Abstract
Body mass in humans and animals is strongly associated with the rate of heat production as defined by resting energy expenditure (REE). Starting with the ancient Greeks up to the present time philosophers and scientists have endeavored to understand the nature and sources of body heat. Today we recognize that body mass consists of organs and tissues, each of which produces a specified amount of heat at rest. An individual organ’s REE can now be estimated in vivo as the product of its assumed mass-specific metabolic rate and its imaging-derived mass; whole-body REE reflects the sum of organ and tissue metabolic rates. The sizes of organs present and total body mass in adults are governed by two main factors, a person’s stature or height, and their level of adiposity. With greater body size, as represented by adult height independent of adiposity, organs remain stable or increase in mass according to distinct “scaling” patterns. Similarly, with greater relative adiposity organs adaptively accommodate to the increase in imposed mechanical and metabolic loading conditions. Through a detailed analysis of these stature and adiposity effects we show how classical statistical REE prediction models can be mechanistically understood at the anatomic body composition level.
INTRODUCTION
Large mammals generate more energy at rest or resting energy expenditure (REE), than do their small counterparts. This is captured by the nine-decade old mathematical relationship, Kleiber’s Law, REE ∝ M0.75 (∝ is the symbol for proportional) [1]. A similar empirical rule is observed for adult men and women: REE ∝ M0.73 [2]. However, when applied in clinical and research settings, REE in adults is typically predicted by not only body mass separately in men and women, but by two additional covariates, height and age [3]. Many such empirical prediction formulas can be found in the biomedical literature and these kinds of statistical equations continue to be developed for use in new populations.
These observations lead us to the question of why body mass is associated with REE, particularly, why in humans is REE most often best predicted with height and age as additional covariates. Examining these questions take us to the frontier of body composition and human energy expenditure research. With our analysis we can connect these widely used REE prediction equations with physiological structure-function relationships at the anatomic body composition level.
BODY MASS-RESTING ENERGY EXPENDITURE DETERMINANTS
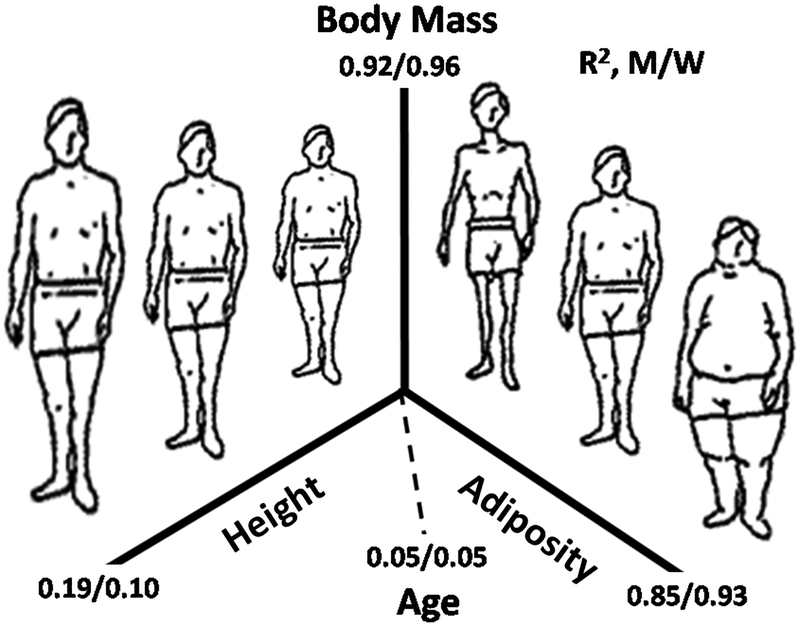
Body mass in Kleiber’s Law and related constructs is assumed to be a homogeneous heat-producing compartment. In humans, however, we can quickly discern that body mass consists of at least two heat-producing components, height-and adiposity, both of which have independent association with REE (Figure 1). A third factor, age, also independently influences body mass after controlling for height and adiposity. The figure shows the R2 values for correlations between each of these three body mass predictors in a large sample of men (n=9385) and women (n=9171) [4]. Taken collectively, height, adiposity, and age account for over 90% of between-individual variation in body mass. The origin of the relation between body mass and REE lies in a detailed analysis of these three determinants.
Figure 1.
Pictorial representation of the major determinants of body mass in healthy men (M) and women (W) participants of the US National Health and Nutrition Examination Survey [4]. Adiposity (fat mass) was evaluated using dual-energy x-ray absorptiometry. R2 values for linear correlations between body mass and each of the three determinants are shown in the figure along with the composite correlations for the determinants derived by multiple regression analysis.
We can narrow our discussion of these three determinants by first considering the relationship between age and body mass. Most people gradually experience loss of lean tissues, notably skeletal muscle mass, as they age [5]. An old person who has the same height and fat mass as a young person will therefore weigh less. After controlling for height and adiposity, the slope of the age term in body mass prediction models developed on the previously mentioned sample is negative, about (X±SE) −0.094±0.003 kg/yr for men and −0.055±0.002 kg/yr for women. That is, according to this cross-sectional sample, men and women lose about ½ to 1 kg body mass per decade after adjusting for height and adiposity. The composition of weight loss with aging is complex and incompletely mapped out; we can therefore simplify our discussion by narrowing our focus now to the age-independent relations between body mass, height, adiposity, and REE.
Organs and Tissues
Body mass represents the sum of all organ and tissue weights. The largest organs and tissues in most adults, in descending order by weight, are skeletal muscle, adipose tissue, bone, skin, gastrointestinal tract, liver, brain, lung, heart, kidneys, and spleen [6]. Field and colleagues in 1939 showed in meticulous animal experiments that REE can largely be accounted for (89%) by summing the heat production rates of 14 individual organs and tissues along with the remaining or “residual” mass [7]. Proof of concept in humans followed when Gallagher et al. [8] in 1998 and then Illner et al. [9] in 2000 showed strong correlations and close absolute agreement between organ mass-derived REE and measured REE in healthy adults. These human REE prediction models are implemented by first measuring organ and tissue volume or mass in vivo using advanced imaging methods and then taking the product of these estimates and corresponding assumed mass-specific metabolic rates (i.e., organ REE/organ mass) [10]. The estimated individual organ and tissue REEs are then summed to derive a value for total body REE. The most recent version of the adult human organ REE model includes ten individual components and is reviewed in detail by Heymsfield et al. [11].
We thus see that body mass represents the sum of individual organ and tissue weights and likewise REE reflects the summated heat production rates of these organs and tissues. This concept, validated in both animal [7] and human models [8, 9, 11], is the foundation for mechanistic organ-tissue energy expenditure prediction models. The next step in our analysis is to show how organs are functionally related to each other and what factors regulate their mass in vivo. These concepts will lead us to a mechanistic understanding at an anatomic level how body mass and REE are related to each other.
Organ Systems.
Organs function within systems, of which ten are generally recognized [11]. These ten systems can be simplified for discussion purposes into four groups that are relevant to the current discussion. The first is the nervous system consisting mainly of brain mass [6]. The human nervous system is responsible for maintaining homeostatic, cognitive, and many other bodily functions. Only weak correlations are present between adult brain mass and body size [12–15]. Since body mass increases across adults as height squared[16], the fraction of body mass as brain is smaller in people who are tall than it is in people who are short [11–13, 15]. That is, any organ that scales to height with a power of less than 2 will be present as a smaller fraction of body mass in people who are tall compared to those who are short. The inverse is also true, that organs scaling to height with powers >2 will comprise a larger fraction of body mass on people who are tall. Brain has a relatively high mass-specific metabolic rate of ~240 kcal/kg/d [10] and accounts for about 350 kcal/d or 20–25% of REE in the average adult. Brain energy expenditure may vary somewhat with age, particularly within some anatomic regions, and with between-individual relative differences in grey and white matter [17].
The second system, musculoskeletal (muscle + bone), facilitates body movements and accounts for the largest percentage (~30–40%) of body mass in non-obese adults. The mass-specific metabolic rate of the musculoskeletal system is ~15–20 kcal/kg/d [11] and this major body component accounts for about 300–400 kcal/d or ~25% of REE in the average adult. Musculoskeletal mass is highly correlated with total body mass and skeletal mass increases as a fraction of body mass in adults who are tall compared to those who are short [11, 14].
The third grouped system consists of digestive, respiratory, cardiovascular, and urinary systems that function to acquire, process, and distribute energy/nutrients and excrete waste products [18]. This composite “metabolic” system includes organs that have relatively high mass-specific metabolic rates, ranging from about 200 to 440 kcal/d [10, 11].
Adipose tissue, technically part of the integument, serves as the fourth system and the body’s main energy store, triglycerides. Adipose tissue has a low mass-specific metabolic rate, 4.5 kcal/kg/d [10]. An important assumption is that in healthy adults who have been well nourished across their lifespan the proportion of body mass as adipose tissue is largely independent of height. That is, the percentage of body fat will remain stable across adults differing in height if fat mass scales as height2, similar to body mass.
Organ System Functions and Integration.
Organ systems and their components do not work in isolation; rather their many functions are highly integrated. Organ growth following birth is closely regulated and synchronized through a wide array of molecular mechanisms [11, 19]. These guiding biological effects provide the delicate balance that maintains the close ties between organ structures and their multiple functions.
The nervous system matures relatively early in life, by about the age of 5–10 years, while the musculoskeletal and metabolic systems continue to increase in mass along with adipose tissue through early adulthood [11]. The sizes of adult organs and their systems are largely constrained by the musculoskeletal system that creates a finite frame within which organs such as liver, kidney and heart must fit. People who are short and who thus have a small musculoskeletal system, also have other organs and tissues proportionally reduced in size. This is a critical observation: a person’s height is a surrogate marker of their maximum linear skeletal dimension and thus their “size” independent of their adiposity. Viewed from another perspective, body mass can be considered as having the two aforementioned portions, one related to a person’s height/size and the other related to their adiposity, leaving aside the body mass effects of aging. Adiposity will be examined in a section that follows.
Organs and their systems in healthy adults must be capable of sustaining maximal mechanical and metabolic loads [11, 20]. Any system “failure” during peak loading conditions will prove detrimental to the host. According to one theory, referred to as symmorphosis [11, 18, 20], systems and their components are “designed” to accommodate maximal mechanical and metabolic loading conditions and these responses are highly integrated within and across systems [13]. A feature of this theory is that organs and their systems can adapt to varying loading conditions, as for example the left ventricle can hypertrophy or atrophy as it adjusts its mass to variations in mechanical and metabolic loads [11, 21]. Given ideal circumstances, according to this theory, the structures and functions of all organs and tissues are highly integrated and their masses are adjusted to be “no more or less” than absolutely needed [13]. Thus, people who are “large”, either through their tall stature or excess adiposity, also have large organ and tissue compartments that increase coordinately with greater body mass. That’s why there are such strong correlations between body mass and REE in adults [11, 18]. In the sections that follow we examine specifically how height and adiposity independently influence the relative amounts of organs and tissues present.
BODY MASS and STATURE
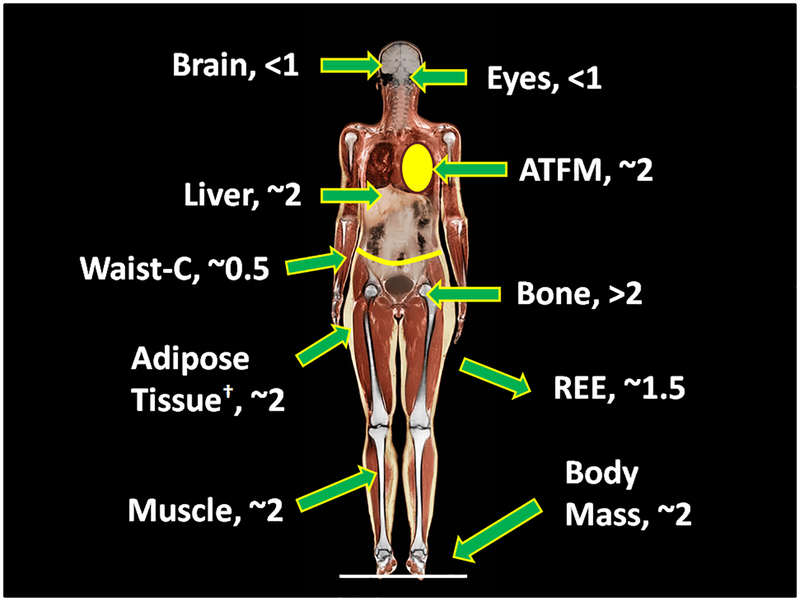
When we assemble a group of healthy adults we observe a relatively large range in height that varies depending on sex and age. Along with body weight, height reflects a person’s constitutional features [22]. Adult height is strongly influenced by environmental factors present during the post-natal growth periods, and we exclude the possibility of nutritional and other deprivations that might influence body size and shape for our more general discussion. When considering healthy adult samples, as noted earlier body mass increases (or “scales”) as height2 in both men and women across most race/ethnic groups evaluated so far [16]. Not all organs and tissues increase in proportion to body mass with greater height, although our current scaling “map” (Figure 2) is still somewhat limited due to the relatively small size of evaluated samples [11, 13–16]. As noted earlier, after adjusting for adiposity and age, the nervous system (i.e., brain mass) scales to height with powers <1 and thus the fraction of body mass as brain is less in people who are tall than in people who are short [11, 13–16]. Ocular volume is also only weakly associated with height [23]. By contrast, the musculoskeletal system, mainly bone, scales to height with powers >2 [11, 13, 15]. Data on metabolic system components is limited, but evidence so far indicates height powers similar to body mass of about 2 [14]. We thus observe that the mass of non-adipose tissue components, with the exception of the nervous system (i.e., brain) and eyeball, are strongly related to body size as defined by height and, accordingly, they are significantly correlated with each other in healthy adults [11]. That is, components of the musculoskeletal and metabolic systems scale roughly the same to height and similar to body mass with powers of about 2 or somewhat higher in the case of bone.
Figure 2.
Scaling powers to height (α, in the equation: component mass or circumference = β × heightα) for selected anatomic compartments and waist circumference (C). †, The noted scaling power for adipose tissue assumes that the percentage of body mass as adiposity is independent of height after controlling for age. ATFM, adipose tissue-free mass (ATFM = body mass-adipose tissue mass) [11–16].
Organs and their systems thus become larger as we move across a population from short to tall stature, and thus like weight and height, reflect a person’s constitution [22]. As we might expect, REE also increases with greater height and related body mass. However, as indicated, greater height is accompanied by relative changes in organ and system proportions. The two best documented of these effects are a relative reduction in the proportion of body mass as brain mass and an increase in the proportion as skeletal mass (Figure 2) [11, 16]. These height-related effects in adults likely explain at an anatomic level why REE scales to height with powers less than 2 (~1.5) [11], why REE/body mass is smaller in people who are tall compared to their short counterparts [12], and why REE scales to body mass with powers less than 1 [11]. These observations help us to understand why most statistical REE prediction equations include height as a significant predictor variable.
BODY MASS and ADIPOSITY
The other main body mass predictor variable, adiposity, also has important effects on body composition and REE. Adipose tissue has a resting mass-specific REE of 4.5 kcal/kg [10] and thus greater total adiposity is accompanied by a predictably larger REE. However, as noted earlier, organ systems must be capable of adapting to maximal metabolic and mechanical loads in order to preserve functionality with a reasonable safety margin. The implication of this concept is that greater adiposity must be accompanied by additional bodily structural support and metabolic management capacity. We can gain a quantitative impression of the magnitude of these effects by examining the association between adiposity and other organs and tissues in a previously reported sample of healthy adults [11].
Men and women in the sample with large differences in BMI and adipose tissue mass (upper and lower fifths) were matched on age (±3 yrs) and height (±2 cm) (Supplementary Material). There were 15 pairs of men and 17 pairs of women (Supplementary Information). Each subject had organ and tissue masses quantified using a combination of imaging methods as reported by Heymsfield et al. [11]; REE was measured by indirect calorimetry and also calculated from the organ-tissue body composition estimates. The organs were grouped into the four aforementioned system and tissue components, nervous (i.e., brain), musculoskeletal (i.e., muscle and bone), metabolic (i.e., liver, heart, and kidneys), and adipose tissue. We refer to the body composition differences between the two groups as “excess” body mass, calculated by subtracting the mean high-adiposity subject values from those of the corresponding low-adiposity group’s values.
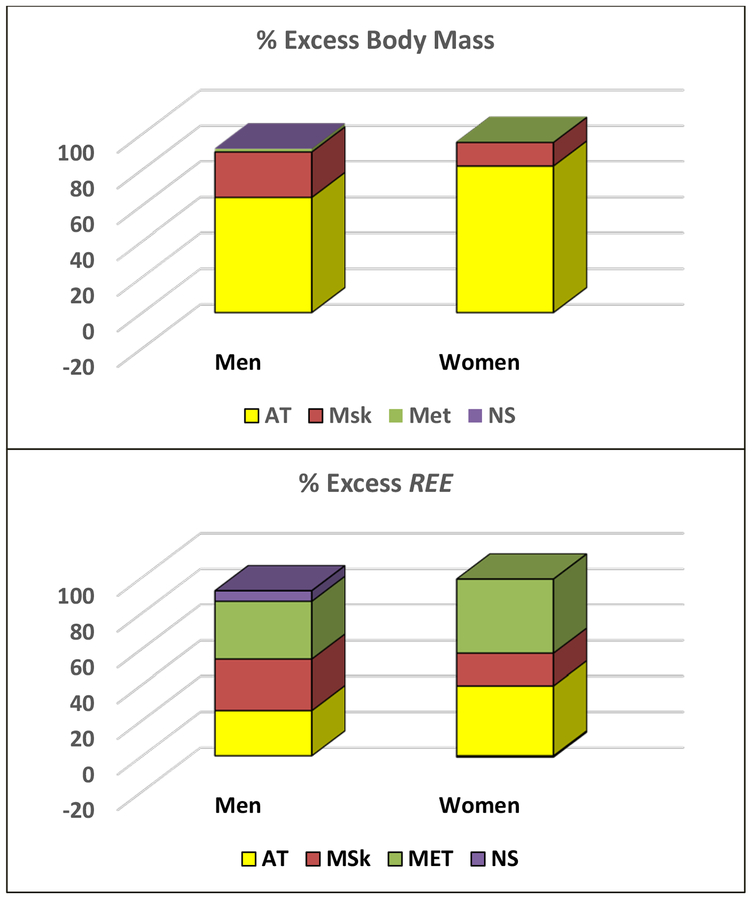
The men and women with excess adiposity weighed 32.8 kg and 46.9 kg more than their respective lean counterparts. As shown in Figure 3, Most of this excess body mass is comprised of adipose tissue (men/women; 64.3% and 82.0%). Of the remainder, the largest portion is accounted for by musculoskeletal mass (25.5% and 13.3%) with only a small proportion related to the metabolic (0.4–1.8%) and nervous systems (-0.2–0.3%). The adaptive responses of musculoskeletal and metabolic system components are brought about through mechanisms related to the mechanical and metabolic loads imposed by the expanded adipose tissue compartment [11, 18].
Figure 3.
Percent (%) of excess body mass (upper panel) and % of excess resting energy expenditure (REE) (lower panel) observed in a sample of healthy men (n=15 pairs) and women (n=17 pairs) matched on age and height but who differed in level of adiposity (upper and lower fifths of the sample) [18]. Between-pair mean differences for body systems and adipose tissue are shown in the figure as a % of body mass or calculated REE [11] differences. AT, adipose tissue; MET, metabolic system (liver, heart, kidneys); MSk, musculoskeletal system; and NS, nervous system (brain).
Since the body mass increase associated with excess adiposity includes lean tissues, the mass-specific metabolic rate of this compartment will be larger than that of adipose tissue (i.e., 4.5 kcal/kg). That prediction is upheld with the mean measured and calculated ΔREE/Δbody mass 13.3/11.4 kcal/d for men and 9.1/9.4 kcal/kg for women. We thus observe that excess body mass in this sample is accompanied by roughly a 10 kcal/kg increase in REE. As shown in Figure 3, the main relative contributions to this increase in REE are approximately equally divided across musculoskeletal and metabolic systems, and adipose tissue.
These observations provide insights into statistical REE prediction models based on fat mass and fat-free mass (FFM) as predictor variables; these molecular-level components are similar to organ-system components adipose tissue and adipose tissue-free mass. Fat-free mass-based models are descendants of the classic REE prediction models that include body mass, height, and age as covariates [3]. Equations based on FFM are founded on the rationale that body mass consists of relatively energetically-very low fat mass (i.e., ~4–5 kcal/kg) and a “metabolically-active” FFM compartment. However, the developed regression models often have a fat mass slope of 8–10 kcal/kg/d, higher than the mass-specific REE of adipose tissue (4.5 kcal/kg/d). This effect is caused largely by the regression model fat mass term capturing the energetic contributions of FFM components. These regression model slopes are very similar to the mass-specific metabolic rate of ~10 kcal/kg for “excess” body mass as observed in the previous example.
CONCLUSIONS
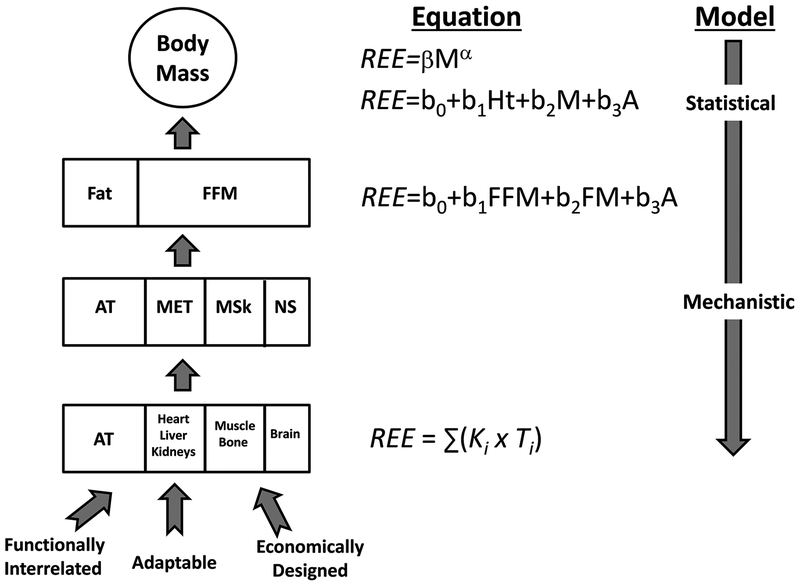
According to our developed perspective, body mass and REE represent the summated mass and heat production rates, respectively, of all organs and tissues (Figure 4). Organs are highly integrated and adaptable within and across systems, in theory creating a system that can collectively respond to maximal metabolic and mechanical demands while maintaining efficient structures with “optimized” mass.
Figure 4.
Relationships between body mass, body composition, and resting energy expenditure (REE) prediction models. Concepts relating organ structure, function, and mass are shown in the figure. A is age; α is the scaling exponent; AT is adipose tissue mass; β is a proportionality coefficient; b0 and bn are regression model intercepts and slopes, respectively; FFM is fat-free mass; Ht is height; Ki is mass-specific metabolic rate; MET is metabolic system; MSk is muscular skeletal system; NS is nervous system; S is sex; and Ti is organ mass.
Height is a measure of overall body size that reflects skeletal dimensions and the associated sizes of organs and their systems. Organs and tissues increase in mass, as does REE, with greater height. However, the nervous system with high-metabolic brain scales weakly to height, less than does body mass. By contrast, relatively low metabolic rate supporting structures such as bone scale to height with powers larger than those of body mass. After controlling for adiposity and age, adults who are tall thus have a relatively lower REE than do people who are short. Similarly, REE scales to body mass in humans with powers <1 [2]. A next step is to link previously reported and potentially new biomechanical and physiological models that provide a mechanistic basis for these stature-related anatomic effects.
The other major contributor to body mass, adipose tissue, is highly variable in adults and through adaptive mechanisms influences the size and function of multiple organs and their systems. Our demonstration reveals some of these trophic effects and their influence on REE.
Statistical REE prediction equations that include body mass, height, fat mass, and FFM as covariates thus capture these coordinated effects of stature and adiposity on relative organ proportions. Our analyses are based on very limited available information at present and are intended to create a framework to guide future research and hypothesis generation. Moving in these directions will take the study of human heat production from its current relatively descriptive state to a more mechanistic level.
Supplementary Material
ACKNOWLEDGEMENTS
The author acknowledges Melanie Peterson for her support in preparing this review.
FUNDING
This work was partially supported by two National Institutes of Health NORC Center Grants P30DK072476, Pennington/Louisiana; and P30DK040561, Harvard; and R01DK109008, Shape UP! Adults.
Abbreviations:
- FFM
fat-free mass
- REE
resting energy expenditure
Footnotes
CONFLICTS OF INTEREST
The author SBH is on the Medical Advisory Boards of Tanita and Medifast Corporations.
REFERENCES
- 1.Kleiber M. Body size and metabolism. Hilgardia. 1932;6(11):315–53. [Google Scholar]
- 2.Food and Agriculture Organization (FAO). Calorie Requirements: Report of the Second Committee on Calorie Requirements (FAO Nutritional Studies No. 15). Rome: FAO of the United Nations; 1957. [Google Scholar]
- 3.Harris JA, Benedict FG. A Biometric Study of Human Basal Metabolism. Proc Natl Acad Sci U S A. 1918;4(12):370–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heymsfield SB, Peterson CM, Thomas DM, Heo M, Schuna JM Jr. Why are there race/ethnic differences in adult body mass index-adiposity relationships? A quantitative critical review. Obes Rev. 2016;17(3):262–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zembron-Lacny A, Dziubek W, Rogowski L, Skorupka E, Dabrowska G. Sarcopenia: Monitoring, Molecular Mechanisms, and Physical Intervention. Physiol Res. 2014;63(6):683–91. [DOI] [PubMed] [Google Scholar]
- 6.International Commission on Radiological Protection. Task Group on Reference Man. Report of the Task Group on Reference Man : a report. Oxford; New York: Pergamon Press; 1975. xix, 480 p. p. [Google Scholar]
- 7.Field J, Belding HS, Martin AW. An analysis of the relation between basal metabolism and summated tissue respiration in the rat I. The post-pubertal albino rat. J Cell Compar Physl. 1939;14(3):143–57. [Google Scholar]
- 8.Gallagher D, Belmonte D, Deurenberg P, Wang Z, Krasnow N, Pi-Sunyer FX, et al. Organ-tissue mass measurement allows modeling of REE and metabolically active tissue mass. Am J Physiol. 1998;275(2 Pt 1):E249–58. [DOI] [PubMed] [Google Scholar]
- 9.Illner K, Brinkmann G, Heller M, Bosy-Westphal A, Muller MJ. Metabolically active components of fat free mass and resting energy expenditure in nonobese adults. Am J Physiol Endocrinol Metab. 2000;278(2):E308–15. [DOI] [PubMed] [Google Scholar]
- 10.Elia M. Organ and tissue contribution to metabolic rate In: Kinney JM, Tucker HN, editors. Energy metabolism: Tissue determinants and cellular corollaries. New York: Raven Press; 1992. p. 61–79. [Google Scholar]
- 11.Heymsfield SB, Bourgeois B, Peterson CM. Body Composition-Energy Expenditure Organ System Model: Initial Evaluation of Predictions. Obes Rev. in press;In press. [Google Scholar]
- 12.Heymsfield SB, Childers D, Beetsch J, Allison DB, Pietrobelli A. Body size and human energy requirements: reduced mass-specific resting energy expenditure in tall adults. J Appl Physiol (1985). 2007;103(5):1543–50. [DOI] [PubMed] [Google Scholar]
- 13.Heymsfield SB, Chirachariyavej T, Rhyu IJ, Roongpisuthipong C, Heo M, Pietrobelli A. Differences between brain mass and body weight scaling to height: potential mechanism of reduced mass-specific resting energy expenditure of taller adults. J Appl Physiol (1985). 2009;106(1):40–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heymsfield SB, Gallagher D, Mayer L, Beetsch J, Pietrobelli A. Scaling of human body composition to stature: new insights into body mass index. Am J Clin Nutr. 2007;86(1):82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heymsfield SB, Muller MJ, Bosy-Westphal A, Thomas D, Shen W. Human brain mass: similar body composition associations as observed across mammals. Am J Hum Biol. 2012;24(4):479–85. [DOI] [PubMed] [Google Scholar]
- 16.Heymsfield SB, Peterson CM, Thomas DM, Heo M, Schuna JM Jr., Hong S, et al. Scaling of adult body weight to height across sex and race/ethnic groups: relevance to BMI. Am J Clin Nutr. 2014;100(6):1455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geisler C, Hubers M, Granert O, Muller MJ. Contribution of structural brain phenotypes to the variance in resting energy expenditure in healthy Caucasian subjects. J Appl Physiol (1985). 2017:jap006902017. [DOI] [PubMed] [Google Scholar]
- 18.Heymsfield SB. Energy Expenditure – Body Size Associations: Molecular Coordination. Eur J Clin Nutr. in press;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lui JC, Baron J. Mechanisms limiting body growth in mammals. Endocr Rev. 2011;32(3):422–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weibel ER. Symmorphosis: on form and function in shaping life. Cambridge, MA: Harvard University Press; 2000. [Google Scholar]
- 21.Ford LE. Heart size. Circ Res. 1976;39(3):297–303. [DOI] [PubMed] [Google Scholar]
- 22.Muller MJ, Langemann D, Gehrke I, Later W, Heller M, Gluer CC, et al. Effect of constitution on mass of individual organs and their association with metabolic rate in humans--a detailed view on allometric scaling. PLoS One. 2011;6(7):e22732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heymsfield SB, Gonzalez MC, Thomas D, Murray K, Jia G, Cattrysse E, et al. Adult Human Ocular Volume: Scaling to Body Size and Composition. Anat Physiol. 2016;6(5). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.