Abstract
Background:
Associations among inflammatory cytokines, erythropoietin, and anemia in critically ill septic patients remain unclear. This study tested the hypothesis that elevated inflammatory cytokines and decreased erythropoietin would be associated with iron-restricted anemia while accounting for operative blood loss, phlebotomy blood loss, and red blood cell (RBC) transfusion volume.
Methods:
Prospective observational cohort study of 42 critically ill septic patients. Hemoglobin (Hb) at sepsis onset and hospital discharge were used to calculate ΔHb. Operative blood loos, phlebotomy blood loss, and RBC transfusion volume were used to calculate adjusted ΔHb (AdjΔHb) assuming 300 mL RBC = 1 g/dL Hb. Patients with AdjΔHb >0 (+AdjΔHb, n=18) were compared to patients with AdjΔHb ≤0 (−AdjΔHb, n=24).
Results:
Plasma TNF-alpha, G-CSF, IL-6, IL-8, and erythropoietin, erythrocyte mean corpuscular volume (MCV), and serum transferrin receptor (sTfR) were measured on days 0, 1, 4, 7, and 14. Patients with −AdjΔHb had significantly higher day 14 levels of IL-6 (37.4 vs. 15.2 pg/mL, p<0.05), IL-8 (39.1 vs. 18.2 pg/mL, p=0.01), and G-CSF (101.3 vs. 60.5 pg/mL, p=0.01), but not erythropoietin. On linear regression analysis, lower AdjΔHb was associated with higher day 14 levels of IL-6 (r2=0.22, p<0.01), IL-8 (r2=0.10, p=0.04), SDF-1 (r2=0.14, p=0.02), and TNF-alpha (r2=0.13, p=0.02), but not erythropoietin. Patients with −AdjΔHb had significantly lower MCV on days 4 (89.6 vs. 93.2 fL/cell, p=0.04), 7 (92.3 vs. 94.9 fL/cell, p=0.04), and 14 (92.1 vs. 96.0 fL/cell, p=0.03), but similar sTfR levels.
Conclusions:
Persistent elevation of inflammatory cytokines was associated with iron-restricted anemia among critically ill septic patients, occurring in the absence of systemic iron deficiency, independent of endogenous erythropoietin.
Level of Evidence:
Level II, Prognostic study
Keywords: Inflammation, erythropoietin, anemia, sepsis, critical illness
Introduction
Anemia affects approximately 95% of all patients who remain critically ill for three or more days (1). Management of severe anemia often involves red blood cell (RBC) transfusion, which places patients at increased risk for infectious complications and mortality. Etiologies of anemia among intensive care unit (ICU) patients include decreased RBC survival, blood loss, dysregulated iron metabolism, and bone marrow suppression (2–5). Increased erythropoietin (EPO) production is an important compensatory mechanism, promoting erythropoiesis in response to anemia and hypoxic stress. Exogenous erythropoietin administration has been investigated as therapeutic strategy for the management of critically ill anemic subjects, with mixed results in large randomized trials (6, 7). Sepsis is associated with increased circulating EPO levels compared to healthy control values, but ICU patients have a smaller increase in EPO than healthy subjects with the same degree of anemia, implicating a blunted EPO response in the pathophysiology of anemia of critical illness.
Several cytokines affect erythropoiesis and EPO physiology. Interleukins 6 and 8 (IL-6 and IL-8) influence erythropoiesis by modulating hepcidin and iron metabolism. Exogenous granulocyte colony-stimulating factor (G-CSF) potentiates anemia among breast cancer patients receiving adjuvant chemotherapy, and elevated G-CSF is associated with persistent anemia following severe traumatic injury in rats and humans (8–10). Plasma stromal cell-derived factor 1 (SDF-1, also known as CXCL12) is elevated following traumatic injury in rodents and humans, and promotes mobilization of hematopoietic progenitors from the bone marrow to sites of injury (11, 12). In vitro and in vivo models have implicated the inflammatory cytokine tumor necrosis factor (TNF) alpha in suppression of EPO and erythropoiesis (13, 14). These cytokines have the propensity to promote iron-restricted erythropoiesis, characterized by functional rather than absolute iron deficiency, occurring secondary to dysregulation of iron metabolism and trafficking.
Associations among inflammatory cytokines, erythropoietin (EPO), and anemia in critically ill septic patients remain unclear. Analysis of these associations in critically ill patients is confounded by the effects of operative blood loss (OBL), phlebotomy blood loss (PBL), and RBC transfusions on hemoglobin (Hb) levels. The purpose of this study was to investigate associations among IL-6, IL-8, and G-CSF, SDF-1, TNF-alpha, and EPO on anemia among critically ill septic patients. We hypothesized that high levels of inflammatory cytokines and decreased EPO would be associated with persistent anemia among critically ill septic patients when adjusting Hb levels for the effects of OBL, PBL, and RBC transfusions, and that critically ill septic patients would exhibit an iron-restricted anemia phenotype.
Methods
We performed a prospective observational cohort analysis of 42 adult (age ≥18 years) patients treated for sepsis, as defined below, in our surgical intensive care units. This study was registered on ClinicalTrials.gov (NCT02276417). Institutional Review Board approval was obtained. All study subjects signed written informed consent. Patients were identified by a modified version of the MEWS early-detection protocol which screened for sepsis based on temperature, heart rate, respiratory rate, blood pressure, and level of consciousness (15). Patients identified by this screening protocol were then assessed for the presence of severe sepsis (sepsis-induced tissue hypoperfusion or organ dysfunction) or septic shock (severe sepsis with persistent arterial hypotension despite volume resuscitation) based on consensus definitions (16–18). Resuscitation and management strategies were standardized according to a computerized clinical decision support protocol (19, 20). Patients with unmeasured blood loss (e.g. traumatic injury, gastrointestinal bleeding, and postoperative hemorrhage) were excluded. Patients with total hospital length of stay <14 days were excluded to ensure that data would not be censored due to patient discharge. Additional exclusion criteria were age <18 years, transfer following outside facility length of stay >2 days, severe traumatic brain injury (CT evidence of neurologic injury and Glasgow Coma Scale score <8), spinal cord injury resulting in permanent sensory and/or motor deficits, sepsis with an uncontrollable source (e.g. unresectable bowel ischemia), NY Heart Association class IV heart failure, Child-Pugh Class B or C liver disease, known HIV infection with CD4 count <200 cells/mm3, organ transplant recipient on an immunosuppressant agent, administration of exogenous EPO within 30 days prior to onset of sepsis or during admission, chemotherapy or radiotherapy within 30 days prior to onset of sepsis, expected lifespan <3 months due to severe pre-existing comorbidities, active Do Not Resuscitate or Do Not Intubate order, pregnancy, incarceration, or institutionalization.
Data regarding baseline patient characteristics, management, and outcomes for each study subject were collected and managed using the REDCap electronic data capture platform (RedCap, Nashville, TN). For all parameters, time zero was recognition of sepsis and initiation of the computerized clinical decision support protocol. Plasma IL-6, IL-8, G-CSF, SDF-1, TNF-alpha, and EPO levels were determined by enzyme linked immunosorbent assay (R&D Systems, Minneapolis, MN) and Luminex (Merck Millipore, Darmstadt, Germany and Luminex Corporation, Madison, WI) performed on samples obtained within 12 hours and on days 1, 4, 7, and 14. Hb and MCV were measured at the same time points. MCV was included in the analysis because unlike many other biomarker used in this study, MCV is measured on standard complete blood count laboratory tests, and therefore readily available to clinicians. Among healthy subjects, MCV equilibrates within approximately six hours of red blood cell transfusion. Serum TfR levels were measured by enzyme linked immunosorbent assay (R&D Systems) within 12 hours and on day 14. Unlike ferritin, soluble transferrin receptor is not an acute-phase reactant, and may be the most reliable marker of iron deficiency during critical illness. sTfR increases during iron deficiency anemia and remains normal during anemia due to inflammation (21, 22). Serum iron was not measured because its diagnostic utility is limited by variability in protein-binding properties and bioavailability during inflammatory states (23).
Adjusted ΔHb was calculated as previously described (24). Hb levels at sepsis onset and hospital discharge were used to calculate change in Hb (ΔHb) from sepsis onset to hospital discharge for each patient. To account for the effects of OBL, PBL, and PRBC transfusions on Hb levels, each of these variables were recorded for each patient. To estimate PBL, the amount of blood drawn for each commonly performed laboratory test was determined. At our institution during the study period, basic metabolic panels and complete blood counts contained 5–7 mL of blood, arterial blood gases contained 2 mL of blood, and blood cultures contained 8–10 mL of blood. Next, the number of laboratory tests performed daily for ICU and floor patients and associated PBL was ascertained from previous reports (3, 25–27). Based on these parameters, phlebotomy blood loss was calculated by multiplying the first 30 ICU days by 55 mL/day, multiplying all additional ICU days by 13 mL/day, multiplying the number of ICU-free days by 9 mL/day, and adding these three values. OBL was obtained from operative notes in the electronic health record. At our institution, general indications for red cell transfusion are Hb < 7 g/dL or hematocrit < 21% for patients with no symptomatic cardiovascular disease, Hb < 10 g/dL or hematocrit < 30% for patients with symptomatic cardiovascular disease, and acute blood loss > 30% of total blood volume. OBL, PBL, and PRBC transfusion volume were recorded in mL. Each 300 mL change in blood volume was expressed as a 1 g/dL change in Hb (e.g., 600 mL blood loss = Hb decrease by 2 g/dL, 900 mL PRBC transfusion = Hb increase by 3 g/dL) based on previous work (28–31). ΔHb was then adjusted to account for the effects of OBL, PBL, and PRBC transfusion volume.
Statistical analysis and figure production were performed in SPSS (version 24, IBM, Armonk, NY) and GraphPad Prism (version 6.05, GraphPad Software, La Jolla, CA). A power analysis was not performed as this was an exploratory study. Base deficit and pH values were missing at random (MAR) for one patient (5%). Base deficit and pH were analyzed using the available data without imputing missing values. Missing data and outliers identified by the ROUT method for each plasma and serum analyte at each time point are illustrated in Supplemental Table (see Table, Supplemental Digital Content 1, http://links.lww.com/TA/B246). Continuous variables were compared by the Kruskal-Wallis test and reported as median [interquartile range]. Discrete variables were compared by Fisher’s Exact test and reported as n (%). Linear regression was performed to assess relationships between day 14 cytokine levels and adjusted ΔHb, reported as r2 coefficients. Significance was set at α = 0.05.
Results
Forty-two patients were included. The study population had advanced age (65 years) and a substantial burden of chronic disease (Charlson comorbidity index 4.5) (Table 1). The most common primary diagnosis was intraabdominal sepsis, affecting half of the study population. Comparing patients with positive versus negative adjusted ΔHb, there were no significant differences in age, sex, chronic disease burden, primary diagnosis, or APACHE II scores at the time of sepsis onset. However, patients with negative adjusted ΔHb had significantly lower core body temperature (36.9 vs. 37.4°C, p = 0.032) at the time of sepsis onset.
Table 1:
Characteristics of the study population.
| Patient characteristics | +AdjΔHb n = 18 | −AdjΔHb n = 24 | p |
|---|---|---|---|
| Age (years) | 68 [62–74] | 62 [53–70] | 0.091 |
| Male | 12 (67%) | 14 (58%) | 0.750 |
| Charlson comorbidity index | 5.0 [3.0–7.0] | 4.0 [2.3–7.8] | 0.848 |
| Primary diagnosis | |||
| Intraabdominal sepsis | 8 (44%) | 13 (54%) | 0.756 |
| Necrotizing soft tissue infection | 5 (28%) | 3 (13%) | 0.256 |
| Pneumonia | 5 (28%) | 3 (13%) | 0.256 |
| Bloodstream infection | 1 (6%) | 4 (17%) | 0.371 |
| Pyelonephritis | 1 (6%) | 3 (13%) | 0.623 |
| Surgical site infection | 2 (11%) | 2 (8%) | >0.999 |
| Tracheobronchitis | 1 (6%) | 1 (4%) | >0.999 |
| Empyema | 0 (0%) | 1 (4%) | >0.999 |
| Extremity wet gangrene | 0 (0%) | 1 (4%) | >0.999 |
| Mediastinitis | 1 (6%) | 0 (0%) | 0.429 |
| Multiple sources | 6 (33%) | 7 (29%) | >0.999 |
| Core body temperature (°C) | 37.4 [36.8–38.7] | 36.9 [36.5–37.3] | 0.032 |
| Heart rate | 106 [98–112] | 111 [103–118] | 0.263 |
| Systolic blood pressure (mmHg) | 110 [96–122] | 108 [93–131] | 0.899 |
| Mean arterial pressure (mmHg) | 71 [61–81] | 75 [62–91] | 0.334 |
| White blood cell count (x109/L) | 12.7 [9.0–17.0] | 14.0 [6.9–18.5] | 0.542 |
| Arterial pH | 7.30 [7.25–7.39] | 7.31 [7.24–7.38] | 0.693 |
| Base deficit (mEq/L) | 3.8 [0.5–5.4] | 3.5 [1.7–8.0] | 0.479 |
| Hemoglobin (mg/dL) | 10.1 [7.9–11.8] | 11.4 [8.6–12.6] | 0.231 |
| Creatinine (mg/dL) | 1.0 [0.8–1.5] | 1.0 [0.6–1.6] | 0.829 |
| APACHE II score | 13 [10–18] | 14 [8–21] | 0.809 |
AdjΔHb: difference in hemoglobin (Hb) from sepsis onset to discharge adjusted for operative blood loss, phlebotomy blood loss, and red cell transfusions, assuming 300 mL red cells = 1 g/dL Hb; APACHE: Acute Physiology and Chronic Health Evaluation. Data are presented as median [interquartile range] or n (%).
Management and outcome parameters are listed in Table 2. Sixty-seven percent of all patients required a source control procedure, without significant differences between cohorts. Operative blood loss, phlebotomy blood loss, and RBC transfusion burden were not significantly different between cohorts, though discharge hemoglobin was significantly lower in the negative adjusted ΔHb cohort (8.4 vs. 9.3 g/dL, p = 0.001). There were two patients in the negative adjusted ΔHb group who developed new dialysis requirements during admission, and no patients in the positive adjusted ΔHb group who developed new dialysis requirements (p = 0.247). Median hospital length of stay was 23 days, with 13 of these days spent in the ICU, and no significant differences between cohorts. Only 15 out of 42 patients (36%) were discharged to their home. There was one inpatient mortality (2%), which occurred in the negative adjusted ΔHb cohort.
Table 2:
Summary of management and outcome parameters.
| Management and outcomes | +AdjΔHb n = 18 | −AdjΔHb n = 24 | p |
|---|---|---|---|
| During admission | |||
| Source control procedure | 11 (61%) | 17 (71%) | 0.530 |
| Number of operations | 1.0 [1.0–2.0] | 2.0 [1.0–3.0] | 0.116 |
| Operative blood loss (mL) | 120 [9–530] | 258 [28–821] | 0.401 |
| Phlebotomy blood loss (mL) | 789 [537–1,115] | 765 [535–1,138] | 0.939 |
| Red blood cell transfusions | 1.0 [0.0–2.0] | 2.5 [0.0–6.5] | 0.137 |
| Discharge hemoglobin (g/dL) | 9.3 [8.5–10.6] | 8.4 [7.6–8.9] | 0.001 |
| Hospital length of stay (days) | 23 [22–35] | 24 [17–30] | 0.731 |
| ICU length of stay (days) | 13 [6–18] | 12 [7–19] | 0.629 |
| Discharge disposition | |||
| Home | 6 (33%) | 9 (38%) | >0.999 |
| Skilled nursing facility | 6 (33%) | 4 (17%) | 0.281 |
| Inpatient rehabilitation | 1 (6%) | 1 (4%) | >0.999 |
| Long term care facility | 3 (17%) | 8 (33%) | 0.299 |
| Hospice | 2 (11%) | 1 (4%) | 0.567 |
| Inpatient mortality | 0 (0%) | 1 (4%) | >0.999 |
AdjΔHb: difference in hemoglobin (Hb) from sepsis onset to discharge adjusted for operative blood loss, phlebotomy blood loss, and red cell transfusions, assuming 300 mL red cells = 1 g/dL Hb; ICU: intensive care unit. Data are presented as n (%) or median [interquartile range].
The strongest associations between cytokine levels and adjusted ΔHb occurred on day 14, as illustrated in Figure 1. Patients with negative adjusted ΔHb had higher day 14 levels of IL-8 (39.1 vs. 18.2 pg/mL, p=0.01), and G-CSF (101.3 vs. 60.5 pg/mL, p=0.01). The negative adjusted ΔHb cohort also had higher levels of day 14 TNF-alpha (22.8 vs. 13.5 pg/mL), though the difference was not statistically significant (p = 0.12). Day 14 EPO was similar between negative and positive adjusted ΔHb cohorts (27.1 vs. 38.5 mIU/mL, respectively, p=0.79).
Figure 1:
Trends in cytokines that influence erythropoiesis measured in the plasma following recognition of sepsis an initiation of a standardized treatment protocol. Initial and discharge hemoglobin were used to calculate Δhemoglobin (ΔHb). Operative blood loss, phlebotomy blood loss, and red blood cell (RBC) transfusion volume were used to calculate adjusted ΔHb (AdjΔHb) assuming 300 mL RBC = 1 g/dL Hb. Values for patients with negative (−) and positive (+) AdjΔHb were compared by the Kruskal-Wallis test, *p < 0.05. IL-6: interleukin 6, IL-8: interleukin 8, G-CSF: granulocyte colony-stimulating factor, SDF-1: stromal cell-derived factor 1, TNF-alpha: tumor necrosis factor alpha, EPO: erythropoietin. Dark gray: negative adjusted ΔHb; light gray: positive adjusted ΔHb.
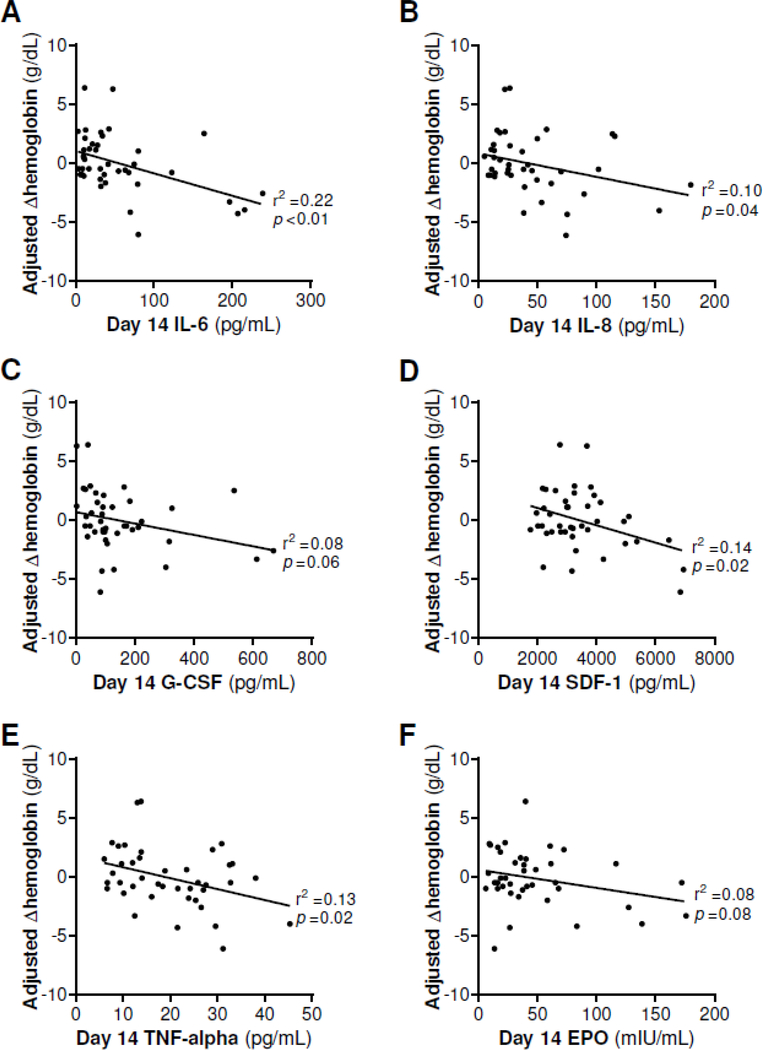
Linear regression was performed to assess the relationship between day 14 cytokine levels and adjusted ΔHb as a continuous variable, as illustrated in Figure 2. Lower AdjΔHb was associated with higher day 14 levels of IL-6 (r2 = 0.22, p < 0.01), IL-8 (r2 = 0.10, p = 0.04), SDF1 (r2 = 0.14, p = 0.02), and TNF-alpha (r2 = 0.13, p = 0.02). However, there was no significant relationship between day 14 EPO and adjusted ΔHb (r2 = 0.08, p = 0.08).
Figure 2:
Cytokines that influence erythropoiesis measured in plasma 14 days after recognition of sepsis an initiation of a standardized treatment protocol. Initial and discharge hemoglobin were used to calculate Δhemoglobin (ΔHb). Operative blood loss, phlebotomy blood loss, and red blood cell (RBC) transfusion volume were used to calculate adjusted ΔHb (AdjΔHb) assuming 300 mL RBC = 1 g/dL Hb. Linear regression was performed to calculate r2. IL-6: interleukin 6, IL-8: interleukin 8, G-CSF: granulocyte colony-stimulating factor, SDF-1: stromal cell-derived factor 1, TNF-alpha: tumor necrosis factor alpha, EPO: erythropoietin.
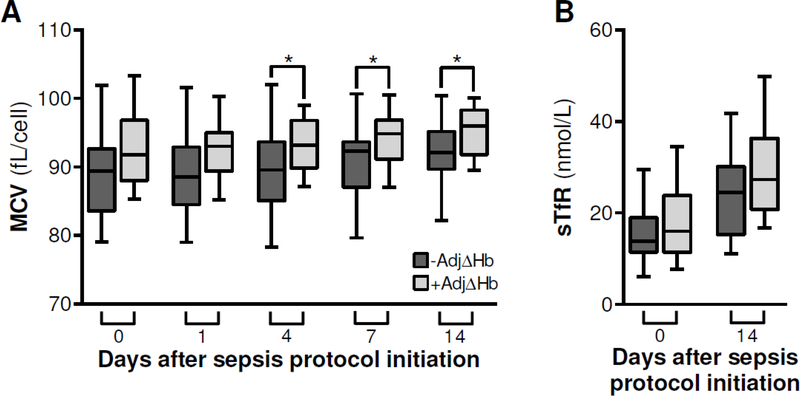
Analysis of MCV and sTfR are illustrated in Figure 3. MCV was initially similar between cohorts, but was significantly lower among patients with negative adjusted ΔHb on days four (89.6 vs. 93.2 fL/cell, p = 0.04), seven (92.3 vs. 94.9 fL/cell, p = 0.04), and 14 (92.1 vs. 96.0 fL/cell, p = 0.03) (Fig. 3A). sTfR levels were similar between cohorts at the time of sepsis protocol initiation and on day 14 (Fig. 3B).
Figure 3:
Trends in mean corpuscular volume (MCV) and serum transferrin receptor (sTfR) following recognition of sepsis an initiation of a standardized treatment protocol. Initial and discharge hemoglobin were used to calculate Δhemoglobin (ΔHb). Operative blood loss, phlebotomy blood loss, and red blood cell (RBC) transfusion volume were used to calculate adjusted ΔHb (AdjΔHb) assuming 300 mL RBC = 1 g/dL Hb. Values for patients with negative (−) and positive (+) AdjΔHb were compared by the Kruskal-Wallis test, *p < 0.05. Dark gray: negative adjusted ΔHb; light gray: positive adjusted ΔHb.
Discussion
In this study, persistent inflammation following septic insult was associated with persistent anemia, independent of EPO levels. Patients with negative adjusted ΔHb developed a microcytic anemia, implicating inflammatory iron-restricted erythropoiesis. sTfR was not significantly different between cohorts, indicating that systemic iron deficiency may not have been responsible for the observed microcytic anemia. Although most clinical parameters were similar between patients with positive and negative adjusted ΔHb, core body temperature at the time of sepsis onset was significantly lower in patients with negative adjusted delta Hb. Hypothermia has been previously associated with impaired erythropoiesis in animal and human studies (32–34). Although the difference in red cell transfusions between groups was not statistically significant, this may represent a false negative analysis attributable to small group sizes. Receipt of 1.0 unit versus 2.5 units of red cells impacts iron availability due to the relatively high concentrations of iron in red cell transfusions, and impacts cytokine profiles. The observation that MCV was lower among subjects with negative adjusted ΔHb despite receiving more red cell transfusions supports the hypothesis that anemia among septic patients is partially attributable to functional iron restriction.
Previous studies have established correlations between inflammatory cytokines and erythropoiesis. Wu et al. (35) demonstrated that plasma from severely injured patients suppresses hematopoietic progenitor cell growth in bone marrow from healthy subjects. The effects appeared to be mediated by the bone marrow stroma, consistent with in vitro studies by Fonseca et al. (35, 36). These effects also occurred early after injury and resolved quickly, suggesting that homeostasis was restored early following hemorrhage control and tissue repair, in contrast to the persistent inflammation often associated with sepsis. In vivo studies by Schubert et al. have demonstrated that anemia develops in mice subjected to septic peritonitis by cecal ligation and puncture (CLP) in both TNF-deficient and wild-type mice, suggesting that anemia following septic insult may not be mediated by TNF-alpha (37). However, several other in vitro and in vivo models have implicated TNF-alpha in suppression of erythropoiesis, consistent with our observations (13, 14). IL-6 and IL-8 may hinder erythropoiesis by modulating hepcidin and iron metabolism. The microcytic erythrocyte phenotype observed in our study is consistent with this hypothesis. Exogenous administration of G-CSF to breast cancer patients receiving adjuvant chemotherapy has been shown to worsen anemia in a dose-dependent manner (8). This effect may have been due to mobilization of erythrocyte progenitors from the bone marrow to the peripheral blood, a phenomenon which has been described among severely injured rodents and critically ill trauma patients (8, 10). Although use of G-CSF for septic patients with neutropenia is widely reported, associations between G-CSF and anemia among septic patients are not well defined.
The lack of correlation between EPO and persistent anemia in the present study may be due to blunting of the EPO response, which has been described during critical illness and sepsis. Several clinical trials have evaluated the efficacy of exogenous EPO for critically ill patients. However, the appropriate role of exogenous EPO in this setting remains controversial. In a large randomized multicenter trial (n = 1,302), exogenous EPO increased Hb levels and decreased RBC transfusion among critically ill patients (6). A subsequent trial of similar design (n = 1,460) substantiated the effects of EPO on Hb concentration but did not detect a difference in RBC transfusion requirements (7). Despite a significant increase in thrombotic events among patients receiving EPO in the second trial which may have been impacted by inconsistent provision of chemoprophylaxis for venous thromboembolic disease, both demonstrated survival advantages among trauma subpopulations. This finding may be attributable to non-hematologic properties of EPO, including anti-inflammatory properties and cytoprotective effects on apoptosis. However, a meta-analysis of nine randomized trials of EPO administration to a broader population of critically ill patients did not identify a significant decrease in mortality with exogenous EPO (OR 0.81, 95% CI 0.61–1.01) (38). In addition, inhibition of the erythropoietin receptor (EPO-R) has been shown to neutralize EPO activity and potentiate anemia (39). It remains plausible that downregulation of EPO-R is partly responsible for abrogating the beneficial effects of EPO among critically ill septic patients. Our findings suggest that EPO may not play a central role in the pathophysiology of anemia among critically ill septic patients.
This study was limited by a small number of subjects (n = 42), heterogeneous patient population, and limited generalizability due to single-institution design. Fortunately, the observed associations between cytokines and hematologic parameters were strong enough that significant effects were observed in this small sample, which would have been underpowered to detect subtle differences. However, analyses which were not statistically significant may represent type II errors with false negative findings, and should be interpreted with caution. Future investigations of anemia among critically septic patients should include patients who are not subjected to ongoing inflammation due to surgical procedures and should include assessment of EPO-R and hepcidin physiology as well as the presence of iron-deficient erythropoiesis by assessment of erythrocyte zinc protoporphyrin levels (40, 41). A deeper understanding of the pathophysiology of anemia among critically ill septic patients may promote the development and implementation of preventative and therapeutic measures targeting iron restriction secondary to persistent inflammation and dysregulated trafficking of available iron stores. Further investigation of the pathophysiology of this condition is especially important given the inconsistent results from trials of iron supplementation and hepcidin antagonism for abrogating anemia among critically ill patients. Because both anemia and red cell transfusion are associated with significantly increased risk for adverse events, mitigating anemia without the need for transfusion harbors the potential to improve outcomes among septic patients.
Conclusions
Among critically ill patients, persistent inflammation following septic insult was associated with microcytic anemia in the absence of systemic iron deficiency, independent of endogenous plasma EPO. These effects were observed while accounting for the effects of OBL, PBL, and RBC transfusions. Future research should focus on the roles of EPO-R and hepcidin in the pathophysiology of anemia among critically ill septic patients.
Supplementary Material
Acknowledgements
The authors thank Brian Bouverat, Jilliane Brakenridge, Ruth Davis, Jennifer Lanz, and the Sepsis and Critical Illness Research Center staff for their valuable contributions to this study.
This work was supported by P50 GM111152–01 awarded by the National Institute of General Medical Sciences (NIGMS). PAE was supported by R01 GM113945–01 from the NIGMS. AMM was supported by R01 GM105893–01A1 from the NIGMS. TJL, JCM, and JAS were supported by a post-graduate training grant (T32 GM-008721) in burns, trauma, and perioperative injury from the NIGMS.
Footnotes
This manuscript is not under consideration elsewhere and the authors have nothing to disclose.
References
- 1.Rodriguez RM, Corwin HL, Gettinger A, Corwin MJ, Gubler D, Pearl RG. Nutritional deficiencies and blunted erythropoietin response as causes of the anemia of critical illness. J Crit Care. 2001;16(1):36–41. [DOI] [PubMed] [Google Scholar]
- 2.Vincent JL, Baron JF, Reinhart K, Gattinoni L, Thijs L, Webb A, Meier-Hellmann A, Nollet G, Peres-Bota D, Investigators ABC. Anemia and blood transfusion in critically ill patients. JAMA. 2002;288(12):1499–507. [DOI] [PubMed] [Google Scholar]
- 3.Corwin HL, Parsonnet KC, Gettinger A. RBC transfusion in the ICU. Is there a reason? Chest. 1995;108(3):767–71. [DOI] [PubMed] [Google Scholar]
- 4.Rogiers P, Zhang H, Leeman M, Nagler J, Neels H, Melot C, Vincent JL. Erythropoietin response is blunted in critically ill patients. Intens Care Med. 1997;23(2):159–62. [DOI] [PubMed] [Google Scholar]
- 5.Elliot JM, Virankabutra T, Jones S, Tanudsintum S, Lipkin G, Todd S, Bion J. Erythropoietin mimics the acute phase response in critical illness. Crit Care. 2003;7(3):R35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corwin HL, Gettinger A, Pearl RG, Fink MP, Levy MM, Shapiro MJ, Corwin MJ, Colton T, Group EPOCCT. Efficacy of recombinant human erythropoietin in critically ill patients: a randomized controlled trial. JAMA. 2002;288(22):2827–35. [DOI] [PubMed] [Google Scholar]
- 7.Corwin HL, Gettinger A, Fabian TC, May A, Pearl RG, Heard S, An R, Bowers PJ, Burton P, Klausner MA, et al. Efficacy and safety of epoetin alfa in critically ill patients. N Engl J Med. 2007;357(10):965–76. [DOI] [PubMed] [Google Scholar]
- 8.Papaldo P, Ferretti G, Di Cosimo S, Giannarelli D, Marolla P, Lopez M, Cortesi E, Antimi M, Terzoli E, Carlini P, et al. Does granulocyte colony-stimulating factor worsen anemia in early breast cancer patients treated with epirubicin and cyclophosphamide? J Clin Oncol. 2006;24(19):3048–55. [DOI] [PubMed] [Google Scholar]
- 9.Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124(2):407–21. [DOI] [PubMed] [Google Scholar]
- 10.Bible LE, Pasupuleti LV, Alzate WD, Gore AV, Song KJ, Sifri ZC, Livingston DH, Mohr AM. Early propranolol administration to severely injured patients can improve bone marrow dysfunction. J Trauma Acute Care Surg. 2014;77(1):54–60; discussion 59–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kavanagh DP, Kalia N. Hematopoietic stem cell homing to injured tissues. Stem Cell Rev. 2011;7(3):672–82. [DOI] [PubMed] [Google Scholar]
- 12.Hannoush EJ, Sifri ZC, Elhassan IO, Mohr AM, Alzate WD, Offin M, Livingston DH. Impact of enhanced mobilization of bone marrow derived cells to site of injury. J Trauma. 2011;71(2):283–9; discussion 9–91. [DOI] [PubMed] [Google Scholar]
- 13.Jelkmann W, Pagel H, Wolff M, Fandrey J. Monokines inhibiting erythropoietin production in human hepatoma cultures and in isolated perfused rat kidneys. Life Sci. 1992;50(4):301–8. [DOI] [PubMed] [Google Scholar]
- 14.Moldawer LL, Marano MA, Wei H, Fong Y, Silen ML, Kuo G, Manogue KR, Vlassara H, Cohen H, Cerami A, et al. Cachectin/tumor necrosis factor-alpha alters red blood cell kinetics and induces anemia in vivo. FASEB J. 1989;3(5):1637–43. [DOI] [PubMed] [Google Scholar]
- 15.Loftus TJ, Mira JC, Ozrazgat-Baslanti T, Ghita GL, Wang Z, Stortz JA, Brumback BA, Bihorac A, Segal MS, Anton SD, et al. Sepsis and Critical Illness Research Center investigators: protocols and standard operating procedures for a prospective cohort study of sepsis in critically ill surgical patients. BMJ Open. 2017;7(7):e015136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muckart DJ, Bhagwanjee S. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference definitions of the systemic inflammatory response syndrome and allied disorders in relation to critically injured patients. Crit Care Med. 1997;25(11):1789–95. [DOI] [PubMed] [Google Scholar]
- 17.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–6. [DOI] [PubMed] [Google Scholar]
- 18.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637. [DOI] [PubMed] [Google Scholar]
- 19.Croft CA, Moore FA, Efron PA, Marker PS, Gabrielli A, Westhoff LS, Lottenberg L, Jordan J, Klink V, Sailors RM, et al. Computer versus paper system for recognition and management of sepsis in surgical intensive care. J Trauma Acute Care Surg. 2014;76(2):311–7; discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 20.McKinley BA, Moore LJ, Sucher JF, Todd SR, Turner KL, Valdivia A, Sailors RM, Moore FA. Computer protocol facilitates evidence-based care of sepsis in the surgical intensive care unit. J Trauma. 2011;70(5):1153–66; discussion 66–7. [DOI] [PubMed] [Google Scholar]
- 21.Pieracci FM, Barie PS. Diagnosis and management of iron-related anemias in critical illness. Crit Care Med. 2006;34(7):1898–905. [DOI] [PubMed] [Google Scholar]
- 22.Beguin Y Soluble transferrin receptor for the evaluation of erythropoiesis and iron status. Clin Chim Acta. 2003;329(1–2):9–22. [DOI] [PubMed] [Google Scholar]
- 23.Burns ER, Goldberg SN, Lawrence C, Wenz B. Clinical utility of serum tests for iron deficiency in hospitalized patients. Am J Clin Pathol. 1990;93(2):240–5. [DOI] [PubMed] [Google Scholar]
- 24.Loftus TJ, Rosenthal MD, Croft CA, Smith RS, Moore FA, Brakenridge SC, Efron PA, Mohr AM. The effects of beta blockade and clonidine on persistent injury-associated anemia. J Surg Res. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chant C, Wilson G, Friedrich JO. Anemia, transfusion, and phlebotomy practices in critically ill patients with prolonged ICU length of stay: a cohort study. Crit Care. 2006;10(5):R140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyon AW, Chin AC, Slotsve GA, Lyon ME. Simulation of repetitive diagnostic blood loss and onset of iatrogenic anemia in critical care patients with a mathematical model. Comput Biol Med. 2013;43(2):84–90. [DOI] [PubMed] [Google Scholar]
- 27.Dolman HS, Evans K, Zimmerman LH, Lavery T, Baylor AE, Wilson RF, Tyburski JG. Impact of minimizing diagnostic blood loss in the critically ill. Surgery. 2015;158(4):1083–7; discussion 7–8. [DOI] [PubMed] [Google Scholar]
- 28.Lumadue JA, Ness PM. Current approaches to red blood cell transfusion. Semin Hematol. 1996;33(4):277–89. [PubMed] [Google Scholar]
- 29.Gould SA, Sehgal LR, Sehgal HL, Moss GS. Hypovolemic shock. Crit Care Clin. 1993;9(2):239–59. [PubMed] [Google Scholar]
- 30.Wiesen AR, Hospenthal DR, Byrd JC, Glass KL, Howard RS, Diehl LF. Equilibration of hemoglobin concentration after transfusion in medical inpatients not actively bleeding. Ann Intern Med. 1994;121(4):278–30. [DOI] [PubMed] [Google Scholar]
- 31.Huber H, Lewis SM, Szur L. The Influence of Anaemia, Polycythaemia and Splenomegaly on the Relationship between Venous Haematocrit and Red-Cell Volume. Br J Haematol. 1964;10:567–75. [DOI] [PubMed] [Google Scholar]
- 32.Maekawa S, Iemura H, Kuramochi Y, Nogawa-Kosaka N, Nishikawa H, Okui T, Aizawa Y, Kato T. Hepatic confinement of newly produced erythrocytes caused by low-temperature exposure in Xenopus laevis. J Exp Biol. 2012;215(Pt 17):3087–95. [DOI] [PubMed] [Google Scholar]
- 33.O’Brien H, Amess JA, Mollin DL. Recurrent thrombocytopenia, erythroid hypoplasia and sideroblastic anaemia associated with hypothermia. Br J Haematol. 1982;51(3):451–6. [DOI] [PubMed] [Google Scholar]
- 34.Lo L, Singer ST, Vichinsky E. Pancytopenia induced by hypothermia. J Pediatr Hematol Oncol. 2002;24(8):681–4. [DOI] [PubMed] [Google Scholar]
- 35.Wu JC, Livingston DH, Hauser CJ, Deitch EA, Rameshwar P. Trauma inhibits erythroid burst-forming unit and granulocyte-monocyte colony-forming unit growth through the production of TGF-beta1 by bone marrow stroma. Ann Surg. 2001;234(2):224–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fonseca RB, Mohr AM, Wang L, Clinton E, Sifri ZC, Rameshwar P, Livingston DH. Adrenergic modulation of erythropoiesis following severe injury is mediated through bone marrow stroma. Surg Infect (Larchmt). 2004;5(4):385–93. [DOI] [PubMed] [Google Scholar]
- 37.Schubert T, Echtenacher B, Hofstadter F, Mannel DN. TNF-independent development of transient anemia of chronic disease in a mouse model of protracted septic peritonitis. Lab Invest. 2003;83(12):1743–50. [DOI] [PubMed] [Google Scholar]
- 38.Zarychanski R, Turgeon AF, McIntyre L, Fergusson DA. Erythropoietin-receptor agonists in critically ill patients: a meta-analysis of randomized controlled trials. Can Med Assoc J. 2007;177(7):725–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hara A, Furuichi K, Higuchi M, Iwata Y, Sakai N, Kaneko S, Wada T. Autoantibodies to erythropoietin receptor in patients with immune-mediated diseases: relationship to anaemia with erythroid hypoplasia. Br J Haematol. 2013;160(2):244–50. [DOI] [PubMed] [Google Scholar]
- 40.van Iperen CE, Gaillard CA, Kraaijenhagen RJ, Braam BG, Marx JJ, van de Wiel A. Response of erythropoiesis and iron metabolism to recombinant human erythropoietin in intensive care unit patients. Crit Care Med. 2000;28(8):2773–8. [DOI] [PubMed] [Google Scholar]
- 41.Pieracci FM, Stovall RT, Jaouen B, Rodil M, Cappa A, Burlew CC, Holena DN, Maier R, Berry S, Jurkovich J, et al. A multicenter, randomized clinical trial of IV iron supplementation for anemia of traumatic critical illness*. Crit Care Med. 2014;42(9):2048–57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.