Summary
Understanding pathways guiding the development of definitive hematopoiesis with lymphoid potential is essential for advancing human pluripotent stem cell (hPSC) technologies for treatment of blood diseases and immunotherapies. In the embryo, lymphoid progenitors and hematopoietic stem cells (HSCs) arise from hemogenic endothelium (HE) lining arteries, but not veins. Here, we show that activation of arterial program through ETS1 overexpression or by modulating MAPK/ERK signaling pathways at the mesodermal stage of development, dramatically enhanced formation of arterial type HE expressing DLL4 and CXCR4. Blood cells generated from arterial HE were more than 100-fold enriched in T cell precursor frequency and possessed the capacity to produce B lymphocytes and red blood cells expressing high levels of BCL11a and β-globin. Together, these findings provide an innovative strategy to aid in the generation of definitive lymphomyeloid progenitors and lymphoid cells from hPSCs for immunotherapy through enhancing arterial programming of HE.
De novo production of hematopoietic and lymphoid cells from in vitro expandable human cells, such as human pluripotent stem cells (hPSCs) represents a promising approach for transplantation and immunotherapies of hematologic diseases and cancers. Although the feasibility of generating engraftable hematopoietic cells and T lymphoid cells from hPSCs has been demonstrated (Ledran et al., 2008; Rahman et al., 2017; Sugimura et al., 2017; Vizcardo et al., 2013; Wang et al., 2005), further translation of these technologies from bench-to-bedside requires developing of clinically safe protocols for scalable production of therapeutic cells in defined physiological conditions. Thus, identifying the proper molecular pathways guiding multipotential lymphomyeloid progenitor specification from hPSCs is essential to advance T lymphoid cell and HSC manufacturing technologies.
During development, blood cells and HSCs arise from hemogenic endothelium (HE) which represent a distinct RUNX1-expressing subset of vascular endothelium with capacity to undergo endothelial-to-hematopoietic transition (EHT) (Boisset et al., 2010; Kissa and Herbomel, 2010; North et al., 1999; Richard et al., 2013). In contrast to the first wave of primitive hematopoiesis lacking of lymphoid and granulocytic potential, definitive hematopoiesis produces the entire spectrum of adult-type erythro-myeloid progenitors (EMP; second wave), lymphoid cells, cells capable of limited engraftment (third wave), and HSCs with the capacity for long-term repopulation of an adult recipient (fourth wave) (reviewed in (Lin et al., 2014; Medvinsky et al., 2011; Tober et al., 2016)). While some definitive hematopoietic cells such as EMPs can be produced from HE in venous vessels and capillaries (Frame et al., 2016; Goldie et al., 2008; Li et al., 2005), production of lymphoid cells and HSCs is mostly restricted to arterial vasculature (de Bruijn et al., 2000; Gordon-Keylock et al., 2013; North et al., 1999; Rybtsov et al., 2016; Yzaguirre and Speck, 2016). The apparent lack of venous contribution to lymphoid cells and HSCs (Burns et al., 2009; Gering and Patient, 2005; Kim et al., 2013; Lawson et al., 2001; Lawson et al., 2002) suggests that arterial specification of HE could be an essential prerequisite for establishing definitive hematopoiesis with lymphoid potential. However, discovery of HSC specification pathways that are uncoupled from arterial patterning (Burns et al., 2009; Monteiro et al., 2016; Robert-Moreno et al., 2008) raises the question whether arterial programming of HE is required for establishing definitive hematopoiesis.
In hPSC cultures, HE can be separated from non-HE based on CD73 expression (Choi et al., 2012; Ditadi et al., 2017). Although previous studies demonstrated arterial commitment within CD73+ non-HE fraction of hPSC-derived endothelium (Ditadi et al., 2015), little is known about the effect of arterial programming on CD73− HE. During vascular development, arterial fate is control by a number of key signaling pathways including Hedgehog, VEGF, NOTCH, MAPK/ERK, Wnt/B-catenin signaling pathways and ETS, SOXF and FOXC1/C2 transcription factors (reviewed in (Fish and Wythe, 2015)). Here, we found that inducing arterial specification of HE by overexpression of ETS family transcription factor, ETS1, or through modulation of MAPK/ERK pathways, led to arterial HE formation with DLL4+CXCR4+/− phenotype and definitive erythroid, T and B lymphoid potentials. Together, these findings suggest that promoting arterial patterning in hPSC cultures could aid in vitro approaches to instruct definitive hematopoiesis with lymphoid potential from hPSCs.
Results
ETS1 induction upregulates SOXF and NOTCH-signaling associated genes and enhances arterial specification
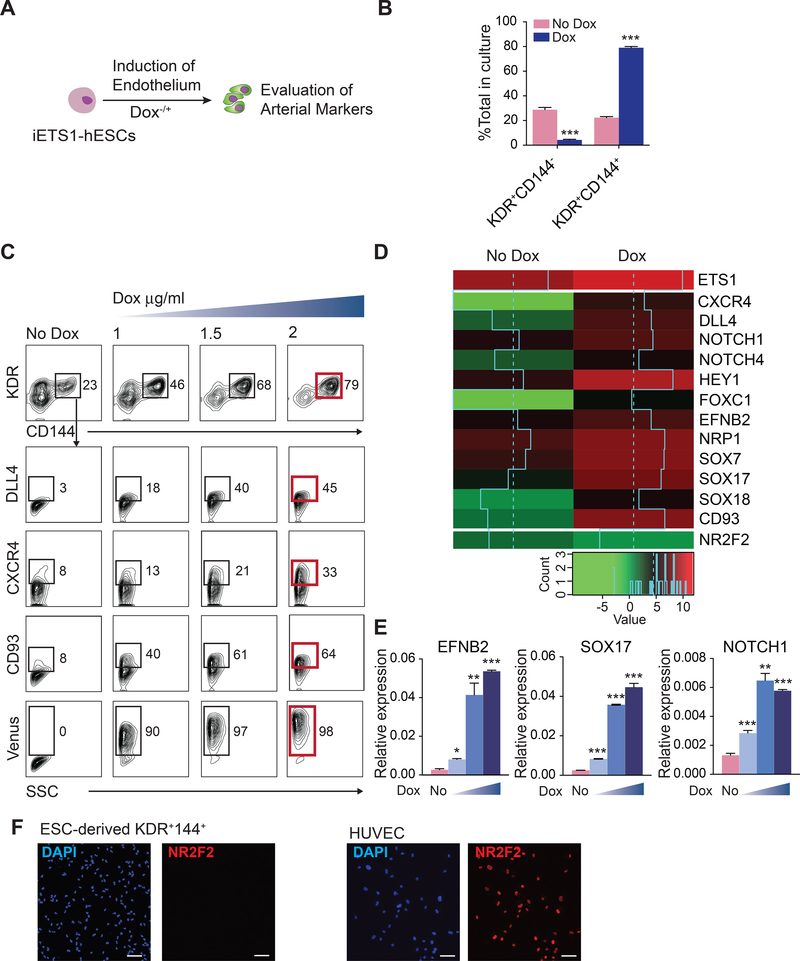
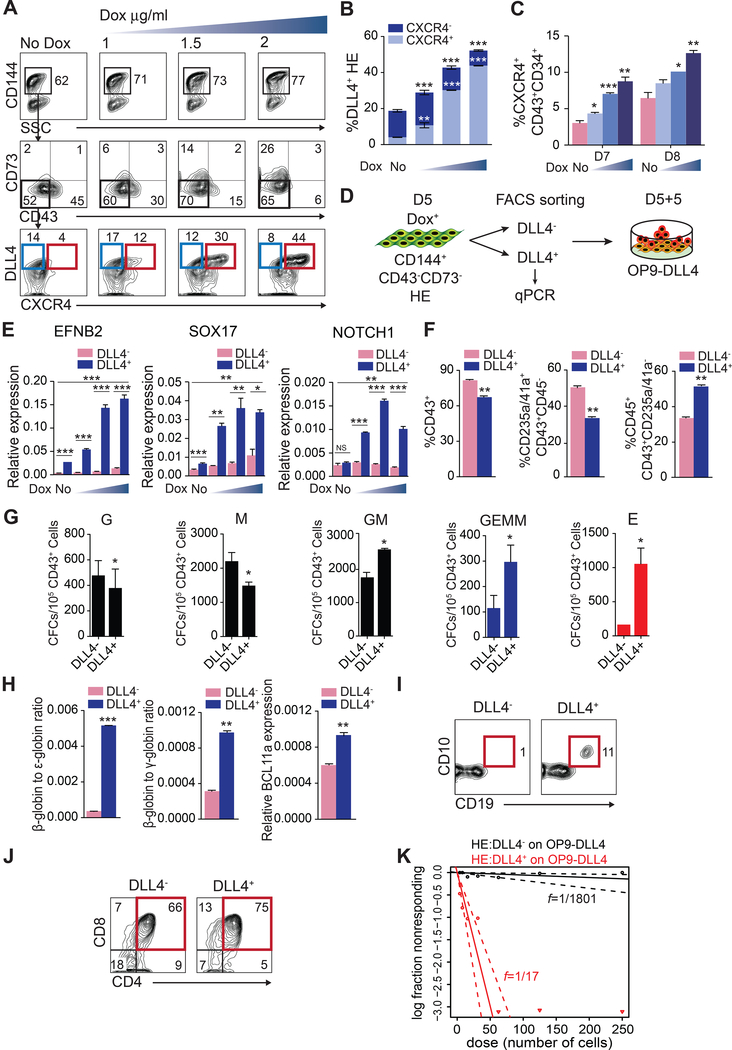
In embryo, arterial fate is specified following induction of DLL4 expression (Chong et al., 2011) initiated by signaling through an arterial-specific enhancer located within the third intron of DLL4 that is controlled by ETS factors (Sacilotto et al., 2013; Wythe et al., 2013). To evaluate whether activation of arterial-specific enhancer affects arterial programming of HE and hematopoiesis from hPSCs, we engineered H1 human embryonic stem cells (hESC) carrying doxycycline (DOX)-inducible ETS1 transgene (iETS1-hESCs; Figure S1) and differentiated them to endothelial and hematopoietic cells in chemically defined conditions (Uenishi et al., 2014). We treated cultures with DOX beginning at mesodermal stage of development (day 2 of differentiation) and analyzed the expression of arterial markers DLL4 and CXCR4 (Chong et al., 2011; Yamamizu et al., 2010) on CD144+ (VE-cadherin+) endothelial cells emerging on day 4 of differentiation (Figure 1A). As shown in Figures 1B and 1C, DOX treatment increased formation of CD144+ endothelial cells and induced expression of DLL4 and CXCR4 in a dose-dependent manner. RNAseq analysis of endothelial cells isolated on day 4 of differentiation, revealed that DOX treatment led to a more than 3-fold upregulation in ETS1 mRNA levels along with a marked increase in arterial specification-associated genes including CXCR4, NOTCH ligand DLL4, NOTCH1, NOTCH4, HEY1, SOXF group genes (SOX7, SOX17, and SOX18) (Corada et al., 2013; Duarte et al., 2004; Gale et al., 2004; Kim et al., 2007; Sacilotto et al., 2013; Villa et al., 2001; Yamamizu et al., 2010; Yurugi-Kobayashi et al., 2006), as well as CD93, a gene associated with emerging HSCs in AGM region (Bertrand et al., 2005), without upregulation of venous marker NR2F2 (Figure 1D). Arterial genes upregulation was confirmed by RT-qPCR (Figure 1E) and the lack of NR2F2 venous marker expression was confirmed by immunofluorescent staining (Figure 1F). Based on these we findings, we concluded that ETS1 upregulation enhances arterial specification from hPSCs.
Figure 1. ETS1 induction enhances arterial specification of hPSCs.
(A) Experimental scheme. iETS1 hESC were differentiated in defined conditions with or without DOX for 4 days and evaluated for expression of arterial markers in CD144+ endothelial cells. (B) The effect of DOX treatment on generation of CD144+ endothelial cells. (C) Flow cytometric analysis of arterial markers expression by hESC-derived endothelial cells following DOX treatment (1, 1.5 or 2 μg/ml) for 2–4 days. Representative data of three independent experiments is shown. (D) Heat map of arterial and venous genes expression in day 4 KDR+CD144+ endothelial cells obtained with or without ETS1 induction as determined by RNAseq analysis. Log2-transformed Transcripts Per Million (log2(TPM)) are used for color mapping. (E) RT-qPCR analysis confirms upregulation of arterial genes following DOX treatment. (F) Immunofluorescent analysis of NR2F2 expression by iETS1-hESCs derived KDR+CD144+ endothelial cells in DOX-treated conditions. Human umbilical vein endothelial cells (HUVECs) served as positive control for NR2F2 expression; scale bar is 50 μm. Bar graphs in (B) and (E) are mean ± SD of at least three independent experiments; *p<0.05; **p<0.01; ***p<0.001. See also Figure S1.
ETS1 induction at mesodermal stage enhances definitive hematopoiesis from hESCs.
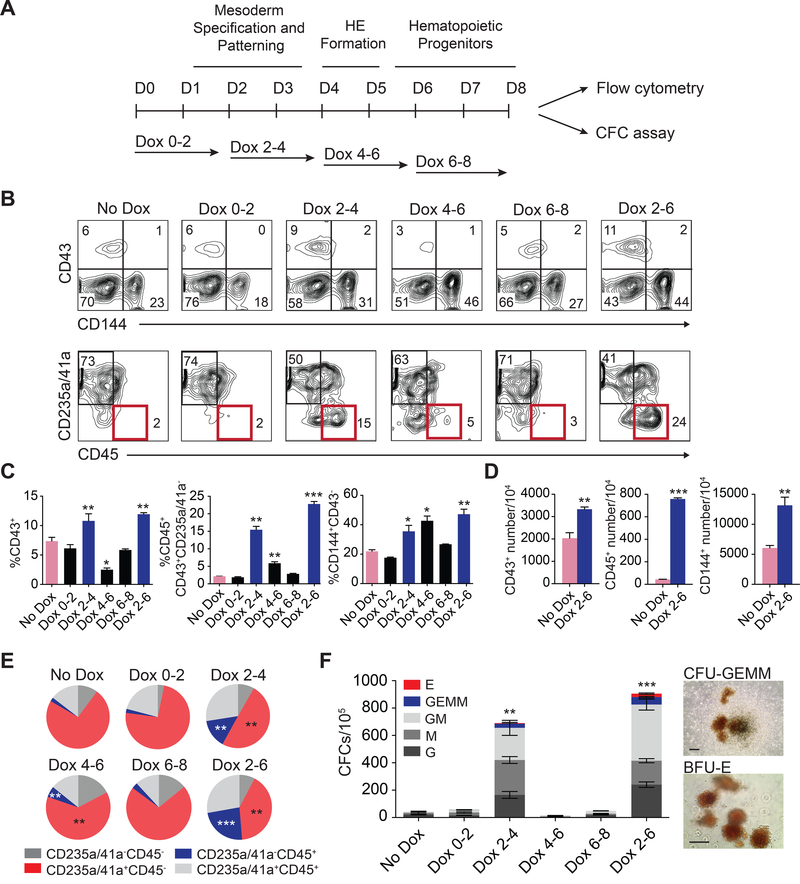
To determine how ETS1 affects hematopoiesis and whether its effect on hematopoiesis is associated with arterial program activation in HE, we treated cells with DOX in a stepwise fashion as depicted in Figure 2A. In our differentiation system, hPSCs undergo a stepwise progression toward APLNR+PDGFRα+ mesoderm with hemangioblast colony forming cells (HB-CFCs) that reflects primitive hematopoiesis, KDRhighPDGFRαlow/− hematovascular mesodermal progenitors with definitive hematopoietic potential, CD144+CD43−CD73− HE and CD43+ hematopoietic progenitors which include CD235a+CD41a+ erythromegakaryocytic progenitors (E-MK) and CD235a/41a−CD45+/− hematopoietic progenitors with lin−CD34+CD90+CD38CD45RA− hematopoietic stem/progenitor cell (HSPC) phenotype (Choi et al., 2012; Choi et al., 2009; Uenishi et al., 2014; Vodyanik et al., 2006) (Figure S2A). Stepwise DOX treatment experiments, revealed that upregulation of ETS1 during hematovascular mesoderm and HE specification on days 2–4 or 2–6 of differentiation produced the most profound effect on generation of CD43+ and CD45+ hematopoietic progenitors (Figures 2B-2E). Importantly, ETS1 upregulation increased the proportion and absolute numbers of multipotential CD235a/CD41a−CD45+ progenitors and CFC-GEMM frequencies (Figures 2C-2F). Typically, colonies from DOX+ were large (Figure 2F). ETS1 induction before mesoderm establishment (days 0–2) or post-HE stage (days 6–8) had minimal effect on blood production (Figures 2B-2F). Thus, we concluded that the window for the optimal effect of ETS1 on hematopoiesis coincided with amplification of arterial program by ETS1.
Figure 2. Stage-specific effect of ETS1 on hematopoietic development.
(A) Experimental scheme. (B) Flow cytometric analysis of the hematopoietic progenitors obtained from iETS1 hESCs treated with DOX at indicated time points. Representative experiment of three independent experiments is shown. (C) The percentage of hematopoietic and endothelial cells in day 8 of differentiation cultures following DOX treatment at indicated time points. (D) Bar graph shows the total number of CD43+, CD45+ and CD144+ generated from 104 iETS1-hESCs in DOX-treated and untreated conditions. (E) Pie charts display the composition of CD43+ subsets. (F) Hematopoietic colony-forming potential of iETS1 hESCs treated with DOX at indicated time points. Photographs show GEMM and BFU-E colonies generated from DOX-treated cultures. Image bar is 200 μm. Bar graphs in (C), (D) and (F) are mean ± SD. for two independent experiments performed in duplicates; *p<0.05; **p<0.01; ***p<0.001 compared to no DOX treatment. See also Figure S2.
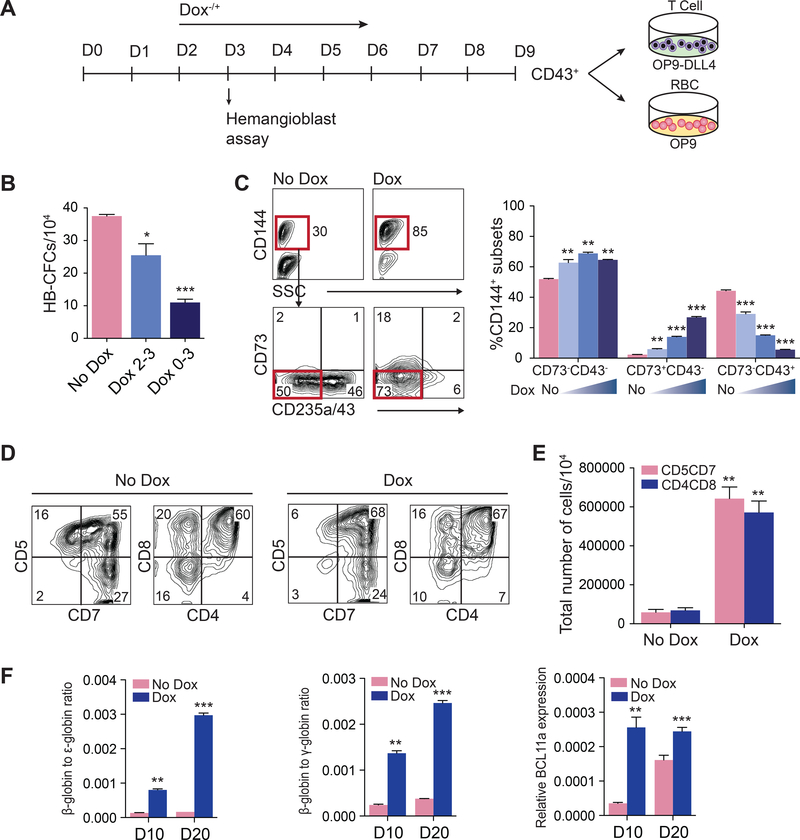
To define which type of hematopoiesis is affected by ETS1 overexpression, we evaluated the effect of DOX treatment on i) hemangioblast (HB) CFCs that reflect the primitive wave of hematopoiesis (Choi et al., 2012; Kennedy et al., 2007) and ii) on T lymphocytes and β-hemoglobin-producing red blood cells that reflect definitive hematopoiesis (Figure 3A) (Choi et al., 2012; Kennedy et al., 2012). When cultures were treated with DOX on day 2 of differentiation, we observed on day 3 a significant decrease in APLNR+PDGFRα+ primitive mesodermal cells (Figure S2B and S2C) and HB-CFCs compared to control (Figure 3B). This effect was more profound when cultures were treated with DOX from day 0 through day 3 to ensure maximum ETS1 overexpression on day 3 (Figure 3B, S2B and S2C). In contrast, DOX treatment starting from day 2 of differentiation increased formation of CD144+CD43−CD73− HE with definitive potential (Choi et al., 2012) on day 5 of differentiation in a dose-dependent manner (Figure 3C). To determine the effect of ETS1 upregulation on T cell potential, we collected CD43+ cells from DOX+ and DOX− cultures and subcultured on OP9-DLL4 stromal cells. Although cells collected from both conditions generated a similar percentage of CD5+CD7+ and CD4+CD8+ T lymphoid cells (Figure 3D), CD43+ cells from DOX+ cultures produced a dramatically (~8 fold) greater number of T lymphoid cells per 104 CD43 cells (Figure 3E). In addition, we found that CD43 cells collected following DOX treatment upregulated adult β-hemoglobin and BCL11a genes associated with definitive erythropoiesis (Sankaran et al., 2008) when cultured in erythroid conditions (Figure 3F); and produced up to 3-fold more megakaryocytes when cultured in megakaryocytic conditions (Figure S2E and S2F). Overall, these studies suggest that ETS1 upregulation suppresses primitive and promotes definitive hematopoiesis from hPSCs, most likely through enhancement of definitive HE specification at the mesodermal stage.
Figure 3. ETS1 induction suppresses primitive while promoting definitive hematopoiesis.
(A) Experimental scheme. (B) Effect of ETS1 induction on hemangioblast (HB)-CFCs. (C) Flow cytometric analysis shows the increase of CD144+CD73−CD235a/43− HE population at day 5 of differentiation of iETS1-hESC cultures treated with DOX for 2–5 days. (D) CD43+ cells generated in DOX-treated and untreated cultures possess T cell potential. Representative experiment of 3 independent experiments shows the expression of T cell markers on CD45-gated cells in T cell differentiation cultures. (E) Bar graph shows the total number of T cells generated from 104 CD43+ cells obtained from iETS1-hESCs in DOX-treated and untreated conditions. (F) Ratio of β/ε and β/γ globin chain and BCL11a gene expression as measured by RT-qPCR in red blood cell cultures generated from CD43+ cells obtained from DOX-treated and untreated iETS1-hESC cultures. Bar graphs in (B), (C), (E) and (F) are mean ± SD of at least three independent experiments. *p<0.05; **p<0.01; ***p<0.001 compared to no DOX treatment. See also Figure S2.
ETS1 overexpression promotes definitive hematopoiesis through NOTCH-mediated signaling
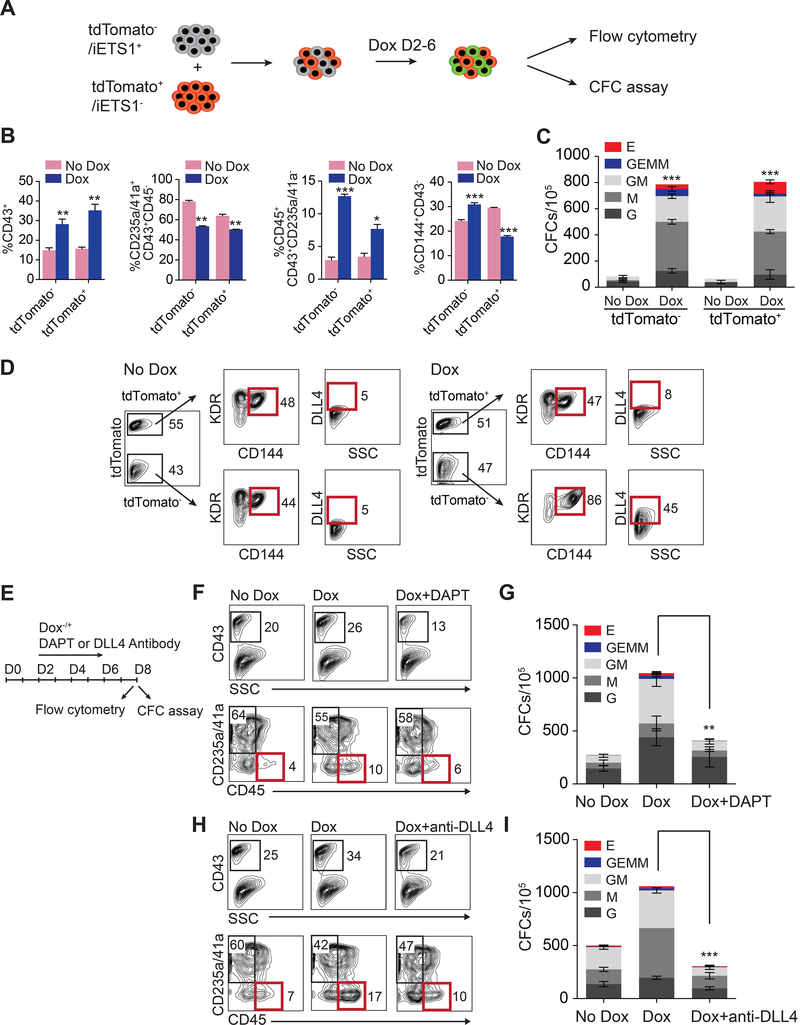
To determine whether ETS1 induction promotes definitive hematopoietic program in a cell-autonomous or non-autonomous manner, we mixed tdTomato (tdT) transgenic H1 hESC with iETS1 H1 hESCs and analyzed hematopoietic development from chimeric cultures with and without induction of ETS1 expression (Figure 4A). These studies revealed that ETS1 upregulation enhanced the production of CD43+ hematopoietic progenitors, including CD45+ progenitors, from both tdT+ and tdT− cells (Figure 4B and S3). When hematopoietic cells were collected on day 8 of differentiation and assayed for CFC potential, the number of hematopoietic colonies in both tdT+ and tdT− were increased following DOX treatment (Figure 4C). Interestingly, after 4 days of culture, endothelial cells in D4 the tdT− fraction expressed greater levels of DLL4 as compared to tdT+ cells (Figure 4D). These results indicate that ETS1 overexpression expands DLL4 expressing CD144+ endothelial cells and suggests that ETS1 could promote definitive hematoendothelial program through upregulation of NOTCH signaling.
Figure 4. ETS1 induction promotes hematopoiesis through upregulation of NOTCH signaling.
(A) Schematic diagram of experiment with chimeric hESC cultures. Mixture of tdTomato− iETS1 and tdTomato+ H1 hESCs were cultured with or without DOX (2 μg/ml) for 2–6 days during hematopoietic differentiation in chemically defined culture conditions. (B) Flow cytometric analysis of hematopoietic development on day 8 of differentiation following gating tdTomato+ or tdTomato− (iETS1) cells in DOX-treated and untreated cultures. (C) Hematopoietic colony-forming potential of tdTomato+ or tdTomato− cells on day 8 of differntiation. (D) Flow cytometric analysis of DLL4 expression in day 4 KDR+CD144+ population following gating tdTomato+ or tdTomato− cells in DOX-treated and untreated cultures. (E) Schematic diagram of experiment to assess the role of NOTCH signaling in ETS1-mediated effect on hematopoiesis. The effect of DAPT treatment on blood production (F) and CFC potential (G) in cultures treated with DOX. The effect of DLL4 neutralizing antibodies on blood production (H) and CFC potential (I) in cultures treated with DOX. Bar graphs in (B), (C), (G) and (I) are mean ± SD of three independent experiments; *p<0.05, **p <0.01; ***p<0.001. See also Figure S3.
To confirm the role of NOTCH activation in promoting definitive hematopoiesis by ETS1, we evaluated hematopoiesis following ETS1 upregulation in the presence of NOTCH signaling inhibitor DAPT, and DLL4 neutralizing antibodies (Figure 4E). As shown in Figures 4F-4I, treatment of hESC cultures with NOTCH signaling inhibitor DAPT, or DLL4 antibodies, abrogated effect of ETS1 upregulation on hematopoiesis, thereby confirming the important role of DLL4 expression and NOTCH activation in ETS1-mediated promotion of definitive hematoendothelial program.
ETS1 overexpression induces HE with DLL4+CXCR4+/− arterial phenotype.
Although previous studies found that DLL4+ endothelial cells in hPSC cultures have reduced hematopoietic potential as compared to DLL4− cells (Ayllon et al., 2015; Ditadi et al., 2015), we observed that increased definitive hematopoietic cell production following ETS1 overexpression was correlated with marked increase in cells expressing arterial markers DLL4 and CXCR4 (Chong et al., 2011; Yamamizu et al., 2010) within the CD144+CD43−CD73− HE population (Figures 5A and 5B). This suggests that enhancement of the definitive hematopoietic program could be attributed to DLL4+ HE population that acquires arterial characteristics, as determined by analysis of EFNB2, SOX17 and NOTCH1 arterial markers by RT-qPCR (Figure 5E). To determine whether DLL4+ arterial type HE has hematopoietic potential, we sorted DLL4+ and DLL4− cells and assessed blood formation from them following 5 days secondary culture on OP9-DLL4 (Figure 5D). Although DLL4+ produced a relatively lower number of CD43+ cells, the proportion of multipotential CD235a/CD41a−CD45+ progenitors was greater in DLL4+ cultures compared to DLL4− (Figure 5F). Hematopoietic progenitors collected from DLL4+ HE also produced a greater number of multipotential CFC-GM and -GEMM (Figure 5G) and generated erythroid cells with substantially higher expression of adult β-hemoglobin and BCL11a (Figure 5H). Importantly, the most significant difference was observed in the lymphoid potentials of DLL4+ and DLL4− HE (Figures 5I-5K). As shown in Figure 5I, only DLL4+ cells had B cell potential. While both DLL4+ and DLL4− cells possessed T cell potential (Figure 5J), the limiting dilution analysis revealed more than a 100-fold enrichment in T cell progenitors in DLL4+ fraction. Interestingly, we have previously shown that in contrast to fetal liver HSCs, PSC-derived hematopoietic progenitors have decreased expression of HSC homing receptor CXCR4 (Salvagiotto et al., 2008). As demonstrated in Figure 5C, following ETS1 induction, not only HE, but CD34+CD43+ hematopoietic progenitors upregulated CXCR4 expression. Together, these data suggest that arterial specification of HE is associated with acquisition of definitive hematopoietic program. To further characterize DLL4+ arterial HE, we evaluated the hematopoietic potential of CXCR4+ and CXCR4− cells (Figure S4A). As shown in Figures S4B-4E, both CXCR4+ and CXCR4− fractions of DLL4+ HE cells generated multipotential CFCs and T cells. However, a more than 3-fold enrichment in T cell progenitors was observed in blood cells generated from CXCR4+ HE cells.
Figure 5. ETS1 promotes specification of arterial type HE.
(A) and (B) DOX treatment enhances specification of DLL4+CXCR4+/− arterial type HE in dose-dependent manner. (C) DOX-treatment enhances production of CD34+CD43+ hematopoietic progenitors expressing CXCR4+ in a dose-dependent manner. (D) Schematic representation of the experimental strategy to assess hematopoietic potential of DLL4+ and DLL4− HE. (E) RT-qPCR analysis of arterial genes in DLL4+ and DLL4− HE. Hematopoietic (F) and CFC (G) potential of DLL4+ and DLL4− HE. (H) Ratio of β/ε and β/γ globin chain and BCL11a gene expression as measured by RT-qPCR in red blood cell cultures generated from DLL4+ and DLL4− HE. (I) B cell potential of DLL4+ and DLL4− HE. (J) T cell potential of DLL4+ and DLL4− HE. Flow cytometry plot depicts percentage of CD4+CD8+ T cells. (K) Limiting dilution assay to determine frequency of T cell progenitors in DLL4+ and DLL4− HE cultures. Bars in (B), (C), (E), (F), (G) and (H) are mean ± SD of at least three independent experiments; *p<0.05, **p <0.01; ***p<0.001. See also Figure S4.
Promotion of arterial specification of HE and definitive hematopoiesis by modulation of MAPK/ERK signaling
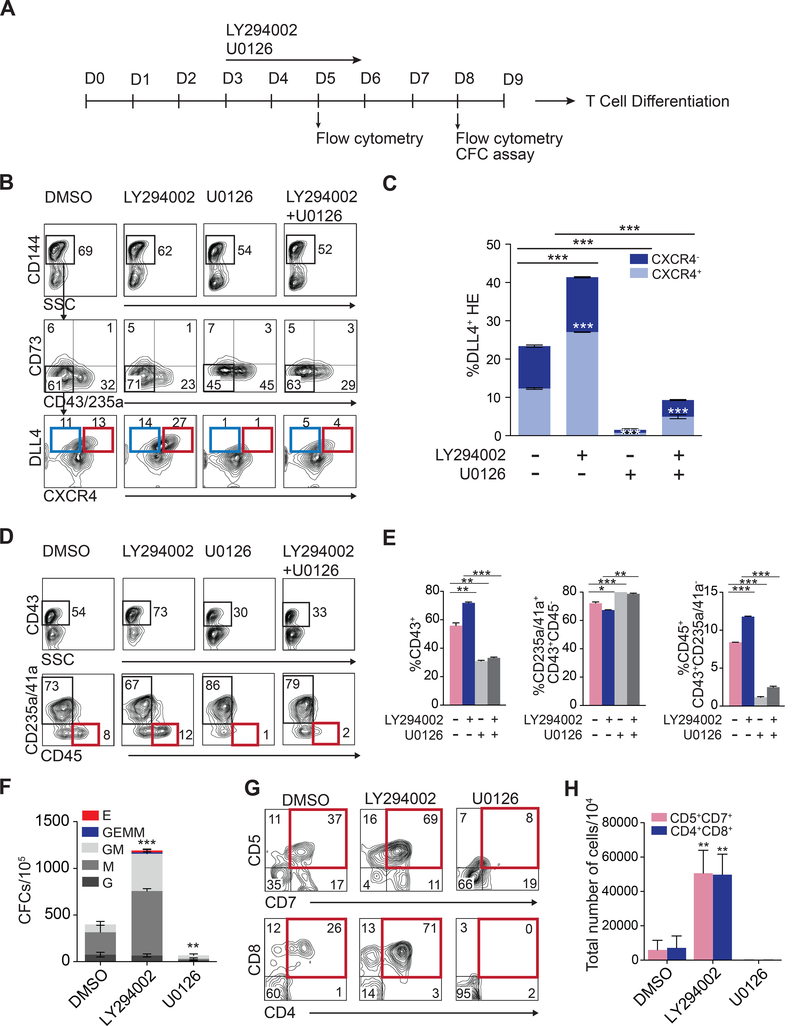
Arterial specification in the embryo is modulated by multiple pathways, including MAPK/ERK signaling. It has been shown that indirect ERK activation through inhibition of Phosphoinositide 3-kinase (PI3K) downstream of VEGF receptor signaling, enhances arterial specification in zebrafish, while inhibition of ERK branch blocks arterial specification (Herbert et al., 2009; Hong et al., 2006). To determine whether modulating MAPK/ERK signaling affects arterial specification of HE from hPSCs, we treated differentiation cultures on days 3 through 6 with PI3K inhibitor LY294002, or MEK1 and MEK2 inhibitor U0126 (Figure 6A). Activation or inhibition of ERK phosphorylation following corresponding treatments in our differentiation system was confirmed by Western blot (Figure S5A and S5B). These studies revealed that treatment with LY294002 enhanced production of DLL4+ arterial type HE, including the DLL4+CXCR4+ fraction, while U0126 treatment markedly abrogated formation of DLL4+ HE in control and LY294002-treated cultures (Figures 6B and 6C). We also observed a direct correlation between definitive hematopoiesis efficacy and arterial specification. When ERK pathways were activated following HE specification, CFC potential and production of multipotent CD235a/CD41a−CD45+ hematopoietic progenitors on day 8 of differentiation was dramatically increased, while ERK inhibition abrogated production of these types of cells (Figures 6D-6F). In addition, T lymphoid potential was significantly increased in cultures treated with LY294002 and entirely abrogated in cultures treated with U0126. Overall, these observations provide additional evidence for our hypothesis that enhancing arterial specification of HE is essential to establishing a definitive hematopoietic program with lymphomyeloid potentials from hPSCs.
Figure 6. Modulation of MAPK/ERK signaling enhances arterial specification and definitive hematopoiesis from hESCs.
(A) Experimental scheme. Effect of LY294002 and U0126 on arterial HE specification (B) and (C), hematopoietic (D)-(E), CFC (F) and T cell development (G) and (H) from hESCs. Bars in (C), (E), (F) and (H) are mean ± SD of at least three independent experiments; *p<0.05, **p <0.01; ***p<0.001. See also Figure S5-S6.
To investigate whether ERK’s effect on arterial HE specification and hematopoietic development is mediated through NOTCH signaling, we treated differentiation cultures with LY294002 in the presence or absence of NOTCH inhibitor DAPT. As shown in Figure S5D-S5G, DAPT treatment abrogated the enhancing effect of LY294002 on formation of DLL4+CXCR4+/− arterial HE and CD235a/CD41a−CD45+ multipotential hematopoietic progenitors, consistent with prior observations in the mouse system that MAPK pathway specifies arterial identity of non-HE through activation of NOTCH signaling (Wythe et al., 2013). It has also been demonstrated that MAPK/ERK pathway promotes phosphorylation of ETS1 and ETS2 (Petrovic et al., 2003; Yang et al., 1996) and other ETS factor members including ELK1 (Yang et al., 1998) and ERG (Fish et al., 2017). ERG phosphorylation is required for p300 recruitment to DLL4 arterial-specific enhancer and its activation (Fish et al., 2017; Wythe et al., 2013). To determine, whether ETS1 activity in our system is controlled by MAPK/ERK signaling, we assessed how ERK inhibition affects HE specification and hematopoietic differentiation in the presence of DOX (Figure S6A). We found that inhibition of ERK phosphorylation with U0126 in DOX-treated cultures essentially abrogated DOX-mediated induction of DLL4+CXCR4+/− arterial HE and CD235a/CD41a−CD45+ multipotential hematopoietic progenitors (Figure S6B-S6F). In aggregate, these results suggest that MAPK/ERK signaling is critical for ETS1 activity leading to NOTCH-mediated arterialization of HE and definitive hematopoiesis.
NOTCH and SOXF-mediated transcriptional program is activated in DLL4+ arterial HE
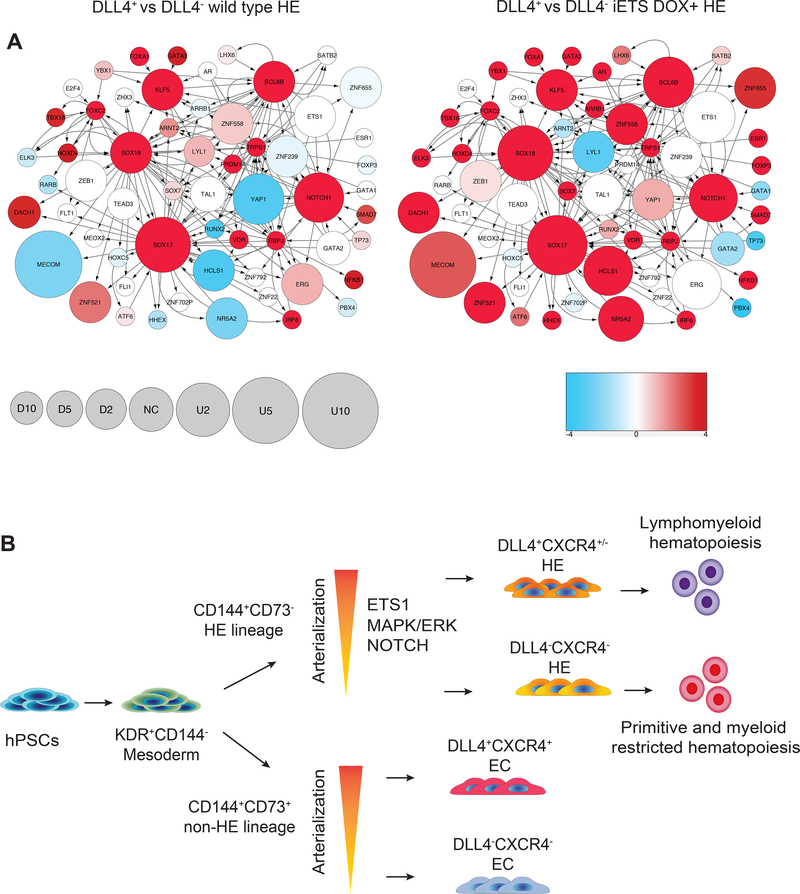
To determine the molecular program associated with establishing arterial HE, we performed RNAseq analysis of DLL4+ and DLL4− HE. As a basis for analysis, genes that were differentially expressed in a 3-way Bayesian model involving DLL4+ versus DLL4− wild type HE and DLL4+ versus DLL4− iETS1 HE from DOX cultures (Supplementary Dataset S1) were used. The transcriptional network relevant to the observed responses was visualized as described in Supplemental Experimental Procedures. Every node of the network reflects both regulon-level signal strength related to a particular transcription factor and the change in mRNA level of the transcript of the gene encoding that transcription factor. The relative abundance of mRNA expression in these networks was coded as node size, while color density represents enrichment (red) or depletion (blue) of known targets of that transcription factor (regulon members) among the differentially expressed genes. As shown in Figure 7A, the increased expression and regulon activity for NOTCH1, SOXF (SOX17, SOX18), KLF5 and BCL6B genes was a distinct feature of DLL4+ arterial HE from wild type and iETS1 hESCs in DOX cultures, although these features were more pronounced in iETS1 DLL4+ HE. As previously shown, proinflammatory signaling plays an important role in HSC development (Espin-Palazon et al., 2014; Li et al., 2014). Interestingly, the regulons of NFKB1 and IRF6 factors were activated in DLL4+ cells suggesting that arterialization of HE is associated with activation of proinflammatory signaling. Despite ETS1 overexpression in DOX cultures, ETS1 regulon signal in iETS1-DLL4+ HE was poor. This is consistent with our findings that the effect of ETS1 is primarily mediated through upregulation of signaling from NOTCH1 and likely SOXF transcription factors, rather than from any immediate activity of ETS1. Overall, these studies suggest that activation of arterial program in HE is primarily driven by the NOTCH and SOXF-driven transcriptional programs.
Figure 7. Gene expression profiling reveals activation of NOTCH and SOXF-mediated transcriptional programs in DLL4+ arterial HE.
(A) Transcriptional regulatory network reconstructed based on analysis of differentially expressed genes in DLL4+ and DLL4− HE cells. Nodes size represents the relative abundance of mRNA of the respective gene, computed as log2 (fold change) in DLL4+ and DLL4− HE cells (see balloon size scale below; U is upregulated, NC is no change and D is downregulated). Color density represents enrichment (red) or depletion (blue) of known targets of that transcription factor (regulon members) among the differentially expressed genes. Color scale: numbers are signed log-transformed FDR values -log10(FDR) for upregulation (positive numbers), log10(FDR) for dowenregulation (negative numbers). Network visualization was performed using Cytoscape ver. 3.4.0 (Shannon et al., 2003). (B) Schematic of arterial HE induction from hPSCs by overexpression of ETS1 or modulation of MAPK/ERK signaling pathways. At least two distinct HE and no-HE endothelial cell lineages are formed in hPSC cultures from mesoderm. As previously shown, CD73+ non-HE specifies into arterial and venous endothelial populations (Ditadi et al., 2015). In present study, we show that similar to non-HE, HE undergoes specification into arterial and venous-type populations with definitive lymphomyeloid and restricted erythro-myeloid hematopoietic potential, respectively. Arterial specification of HE can be promoted by overexpression of ETS1 or by modulation of MAPK/ERK signaling pathways.
Discussion
In present studies, we demonstrated that definitive hematopoiesis from hPSCs could be promoted through activation of arterial program in HE through two mechanisms: overexpression of transcription factor ETS1, which has the capacity to activate arterial-specific enhancer in the third intron of DLL4 gene (Sacilotto et al., 2013; Wythe et al., 2013), or through modulation of MAPK/ERK signaling by small molecules. Both of these approaches induced formation of DLL4+CXCR4+/− arterial type HE that is highly enriched in definitive hematopoietic progenitors with T and B lymphoid potentials. In addition, arterial program activation enhanced production of CD34+CD43+ hematopoietic progenitors expressing HSC homing receptor CXCR4, which is typically not present in hematopoietic progenitors in traditional hESC differentiation cultures (Salvagiotto et al., 2008). Thus, our studies suggest that HE lineage similar to non-HE lineage can undergo arterialization (Figure 7B). These in vitro findings correlate with in vivo observations that demonstrated expression of DLL4 arterial marker by HE underlying intra-aortic hematopoietic clusters in the avian AGM (Richard et al., 2013), significant enrichment in pre-HSCs in the DLL4+ fraction of mouse AGM HE (Hadland et al., 2017) and selective impairment of hematopoiesis in the dorsal aorta, but not yolk sac, in EfnB2−/− mice (Chen et al., 2016).
The effect of ETS1 and MAPK/ERK signaling on arterial HE specification was primarily mediated through upregulation of DLL4 expression and activation of NOTCH signaling. In addition, epistasis experiments revealed a strong dependence of ETS1 activity on MAPK/ERK signaling during HE development. Prior studies in zebra fish revealed that VEGF/ERK signaling induces phosphorylation ETS transcription factor ERG in non-HE (Fish et al., 2017; Wythe et al., 2013). This phosphorylation enhances DNA binding activity of ERG leading to activation of arterial-specific DLL4 enhancer. Since ETS1 is phosphorylated by MAPKs (Petrovic et al., 2003; Yang et al., 1996), it is highly likely that ETS1 effect on arterial specification of HE is controlled by VEGF/MAPK signaling in a similar manner.
Discovering the important role of arterial programming in lymphoid development from PSCs, allowed us to significantly improve T cell progenitor production in defined conditions by applying small molecules to enhance formation of arterial type HE. Scalable T cell production is essential to advance translation of iPSC-based immunotherapies into the clinic. However, in vivo studies from ETS1-induced cultures have failed to show evidence of long or short-term engraftable cells (data not shown). As we demonstrated the effect of ETS1 or MAPK/ERK signaling on hematopoiesis is mostly mediated through activation of NOTCH signaling. NOTCH signaling must be downregulated following EHT (Lizama et al., 2015), most likely through cis-inhibition (Hadland et al., 2017), and fine tuning of NOTCH signaling is required for the appropriate pre-HSC type I HSC development and transition into pre-HSC type II (Souilhol et al., 2016). Thus, excessive activation of arterial program and NOTCH following ETS1 overexpression in our study may not allow for appropriate calibration of NOTCH signaling during EHT and establishment of the self-renewal program required for engraftment. It is also possible that arterial program activation is required, but not sufficient for HSC induction ex vivo. Studies in zebra fish revealed that HSC specification is also regulated by mechanisms uncoupled from arterial patterning (Burns et al., 2009; Monteiro et al., 2016). Molecular profiling of PSC-derived phenotypical HSCs in human and mouse systems has revealed that in addition to a deficient Notch-signaling signature (McKinney-Freeman et al., 2012), ex vivo generated cells compared to their in vivo counterparts, demonstrated deficiency in HOXA and AP-1 complex gene expression that are functioning independently of arterial programming (Dou et al., 2016; Ng et al., 2016; Salvagiotto et al., 2008; Sugimura et al., 2017). Our RNAseq studies found no sunstantial effect of ETS1 on the expression of HOXA and AP-1 complex genes, suggesting that ETS1-activated arterial programming has a major effect on NOTCH-mediated developmental pathways, but fails to affect molecular programs uncoupled from arterial development. Thus, further exploration of the interplay between mechanisms coupled and uncoupled with arterial specification and deciphering kernels for the gene regulatory network required for HSC development, will be essential to further advance HSC generation for clinical purposes.
Experimental Procedures
hESC lines maintenance and hematopoietic differentiation
H1 hESCs were obtained from WiCell Research Institute (Madison, WI). H1 hESC line, the iETS1 H1 hESC line and the tdTomato H1 hESC lines were maintained on matrigel in mTeSR1 medium. Cells were passaged every 3–4 days using 0.5 mM EDTA in PBS. The hESC lines were differentiated on ColIV coating plate (Uenishi et al., 2014). Singular iETS1 hESCs were plated 10,000 cells/cm2 to 6 well plates coated with ColIV (Sigma). After 1 day, media was changed to IF9S media with 50 ng/ml FGF (Peprotech), 50 ng/ml BMP4 (Peprotech), 15 ng/ml Activin A (Peprotech) and 2 mM LiCl (Sigma). On day 2, media was changed to IF9S media with 50 ng/ml FGF and 50 ng/ml VEGF (Peprotech). On day 4, media was changed to IF9S media with 50 ng/ml FGF, 50 ng/ml VEGF, 50 ng/ml TPO (Peprotech), 50 ng/ml SCF (Peprotech), 50 ng/ml IL-6 (Peprotech) and 10 ng/ml IL-3 (Peprotech). On day 6, IF9S media with cytokines the same as day 4 media was added to the culture.
Assessment of hematopoietic potential of DLL4− and DLL4+CXCR4+/− HE.
The iETS1 DOX-treated cells from day 5 of culture were dissociated into single cells by treatment with 1X TrypLE and stained with DLL4-PE, CD144-PerCPVio700, CD43-APC and CD73-BV421 antibodies and then sorted using a FACSAria II cell sorter (BD Biosciences) for isolation of DLL4+ and DLL4− HE. For isolation of CXCR4+ and CXCR4-DLL4+ HE, cells were stained with DLL4-PE, CD144-PerCPVio700, CXCR4-PEVio770, CD73-APC and CD43-APCVio770 antibodies and sorted using a FACSAria II (BD Biosciences). Isolated day 5 DLL4+CD144+CD73−CD43− and DLL4−CD144+CD73−CD43− HE, or CXCR4+DLL4+CD144+CD73−CD43− and CXCR4-DLL4+CD144+CD73−CD43− HE were cultured at a concentration of 4×104 cells per well on a monolayer of Mitomycin C (Cayman Chemicals)-pretreated OP9 cells expressing human DLL4 (OP9-DLL4) in medium with SCF (50 ng/ml), TPO (50 ng/ml), IL-3 (10 ng/ml) and IL-6 (20 ng/ml) (PeproTech) in 6-well plates as described previously (Choi et al., 2012). After 5 days of cultures on OP9-DLL4, cells were harvested and analyzed by flow cytometry. The floating CD43+ cells were collected for T cell or RBC differentiation.
T and B cell differentiation
T cell differentiation of hESC-derived hematopoietic precursors was performed on OP9-DLL4 in T cell differentiated medium consisting of α-MEM (Gibco) supplemented with 20% FBS (HyClone), 5 ng/ml FLT3L, 5 ng/ml IL-7 and 10 ng/ml SCF (PeproTech) as described previously (Uenishi et al., 2014). For B cell differentiation, sorted DLL4+ and DLL4− HE cells were cocultured on OP9 for 4 weeks in αMEM medium containing 20% FBS, FLT3L (5 ng/ml) and IL7 (5 ng/ml) (PerproTech). Cultures were fed with complete media changes weekly. Presence of B cells was confirmed via staining with CD19 APC (Miltenyl Biotech) and CD10 PE (BD Biosciences) antibodies.
Limiting dilution assay to determine frequency of T cell progenitors
Limiting Dilution Assays were conducted with the floating blood cells collected from DLL4+ and DLL4− HE or CXCR+ and CXCR−DLL4+ HE cultured on OP9-DLL4 for 5 days. Row A of a 96-well plate received 500 cells/well, and each subsequent row afterwards received half the previous row (e.g. Row B contained 250, Row C contained 125, etc, until eventually Row H contained 3–4 cells). Wells were assessed 2 weeks later under microscope for presence of floating blood cells and by flow-cytometry for CD5+CD7+ expressing cells. Extreme limiting dilution analysis was conducted using a previously established algorithm (Hu and Smyth, 2009).
Flow cytometry
Cells were analyzed using MACSQuant Analyzer 10 (Miltenyi Biotec) and FlowJo software (Tree star). Cell surface staining utilized the antibodies listed in Table S1. FACS plots with FMO controls and graduations used to identify and gate hematopoietic progenitor and HE subpopulations are presented in Supplementary Figure S7.
DAPT and DLL4 antibody treatment
Notch signaling was blocked by DAPT (γ-secretase inhibitor/GSI, 10 μM, Cayman Chemicals) or DLL4 blocking antibody (10 μg/ml, Creative BioLabs).
LY294002 and U0126 treatment
MAPK/ERK pathway was activated using LY294002 (2–3 μM, Cayman Chemicals) and was inhibited using U0126 (1–2 Mm, Cayman Chemicals)
Quantitative real time PCR
Total RNA was isolated using the RNeasy Plus Micro Kit (Qiagen). RNA was reverse transcribed into cDNA using Oligo(dT) with ImProm-II Reverse Transcriptase (Promega). Real time quantitative PCR was performed in triplicate using SYBR Advantage qPCR Premix (Clontech) on Mastercycler ep realplex (Eppendorf). Gene expression was evaluated as DeltaCt relative to the RPL13a gene. Primer sequences are listed in Table S2.
Supplementary Material
Data Set S1. Genes differentially expressed in DLL4+ and DLL4− HE generated from wild type H1 hESCs or iETS1-hESCs in presence of DOX. Related to Figure 7. Column A, Gene ID; B, Posterior Probability of Equal Expression; C-D, tpm values for the libraries representing DLL4+ and DLL4− HE; E, signed tpm ratio of DLL4+/DLL4− populations, negative if DLL4− population had higher expression; F, Gene name. Tab WT, wild type hESCs differentiated without DOX; DOX1.5, iETS1 hESCs differentiated in presence of 1.5 μg/ml DOX.
Acknowledgments
We thank Matthew Raymond for editorial assistance. This work was supported by funds from the National Institute of Health (R01HL116221, U01HL099773, U01HL134655, P51 RR000167), and The Charlotte Geyer Foundation.
Footnotes
Declaration of Interests
M.A.P., A.K. and I.I.S. filed patent applications related to use of ETS1 and arterial programming to increase definitive lymphomyeloid hematopoiesis. Remaining authors declare no competing interests.
ACCESSION NUMBERS
The accession number for the RNA-seq data reported in this paper is GEO: GSE96815 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE96815).
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures, seven figures, two tables and one data file and can be found with this article online at https://doi.org/10.1016/j.celrep.2018.04.092
References
- Ayllon V, Bueno C, Ramos-Mejia V, Navarro-Montero O, Prieto C, Real PJ, Romero T, Garcia-Leon MJ, Toribio ML, Bigas A, et al. (2015). The Notch ligand DLL4 specifically marks human hematoendothelial progenitors and regulates their hematopoietic fate. Leukemia 29, 1741–1753. [DOI] [PubMed] [Google Scholar]
- Bertrand JY, Giroux S, Golub R, Klaine M, Jalil A, Boucontet L, Godin I, and Cumano A (2005). Characterization of purified intraembryonic hematopoietic stem cells as a tool to define their site of origin. Proceedings of the National Academy of Sciences of the United States of America 102, 134–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisset JC, van Cappellen W, Andrieu-Soler C, Galjart N, Dzierzak E, and Robin C (2010). In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature 464, 116–120. [DOI] [PubMed] [Google Scholar]
- Burns CE, Galloway JL, Smith AC, Keefe MD, Cashman TJ, Paik EJ, Mayhall EA, Amsterdam AH, and Zon LI (2009). A genetic screen in zebrafish defines a hierarchical network of pathways required for hematopoietic stem cell emergence. Blood 113, 5776–5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen II, Caprioli A, Ohnuki H, Kwak H, Porcher C, and Tosato G (2016). EphrinB2 regulates the emergence of a hemogenic endothelium from the aorta. Sci Rep 6, 27195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KD, Vodyanik MA, Togarrati PP, Suknuntha K, Kumar A, Samarjeet F, Probasco MD, Tian S, Stewart R, Thomson JA, et al. (2012). Identification of the hemogenic endothelial progenitor and its direct precursor in human pluripotent stem cell differentiation cultures. Cell Rep 2, 553–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KD, Yu J, Smuga-Otto K, Salvagiotto G, Rehrauer W, Vodyanik M, Thomson J, and Slukvin I (2009). Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem Cells 27, 559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong DC, Koo Y, Xu K, Fu S, and Cleaver O (2011). Stepwise arteriovenous fate acquisition during mammalian vasculogenesis. Dev Dyn 240, 2153–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corada M, Orsenigo F, Morini MF, Pitulescu ME, Bhat G, Nyqvist D, Breviario F, Conti V, Briot A, Iruela-Arispe ML, et al. (2013). Sox17 is indispensable for acquisition and maintenance of arterial identity. Nat Commun 4, 2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruijn MF, Speck NA, Peeters MC, and Dzierzak E (2000). Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo. EMBO J 19, 2465–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditadi A, Sturgeon CM, and Keller G (2017). A view of human haematopoietic development from the Petri dish. Nat Rev Mol Cell Biol 18, 56–67. [DOI] [PubMed] [Google Scholar]
- Ditadi A, Sturgeon CM, Tober J, Awong G, Kennedy M, Yzaguirre AD, Azzola L, Ng ES, Stanley EG, French DL, et al. (2015). Human definitive haemogenic endothelium and arterial vascular endothelium represent distinct lineages. Nat Cell Biol 17, 580–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou DR, Calvanese V, Sierra MI, Nguyen AT, Minasian A, Saarikoski P, Sasidharan R, Ramirez CM, Zack JA, Crooks GM, et al. (2016). Medial HOXA genes demarcate haematopoietic stem cell fate during human development. Nat Cell Biol 18, 595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte A, Hirashima M, Benedito R, Trindade A, Diniz P, Bekman E, Costa L, Henrique D, and Rossant J (2004). Dosage-sensitive requirement for mouse Dll4 in artery development. Genes Dev 18, 2474–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espin-Palazon R, Stachura DL, Campbell CA, Garcia-Moreno D, Del Cid N, Kim AD, Candel S, Meseguer J, Mulero V, and Traver D (2014). Proinflammatory signaling regulates hematopoietic stem cell emergence. Cell 159, 1070–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish JE, Cantu Gutierrez M, Dang LT, Khyzha N, Chen Z, Veitch S, Cheng HS, Khor M, Antounians L, Njock MS, et al. (2017). Dynamic regulation of VEGF-inducible genes by an ERK/ERG/p300 transcriptional network. Development 144, 2428–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish JE, and Wythe JD (2015). The molecular regulation of arteriovenous specification and maintenance. Dev Dyn 244, 391–409. [DOI] [PubMed] [Google Scholar]
- Frame JM, Fegan KH, Conway SJ, McGrath KE, and Palis J (2016). Definitive Hematopoiesis in the Yolk Sac Emerges from Wnt-Responsive Hemogenic Endothelium Independently of Circulation and Arterial Identity. Stem Cells 34, 431–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale NW, Dominguez MG, Noguera I, Pan L, Hughes V, Valenzuela DM, Murphy AJ, Adams NC, Lin HC, Holash J, et al. (2004). Haploinsufficiency of delta-like 4 ligand results in embryonic lethality due to major defects in arterial and vascular development. Proc Natl Acad Sci U S A 101, 15949–15954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gering M, and Patient R (2005). Hedgehog signaling is required for adult blood stem cell formation in zebrafish embryos. Dev Cell 8, 389–400. [DOI] [PubMed] [Google Scholar]
- Goldie LC, Lucitti JL, Dickinson ME, and Hirschi KK (2008). Cell signaling directing the formation and function of hemogenic endothelium during murine embryogenesis. Blood 112, 3194–3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon-Keylock S, Sobiesiak M, Rybtsov S, Moore K, and Medvinsky A (2013). Mouse extraembryonic arterial vessels harbor precursors capable of maturing into definitive HSCs. Blood 122, 2338–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadland BK, Varnum-Finney B, Mandal PK, Rossi DJ, Poulos MG, Butler JM, Rafii S, Yoder MC, Yoshimoto M, and Bernstein ID (2017). A Common Origin for B-1a and B-2 Lymphocytes in Clonal Pre- Hematopoietic Stem Cells. Stem cell reports 8, 1563–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert SP, Huisken J, Kim TN, Feldman ME, Houseman BT, Wang RA, Shokat KM, and Stainier DY (2009). Arterial-venous segregation by selective cell sprouting: an alternative mode of blood vessel formation. Science 326, 294–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong CC, Peterson QP, Hong JY, and Peterson RT (2006). Artery/vein specification is governed by opposing phosphatidylinositol-3 kinase and MAP kinase/ERK signaling. Curr Biol 16, 1366–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, and Smyth GK (2009). ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J Immunol Methods 347, 70–78. [DOI] [PubMed] [Google Scholar]
- Kennedy M, Awong G, Sturgeon CM, Ditadi A, LaMotte-Mohs R, Zuniga-Pflucker JC, and Keller G (2012). T lymphocyte potential marks the emergence of definitive hematopoietic progenitors in human pluripotent stem cell differentiation cultures. Cell Rep 2, 1722–1735. [DOI] [PubMed] [Google Scholar]
- Kennedy M, D’Souza SL, Lynch-Kattman M, Schwantz S, and Keller G (2007). Development of the hemangioblast defines the onset of hematopoiesis in human ES cell differentiation cultures. Blood 109, 2679–2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Saunders TL, and Morrison SJ (2007). Sox17 dependence distinguishes the transcriptional regulation of fetal from adult hematopoietic stem cells. Cell 130, 470–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim PG, Albacker CE, Lu YF, Jang IH, Lim Y, Heffner GC, Arora N, Bowman TV, Lin MI, Lensch MW, et al. (2013). Signaling axis involving Hedgehog, Notch, and Scl promotes the embryonic endothelial-to-hematopoietic transition. Proc Natl Acad Sci U S A 110, E141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissa K, and Herbomel P (2010). Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature 464, 112–115. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, and Weinstein BM (2001). Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development 128, 3675–3683. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Vogel AM, and Weinstein BM (2002). sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell 3, 127–136. [DOI] [PubMed] [Google Scholar]
- Ledran MH, Krassowska A, Armstrong L, Dimmick I, Renstrom J, Lang R, Yung S, Santibanez-Coref M, Dzierzak E, Stojkovic M, et al. (2008). Efficient hematopoietic differentiation of human embryonic stem cells on stromal cells derived from hematopoietic niches. Cell Stem Cell 3, 85–98. [DOI] [PubMed] [Google Scholar]
- Li W, Ferkowicz MJ, Johnson SA, Shelley WC, and Yoder MC (2005). Endothelial cells in the early murine yolk sac give rise to CD41-expressing hematopoietic cells. Stem Cells Dev 14, 44–54. [DOI] [PubMed] [Google Scholar]
- Li Y, Esain V, Teng L, Xu J, Kwan W, Frost IM, Yzaguirre AD, Cai X, Cortes M, Maijenburg MW, et al. (2014). Inflammatory signaling regulates embryonic hematopoietic stem and progenitor cell production. Genes Dev 28, 2597–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Yoder MC, and Yoshimoto M (2014). Lymphoid progenitor emergence in the murine embryo and yolk sac precedes stem cell detection. Stem Cells Dev 23, 1168–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizama CO, Hawkins JS, Schmitt CE, Bos FL, Zape JP, Cautivo KM, Borges Pinto H, Rhyner AM, Yu H, Donohoe ME, et al. (2015). Repression of arterial genes in hemogenic endothelium is sufficient for haematopoietic fate acquisition. Nat Commun 6, 7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney-Freeman S, Cahan P, Li H, Lacadie SA, Huang HT, Curran M, Loewer S, Naveiras O, Kathrein KL, Konantz M, et al. (2012). The transcriptional landscape of hematopoietic stem cell ontogeny. Cell Stem Cell 11, 701–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvinsky A, Rybtsov S, and Taoudi S (2011). Embryonic origin of the adult hematopoietic system: advances and questions. Development 138, 1017–1031. [DOI] [PubMed] [Google Scholar]
- Monteiro R, Pinheiro P, Joseph N, Peterkin T, Koth J, Repapi E, Bonkhofer F, Kirmizitas A, and Patient R (2016). Transforming Growth Factor beta Drives Hemogenic Endothelium Programming and the Transition to Hematopoietic Stem Cells. Dev Cell 38, 358–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng ES, Azzola L, Bruveris FF, Calvanese V, Phipson B, Vlahos K, Hirst C, Jokubaitis VJ, Yu QC, Maksimovic J, et al. (2016). Differentiation of human embryonic stem cells to HOXA(+) hemogenic vasculature that resembles the aorta-gonad-mesonephros. Nat Biotechnol 34, 1168–1179. [DOI] [PubMed] [Google Scholar]
- North T, Gu TL, Stacy T, Wang Q, Howard L, Binder M, Marin-Padilla M, and Speck NA (1999). Cbfa2 is required for the formation of intra-aortic hematopoietic clusters. Development 126, 2563–2575. [DOI] [PubMed] [Google Scholar]
- Petrovic N, Bhagwat SV, Ratzan WJ, Ostrowski MC, and Shapiro LH (2003). CD13/APN transcription is induced by RAS/MAPK-mediated phosphorylation of Ets-2 in activated endothelial cells. J Biol Chem 278, 49358–49368. [DOI] [PubMed] [Google Scholar]
- Rahman N, Brauer PM, Ho L, Usenko T, Tewary M, Zuniga-Pflucker JC, and Zandstra PW (2017). Engineering the haemogenic niche mitigates endogenous inhibitory signals and controls pluripotent stem cell-derived blood emergence. Nat Commun 8, 15380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard C, Drevon C, Canto PY, Villain G, Bollerot K, Lempereur A, Teillet MA, Vincent C, Rossello Castillo C, Torres M, et al. (2013). Endothelio-mesenchymal interaction controls runx1 expression and modulates the notch pathway to initiate aortic hematopoiesis. Dev Cell 24, 600–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert-Moreno A, Guiu J, Ruiz-Herguido C, Lopez ME, Ingles-Esteve J, Riera L, Tipping A, Enver T, Dzierzak E, Gridley T, et al. (2008). Impaired embryonic haematopoiesis yet normal arterial development in the absence of the Notch ligand Jagged1. EMBO J 27, 1886–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybtsov S, Ivanovs A, Zhao S, and Medvinsky A (2016). Concealed expansion of immature precursors underpins acute burst of adult HSC activity in foetal liver. Development 143, 1284–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacilotto N, Monteiro R, Fritzsche M, Becker PW, Sanchez-Del-Campo L, Liu K, Pinheiro P, Ratnayaka I, Davies B, Goding CR, et al. (2013). Analysis of Dll4 regulation reveals a combinatorial role for Sox and Notch in arterial development. Proc Natl Acad Sci U S A 110, 11893–11898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvagiotto G, Zhao Y, Vodyanik M, Ruotti V, Stewart R, Marra M, Thomson J, Eaves C, and Slukvin I (2008). Molecular profiling reveals similarities and differences between primitive subsets of hematopoietic cells generated in vitro from human embryonic stem cells and in vivo during embryogenesis. Exp Hematol 36, 1377–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaran VG, Menne TF, Xu J, Akie TE, Lettre G, Van Handel B, Mikkola HK, Hirschhorn JN, Cantor AB, and Orkin SH (2008). Human fetal hemoglobin expression is regulated by the developmental stage-specific repressor BCL11A. Science 322, 1839–1842. [DOI] [PubMed] [Google Scholar]
- Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, and Ideker T (2003). Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13, 2498–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souilhol C, Gonneau C, Lendinez JG, Batsivari A, Rybtsov S, Wilson H, Morgado-Palacin L, Hills D, Taoudi S, Antonchuk J, et al. (2016). Inductive interactions mediated by interplay of asymmetric signalling underlie development of adult haematopoietic stem cells. Nat Commun 7, 10784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimura R, Jha DK, Han A, Soria-Valles C, da Rocha EL, Lu YF, Goettel JA, Serrao E, Rowe RG, Malleshaiah M, et al. (2017). Haematopoietic stem and progenitor cells from human pluripotent stem cells. Nature 545, 432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tober J, Maijenburg MW, and Speck NA (2016). Taking the Leap: Runx1 in the Formation of Blood from Endothelium. Curr Top Dev Biol 118, 113–162. [DOI] [PubMed] [Google Scholar]
- Uenishi G, Theisen D, Lee JH, Kumar A, Raymond M, Vodyanik M, Swanson S, Stewart R, Thomson J, and Slukvin I (2014). Tenascin C promotes hematoendothelial development and T lymphoid commitment from human pluripotent stem cells in chemically defined conditions. Stem cell reports 3, 1073–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa N, Walker L, Lindsell CE, Gasson J, Iruela-Arispe ML, and Weinmaster G (2001). Vascular expression of Notch pathway receptors and ligands is restricted to arterial vessels. Mech Dev 108, 161–164. [DOI] [PubMed] [Google Scholar]
- Vizcardo R, Masuda K, Yamada D, Ikawa T, Shimizu K, Fujii S, Koseki H, and Kawamoto H (2013). Regeneration of human tumor antigen-specific T cells from iPSCs derived from mature CD8(+) T cells. Cell Stem Cell 12, 31–36. [DOI] [PubMed] [Google Scholar]
- Vodyanik MA, Thomson JA, and Slukvin II (2006). Leukosialin (CD43) defines hematopoietic progenitors in human embryonic stem cell differentiation cultures. Blood 108, 2095–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Menendez P, Shojaei F, Li L, Mazurier F, Dick JE, Cerdan C, Levac K, and Bhatia M (2005). Generation of hematopoietic repopulating cells from human embryonic stem cells independent of ectopic HOXB4 expression. J Exp Med 201, 1603–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wythe JD, Dang LT, Devine WP, Boudreau E, Artap ST, He D, Schachterle W, Stainier DY, Oettgen P, Black BL, et al. (2013). ETS factors regulate Vegfdependent arterial specification. Dev Cell 26, 45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamizu K, Matsunaga T, Uosaki H, Fukushima H, Katayama S, Hiraoka-Kanie M, Mitani K, and Yamashita JK (2010). Convergence of Notch and beta-catenin signaling induces arterial fate in vascular progenitors. J Cell Biol 189, 325–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang BS, Hauser CA, Henkel G, Colman MS, Van Beveren C, Stacey KJ, Hume DA, Maki RA, and Ostrowski MC (1996). Ras-mediated phosphorylation of a conserved threonine residue enhances the transactivation activities of c-Ets1 and c-Ets2. Mol Cell Biol 16, 538–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Whitmarsh AJ, Davis RJ, and Sharrocks AD (1998). Differential targeting of MAP kinases to the ETS-domain transcription factor Elk-1. EMBO J 17, 1740–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurugi-Kobayashi T, Itoh H, Schroeder T, Nakano A, Narazaki G, Kita F, Yanagi K, Hiraoka-Kanie M, Inoue E, Ara T, et al. (2006). Adrenomedullin/cyclic AMP pathway induces Notch activation and differentiation of arterial endothelial cells from vascular progenitors. Arterioscler Thromb Vasc Biol 26, 1977–1984. [DOI] [PubMed] [Google Scholar]
- Yzaguirre AD, and Speck NA (2016). Insights into blood cell formation from hemogenic endothelium in lesser-known anatomic sites. Dev Dyn 245, 1011–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Set S1. Genes differentially expressed in DLL4+ and DLL4− HE generated from wild type H1 hESCs or iETS1-hESCs in presence of DOX. Related to Figure 7. Column A, Gene ID; B, Posterior Probability of Equal Expression; C-D, tpm values for the libraries representing DLL4+ and DLL4− HE; E, signed tpm ratio of DLL4+/DLL4− populations, negative if DLL4− population had higher expression; F, Gene name. Tab WT, wild type hESCs differentiated without DOX; DOX1.5, iETS1 hESCs differentiated in presence of 1.5 μg/ml DOX.