Abstract
Objective.
To determine whether temporal trends exist for short-acting beta agonist (SABA), oral corticosteroid (OCS), and anti-inflammatory prescription fills in children with persistent asthma.
Method.
This was a longitudinal analysis of pharmacy record data and health information data obtained by parent report over 12 months for children with persistent asthma 2 to 9 years of age. Eligible children had to report current nebulizer use and one or more emergency department visits or hospitalizations within the past 12 months.
Results.
Children were primarily African-American (89%), male (64%), received Medicaid health insurance (82%), and were a mean age of 4.5 years (SD 2.1). Few families (11%) reported any problems paying for their child’s asthma medications at baseline or at the 12-month follow-up. There was a high degree of association between filling a rescue (SABA or OCS) and controller (leukotriene modifier, inhaled corticosteroid, cromolyn) medication during the same month for all months with Pearson’s correlation coefficients ranging from a low of 0.28 for October to a high of 0.53 in September. Short-acting beta agonist fills were significantly more likely to be filled concurrently with inhaled corticosteroid fills. However, significantly fewer prescription fills were obtained in the summer months with an acceleration of medication fills in September through December and an increase in early spring.
Conclusions.
There was a summer decline in both inhaled corticosteroid and SABA fills. Timing of asthma monitoring visits to occur before peak prescription fill months, i.e., August and December for an asthma “tune-up,” theoretically could improve asthma control. During these primary care visits children could benefit from more intensive monitoring of medication use including monitoring lung function, frequency of prescription refills, and assessment of medication device technique to ensure that an effective dose of medication is adequately delivered to the respiratory tract. Additionally, scheduling non-urgent asthma care visits at pre-peak prescription fill months can take advantage of “step down” during decreased symptom periods and when appropriate restart daily controller medications to “step up” prior to peak asthma periods.
Keywords: asthma, medication use, pediatric
INTRODUCTION
Asthma is a major cause of childhood disability in the United States and is one of the leading causes of pediatric emergency department (ED) visits (1–5). Seasonal patterns have been noted not only in bronchial hyper-reactivity changes corresponding to allergen (6) but also in pediatric asthma hospitalizations (7) and ED visits (8). Allergens, air pollution, and viral infections have been temporally associated with asthma exacerbations as well as seasonal trends in anti-inflammatory medication use for asthma (9). Temporal patterns of pediatric asthma-related ED and hospital utilization indicate winter-spring and autumn peaks (8, 10, 11). Moreover, a September “epidemic” of asthma exacerbations in children is well described (9, 12) and may be due to the increased exposure to Rhinovirus infection upon return to school, fall allergen peaks, and low inhaled corticosteroid (ICS) fills in late summer (9). Low ICS and short-acting beta agonist (SABA) fills in summer months followed by a rapid acceleration of fills in the fall, noted in Canadian children, suggest that prescriptions are filled after children are symptomatic (12) rather than for prevention of exacerbations.
Longstanding national and international asthma guidelines recommend daily ICSs as the cornerstone of treatment for patients with persistent asthma and to prevent exacerbations caused by bronchial inflammation (13–16). However, several studies demonstrate an underutilization of ICS in children with asthma (17–20), resulting in substandard, non-guideline–based care. Furthermore, underutilization of ICS may result in over-dependence on SABA or rescue medications as previously reported (20–21).
The purpose of this study was to determine whether temporal trends exist for anti-inflammatory, SABA and oral corticosteroid prescription fills in a group of young, inner-city children with primarily persistent asthma. Data are based on pharmacy records indicating the number of prescription fills by type of medication for each month by children enrolled in an asthma educational randomized clinical trial. We hypothesized that prescription fill rates for SABAs and oral corticosteroids would vary by time of year and that controller medication fills including ICS and leukotriene modifiers would show less variation by time of year based on national asthma guideline treatment recommendations.
METHODS
This study was a longitudinal analysis of survey and pharmacy record data collected from all subjects enrolled in a randomized controlled trial testing the effectiveness of a nebulizer educational intervention and receiving follow-up for 12 months. All participants were enrolled in a randomized clinical trial to evaluate the effectiveness of an educational intervention focused on parent- and child-appropriate home treatment of symptoms and nebulizer practice on asthma morbidity, medication use, and health care utilization outcomes (19). No intervention effects were noted for pattern of medication use or number of refills over the 12-month follow-up as previously reported (19). Parents were administered a questionnaire face-to-face in the ED or clinic at the time of recruitment and via telephone at 6 and 12 months. At each data collection time point, parents were asked to recall asthma morbidity and health care utilization items over the past 6 months. For this analysis, all participants who had complete pharmacy and questionnaire data available at 12 months were pooled to examine seasonal patterns of medication fills.
Participants
Children 2 to 9 years of age diagnosed with persistent asthma were recruited from pediatric practices affiliated with two large urban university hospitals, including pediatric primary care (30%), pulmonary/allergy specialty (50%), and ED (20%) practices between October 2001 and May 2003. All participants were enrolled in a randomized clinical trial of young children with persistent asthma to evaluate the effectiveness of a nebulizer educational intervention on symptom frequency, asthma medication use, and health care utilization (19). All children were followed for 12 months using parent interviews on surveys of asthma health status at 6 and 12 months follow-up interviews. Pharmacy record data were collected at 12 months. Children with complete survey and pharmacy record data at 12 months were included in the analysis to examine for seasonal patterns of medication fills over the 12 month follow-up.
The Institutional Review Boards of the Johns Hopkins University Medical Institutions and the University of Maryland School of Medicine approved the study protocols. We obtained informed consent from all participating parents including authorization for collection of the child’s pharmacy records.
Measures
Asthma Severity.
Children were assigned an asthma severity level based on national asthma guidelines (13–15) using frequency of child day and night symptoms based on caregiver responses to two questions regarding symptom frequency. Children who met National Asthma Education Prevention Program (NAEPP) criteria for mild, moderate, or severe persistent asthma who reported daytime symptoms (more than two times per week) or nighttime symptoms (more than two times per month) (13–15) at enrollment were included in the study. Children classified as having mild intermittent disease based on symptom frequency and who also reported daily anti-inflammatory medication use were reclassified as mild persistent based on pre-medication severity (16). Children classified with mild intermittent disease based on symptom frequency who also reported a recent ED visit (N = 8) remained in the study sample owing to the potential for underclassification of severity based only on current symptom frequency.
Asthma Prescription Fills.
Pharmacy records for each child were obtained from all pharmacies reported by the parent/caregiver as being used over the 12-month follow-up period, including pharmacies used if travel occurred outside of study area, as previously described (22, 23). Pharmacy records included product name, dosage form, strength, quantity, and date dispensed. Pharmacy records were considered complete if the pharmacy submitted data for the specified period and indicated that they had no record of the child or had no prescriptions during the specified period requested. A database was created to capture pharmacy record data, and before double key entry in the database, all pharmacy records were reviewed by an asthma nurse specialist to ensure complete data retrieval. Discrepancies in data entries were adjudicated. Quick relief medications were defined as short-acting beta agonists (SABA) and oral corticosteroids (OCS) (24) while controllers included inhaled corticosteroids (ICS), leukotriene modifiers (LM), mast cell stabilizers, and longacting beta agonists. The most prevalent SABAs encountered was albuterol in nebulizer and metered dose inhaler formulations. Common ICSs encountered were fluticasone (Flovent; GlaxoSmithKline, Research Triangle Park, NC), budesonide (Pulmicort and Respules; AstraZeneca LP, Wilmington, DE), and fluticasone/salmeterol (Advair; Glaxo-KlineSmith, Research Triangle Park, NC) Diskus. Inhaled corticosteroids and LMs were selected as the controller medications to examine by month because they were the most common anti-inflammatory medications as compared with very few mast cell stabilizer fills that were dispensed for this sample of children.
Asthma Morbidity and Health Care Utilization Factors
Questionnaires were administered to parents at baseline, 6 and 12 months post-randomization, to ascertain symptom frequency, activity limitation, health care utilization, receipt of any samples of asthma medications, difficulty obtaining medications, new pharmacies used, and use of asthma action plans in the home during the prior 6 months. Number of hospitalizations, ED visits, preventive asthma care and specialty care visits, difficulty paying for medications, and having an asthma action plan during the prior 6 months were ascertained at baseline, 6 and 12 months follow-up.
Data Analysis
Baseline sociodemographic and health characteristics of all subjects (N = 221) and subjects with complete pharmacy record data (N = 180) were compared using the chi-square test of independence for categorical data and Student’s t test for continuous data. Prescription information for each child was calculated for each type of medication (ICS, LM, OCS, SABA) for each month over the 12-month study period. Proportion of children filling ICS, LM, OCS, and SABA prescriptions was calculated by month for all children across the 2-year study. Odds ratios were calculated for likelihood of a child filling a prescription for each type of medication for each month using August as the reference month for ICS, LM, and OCS due to the exceptionally low number of fills during August. For SABA fills, July was selected as the reference month due to the exceptionally low use of SABA in July detected in the fill data and based on low health care utilization in children with asthma in July (6, 7). Proportions of children filling each type of medication were then plotted using a smoothing lowess spline fit (25) to the data to smooth out the plot of proportions over the 12 months. Mean number of fills by drug type and by month were analyzed by using a Poisson model for the data as a comparison along with original logistic models of children having fills (Yes/No) for each medication by month. To account for within-person correlations over time, the logistic and Poisson regression models were created using the method of General Estimating Equations (GEE). To examine the association between filling rescue and controller medication, an additional set of analyses were conducted to examine the correlation between (1) the refills for rescue medications (SABA or OCS) and controller (ICS, LM) medications in the same month and (2) filling a rescue medication in 1 month and then subsequently filling a controller medication the next month. Statistical association was tested using Pearson’s correlation coefficient (p) and fills were considered as continuous data. Using logistic regression we examined filling rescue medication as a predictor of filling a controller medication during the same month and the following month. Two-sided tests were used and p values < 0.05 were considered significant. All data analyses were performed using SAS Version 8.0 (Cary, NC) (26) and Stata Version 9.0 (StataCorp, College Station, TX) (27) software.
RESULTS
Characteristics of Patient Population
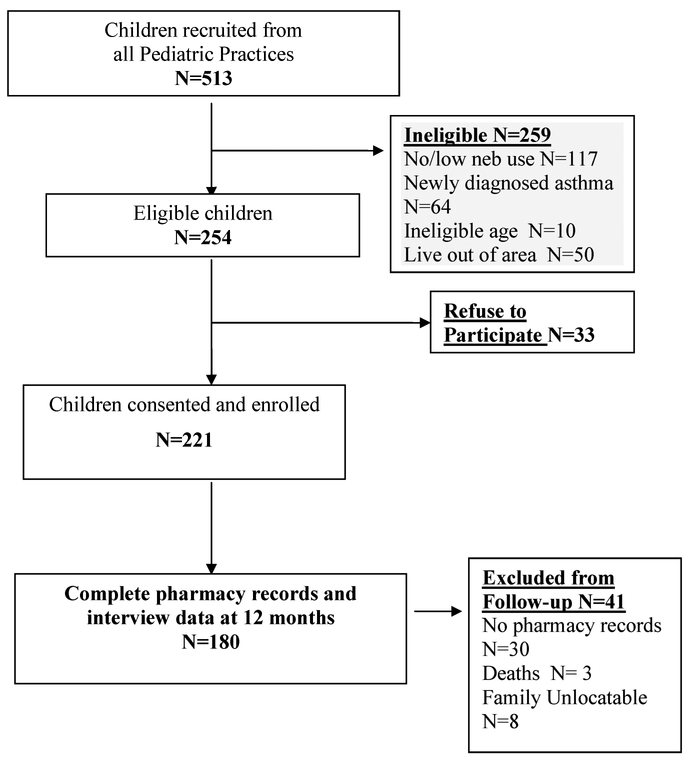
A total of 513 children were recruited, of which 259 were ineligible and 33 refused to participate resulting in 221 of 254 (85%) children and their parents consented and enrolled in the study (Figure 1). All caregivers of the 221 children completed the baseline interview questionnaire and consented to release of pharmacy records. At the 12-month follow-up, 180 (81%) children had complete pharmacy records and interview data and were included in the analysis (Table 1). Exclusion from the follow-up analysis was primarily due to incomplete pharmacy records (N = 30) or lost to follow-up (N = 8) as shown in Figure 1. There were no significant differences noted for all sociodemographic and health characteristics between children with complete follow-up data (N = 180) and the total baseline sample (N = 221). Children included in this analysis were primarily African-American (89%), male (64%), received Medicaid health insurance over entire study period (82%) and were young, with a mean age of 4.5 years (SD 2.1). Most caregivers reported a high school education or higher (76%), and almost half (47%) reported annual household incomes of less than $20,000. Few families (11%) reported any problems paying for their child’s asthma medications at baseline or at the 12-month follow-up.
FIGURE 1.—
Recruitment and retention flow of study participants.
TABLE 1.—
Baseline sociodemographic and health characteristics for children with complete follow-up data (N = 180).
| Sociodemographic characteristic |
Children with complete pharmacy and questionnaire data at follow-up N = 180* Number (%) |
|---|---|
| Race | |
| African-American | 160 (88.9) |
| White | 18 (10.0) |
| other | 2 (1.1) |
| Gender | |
| Male | 115 (63.9) |
| Child age (mean, SD) | |
| Range 2–9 years | 4.5 (2.1) |
| Parent/guardian educational level | |
| Some HS | 42 (23.3) |
| HS graduate or GED | 65 (36.1) |
| Some college/trade school | 58 (32.2) |
| 4-year college or college grad | 15 (8.3) |
| Household income | |
| < $20,000 | 84 (46.7) |
| ≥$20,000 | 70 (38.9) |
| Missing | 26 (14.4) |
| Type of medical insurance for child | |
| Medical Assistance | 147 (82.1) |
| Private | 31 (17.3) |
| Self-pay | 1 (0.6) |
| Problems paying for asthma | |
| Medications in last 6 months | |
| Yes | 20 (11.1) |
| Asthma morbidity and health care | |
| utilization characteristics | |
| Severity level | |
| Mild intermittent | 8 (4.4) |
| Mild persistent | 114 (63.3) |
| Moderate persistent | 33 (18.3) |
| Severe persistent | 25 (13.9) |
| Number of ED visits last 6 months | |
| Mean (SD) | 1.83 (2.55) |
| (Range 0–20 visits) | |
| Number hospital admissions for | |
| asthma last 6 months | |
| Mean (SD) | 0.36 (0.67) |
| Limitation of activity due to asthma | |
| Yes | 116 (64) |
| Receives specialty care | 90(50.0) |
| Yes | |
| Asthma Action Plan in the home | |
| Yes | 74 (41.1) |
No significant differences between groups for all variables.
Asthma morbidity in this group of children with complete pharmacy data (N = 180) was high (Table 1) with 68% categorized with mild persistent and almost one third (32%) categorized with moderate to severe persistent asthma. Mean asthma-related emergency department (ED) visits and hospitalizations reported during the prior 6 months for this group of children were 1.83 (SD 2.55) ED visits and 0.36 (SD 0.67) hospitalizations. Child asthma-related activity limitation was reported by almost two thirds (64%) of parents and only half of parents reported that their child received specialty care, defined as receiving an evaluation by an allergist or pulmonologist within the past 2 years. Only 41% of parents reported receiving a written asthma action plan from the child’s primary care provider.
Medication Fills by Type of Medication across 12-Month Study Period
Among the 180 children with complete data, there were 1,896 prescriptions filled and analyzed over the study period. Only 20% of children obtained 6 or more ICS fills and 40% received 3 or more ICS fills over 12 months. Despite the asthma severity of this sample, 28% of children did not have any ICS fills during the study period. The frequency of SABA fills was comparable to ICS fills with 20% obtaining 6 or more SABA fills over 12 months. The number of SABA fills ranged from 0 to 26 fills. OCS fills were less frequent with most children receiving two or fewer OCS fills over 12 months. Twelve children (6.7%) obtained no fills for any asthma medication over the 12-month study period. Use of sample medications was reported by only 38 (21%) of parents and were primarily for albuterol or levalbuterol (32%), fluticasone or budesonide (29%), or montelukast (29%).
Medication Fills by Month
Children were almost twice as likely to fill an ICS prescription in January, March, or December (January OR: 2.23, CI 1.4–3.6; March OR: 2.10, CI 1.3,3.4; December OR: 2.04, CI 1.3,3.3) as compared to the reference month of August (Table 2). There were no significant differences in ICS fills for February, July, November as compared to August. Leukotriene modifier (LM) fills remained constant across all months except for a higher likelihood of receiving an LM fill in February (OR: 1.6, CI 1.1, 2.4). Short-acting beta-agonist (SABA) fills were most likely received during the spring months of March through June and fall months (September, November, and December) as compared with the reference month of July. The likelihood of receiving a SABA fill by month were comparable when using August as the reference month (data not presented). Oral corticosteroids were most often filled during the spring months (March and April) and early fall (September and October). Of note, children were significantly less likely to fill a prescription for any type of asthma medication during the summer months of July and August. Examination of Poisson models indicated the mean number of fills by drug type for each month were consistent with the logistic models of fills (Yes/No) by drug type for each month (data not reported).
TABLE 2.—
Odds ratios for medication fill for ICS, LM, OCS, or SABA by month (N = 180 Subjects, N = 1,896 prescriptions).
| Month | ICS OR (95% CI) | LM OR (95% CI) | OCS OR (95% CI) | SABA OR (95% CI) |
|---|---|---|---|---|
| January | 2.23 (1.4, 3.6)** | 1.04 (0.7, 1.6) | 1.98 (0.9, 4.5) | 1.33 (0.8,2.2) |
| February | 1.63 (1.0, 2.7) | 1.60 (1.1, 2.4)* | 1.85 (0.8, 4.2) | 1.43 (0.9,2.4) |
| March | 2.10 (1.3, 3.4)** | 1.36 (0.9, 2.1) | 2.38 (1.1, 5.2)* | 1.69(1.0, 2.8)* |
| April | 1.80 (1.1, 2.9)* | 1.36 (0.9, 2.1) | 2.65 (1.2, 5.8)* | 2.15 (1.3, 3.5)** |
| May | 1.86 (1.1, 3.0)* | 1.09 (0.7, 1.7) | 1.60 (0.7, 3.7) | 1.97 (1.2,3.3)** |
| June | 1.68 (1.0, 2.8)* | 1.31 (0.9, 2.0) | 1.60 (0.7,3.7) | 1.97 (1.2, 3.3)** |
| July | 1.10 (0.6,1.9) | 1.09 (0.7, 1.7) | 0.32 (0.1, 1.2) | —-reference— |
| August | —-reference— | —-reference— | —-reference— | 1.09 (0.6, 1.9) |
| September | 1.68 (1.0, 2.8)* | 1.31(0.9, 2.0) | 2.38 (1.1,5.2)* | 1.92 (1.2, 3.2)* |
| October | 1.74 (1.1, 2.9)* | 1.00 (0.6, 1.6) | 2.65 (1.2, 5.8)* | 1.53 (0.9, 2.6) |
| November | 1.63 (1.0, 2.7) | 1.04 (0.7, 1.6) | 1.85 (0.8, 4.2) | 2.09 (1.3, 3.4)** |
| December | 2.04 (1.3, 3.3)** | 1.17 (0.8, 1.8) | 1.73 (0.8, 4.0) | 2.28 (1.4,3.7)** |
p ≤ 0.05.
p ≤ 0.01.
There was a high degree of association between filling a rescue (SABA or OCS) and controller (LM, ICS, cromolyn) medication during the same month for all months with Pearson’s correlation coefficients ranging from a low of 0.28 for October to a high of 0.53 in September (Table 3). All coefficients were highly significant (p < 0.0001). Odds ratios (ORs) for filling a rescue and a controller medication in the same month confirm the high association (ORs: 3.58–13.32). During the months of August and December there were very low fill rates for either rescue or controller medication and this may have contributed to the high ORs for these months. There was low correlation for filling a rescue medication in one month and subsequently filling a controller medication the following month for some months of the year (ORs: 1.03–2.59). However, significant associations were noted for filling a rescue medication in March, May, August, and October with subsequent controller medication fills in April, June, September, and November.
TABLE 3.—
Pearson’s correlation coefficients and odds ratios using logistic regression for filling rescue and controller medications within same month and one month later (N = 180 Subjects, N = 1,896 prescriptions).
| Same month (Rescue medication fill and controller medication fill within same month)a |
One month later (Rescue medication filled one month and controller medication fill during subsequent month) |
|||
|---|---|---|---|---|
| Month | Pearson’s Correlation coeffaOR |
(95%CI)b | Pearson’s Correlation coeffOR |
(95% CI) |
| January | 0.33 | 4.56 (2.2,9.4) | 0.06 | 1.17(0.6,2.4) |
| February | 0.40 | 4.68 (2.3,9.7) | 0.09 | 1.45 (0.7, 3.0) |
| March | 0.34 | 6.23 (3.0, 12.9) | 0.16 | 2.59 (1.3,5.2)c |
| April | 0.48 | 7.87 (3.9,19.0) | 0.07 | 1.03(0.5, 2.0) |
| May | 0.50 | 6.78 (3.3,13.9) | 0.15 | 2.30 (1.2,4.5)c |
| June | 0.41 | 8.01 (3.8, 16.9) | 0.04 | 1.94(0.9,4.0) |
| July | 0.40 | 7.20 (2.9, 17.6) | 0.04 | 1.25 (0.5,3.2) |
| August | 0.44 | 10.50 (4.4,25.0) | 0.15 | 2.32 (1.0,5.2)c |
| September | 0.53 | 5.77 (2.8,11.8) | 0.18 | 1.41(0.7, 2.8) |
| October | 0.28 | 3.58 (1.8,7.2) | 0.04 | 2.23 (1.1,4.5)c |
| November | 0.41 | 6.78 (3.3,14.0) | −0.03 | 1.42(0.7,2.8) |
| December | 0.40 | 13.32 (6.2,28.7) | — | — |
All correlations p < 0.0001;
all ORs p < 0.0001;
p < 0.05.
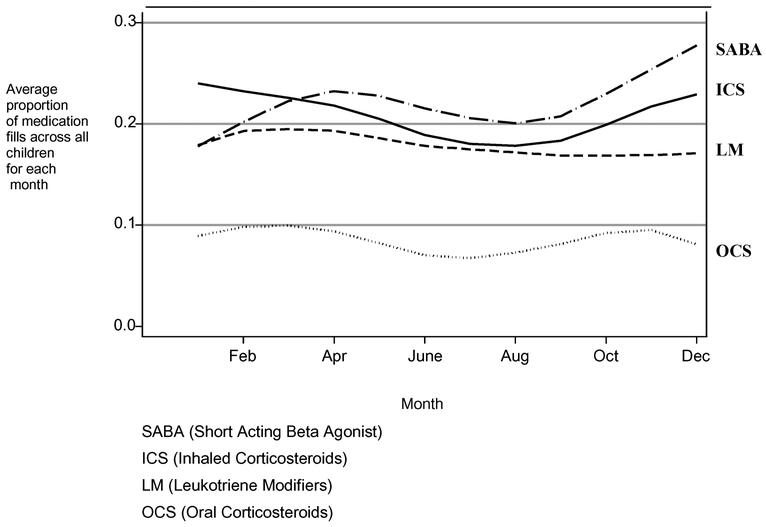
With the exception of LM, fewer children received fills in the summer months. An acceleration of fills in early fall was evident coinciding with a return to school in September (Figure 2). The acceleration in fills for ICS and SABA continued throughout the fall. The proportion of ICS fills remained consistently high through winter and started to decrease in the spring months. SABA fills were low during the months of January and February.
FIGURE 2.—
Average proportion of children with a medication fill by months across 12 months. (2001–2003) N = 180 subjects and N = 1896 prescriptions.
DISCUSSION
Our prescription dispensing data for this group of inner-city minority children with persistent asthma indicate distinct temporal patterns of both rescue and preventive asthma medication fills, indicating significantly fewer prescription fills in the summer with an acceleration of fills in September through December and a second increase in fills during early spring. This pattern suggests that the children in this sample may have been less symptomatic during the summer months with parents less likely to fill any asthma medications. The summer decline in both ICS and SABA fills is consistent with prior reports of prescription filling demonstrated in Canadian adults and children with asthma (12)but contradictory to the national asthma guideline recommendations of consistent daily ICS use for persistent asthma. It is encouraging that in 8 of 12 months, the likelihood of ICS fills was significantly higher than August (the reference month); however, the likelihood of SABA fills was also frequent (7 of 12 months) and is consistent with the mean of 6.5 SABA fills per year previously reported in an adult Medicaid population (28). The association between beta-agonist prescription fills and subsequent asthma exacerbations has been recognized (28) as well as the over dependence of low-income children on rescue medications (21).
Temporally, our data support the fall “epidemic” of acute asthma exacerbations (9) and the reported autumn peak in ED and hospital utilization (7, 10, 11) noted in national and international sites (12)based on the acceleration of SABA and ICS fills starting in September and continuing through out the fall. Peak hospitalizations for asthma occurred in September (weeks 36–39) in a prior study using hospital discharge data (7). This seasonal pattern is consistent with the early fall increase in SABA and ICS fills noted in our data. The decline in SABA and ICS fills in early summer may be related to overall decrease in asthma symptoms possibly due to decreased exposure to viral triggers and/or decreased exposure to indoor allergens and irritants with increased outdoor activities during the summer months or not filling prescriptions in the summer months if traveling. Alternatively, adherence to asthma medications may be decreased in the summer, yet if children were symptomatic we would expect to see higher fill rates of SABA in the summer months. It is possible that good asthma control was achieved during summer months and children underwent step down therapy.
The low likelihood for SABA and ICS fills during the summer is surprising in light of poor air quality during that time of year including increased ambient air ozone levels. This is in contrast to the report that increased exposure to ambient air ozone has been shown to be associated with increased asthma morbidity, ED visits, hospitalizations, and rescue medication use in children with asthma (29). Filling a rescue medication was highly correlated with filling a controller medication during the same month, but not as highly correlated with filling a controller in the subsequent month. It appears that treating acute symptoms may be a motivator for controller medication fills. However, as rescue medication fills decreased, controller medication fills appeared to decrease.
It has been proposed that in some patients with adequate asthma control, intermittent ICS fills may be sufficient to maintain control. Perhaps there is a subgroup of children who fill their ICS for 6 to 8 months a year with two or fewer SABA or OCS fills a year and are able to keep their asthma in control. Alternatively, the concurrent fill patterns of SABA and ICS support the idea that families may not believe asthma exacerbations can be prevented, but rather they believe that these exacerbations require treatment with both types of medications.
The most recent national asthma guidelines recommend a minimum of two clinic visits over 12 months to assess asthma control for children with persistent asthma (15). Timing of asthma monitoring visits to occur before peak prescription fill months, i.e., August and December, for an asthma “tune-up” theoretically could improve asthma control. In fact, addition of montelukast has recently been shown to reduce the “September epidemic” of asthma in young boys and adolescent girls (30). During these primary care visits children could benefit from more intensive monitoring of medication use including monitoring lung function, frequency of prescription refills, and assessment of medication device technique to ensure that an effective dose of medication is adequately delivered to the respiratory tract. Alternatively, referral of children with more severe asthma to a specialist, as suggested in the current asthma guidelines (15), may allow more time for monitoring symptoms and medication use, asthma education, and additional diagnostic testing such as allergy skin testing and pulmonary function studies.
Limitations
Although we were able to obtain pharmacy records with the number of prescriptions dispensed over a 12-month period, we are unable to determine actual medication use by the child, sharing of medication among household members, or the number of family members with asthma residing in each household. However, prescription records do indicate drug availability and have been shown to be a reliable source of drug exposure (31). We did not attempt to convert prescription fills to monthly medication supply because we did not have information on the actual dose prescribed to make those estimations accurately. However, 95% of prescriptions filled were for quantities of one canister of medication (or equivalent amount of nebulized or oral LM) rather than quantities that appeared to be intended for multiple months. Additionally, we are unable to generalize our findings beyond this group of children due to the selection bias of this sample. Similar seasonal patterns in asthma-related ED visits and hospitalizations have been reported and support our results. This sample was selected from a base of high-risk inner-city children with persistent asthma who attended both primary and specialty care sites. Because almost half received specialty care in addition to primary care, the likelihood of receiving an anti-inflammatory prescription is most likely higher than in the primary care settings that treat most children with asthma.
In conclusion, prescription fills for children with persistent asthma indicate significantly fewer prescription fills in the summer months and an acceleration of fills in September through December. Optimally, we recommend that back-to-school medical visits include taking advantage of opportunities to reinforce asthma action plan use and when appropriate restarting daily controller medications to prevent predictable asthma episodes. Awareness of seasonal peaks by healthcare providers should also affect decisions to “step down” therapy during times of decreased asthma symptoms, such as the summer months, and to “step up” or prepare children for peak asthma periods with adequate preventive asthma medications.
ACKNOWLEDGEMENT
This research was supported by the National Institute of Nursing Research, NIH NR05060. The authors thank the families for their willingness to participate in this study, Amanda Manning for retrieving the pharmacy records, and the pharmacists for their cooperation in providing pharmacy records.
REFERENCES
- 1.Bloom B, Dey AN. Summary health statistics for U.S. children: National Health Interview Survey, 2004. National Center for Health Statistics. Vital Health Statistics 2006; 10(227):4–5. [PubMed] [Google Scholar]
- 2.Akinbami LJ, Schoendorf KC. Trends in childhood asthma: Prevalence, health care utilization, and mortality. Pediatrics 2002; 110:315–322. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control. National Surveillance for Asthma- United States. 1980–2004 MMWR 2007; 56 (SS09):1–14; 18–54. [Google Scholar]
- 4.Newacheck PW, Halfon N. Prevalence, impact and trends in childhood disability due to asthma. Arch Pediatr Adolesc Med 2000; 154:287–293. [DOI] [PubMed] [Google Scholar]
- 5.McCaig LF, Nawar EN. National Hospital Ambulatory Medical Care Survey: 2004 Emergency Department Summary. Adv Data Vital Health Stat 2006; 372:5,18. [PubMed] [Google Scholar]
- 6.Tilles SA, Bardana EJ. Seasonal variation in bronchial hyperactivity (BHR) in allergic patients. Clin Rev Allergy Immunol 1997; 15:169–185. [DOI] [PubMed] [Google Scholar]
- 7.Blaisdell CJ, Weiss S, Timmins S, Kimes DS, Levine ER, Myers M, Bollinger MB. Using seasonal variations in asthma hospitalizations in children to predict hospitalization frequency. J Asthma 2002; 39:567–575. [DOI] [PubMed] [Google Scholar]
- 8.Wang HC, Yousef E. Air quality and pediatric asthma-related emergencies. J Asthma 2007; 44:839–841. [DOI] [PubMed] [Google Scholar]
- 9.Johnston NW, Johnston SL, Duncan JM, Greene JM, Kebadze T, Keith PK, Roy M, Waserman S, Sears MR. The September epidemic of asthma exacerbations in children: A search for etiology. J Allergy Clin Immunol 2005; 115:132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimes D, Levine E, Timmins S, Weiss SR, Bollinger ME, Blaisdell C. Temporal dynamics of emergency department and hospital admissions of pediatric asthmatics. Environ Res 2004; 94:7–17. [DOI] [PubMed] [Google Scholar]
- 11.Kimball-Dun M, Pearce N, Beasley R. Seasonal variation in asthma hospitalizations and death rates in New Zealand. Respirology 2005:241–246. [DOI] [PubMed] [Google Scholar]
- 12.Johnston NW, Sears MR. Asthma exacerbations.1: Epidemiology. Thorax 2006; 61:722–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.U.S. Department of Health and Human Service. PHS, NIH, NHLBI. Expert Panel Report 2. Guidelines for the Diagnosis and Management of Asthma. National Asthma Education and Prevention Program (NAEPP), 1997. NIH Publication; 97–4051. [Google Scholar]
- 14.U.S. Department of Health and Human Service. PHS, NIH, NHLBI. Guidelines for the Diagnosis and Management of Asthma: Update on Selected Topics, 2002. National Asthma Education and Prevention Program (NAEPP), 2002. NIH Publication; 97–4051. [Google Scholar]
- 15.U.S. Department of Health and Human Service. NIH, NHLBI. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Full Report 2007. National Asthma Education and Prevention Program (NAEPP), 2007. NIH Publication; 07–4051. August 2007. [Google Scholar]
- 16.Global Initiative for Asthma (GINA): Global Strategy for Asthma Management and Prevention: Revised 2002. Bethesda, MD: National Heart, Lung and Blood Institute, National Institutes of Health: 2002. [Google Scholar]
- 17.Diaz T, Sturm T, Matte T, Bindra M, Lawler K, Findley S, Maylahn C Medication use among children with asthma in East Harlem. Pediatrics 2000; 105:1188–1193. [DOI] [PubMed] [Google Scholar]
- 18.Halterman JS, Yoos HL, Sidora K, Kitzman H, McMullen A. Medication use and health care contacts among symptomatic children with asthma. Ambul Pediatr 2001; 1:275–279. [DOI] [PubMed] [Google Scholar]
- 19.Butz AM, Tsoukleris MG, Donithan M, Hsu VD, Zuckerman I, Mudd K, Thompson RE, Rand C, Bollinger ME. Effectiveness of Home Nebulizer Education Intervention in Young Minority Children with Asthma. Arch Pediatric Adolesc Med 2006; 160:622–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Am Butz, Tsoukleris MG, Donithan M, HSU VD, Zuckerman I, Mudd K, Bollinger ME Patterns of inhaled anti-inflammatory medication use in young underserved children with asthma. Pediatrics 2006; 118:2504–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonner S, Matte T, Rubin M, Fagan JK, Ahern J, Evans D. Oral beta2-agonist use by preschool children with asthma in East and Central Harlem, New York. J Asthma 2006; 43:31–35. [DOI] [PubMed] [Google Scholar]
- 22.Mudd K, Bollinger ME, Hsu VD, Donithan M, Butz A. Pharmacy fill patterns in young urban children with persistent asthma. J Asthma 2006; 43:597–600. [DOI] [PubMed] [Google Scholar]
- 23.Mudd K, Bollinger ME, Hsu VD, Manning A, Tsoukleris MG, Butz AM. Concordance of Medicaid and pharmacy record data in inner-city children with asthma. Contemp Clin Trials 2008; 29:13–20. [DOI] [PubMed] [Google Scholar]
- 24.Carlton BG, Lucas DO, Ellis EF, Conboy-Ellis K, Schoheiber O, Stempel D. The status of asthma control and asthma prescribing practices in the United States: Results of a large prospective asthma control survey of primary are practices. J Asthma 2005; 42:529–535. [DOI] [PubMed] [Google Scholar]
- 25.Hastie TJ, Tibshirani RJ. Generalized Additive Models. New York, NY: Chapman & Hall/CRC, 1990:29–31. [Google Scholar]
- 26.SAS Institute.SAS/STAT User’s Guide (version 8.0 ed). Cary, NC: SAS Institute, Inc, 2000. [Google Scholar]
- 27.Stata Statistical Software: Release 9.0. College Station, TX: Stata Corporation, 2005. [Google Scholar]
- 28.Naureckas ET, Dukic V, Bao X, Rathouz P. Short-acting B agonist prescription fills as a marker for asthma morbidity. Chest 2005; 128: 602–608. [DOI] [PubMed] [Google Scholar]
- 29.Bernstein JA, Alexis N, Barnes C, Bernstein IL, Bernstein JA, Nel A, Peden D, Diaz-Sanchez D, Tarlo SM, Williams PB. Health effects of air pollution. J Allergy Clin Immunol 2004; 114:1116–1123. [DOI] [PubMed] [Google Scholar]
- 30.Johnston NW, Mandhane PJ, Dai J, Duncan JM, Greene JM, Lambert K, Sears MR. Attenuation of the September epidemic of asthma exacerbations in children: A Randomized, controlled trial of Montelukast added to usual therapy. Pediatrics 2007; 120:e702–e712. [DOI] [PubMed] [Google Scholar]
- 31.Grymonpre R, Cheang M, Fraser M, Metge C, Sitar DS. Validity of prescription claims database to estimate medication adherence in older persons. Med Care 2006; 44:471–477. [DOI] [PubMed] [Google Scholar]