | 84% (62/74, 95% CI: 73–91%) | 93% (27/29, 95% CI: 77–99%) | 93% (28/30, 95% CI: 78–99%) | 47% (7/15, 95% CI: 21–73%) |
| Data Partner type | ||||
| Insurer/claims-based | 71% (49/69, 95% CI: 59–81%) | 92% (22/24, 95% CI: 73–99%) | 89% (17/19, 95% CI: 67–99%) | 38% (10/26, 95% CI: 20–59%) |
| Integrated care delivery systems | 90% (18/20, 95% CI: 68–99%) | 100% (6/6, 95% CI: 54–100%) | 86% (12/14, 95% CI: 57–98%) | -- |
| By whether an AMI code was observed in prior 183 days | ||||
| No prior AMI | 78% (54/69, 95% CI: 67–87%) | 92% (23/25, 95% CI: 74–99%) | 96% (22/23, 95% CI: 78–100%) | 43% (9/21, 95% CI: 22–66%) |
| Prior AMI | 65% (13/20, 95% CI: 41–85%) | 100% (5/5, 95% CI: 48–100%) | 70% (7/10, 95% CI: 35–93%) | 20% (1/5, 95% CI: 1–72%) |
Statistics reported in this table reflect the PPV of administrative ICD-9-CM AMI diagnosis codes for a confirmed (definite, probable, or possible) acute AMI. Patients with a classification of insufficient information / acute TEE status unknown were removed from the denominator for the PPV calculations and not included in this table.
Within the Sentinel Distributed Database, a position-unspecified inpatient diagnosis is a non-facility professional/provider claim associated with an inpatient encounter.
While data on suspected AMI etiology were not collected systematically during the adjudication process, the adjudicators noted that a substantial number of the confirmed AMIs (46%) were likely to be type 2 AMIs (i.e., attributable to demand ischemia).24 The majority of these type 2 AMIs occurred in the setting of anemia, respiratory insufficiency, and/or septic shock. Such conditions are more common in certain IVIG user subgroups than in the general population. Demand ischemia was the suspected etiology in 21%, 72%, and 40% of confirmed AMIs with principal, secondary, and unspecified coding positions, respectively.
DISCUSSION
In this chart validation study, which relied on data from a protocol-based assessment of the risk of thromboembolic events following IGIV treatment,19 we evaluated the validity of inpatient administrative diagnosis codes for AMI within the SDD. PPVs were high for principal (93%) and secondary diagnoses (88%), and lower for position-unspecified diagnoses (38%). The PPV estimates from our study are broadly consistent with those reported in prior chart validation studies conducted in the U.S. and Canada over the last two decades.2–11
Previous validation studies have reported that principal hospital discharge diagnoses of AMI are associated with PPVs of 80–95%.2–10 Between-study variation in these estimates may be attributable to differences in coding algorithms used to identify potential cases, chart validation criteria, patient populations, and coding practices. In an earlier SDD validation study, chart validation was conducted for a random sample of 153 patients with principal inpatient diagnoses of AMI (ICD-9-CM 410.x0 or 410.x1) drawn from four Sentinel Data Partners.11 They reported a PPV of 86% (95% CI: 79–91%), slightly lower than our estimate of 93% (95% CI: 78–99%) for principal-position diagnoses. In their study, cases with insufficient chart information were counted as false positives rather than as missing data. If these cases were removed from the PPV denominator, as was done in our calculations, their PPV estimate would be 95%, closer to ours.
The main contribution of our study to the existing literature was to provide PPV estimates for secondary and position-unspecified inpatient AMI diagnosis codes recorded in the SDD. Relative to principal diagnoses, PPVs were only slightly lower for secondary diagnoses (88%) in our sample. However, it was noteworthy that confirmed AMIs coded as secondary diagnoses were more likely to be type 2 AMIs (72%) than were AMIs coded as principal diagnoses (21%). This difference can likely be attributed to the greater severity and complexity of illness among hospitalized patients, as secondary diagnoses may represent conditions that develop during a hospital stay.23 As noted before, type 2 AMIs may be more common in our sample of IGIV users due to the higher prevalence of hematopoietic dysfunction, infection and sepsis among some IGIV patient subgroups.
PPVs were significantly lower for position-unspecified diagnoses (38%). In our study, restricting to principal and secondary AMI diagnoses would have improved the overall PPV of the endpoint definition from 75% to 90%, at the cost of missing 10 of 67 confirmed AMIs (15%). In future studies of AMI where chart confirmation of outcome is not possible, investigators may consider excluding position-unspecified diagnosis codes from their endpoint definitions after weighing the tradeoff between sensitivity and a higher PPV.
A limitation of our study was that medical charts were unobtainable for 26% of potential post-IGIV cases identified in the SDD. However, the typical reasons that charts were unavailable (e.g., unable to link SDD records to patient or provider identifiers) did not give us reason to suspect that our analyzable sample was systematically different than the total set of potential cases identified. Another limitation—referred to above in the discussion of the high prevalence of type 2 AMIs—is that our sample was limited to patients receiving IGIV, and thus may not be representative of the total population of health plan beneficiaries included in the SDD. An additional limitation of our study was that each case was reviewed by a single physician due to cost constraints; a number of prior validation studies have had each case reviewed independently by two physician-adjudicators. This may have reduced the accuracy associated with the clinical diagnoses that served as the gold standard in our study.
Combined with findings from earlier research, our results indicate that inpatient diagnosis codes can be used for the identification of AMI within the SDD. While PPVs for both principal and secondary diagnosis codes are high, investigators should be aware that the latter may often represent AMIs arising in hospitalized, complex patients due to causes other than an intracoronary thrombus.
Supplementary Material
Key points:
The PPV for principal AMI diagnoses was 93% (95% CI: 78–99%)
The PPV for secondary AMI diagnoses was 88% (95% CI: 72–97%)
The PPV for position-unknown diagnoses was 38% (95% CI: 20–59%)
Secondary and position-unknown diagnoses were more often due to demand ischemia
AMI PPVs were similar to estimates from other studies
Acknowledgements:
We thank contributors from the University of Iowa (Angela Overton, Erin Rindels, Michael Mueller, Nicholas Rudzianski, and James Torner), Harvard Medical School and Harvard Pilgrim Health Care Institute (Madelyn Pimentel, Meghan Baker, and Casey Covarrubias), Telligen- West Des Moines (Lois Pedelty and Kim Price), and Kaiser Permanente (Bruce Fireman). The results reported herein correspond to the objectives of Mini-Sentinel contract HHSF223200910006I from the U.S. Food and Drug Administration (FDA) and Department of Health and Human Services (HHS). This work was also supported by the Sentinel Coordinating Center, which is funded by the FDA through HHS contract number HHSF223201400030I.
Sponsors/Grant Numbers: The results reported herein correspond to the objectives of Mini-Sentinel contract HHSF223200910006I from the U.S. Food and Drug Administration (FDA) and Department of Health and Human Services (HHS). This work was also supported by the Sentinel Coordinating Center, which is funded by the FDA through HHS contract number HHSF223201400030I.
Footnotes
Prior Postings and Presentation: As with the study protocol, the final study report—a non-peer-reviewed document—on intravenous immune globulin and thromboembolic adverse events will be posted to the Sentinel Initiative website. In addition, a high-level overview of these results was presented at ICPE 2017.
References
- 1.Snapshot of Database Statistics Sentinel Coordinating Center, 2017. (Accessed September 10, 2017, at https://www.sentinelinitiative.org/sentinel/snapshot-database-statistics.) [Google Scholar]
- 2.Wahl PM, Rodgers K, Schneeweiss S, et al. Validation of claims-based diagnostic and procedure codes for cardiovascular and gastrointestinal serious adverse events in a commercially-insured population. Pharmacoepidemiol Drug Saf 2010;19:596–603. [DOI] [PubMed] [Google Scholar]
- 3.McCormick N, Lacaille D, Bhole V, Avina-Zubieta JA. Validity of myocardial infarction diagnoses in administrative databases: a systematic review. PLoS ONE 2014;9:e92286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burwen DR, Galusha DH, Lewis JM, et al. National and state trends in quality of care for acute myocardial infarction between 1994–1995 and 1998–1999: the medicare health care quality improvement program. Arch Intern Med 2003;163:1430–9. [DOI] [PubMed] [Google Scholar]
- 5.Rosamond WD, Chambless LE, Sorlie PD, et al. Trends in the sensitivity, positive predictive value, false-positive rate, and comparability ratio of hospital discharge diagnosis codes for acute myocardial infarction in four US communities, 1987–2000. Am J Epidemiol 2004;160:1137–46. [DOI] [PubMed] [Google Scholar]
- 6.Varas-Lorenzo C, Castellsague J, Stang MR, Tomas L, Aguado J, Perez-Gutthann S. Positive predictive value of ICD-9 codes 410 and 411 in the identification of cases of acute coronary syndromes in the Saskatchewan Hospital automated database. Pharmacoepidemiol Drug Saf 2008;17:842–52. [DOI] [PubMed] [Google Scholar]
- 7.Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J 2004;148:99–104. [DOI] [PubMed] [Google Scholar]
- 8.Heckbert SR, Kooperberg C, Safford MM, et al. Comparison of self-report, hospital discharge codes, and adjudication of cardiovascular events in the Women’s Health Initiative. Am J Epidemiol 2004;160:1152–8. [DOI] [PubMed] [Google Scholar]
- 9.Petersen LA, Wright S, Normand SL, Daley J. Positive predictive value of the diagnosis of acute myocardial infarction in an administrative database. J Gen Intern Med 1999;14:555–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Metcalfe A, Neudam A, Forde S, et al. Case definitions for acute myocardial infarction in administrative databases and their impact on in-hospital mortality rates. Health Serv Res 2013;48:290–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cutrona SL, Toh S, Iyer A, et al. Validation of acute myocardial infarction in the Food and Drug Administration’s Mini-Sentinel program. Pharmacoepidemiol Drug Saf 2013;22:40–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hlatky MA, Ray RM, Burwen DR, et al. Use of Medicare data to identify coronary heart disease outcomes in the Women’s Health Initiative. Circ Cardiovasc Qual Outcomes 2014;7:157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng CL, Lee CH, Chen PS, Li YH, Lin SJ, Yang YH. Validation of acute myocardial infarction cases in the national health insurance research database in taiwan. J Epidemiol 2014;24:500–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrett E, Shah AD, Boggon R, et al. Completeness and diagnostic validity of recording acute myocardial infarction events in primary care, hospital care, disease registry, and national mortality records: cohort study. BMJ 2013;346:f2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Austin PC, Daly PA, Tu JV. A multicenter study of the coding accuracy of hospital discharge administrative data for patients admitted to cardiac care units in Ontario. Am Heart J 2002;144:290–6. [DOI] [PubMed] [Google Scholar]
- 16.Madsen M, Davidsen M, Rasmussen S, Abildstrom SZ, Osler M. The validity of the diagnosis of acute myocardial infarction in routine statistics: A comparison of mortality and hospital discharge data with the Danish MONICA registry. J Clin Epidemiol 2003;56:124–30. [DOI] [PubMed] [Google Scholar]
- 17.Mini-Sentinel Assessment Protocol: Thromboembolic Events After Immunoglobulin Administration: Version 2.0. Mini-Sentinel Coordinating Center, 2014. (Accessed 20 August 2014, at http://www.mini-sentinel.org/work_products/Assessments/Mini-Sentinel_Thromboembolic-Events-After-Immunoglobulin-Administration-Protocol.pdf.)
- 18.Orange JS, Hossny EM, Weiler CR, et al. Use of intravenous immunoglobulin in human disease: a review of evidence by members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol 2006;117:S525–53. [DOI] [PubMed] [Google Scholar]
- 19.Mini-Sentinel Assessment Protocol: Thromboembolic Events After Immunoglobulin Administration: Version 3.0. Mini-Sentinel Coordinating Center, 2015. (Accessed September 10, 2017, at https://www.sentinelinitiative.org/sites/default/files/Drugs/Assessments/Mini-Sentinel_Thromboembolic-Events-After-Immunoglobulin-Administration-Protocol_0.pdf.)
- 20.McGraw D, Rosati K, Evans B. A policy framework for public health uses of electronic health data. Pharmacoepidemiol Drug Saf 2012;21 Suppl 1:18–22. [DOI] [PubMed] [Google Scholar]
- 21.Rosati K, Evans B, McGraw D. HIPAA and Common Rule Compliance in the Mini-Sentinel Pilot Unpublished White Paper. 2012.
- 22.Forrow S, Campion DM, Herrinton LJ, et al. The organizational structure and governing principles of the Food and Drug Administration’s Mini-Sentinel pilot program. Pharmacoepidemiol Drug Saf 2012;21 Suppl 1:12–7. [DOI] [PubMed] [Google Scholar]
- 23.Health information policy council; 1984 revision of the Uniform Hospital Discharge Data Set--HHS. Notice. Fed Regist 1985;50:31038–40. [PubMed] [Google Scholar]
- 24.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD. Third universal definition of myocardial infarction. Glob Heart 2012;7:275–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


