Abstract
BACKGROUND
The immunopathogenesis of type 1 diabetes mellitus is associated with T-lymphocyte autoimmunity. However, there is growing evidence that B lymphocytes play a role in many T-lymphocyte–mediated diseases. It is possible to achieve selective depletion of B lymphocytes with rituximab, an anti-CD20 monoclonal antibody. This phase 2 study evaluated the role of B-lymphocyte depletion in patients with type 1 diabetes.
METHODS
We conducted a randomized, double-blind study in which 87 patients between 8 and 40 years of age who had newly diagnosed type 1 diabetes were assigned to receive infusions of rituximab or placebo on days 1, 8, 15, and 22 of the study. The primary outcome, assessed 1 year after the first infusion, was the geometric mean area under the curve (AUC) for the serum C-peptide level during the first 2 hours of a mixed-meal tolerance test. Secondary outcomes included safety and changes in the glycated hemoglobin level and insulin dose.
RESULTS
At 1 year, the mean AUC for the level of C peptide was significantly higher in the rituximab group than in the placebo group. The rituximab group also had significantly lower levels of glycated hemoglobin and required less insulin. Between 3 months and 12 months, the rate of decline in C-peptide levels in the rituximab group was significantly less than that in the placebo group. CD19+ B lymphocytes were depleted in patients in the rituximab group, but levels increased to 69% of baseline values at 12 months. More patients in the rituximab group than in the placebo group had adverse events, mostly grade 1 or grade 2, after the first infusion. The reactions appeared to be minimal with subsequent infusions. There was no increase in infections or neutropenia with rituximab.
CONCLUSIONS
A four-dose course of rituximab partially preserved beta-cell function over a period of 1 year in patients with type 1 diabetes. The finding that B lymphocytes contribute to the pathogenesis of type 1 diabetes may open a new pathway for exploration in the treatment of patients with this condition. (ClinicalTrials.gov number, NCT00279305.)
THE AUTOIMMUNE DESTRUCTION OF BETA cells in patients with type 1 diabetes mellitus begins before the onset of hyperglycemia, but measurement of C-peptide responses at the time of diagnosis indicates that patients retain some beta-cell function at this stage. Furthermore, the persistence of residual beta-cell function is associated with reductions of severe hypoglycemic episodes and complications.1 Thus, an intervention that maintains endogenous insulin production might improve the management of type 1 diabetes and reduce long-term complications.
Although the presence of autoantibodies is a diagnostic criterion, the immunopathogenesis of beta-cell destruction in type 1 diabetes is typically associated with T-lymphocyte autoimmunity. Immunosuppressive or immunomodulatory drugs have been administered early in the course of type 1 diabetes in some clinical trials,2–7 including trials testing the effectiveness of humanized anti-CD3 antibody and glutamic acid decarboxylase.5–7
Many T-lymphocyte–mediated diseases include a B-lymphocyte component. B lymphocytes can play a crucial role as antigen-presenting cells,8,9 expressing high levels of class II major-histocompatibility-complex antigens10–13 and generating cryptic peptides to which T lymphocytes are not tolerant.14 For example, in nonobese mice with diabetes, the disease can be inhibited by depletion of B lymphocytes.8,15,16 In contrast to these findings in animal models, a report of type 1 diabetes in a child with X-linked agammaglobulinemia suggests that the presence of B lymphocytes is not essential to the development of type 1 diabetes.17
B lymphocytes can be selectively depleted with the anti-CD20 monoclonal antibody rituximab (Rituxan, Genentech and Biogen Idec).18–20 We tested the hypothesis that transient elimination of B lymphocytes with rituximab would decrease immune-mediated destruction of beta cells and result in preserved beta-cell function in patients with type 1 diabetes of recent onset.
METHODS
PATIENTS AND STUDY DESIGN
A total of 126 patients between the ages of 8 and 45 years who had type 1 diabetes underwent screening at 12 sites in the United States and Canada for the presence of at least one type of detectable diabetes autoantibody: microinsulin autoantibody (in patients who had started taking insulin in the preceding 7 days), glutamic acid decarboxylase 65, islet-cell antigen 512, or islet-cell autoantibody. Enrollment occurred between May 2006 and August 2007; all patients had completed 1 year of follow-up by August 2008. Patients underwent mixed-meal tolerance testing within 3 weeks to 3 months after the diagnosis had been established and were required to have stimulated peak C-peptide levels of at least 0.2 pmol per milliliter to be eligible for enrollment. The initial patients enrolled were 12 years of age or older. After a safety assessment by the data and safety monitoring board, children as young as 8 years of age were included. Eligible patients underwent randomization in a 2:1 ratio of active treatment to placebo, stratified according to clinical center. Both participants and research staff were unaware of the treatment assignments. However, clinical blinding was difficult because of the infusion reactions that predominated in the rituximab group.
The protocol and consent documents were approved by an independent ethics committee or institutional review board at each participating center. All patients (or their parents) provided written informed consent; patients under 18 years of age provided written assent. An independent data and safety monitoring board met every 6 months and conducted quarterly summary safety reviews. A TrialNet medical monitor (who was unaware of the treatment assignments) reviewed all adverse events.
Genentech and Biogen Idec provided the rituximab and the matching placebo but had no involvement with the design, conduct, or management of the study; the collection, analysis, or interpretation of the data; or the preparation of the manuscript. All the authors were involved in the conduct of the study and the collection and review of study data. The principal investigator wrote the first draft of the manuscript. The writing group, which consisted of five of the authors, made the decision to submit the manuscript for publication. The other authors reviewed and commented on various versions of the manuscript and suggested revisions. The members of the writing group assume responsibility for the overall content and integrity of the article. There are no agreements concerning confidentiality of the data between the sponsor and the authors or their institutions. The authors did provide Genentech a copy of the original manuscript before submission.
One intravenous infusion of rituximab (375 mg per square meter of body-surface area) in a solution (pH 6.5) with 9 mg of sodium chloride per milliliter, 7.35 mg of sodium citrate dihydrate per milliliter, 0.7 mg of polysorbate 80 per milliliter, and water for injection or of an identical placebo solution without the rituximab was given on study days 1, 8, 15, and 22. The four doses constituted one course of treatment. The patients received acetaminophen and diphenhydramine before each infusion to attenuate infusion-related events. Corticosteroids were not administered, in order to avoid any potential effect on plasma glucose levels.
During the study, intensive diabetes management was provided, with the goal of maintaining glycated hemoglobin levels within the age-specific ranges recommended by the American Diabetes Association. The treating or referring physician had the primary responsibility for diabetes management. The use of noninsulin medications for glycemic control was prohibited.
The primary outcome was the mean area under the curve (AUC) for the stimulated C-peptide response during the first 2 hours of a 4-hour mixed-meal tolerance test conducted at 12 months, with the response expressed in picomoles per milliliter.21 These assessments were completed in August 2008, at which time analysis of 1-year outcomes was conducted. Safety outcomes focused on infusion reactions (occurring within 24 hours after an infusion), infections, and laboratory assessments, particularly assessments of white-cell counts and immunoglobulin levels. Patients were to continue follow-up for an additional year, with double blinding maintained.
After 21 patients had been enrolled, the Food and Drug Administration (FDA) reported progressive multifocal leukoencephalopathy (PML) in 2 people treated with rituximab for systemic lupus erythematosus. TrialNet immediately halted infusions in 5 patients who had recently undergone randomization and suspended enrollment for a period of approximately 2 months (between December 2006 and February 2007). After consultation with experts in PML and with representatives of the FDA, the data and safety monitoring board allowed enrollment to resume but required the addition of careful neurologic examinations to the protocol.
LABORATORY TESTS
Blood samples were sent to TrialNet core laboratories for analysis. A 4-hour mixed-meal tolerance test was conducted at baseline and 1 year, and 2-hour mixed-meal tolerance testing, with samples obtained at intervals of 15 to 30 minutes, was performed at 3 months and 6 months.21,22 Levels of antibodies to glutamic acid decarboxylase 65, islet-cell antigen 512, and microinsulin (by microassay) were measured with the use of radio-immunobinding assays, and levels of islet-cell autoantibodies were measured with the use of indirect immunofluorescence. The presence of serum antibodies to hepatitis B surface antigen, hepatitis C, or the human immunodeficiency virus excluded potential participants. Human leukocyte antigen class II alleles (DRB1, DQA1, DQB1) were assessed using polymerase-chainreaction amplification and sequence-specific hybridization. Levels of CD19+ cells were determined with the use of multiparameter flow cytometry. Complete blood counts were performed at each clinical center. Serum levels of rituximab and of human antichimeric antibodies were determined with the use of enzyme-linked immunosorbent assay (ELISA) techniques at Genentech.23 (For additional information concerning methods, see the Supplementary Appendix, available with the full text of this article at NEJM.org)
STATISTICAL ANALYSIS
The effectiveness analyses were based on the prespecified intention-to-treat cohort of 81 patients that excluded 1 patient whose consent was withdrawn before administration of the initial infusion and 5 patients whose infusions were stopped as a result of the PML safety alert. The safety cohort consists of all 87 patients who underwent randomization. The prespecified primary analysis was a comparison of the C-peptide levels, calculated as loge([mean C peptide]+1) at baseline and at 12 months in an analysis of covariance adjusted for age and sex. Baseline age, sex, C-peptide level, and glycated hemoglobin level were prespecified subgroup factors. Secondary analyses included the overall difference between groups in a normal errors repeated-measures model. The geometric mean C-peptide level was obtained from the inverse transformation. The mean rate of change over a period of 3 to 12 months was estimated with the use of a mixed-effects random coefficient model (for details, see the Supplementary Appendix).24
The originally planned enrollment target was 66 patients, which would provide 85% power to detect a 65% between-group difference in the geometric mean for the level of C peptide at the 0.05 level (one-sided test), assuming a 10% rate of loss to follow-up and a 2:1 ratio for assignments to the rituximab and placebo groups.21 Screening was closed after this target was reached, allowing for the PML interruptions, but additional patients who had already begun the screening process were allowed to enroll.
Results
PATIENTS
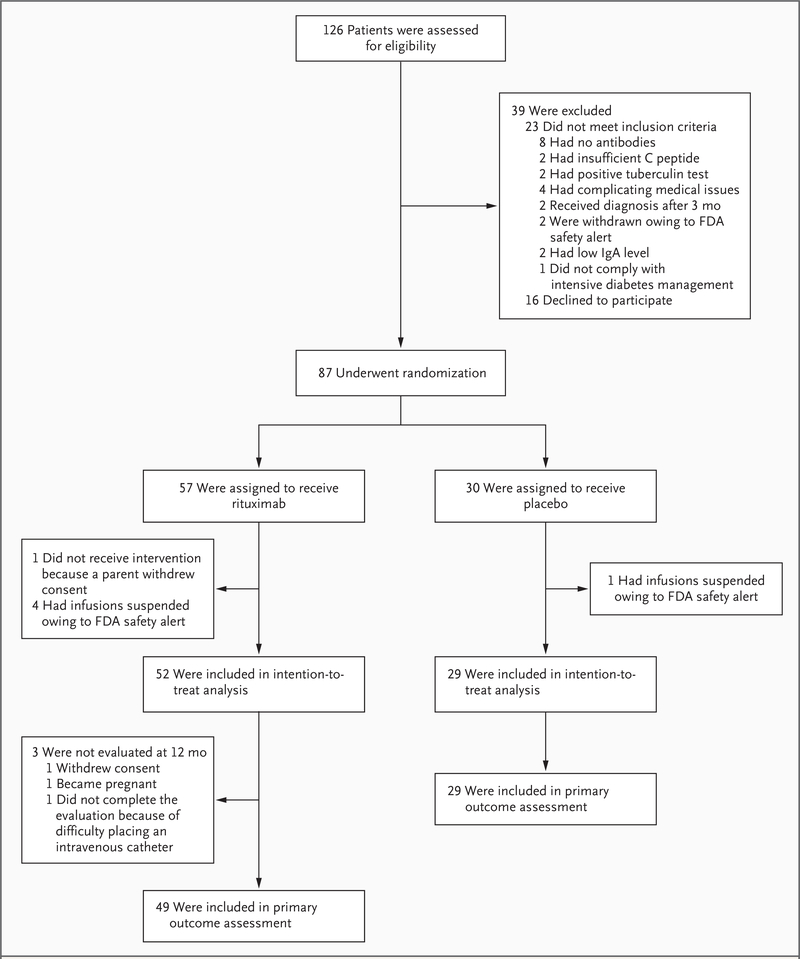
Of the 87 patients who underwent randomization, the predefined intention-to-treat cohort included 81 patients (Fig. 1 and Table 1). Three patients did not complete the 1-year mixed-meal tolerance test (1 because of withdrawal of consent, 1 because of pregnancy, and 1 because of difficulty with placement of the intravenous catheter); thus, 78 patients were included in the analysis of the primary outcome.
Figure 1. Enrollment, Randomization, and Follow-up of Study Participants.
Between May 2006 and August 2007, a total of 126 patients were screened and 87 underwent randomization. Of the 87 patients who underwent randomization, 81 composed the intention-to-treat cohort, of whom 78 contributed to the primary effectiveness analyses at 12 months. In 5 of the 6 patients who were excluded, infusions were stopped after an FDA safety alert concerning the development of progressive multifocal leukoencephalopathy in other study populations receiving rituximab; in the other patient, parental consent was withdrawn before administration of the initial infusion.
Table 1.
Characteristics of the Study Groups.*
| Rituximab (N = 57) | Placebo (N = 30) | |
|---|---|---|
| Age — yr | ||
| Mean | 19.0±8.6 | 17.3±7.8 |
| Median | 16 | 14 |
| Range | 8–40 | 9–38 |
| Male sex — no. of patients (%) | 36 (63) | 18 (60) |
| Race or ethnic group — no. of patients (%)† | ||
| White | 55 (96) | 29 (97) |
| Non-Hispanic | 54 (95) | 27 (90) |
| No. of autoantibodies — no. of patients (%) | ||
| 1 | 10 (18) | 3 (10) |
| 2 | 18 (32) | 7 (23) |
| 3 | 17 (30) | 9 (30) |
| 4 | 12 (21) | 11 (37) |
| No. of days since diagnosis | 80±22 | 83±19 |
| No. of days from diagnosis to first infusion | ||
| Median | 81 | 91 |
| Range | 37–137 | 34–109 |
| Weight (kg) | 66.0±21.9 | 58.0±16.8 |
| Body-mass index | 23.2±5.2 | 21.2±4.3 |
| Total white cells — per mm3 | 5500±1500 | 4900±1200 |
| CD19+ B cells lymphocytes — per mm3 | 275.23±134.74 | 338.02±226.13 |
| CD20+ B cells lymphocytes — per mm3 | 268.58±127.41 | 295.85±122.15 |
| Mean AUC for C peptide — pmol/ml | 0.75±0.39 | 0.74±0.37 |
| Glycated hemoglobin at baseline — % | 7.31±1.46 | 7.08±1.28 |
| Total insulin dose (units/kg) | 0.37±0.24 | 0.38±0.22 |
| Diabetes-associated HLA alleles present — no. of patients (%) | ||
| DR3–DQ2 | 25 (44) | 12 (40) |
| DR4–DQ8 | 19 (33) | 14 (47) |
| DR3–DQ2 and DR4–DQ8 | 12 (21) | 7 (23) |
| Received all 4 full infusions — no. of patients (%) | 46 (81) | 28 (93) |
Plus–minus values are means ±SD. The body-mass index is the weight in kilograms divided by the square of the height in meters. AUC denotes area under the curve.
Race was self-reported.
EFFECTIVENESS
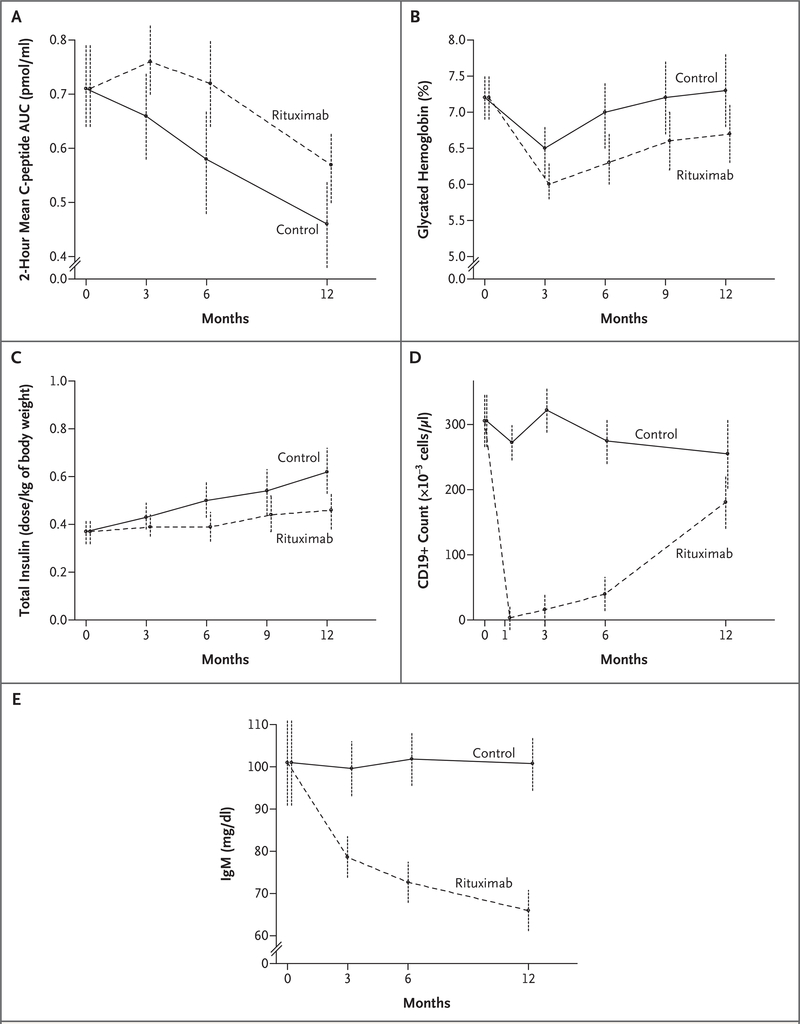
At 1 year, the mean AUC for the level of C peptide at 2 hours in the rituximab group was 0.56 pmol per milliliter (95% confidence interval [CI], 0.50 to 0.63), as compared with 0.47 pmol per milliliter (95% CI, 0.39 to 0.55) in the placebo group (Fig. 2A), making the mean level for the treatment group 20% higher than that for the placebo group (95% CI, >2 to infinity; P = 0.03). The results were similar at 4 hours (P = 0.009) and among the 71 patients who received all four infusions (P = 0.004). The patients receiving rituximab also had higher absolute levels of C peptide at 3, 6, and 12 months, with the greatest differences at 6 and 12 months (nominal P = 0.03 for both comparisons) and over all the time points in aggregate (P<0.001).
Figure 2. Effects of Rituximab on C-Peptide Level, Glycated Hemoglobin Level, Insulin Dose Required, CD19+ Cell Counts, and IgM Level.
For each panel, 95% confidence limits are shown at each time point within each group.
In the rituximab group, the geometric mean C-peptide value initially improved from baseline (Fig. 2A). Starting with the value at 3 months, the average rate of decline in C-peptide levels calculated as loge([mean C peptide]+1) was similar in the two groups (P = 0.27), as shown in Figure 2A. However, in an analysis of the log values alone (without +1), the level of C peptide in the rituximab group declined at a rate of 37.7% per year (95% CI, 23.5 to 49.4), whereas that in the placebo group declined at a rate of 55.8% per year (95% CI, 42.1 to 66.2; P = 0.03).
As compared with the placebo group, the rituximab group had lower levels of glycated hemoglobin over the 12-month period (6.76±1.24% vs. 7.00±1.30%, P<0.001) (Fig. 2B) and required lower doses of insulin (0.39±0.22 U per kilogram of body weight vs. 0.48±0.23 U per kilogram, P<0.001) (Fig. 2C). Using the change in the t-test statistic value in the repeated-measures model without, and then with, adjustment for the overall and baseline C-peptide values, we found that over the 12-month period, 34% of the between-group difference in the change in the glycated hemoglobin level and 34% of the between-group difference in the change in the insulin dose were explained by the difference in C-peptide levels between the two groups.
CD19+ cells were depleted by rituximab (P<0.001 for the comparison with placebo), indicating B-cell depletion, and gradually recovered over the course of 12 months (Fig. 2D); CD19+ cells were relatively stable in the placebo group. The difference between the two study groups in the number of CD19+ cells at 3 months explained 97% of the difference between groups in C-peptide levels at 12 months, the difference in the number of CD19+ cells at 6 months explained 88% of the difference in C-peptide levels at 12 months, and the difference in the number of CD19+ cells at 12 months explained only 8% of the difference in C-peptide levels at 12 months.
The ratio of the geometric mean AUC for the C-peptide levels at 12 months for the rituximab group to that of the control group was similar within subgroups (see Fig. A in the Supplementary Appendix). There was no significant heterogeneity among subgroups.
ADVERSE EVENTS AND SAFETY
During the first infusion, 93% of patients given rituximab had adverse reactions, as compared with 23% of those given placebo (Table 2). For subsequent infusions, the rate of reactions was similar in the two study groups, although the specific types of infusion reactions differed between the groups (for more information, see the Supplementary Appendix).
Table 2.
Number of Patients with Infusion-Related Events According to Study Group.*
| Event | Rituximab (N = 57) | Control (N = 30) | P Value† |
|---|---|---|---|
| number (percent) | |||
| Patients receiving first infusion | 56 (98) | 30 (100) | |
| Patients with one or more events | 52 (93) | 7 (23) | <0.001 |
| Fever | 12 (21) | 1 (3) | 0.02 |
| Cough | 3 (5) | 0 | 0.27 |
| Shortness of breath | 4 (7)‡ | 1 (3) | 0.43 |
| Hypotension | 13 (23) | 1 (3) | 0.01 |
| Hypertension | 6 (11) | 0 | 0.07 |
| Tachycardia | 12 (21) | 0 | 0.004 |
| Rash | 21 (38)‡ | 0 | <0.001 |
| Pruritus | 18 (32) | 0 | <0.001 |
| Vomiting | 10 (18) | 0 | 0.01 |
| Nausea | 18 (32) | 1 (3) | 0.001 |
| Other | 22 (39) | 3 (10) | 0.003 |
| Patients receiving more than one infusion | 51 (89) | 29 (97) | |
| Patients with one or more events during second, third, or fourth infusion | 14 (27) | 8 (28) | 0.61 |
| Fever | 1 (2) | 1 (3) | 0.88 |
| Cough | 0 | 1 (3) | 1.00 |
| Shortness of breath | 0 | 0 | NA |
| Hypotension | 7 (14) | 5 (17) | 0.78 |
| Hypertension | 0 | 0 | NA |
| Tachycardia | 1 (2) | 0 | 0.64 |
| Rash | 1 (2) | 0 | 0.64 |
| Pruritus | 1 (2) | 0 | 0.64 |
| Vomiting | 0 | 0 | NA |
| Nausea | 0 | 0 | NA |
| Other | 4 (8) | 4 (14) | 0.89 |
Rituximab or placebo was administered at a rate of 50 mg per hour. If infusion-related events did not occur, the infusion rate could be increased in increments of 50 mg per hour every 30 minutes to a maximum of 400 mg per hour. If an infusion rate of 400 mg per hour was tolerated, subsequent infusions started at a rate of 100 mg per hour and were increased by 100 mg per hour every 30 minutes until a rate of 400 mg per hour was reached. For mild infusion reactions, the rate of infusion was slowed or temporarily interrupted and was resumed at half the previous rate on alleviation of symptoms. For moderate or severe infusion reactions, the infusion was not restarted and subsequent infusions were not administered. One patient could have multiple events. NA denotes not applicable.
P values are one-sided and were calculated with the use of Fisher’s exact test.
One patient had a grade 3 infusion reaction consisting of shortness of breath and rash.
In all, 57 patients in the rituximab group reported 392 adverse events, whereas 30 patients in the placebo group reported 148 adverse events. Most adverse events were grade 1 or grade 2; none were grade 4. For adverse events that were grade 2 or higher, the rate ratios and odds ratios were close to 1.0, except in the case of leukopenia, which occurred more frequently in the rituximab group; given the small numbers of patients, this difference was not significant. Adverse events of grade 3 occurred in six patients in the rituximab group and two in the placebo group (see the Supplementary Appendix), with risk ratios close to 1.0. Neutrophil counts were low in several patients at baseline, but neither the rate of neutropenia nor the absolute neutrophil count differed significantly between the groups at any time.
IgM levels fell in the rituximab group, an effect that persisted at 1 year (P<0.001 for the comparison with the placebo group) (Fig. 2E). After the recovery of B cells, an IgM response to immunization with a neoantigen (phiX174) was noted (see Table A in the Supplementary Appendix). At 3 months, the IgG levels in patients who received rituximab were an average of 45.8 mg per deciliter higher than those in patients who received placebo (a difference of 5%; P = 0.048); neither the difference after adjustment for multiple comparisons nor the differences at 6 months and at 12 months were significant. None of the patients required treatment for hypogammaglobulinemia, and no adverse effects on routine laboratory measures were noted. (Additional data on adverse events are available in Tables B, C, and D in the Supplementary Appendix.)
At 6 months, the mean serum rituximab level was higher in the subgroup of patients who had received all four infusions than in the overall rituximab group (4363±13417 ng per milliliter [34 patients] vs. 3968±12725 ng per milliliter [38 patients]); however, the levels were similar at 9 months (577±288 ng per milliliter [22 patients] and 567±215 ng milliliter [25 patients], respectively). Three patients, none of whom had human antichimeric antibodies at baseline, had measurable levels of these antibodies at 6 months.
DISCUSSION
The results of our study support the hypothesis that B lymphocytes play a role in the pathogenesis of type 1 diabetes mellitus. A single course of rituximab administered soon after diagnosis appears to preserve insulin secretion in part for at least 1 year. In addition to the improvement in C-peptide levels, the glycated hemoglobin level and the insulin dose were both significantly lower in the rituximab group than in the placebo group. Analyses suggest that the effects of treatment on the glycated hemoglobin level and insulin dose were related to the preservation of C-peptide levels.
The most recent and comparable trials assessing the use of antibodies to treat early type 1 diabetes used humanized anti-CD3 antibodies.5,6 The study reported by Herold et al.6 was most similar to ours in that the investigators used a mixed-meal tolerance test to assess levels of C peptide; they noted that the mean AUC for C-peptide level fell in the placebo group from 133.2 nmol per liter at baseline to 66.7 nmol per liter at 12 months, a decline of approximately 50% per year, whereas it rose slightly in the treatment group, from 111.5 nmol per liter to 114.2 nmol per liter. At 12 months, the level of C peptide was 71% higher in patients receiving treatment than in those receiving placebo. We observed a difference of 20% in our trial. The glycated hemoglobin level and the insulin dose required also improved,6 but slightly less than in our trial. In the study by Herold et al., as in ours, the average insulin dose at 12 months was less than 0.5 units per kilogram of body weight, indicating a clinical remission, as defined in the study by Herold et al. and in another study.25
It is difficult to make a true comparison between the studies of anti-CD3 antibody and our study of anti-CD20 antibody because of some critical differences in design. In the study by Her-old et al.,6 patients were younger than in our study, and they started treatment earlier (within 6 weeks after diagnosis vs. approximately 11 weeks in our study); in the study by Keymeulen et al.,5 patients began treatment even earlier (within 4 weeks after diagnosis). Although blinding was incomplete in our trial, there was no attempt at blinding in the study by Herold et al. The high incidence of anti-idiotype antibodies reported by Herold et al. (50% at 1 month, although the antibodies had disappeared by 12 months) might affect future courses of treatment. An important aspect of the study by Herold et al. was hospitalization for a 14-day course of infusion; there was also a higher frequency of adverse events than in the present study, which may mean that their approach was more arduous for patients. A direct comparison would be needed to assess the merits of each approach.
Subgroup analyses in our trial hint at a greater response in children and adolescents than in adults, although the difference was not significant (for details, see the Supplementary Appendix). This finding might be due to a more rapid reduction in the AUC for C peptide in younger patients. Until such results are confirmed, we believe that in future trials that examine the effect of treatment on C-peptide levels it would be prudent to stratify patients according to age or adjust analyses for age.
The actual mechanism of the effect of rituximab in type 1 diabetes is uncertain. We speculate that rituximab may reduce the production of cytokines that augment the immune response locally within the pancreas or the peripancreatic lymph nodes. It is also possible that the antigen presentation provided by B lymphocytes, which is required for continued T-lymphocyte action, is altered by rituximab.
Human antichimeric antibodies developed in 3 of the 56 patients who received rituximab (5%) — a lower rate than those reported among patients with other diagnoses who have received rituximab (24.6% in patients with multiple sclerosis and 35% in patients with systemic lupus)26; however, the rate in our study is similar to the reported rates in patients with cancer and those with rheumatoid arthritis.27 Our three patients in whom human antichimeric antibodies developed had vigorous reactions to the first dose of rituximab and did not complete the initial dose or receive subsequent doses; consequently, all had subtherapeutic levels of rituximab.
Infusion reactions were the most frequent adverse events in our study, and they were limited to the first infusion in 38 patients. The reaction to the first dose is primarily a consequence of increased release of cytokines.28 One patient in our study had an adverse event reported as anaphylaxis, which was consistent with the cytokine-release syndrome, and resolved without incident. Although corticosteroids are reported to reduce the incidence and severity of infusion reactions with rituximab,29 we did not use corticosteroids in our study; however, infusion reactions were generally mild and dissipated with a slowing of the infusion rate. The reactions we observed were similar to those seen in a recent trial of rituximab for the treatment of multiple sclerosis, in which corticosteroids were also not used.30
Increased rates of neutropenia and infection were not observed in patients in the rituximab group as compared with those in the placebo group. The vehicle in which rituximab was administered was a saline solution without additional protein. It is conceivable that the neutropenia reported in other trials was caused by therapeutic agents that patients received along with the rituximab.31 The absence of an increase in rituximab-associated infections in our study is consistent with the findings in other studies that avoided the use of both immunosuppressive agents and corticosteroids.30 However, our sample was too small to detect rare infections, such as PML, and other uncommon adverse events. Careful attention, larger studies, and longer periods of follow-up will be needed to obtain better estimates of the risks of adverse events.
The reduction in IgM levels, which is consistent with the findings in previous studies of rituximab in which IgM levels were measured,30,32 suggests that rituximab is more effective in reducing the B-lymphocyte population than in reducing the number of cells that secrete IgG. The IgM response reported in patients given the neoantigen phiX174 at the time of B-cell recovery suggests that the immune system recovers.33,34 We have no explanation for the slight increase we observed in IgG levels, but we believe it is unlikely to be clinically meaningful; in other studies of rituximab there has been no change in IgG levels.30,32
This phase 2 study shows that a therapy that targets B cells may have a beneficial effect on beta-cell function in early type 1 diabetes. It is unlikely that treatment with rituximab as administered in this study would be optimal. We observed an initial improvement shortly after the administration of rituximab but with a subsequent resumption in the decline of the AUC for C peptide. Neither repeat nor long-term administration of rituximab was attempted, since information concerning the relative efficacy or safety of such an approach was lacking. Given our results, we believe that other anti–B-lymphocyte agents should be tested — for example, humanized anti-CD20 antibodies.35 Whether anti–B-lymphocyte therapy would prevent or delay diabetes in patients with autoantibodies, dysglycemia, or both is currently unknown.
Supplementary Material
Acknowledgments
Supported by the Type 1 Diabetes TrialNet Study Group, a clinical-trials network funded by the National Institutes of Health through the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, the National Institute of Child Health and Human Development, and the General Clinical Research Centers Program; the Juvenile Diabetes Research Foundation International; and the American Diabetes Association.
Dr. Pescovitz reports serving on advisory boards for Genentech, Roche, Biogen Idec, and Eli Lilly and receiving lecture fees and grant support from Roche; Dr. Gitelman, serving on an advisory board for Genentech; Dr. Goland, receiving grant support from Tolerx; Dr. Gottlieb, receiving grant support from Bayhill Therapeutics, MacroGenics, and Tolerx; Dr. Marks, serving on advisory boards for Genentech, Roche, and Biogen Idec and receiving grant support from Eli Lilly; Dr. Rodriguez, serving on advisory boards for Genentech and Biogen Idec and on the speakers bureau for Eli Lilly and receiving grant support from MacroGenics and Eli Lilly; Dr. Schatz, serving on advisory boards for Genentech and Roche; Dr. Wherrett, receiving lecture fees from Eli Lilly; Dr. Wilson, serving on an advisory board for Genentech and receiving grant support from the Glaser Pediatric Research Network; Dr. Lachin, receiving consulting fees from Tolerx, Bayhill Therapeutics, and Andromeda Biotech; and Dr. Skyler, receiving grant support from Bayhill Therapeutics and Osiris Therapeutics. No other potential conflict of interest relevant to this article was reported.
Contributor Information
Mark D. Pescovitz, Indiana University School of Medicine, Indianapolis
Carla J. Greenbaum, Benaroya Research Institute, Seattle
Heidi Krause-Steinrauf, George Washington University Biostatistics Center, Rockville, MD
Dorothy J. Becker, University of Pittsburgh, Pittsburgh
Stephen E. Gitelman, University of California, San Francisco, San Francisco
Robin Goland, Columbia University, New York
Peter A. Gottlieb, University of Colorado Barbara Davis Center for Childhood Diabetes, Aurora
Jennifer B. Marks, University of Miami Diabetes Research Institute, Miami
Paula F. McGee, George Washington University Biostatistics Center, Rockville, MD
Antoinette M. Moran, University of Minnesota, Minneapolis
Philip Raskin, University of Texas Southwestern Medical School, Dallas
Henry Rodriguez, Indiana University School of Medicine, Indianapolis
Desmond A. Schatz, University of Florida, Gainesville
Diane Wherrett, Hospital for Sick Children, University of Toronto, Toronto
Darrell M. Wilson, Stanford University, Stanford, CA
John M. Lachin, George Washington University Biostatistics Center, Rockville, MD
Jay S. Skyler, University of Miami Diabetes Research Institute, Miami
REFERENCES
- 1.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329: 977–86. [DOI] [PubMed] [Google Scholar]
- 2.Schernthaner G Progress in the immunointervention of type-1 diabetes mellitus. Horm Metab Res 1995;27:547–54. [DOI] [PubMed] [Google Scholar]
- 3.Mahon JL, Dupre J, Stiller CR. Lessons learned from the use of cyclosporine for insulin-dependent diabetes mellitus: the case for immunotherapy for insulin-dependent diabetics having residual insulin secretion. Ann N Y Acad Sci 1993;696:351–63. [DOI] [PubMed] [Google Scholar]
- 4.Feutren G, Mihatsch M. Risk factors for cyclosporine-induced nephropathy in patients with autoimmune diseases. N Engl J Med 1992;326:1654–60. [DOI] [PubMed] [Google Scholar]
- 5.Keymeulen B, Vandemeulebroucke E, Ziegler AG, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med 2005;352:2598–608. [DOI] [PubMed] [Google Scholar]
- 6.Herold KC, Hagopian W, Auger JA, et al. Anti-CD3 monoclonal antibody in newonset type 1 diabetes mellitus. N Engl J Med 2002;346:1692–8. [DOI] [PubMed] [Google Scholar]
- 7.Ludvigsson J, Faresjö M, Hjorth M, et al. GAD treatment and insulin secretion in recent-onset type 1 diabetes. N Engl J Med 2008;359:1909–20. [DOI] [PubMed] [Google Scholar]
- 8.Noorchashm H, Lieu YK, Noorchashm N, et al. I-Ag7-mediated antigen presentation by B lymphocytes is critical in overcoming a checkpoint in T cell tolerance to islet beta cells of nonobese diabetic mice. J Immunol 1999;163:743–50. [PubMed] [Google Scholar]
- 9.Rivera A, Chen CC, Ron N, Dougherty JP, Ron Y. Role of B cells as antigen-presenting cells in vivo revisited: antigen-specific B cells are essential for T cell expansion in lymph nodes and for systemic T cell responses to low antigen concentrations. Int Immunol 2001;13:1583–93. [DOI] [PubMed] [Google Scholar]
- 10.Lapointe R, Bellemare-Pelletier A, Housseau F, Thibodeau J, Hwu P. CD40stimulated B lymphocytes pulsed with tumor antigens are effective antigen-presenting cells that can generate specific T cells. Cancer Res 2003;63:2836–43. [PubMed] [Google Scholar]
- 11.Serreze DV, Silveira PA. The role of B lymphocytes as key antigen-presenting cells in the development of T cell-mediated autoimmune type 1 diabetes. Curr Dir Autoimmun 2003;6:212–27. [DOI] [PubMed] [Google Scholar]
- 12.Takemura S, Klimiuk PA, Braun A, Goronzy JJ, Weyand CM. T cell activation in rheumatoid synovium is B cell dependent. J Immunol 2001;167:4710–8. [DOI] [PubMed] [Google Scholar]
- 13.Kim H-J, Krenn V, Steinhauser G, Berek C. Plasma cell development in synovial germinal centers in patients with rheumatoid and reactive arthritis. J Immunol 1999; 162:3053–62. [PubMed] [Google Scholar]
- 14.Davidson A, Diamond B. Autoimmune diseases. N Engl J Med 2001;345:340–50. [DOI] [PubMed] [Google Scholar]
- 15.Noorchashm H, Greeley SA, Naji A. The role of T/B lymphocyte collaboration in the regulation of autoimmune and alloimmune responses. Immunol Res 2003; 27:443–50. [DOI] [PubMed] [Google Scholar]
- 16.Noorchashm H, Noorchashm N, Kern J, Rostami SY, Barker CF, Naji A. B-cells are required for the initiation of insulitis and sialitis in nonobese diabetic mice. Diabetes 1997;46:941–6. [DOI] [PubMed] [Google Scholar]
- 17.Martin S, Wolf-Eichbaum D, Duinkerken G, et al. Development of type 1 diabetes despite severe hereditary B-lymphocyte deficiency. N Engl J Med 2001; 345:1036–40. [DOI] [PubMed] [Google Scholar]
- 18.Reff ME, Carner K, Chambers KS, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood 1994;83:435–45. [PubMed] [Google Scholar]
- 19.Pescovitz MD. Rituximab, an anti-CD20 monoclonal antibody: history and mechanism of action. Am J Transplant 2006;6:859–66. [DOI] [PubMed] [Google Scholar]
- 20.Idem. The use of rituximab, anti-CD20 monoclonal antibody, in pediatric transplantation. Pediatr Transplant 2004;8:9–21. [DOI] [PubMed] [Google Scholar]
- 21.Palmer JP, Fleming GA, Greenbaum CJ, et al. C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve beta-cell function: report of an ADA workshop, 21–22 October 2001. Diabetes 2004;53:250–64. [Erratum, Diabetes 2004;53:1934.] [DOI] [PubMed] [Google Scholar]
- 22.Greenbaum CJ, Mandrup-Poulsen T, McGee PF, et al. Mixed-meal tolerance test versus glucagon stimulation test for the assessment of beta-cell function in therapeutic trials in type 1 diabetes. Diabetes Care 2008;31:1966–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fervenza FC, Cosio FG, Erickson SB, et al. Rituximab treatment of idiopathic membranous nephropathy. Kidney Int 2008;73:117–25. [DOI] [PubMed] [Google Scholar]
- 24.Demidenko E Mixed models: theory and applications. Hoboken, NJ: John Wiley, 2004. [Google Scholar]
- 25.Yilmaz MT, Devrim AS, Biyal F, et al. Immunoprotection in spontaneous remission of type 1 diabetes: long-term follow-up results. Diabetes Res Clin Pract 1993;19: 151–62. [DOI] [PubMed] [Google Scholar]
- 26.Looney RJ, Anolik JH, Campbell D, et al. B cell depletion as a novel treatment for systemic lupus erythematosus: a phase I/II dose-escalation trial of rituximab. Arthritis Rheum 2004;50:2580–9. [DOI] [PubMed] [Google Scholar]
- 27.Piro LD, White CA, Grillo-Lopez AJ, et al. Extended rituximab (anti-CD20 mono-clonal antibody) therapy for relapsed or refractory low-grade or follicular non-Hodgkin’s lymphoma. Ann Oncol 1999; 10:655–61. [DOI] [PubMed] [Google Scholar]
- 28.Agarwal A, Vieira CA, Book BK, Sidner RA, Fineberg NS, Pescovitz MD. Rituximab, anti-CD20, induces in vivo cytokine release but does not impair ex vivo T-cell responses. Am J Transplant 2004;4:1357–60. [DOI] [PubMed] [Google Scholar]
- 29.Emery P, Fleischmann RM, Filipo-wicz-Sosnowska A, et al. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIB randomized, double-blind, placebo-controlled, dose-ranging trial. Arthritis Rheum 2006; 54:1390–400. [DOI] [PubMed] [Google Scholar]
- 30.Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing–remitting multiple sclerosis. N Engl J Med 2008;358:676–88. [DOI] [PubMed] [Google Scholar]
- 31.Cattaneo C, Spedini P, Casari S, et al. Delayed-onset peripheral blood cytopenia after rituximab: frequency and risk factor assessment in a consecutive series of 77 treatments. Leuk Lymphoma 2006;47: 1013–7. [DOI] [PubMed] [Google Scholar]
- 32.Cambridge G, Leandro MJ, Edwards JC, et al. Serologic changes following B lymphocyte depletion therapy for rheumatoid arthritis. Arthritis Rheum 2003; 48:2146–54. [DOI] [PubMed] [Google Scholar]
- 33.Bearden CM, Agarwal A, Book BK, et al. Rituximab inhibits the in vivo primary and secondary antibody response to a neoantigen, bacteriophage phiX174. Am J Transplant 2005;5:50–7. [DOI] [PubMed] [Google Scholar]
- 34.Ochs HD, Davis SD, Wedgwood RJ. Immunologic responses to bacteriophage phi-X 174 in immunodeficiency diseases. J Clin Invest 1971;50:2559–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Genovese MC, Kaine JL, Lowenstein MB, et al. Ocrelizumab, a humanized antiCD20 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: a phase I/II randomized, blinded, placebo-controlled, dose-ranging study. Arthritis Rheum 2008;58:2652–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.