Abstract
This article summarizes the presentations and recommendations of the tenth annual American Geriatrics Society and National Institute on Aging Bench-to-Bedside research conference, “Sensory Impairment and Cognitive Decline,” on October 2–3, 2017, in Bethesda, Maryland. The risk of impairment in hearing, vision, and other senses increases with age, and almost 15% of individuals aged 70 and older have dementia. As the number of older adults increases, sensory and cognitive impairments will affect a growing proportion of the population. To limit its scope, this conference focused on sensory impairments affecting vision and hearing. Comorbid vision, hearing, and cognitive impairments in older adults are more common than would be expected by chance alone, suggesting that some common mechanisms might affect these neurological systems. This workshop explored the mechanisms and consequences of comorbid vision, hearing, and cognitive impairment in older adults; effects of sensory loss on the aging brain; and bench-to-bedside innovations and research opportunities. Presenters and participants identified many research gaps and questions; the top priorities fell into 3 themes: mechanisms, measurement, and interventions. The workshop delineated specific research questions that provide opportunities to improve outcomes in this growing population. J Am Geriatr Soc 66:2052–2058, 2018.
Keywords: vision, hearing, cognition, dementia, comorbidity
This article summarizes presentations and recommendations from the tenth annual American Geriatrics Society and National Institute on Aging Bench-to-Bedside research conference, “Sensory Impairment and Cognitive Decline,” on October 2–3, 2017, in Bethesda, Maryland.
The risk of impairment of hearing, vision, and other senses increases with age, and almost 15% of individuals older than 70 years have dementia.1–3 As the number of older adults increases, sensory and cognitive impairments will affect a growing proportion of the population. To limit its scope, this conference focused on concurrent impairments in cognition, vision, and hearing.
Rates of impairment in near and distance visual acuity increase with age. The most common vision problems in older adults are impaired spatial contrast sensitivity (important for pattern recognition), scotopic dysfunction (slower adaptation to darkness and impaired light sensitivity at night), and slow visual processing.4
The link between visual impairment and cognitive decline is complex. For example, impaired vision could affect cognitive function,5 and improving near vision with eyeglasses can reduce depressive symptoms,6 which can be manifested as cognitive deficits. Cognition could also affect vision. People locate and fixate on visual targets in semantically appropriate locations that are not visually salient, showing that they use cognitive factors, not only image characteristics, to guide their visual attention. Visual impairment and cognitive decline might also have common causes.
Hearing loss is the third most common health disorder in older adults.7 Peripheral hearing loss in this population is associated with 30% to 40% faster cognitive decline than in those without hearing loss and with a 24% higher risk of cognitive impairment.8 Hearing impairment is also related to faster decreases in the volume of brain regions that are important for spoken language processing and other cognitive functions.9
Cognitive impairment could also contribute to hearing-related difficulties in challenging listening conditions. For example, changes throughout the central auditory pathway make it more difficult for older adults with hearing loss to separate speech from background noise, leading to communication difficulties.10 This challenge is particularly acute in those with a decrease in cognitive function. Cognitive auditory training could potentially improve the neural processing needed to understand speech in noisy environments.
COMORBID SENSORY AND COGNITIVE IMPAIRMENT
Mechanisms
The co-occurrence of sensory impairments (e.g., vision and hearing) is more common than expected by chance alone, and vision and hearing impairments are associated with greater risk of cognitive impairment.11 One cross-sectional study in community-dwelling older adults who did not have dementia found that more than 2% of this population reported both vision and hearing impairments and screened positive for cognitive impairment.12 Hearing and vision rely on cognitive processes, and cognitive testing involves visual or auditory tasks, so these relationships may be multifactorial.
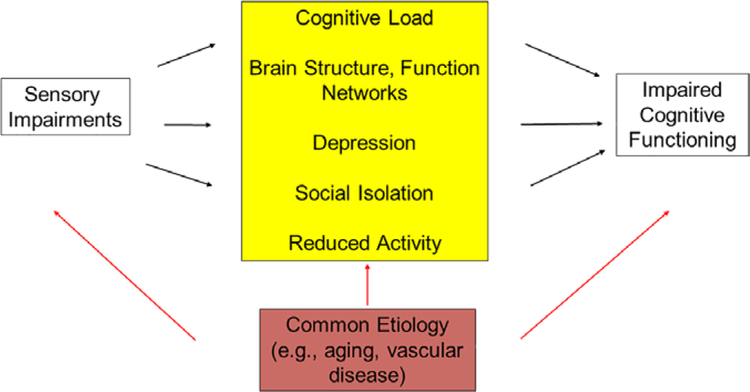
A common pathological process (e.g., vascular disease) might be responsible for sensory and cognitive impairments in older adults (Figure 1). According to some studies, amyloid-β, a pathological hallmark of Alzheimer’s disease, is present in the ocular lens (where it can cause supranuclear cataracts) and retina, and its presence in the retina could be a biomarker for Alzheimer’s disease, which is also associated with changes in retinal ganglion cells and optic neuropathy.13
Figure 1.
Hypothesized relationships that may explain the epidemiological link that has been described between sensory (vision, hearing) impairments and cognitive impairment. The yellow box depicts possible causative pathways by which sensory loss leads to reduced cognitive performance. For example, sensory impairment may increase “cognitive load,” or the total amount of demand placed on the brain at any given time. Assuming cognitive capacity is a fixed resource, high cognitive load could impair performance on cognitive tests. Another possibility is that sensory impairment interferes with physical or cognitively stimulating activities (e.g., exercise, reading) and that the lack of activity has a detrimental effect on cognitive function. The red box depicts the possibility that the relationship between sensory impairment and cognitive impairment is correlational. If this is the case, the impairments may co-occur more frequently than expected because they share common risk factors (e.g., age, smoking, genetics). Any or all of the pathways depicted here may explain a link between sensory and cognitive impairment.
Alternatively, sensory impairments might have a causal role in cognitive impairment. By increasing cognitive load, these impairments might limit the neural resources needed for optimal performance of cognitive tasks. In addition, loss of sensory input could directly alter brain structure and function (e.g., long-term deafferentation or overloaded brain circuity from resource demands to address poor signal-to-noise ratios). Finally, sensory loss may lead to depression, social isolation, and lack of physical activity, which could cause cognitive impairment.
Consequences of Comorbid Cognitive and Sensory Impairment
Persons with comorbid sensory and cognitive impairment are at greater risk of depression and death. Whether these are simply additive effects is not clear. Improving vision in persons with cognitive impairment may improve cognition or reduce cognitive decline, but cognitive impairment can reduce the effectiveness of visual rehabilitation.14 There has been limited research on the functional consequences of combined sensory and cognitive impairments despite the high prevalence of these conditions in older adults. Table 1 summarizes conclusions and key research gaps identified during the “Comorbid Sensory and Cognitive Impairment” session.
Table 1.
Comorbid Sensory and Cognitive Impairment: Current Knowledge and Gaps
What we know:
Knowledge gaps:
|
SENSORY LOSS AND THE AGING BRAIN
Age-related changes in the central nervous system (CNS) can drive functional decline directly or indirectly (by weakening the potential for plasticity), and CNS changes (e.g., in hippo-campal volume and artery and vein radius) are related to cognitive impairment.15
Noninvasive techniques are invaluable for understanding the neural mechanisms of age-related changes in human sensory perception, but they lack the spatial and temporal resolution needed to fully characterize the sensory processes throughout the CNS or how aging affects these processes. Animal models can help meet this need. Even though the age at which a given species becomes old varies, each species exhibits brain and cognitive changes near the end of the lifespan. Animal models have been used to study the roles of the hippocampus, prefrontal cortex, and perirhinal cortex in cognition and the ability to regulate neuronal responses to sound.
Visual acuity, contrast sensitivity, dark adaptation, and visual processing speed change with aging, even in people who do not have cataracts, glaucoma, or age-related macular degeneration, and these changes have been associated with cognitive impairments.4 Impaired vision compromises visual cognition, and cognitive deficits can also impair visual perceptual processes. Interventions that enhance visual stimuli (e.g., by increasing contrast) can improve visual search by decreasing reaction time.16 Other approaches to enhance visual stimuli include medical and surgical ophthalmic interventions (e.g., cataract surgery) and improving the visual environment in the home by, for example, reducing glare and removing visual clutter. Certain methods, including some that use concurrent brain stimulation, can amplify and clarify visual information in adults with cognitive deficits.17
Vision is particularly important to adults with hearing impairment, who depend more on visual cues. Vision recruits and repurposes auditory areas of the brain for visual processing in individuals with hearing loss.18 This reorganization appears to enhance the use of multiple senses in speaking and listening to speech, which is particularly important for people with age-related hearing loss when listening becomes effortful. In difficult listening situations (e.g., noisy environments), older adults also recruit additional brain processes to help them understand conversational speech.19 Table 2 summarizes conclusions and key research gaps identified during the “Sensory Loss and the Aging Brain” session.
Table 2.
Sensory Loss and the Aging Brain: Current Knowledge and Gaps
What we know:
Knowledge gaps:
|
BENCH-TO-BEDSIDE INNOVATIONS AND OPPORTUNITIES
As the population ages, comorbid sensory loss and cognitive impairment will become even more common. The number of centenarians will grow, and it will be important to know what normal cognitive and sensory aging means in these older adults so that sensory measurements can be adjusted to age and cognitive status. According to the Framework for Understanding Effortful Listening, listening requires effort and is tiring for those with impaired hearing, which can reduce motivation to listen, and a better understanding is needed of effortful listening.20 The same is likely to be true for vision.
Although it is unclear whether sensory loss drives accelerated brain aging or brain aging drives accelerated sensory loss (or both), tools are available to track these changes prospectively in large-scale longitudinal studies. One such study used a new approach to medical image analysis for a cross-sectional analysis of associations between aging and brain changes in 2,705 adults.21 The results showed varied rates of changes in different brain regions associated with cognitive function. Furthermore, participants in the Baltimore Longitudinal Study of Aging had differences according to sex on most tests of cognitive ability, with better performance in women for verbal memory tasks and in men for visual spatial abilities.22 Amyloid and tau positron emission tomography has revolutionized research on Alzheimer’s disease, making it possible to track the progress of the disease over time.
Debate continues about which interventions are most effective for influencing cognition later in life. For example, moderately strong evidence supports the benefits of exercise (e.g., brisk walking) for improving cognitive and brain function in older adults, and exercise holds promise as an effective treatment approach.23 Although exercise affects every part of the body, the mechanisms underlying the link between exercise and cognitive function need to be better understood. Potential explanations include cellular or molecular mechanisms, psychosocial changes, and effects on sleep. The evidence supporting cognitive training interventions is mixed, and it is not clear whether learning during training in one task is transferable to other tasks. There has been little discussion of the potential effect of these interventions on sensory function or how sensory status might modify their effects on cognition.
In addition to treating cognitive impairment directly, treating hearing and vision loss in older adults is important for ensuring healthy aging and continued active participation and engagement by adults with sensory and cognitive impairment. Restorative sensory care can consist of pharmacological interventions, surgery (e.g., cochlear implants, cataract surgery), or rehabilitation (e.g., assistive devices (e.g., hearing aids or portable magnifiers), strategies (e.g., speech reading, improved lighting)). Treatment for hearing loss reduces caregiver-identified problem behaviors, depressive symptoms, and neuropsychiatric symptoms.26 Similarly, in addition to improving visual acuity, low-vision rehabilitation can improve health-related quality of life, memory, and independence in older adults with cognitive deficits.27
Rehabilitation for age-related hearing loss and low vision is underused. For example, fewer than 20% of the more than half of adults aged 70 to 79 with hearing loss use hearing aids.28 Simple, low-cost, community-delivered solutions for hearing and vision care are critical for low-income and minority older adults.
A variety of office-based sensory system evaluation tools, including questionnaires and clinical techniques, are available. The challenges of using these tools in individuals with cognitive impairment include the uncertainty of test characteristics (e.g., their sensitivity and specificity might not have been determined in populations with cognitive impairments), lack of time to administer the tests, and logistics of arranging multiple clinic visits for follow-up assessments. Furthermore, the U.S. Preventive Services Task Force has determined that the evidence is insufficient to support vision or hearing screening in the primary care setting in adults aged 50 and older, resulting in less impetus for screening.29,30
It is possible to modify a standardized cognitive test to accommodate individuals with visual impairment by, for example, asking them to say a sentence aloud instead of writing it down, and individuals with visual impairment do better on vision-independent test versions. Standardized protocols to ensure that sensory impairments do not directly confound cognitive test administration (someone with hearing loss misunderstands a spoken word in a verbal memory task) are not routinely used despite the high prevalence of hearing and vision loss in older adults. Table 3 summarizes conclusions and key research gaps identified during the “Bench to Bedside Innovations and Opportunities” session.
Table 3.
Bench-to-Bedside Innovations and Opportunities: Current Knowledge and Gaps
What we know:
Knowledge gaps:
|
FUTURE RESEARCH PRIORITIES
Mechanisms Underlying the Sensory–Cognitive Interface
Effect of Sensory Loss on the Aging Brain
High-priority research questions include whether changes in sensory systems drive cognitive changes or whether any causal relationships between sensory and cognitive function may be bidirectional. We need to understand the roles of cardiovascular disease, metabolic dysregulation, genetics, and inflammation as common pathways to cognitive and sensory impairment. We also need to learn when the response to cognitive load becomes maladaptive and whether this differs according to system.
Another research priority is to determine whether sensory treatment has a downstream effect on cognition, including elucidation of the temporal sequence of peripheral and sensory changes and cognitive decline, and whether comorbid sensory impairment accelerates the downward trajectory of cognitive decline in dementia.
Neural Mechanisms
Major gaps in knowledge about brain pathways and networks at the interface of cognitive and sensory research include how top-down processes and bottom-up activity intersect and how generalizable these processes are across sensory systems. Studies could include comparisons of human and animal models of the same sensory and cognitive functions and between different sensory systems to assess shared top-down cognitive processes. Another research gap is the lack of knowledge as to which genetic and biological factors contribute to good sensory and cognitive function in older adults.
More research is needed to understand how senses beyond audition and vision (e.g., vestibular and olfactory systems) influence cognitive health and what happens when more than one sensory system is impaired. Small-vessel disease, a reasonable common pathway for vision, hearing, and cognitive impairment, has been understudied. Leveraging large population studies and existing study data is one potential means of addressing this question. Research gaps also exist regarding the influence of inflammation on sensory and cognitive function. Animal models of human sensory and cognitive processing would help advance this research.
Research is needed to identify the primary and secondary mechanisms of the benefits of exercise and healthy lifestyle, genetics, and other factors for cognitive and sensory health and the biology that these activities affect. Research should be conducted to determine whether exercise provides additional benefits, such as enhancing directed attention in background noise, visual acuity, or executive function. More collaboration is needed between those who study the interaction of sensory and cognitive processes and those seeking mechanisms that affect sensory and cognitive function.
Disparities
Most of our knowledge about prevention and treatment of cognitive impairment is based on white populations of higher socioeconomic status. Knowledge is limited on visual, hearing, and cognitive impairment in older under-represented minorities and other underserved populations.
Research should be conducted to determine the trajectory of sensory and cognitive function in older adults without depending on self-reports\. These data need to be stratified according to sex, race and ethnicity, socioeconomic status, education level, and healthcare use. This research needs to include the oldest, most vulnerable adults who live in assisted living and retirement communities.
Few epidemiological datasets are available with minority representation that are not based on self-reported measures. When there is sample bias, the co-prevalence of sensory and cognitive impairments might be underestimated because individuals with sensory impairments might be less likely to participate in research or be excluded.
Measurement
How to Measure
Portable, cost-effective, user-friendly measures of sensory and cognitive function that can be used in different populations (e.g., those who speak different languages and are of different ages, sexes, and races and ethnicities) must be developed. These new measures will be more successful if they are freely available to clinicians and researchers, so they should be usable in research environments and transferable to clinical environments.
Research should be conducted to determine how best to assess cognition in people with sensory loss, given the need to maintain fidelity with validated cognitive tests. To distinguish cognitive from sensory deficits, researchers might develop standardized test administration protocols that can ensure that a person’s inability to perceive the stimuli or understand the instructions correctly does not directly confound existing cognitive tests with auditory or visual stimuli.
Traumatic brain injury and chronic traumatic encephalopathy affect central visual and auditory processing, but peripheral sensory is often minimally affected. Studies of traumatic brain injury function and chronic traumatic encephalopathy might provide opportunities to study the effect on cognition of changes in vision and hearing function in the absence of peripheral vision or hearing loss.
Although cognitive function is traditionally assessed by observing behavior, the workshop considered the role of a theoretical biomarker of cognitive or brain function. Such a biomarker might be valuable in individuals whose cognitive function is difficult to measure because of sensory deficits.
What to Measure
Surveillance research is needed to describe cognitive, hearing, and vision over the lifespan in different populations. Measurements and analytical tools are required to assess cognition fairly and accurately in the presence of sensory loss, including dual sensory impairment. We need cognitive and sensory tests that are more objective (e.g., brain activation) and more ecologically valid. We also need to collect information on social network, personality, occupational, and other factors that could affect cognitive and sensory health and treatment patterns.
Valid measures of sensory function are needed for animal studies, as are cross-species models of cognition in individuals with hearing and vision loss.
Interventions
Intervention Development Research
More data are needed on individual differences in treatment effect and differences between healthy older adults and those with impaired sensory function, impaired cognition, impaired sensory and cognitive interactions, and poor effort and motivation. Intervention development research should be conducted to examine adults who are and are not aging “successfully.”
Intervention development requires multidisciplinary, long-term longitudinal studies to establish trajectories of changes in sensory function and cognition and their interactions. Studies are also needed to determine the mechanisms of successful interventions and their clinical relevance to people.
Triggers of decline and protective factors should be identified. A precision medicine type of approach could be developed to provide individualized care because the cognitive and functional limitations of older adults probably differ in degree and type of sensory impairment. Research is also needed to identify specific sensory (within domain) or cognitive impairments and address functional problems associated with these deficits.
Mixed-methods approaches to implementation science are critical for ensuring that interventions that are developed are actually used. The best timing for interventions must be determined, as must strategies to ensure feasibility and uptake.
Health Services and Comparative Effectiveness Research
Research is needed to establish the efficacy of assistive devices, such as hearing aids and low-vision assistive devices, including training in their use, for functional outcomes in individuals with visual, hearing, and cognitive impairments. Policymakers, funding agencies, researchers, and training programs are needed to consider vision, hearing, and cognition in an integrated system of health, not in discrete silos.
Comparative effectiveness research could be conducted to determine the efficacy of vision and hearing screening for older adults in primary care for improving individual and population health outcomes, including cognitive outcomes. Such research could compare treatments, including usual care, disease treatment, and rehabilitation interventions, and assess multiple outcomes (e.g., vision, hearing, cognitive function, quality of life, activities of daily living, cost effectiveness, caregiver concerns).
Researchers should also exploit medical specialty registries that collect electronic health records (e.g., IRIS [Intelligent Vision in Sight] and the Reg-ent ear, nose, and throat data registries) to address research questions.
Table 4 provides a summary of the highest-priority research gaps.
Table 4.
Top Research Gaps in Sensory and Cognitive Impairment in Older Adults
Mechanisms underlying the sensory cognition interface
Measurement
Interventions (including screening)
|
CONCLUSION
The evidence reviewed in the tenth annual Bedside-to-Bench research conference, “Sensory Impairment and Cognitive Decline,” indicates that vision, hearing, and cognitive impairments co-occur in older adults more often than would be expected by chance alone. Presenters and participants identified many research gaps and questions, and the top priorities fell into 3 themes: mechanisms, measurement, and interventions. This workshop delineated specific research questions that provide opportunities to improve outcomes in this growing population. The full report of the Conference is included as a Supplement Appendix to this Executive Summary.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank science writer Deborah Berlyne, PhD, and staff at the American Geriatrics Society, especially Anna Mikhailovich and Elisha Medina-Gallagher, for outstanding support. We also thank all conference speakers and participants. We are grateful to the sponsors of the conference, including the National Institute on Aging (NIA), American Academy of Audiology Foundation, Cochlear Ltd., EyeSight Foundation of Alabama, Lighthouse Guild, National Eye Institute, MED-EL, Research to Prevent Blindness, and Retirement Research Foundation.
Financial Disclosure: Funding was provided by National Institutes of Health (NIH) Grant U13 AG054139-01. Dr. Whitson’s efforts and contributions were supported by R01AG043438, R24AG045050, UH2AG056925, and 5P30AG028716. Dr. Lin’s effort and contributions were also supported by R01AG055426, R01HL096812, and R33DC015062.
The information and views in this manuscript do not necessarily reflect those of the sponsors; the authors retained full independence in the planning of the conference agenda and the summary provided herein.
Footnotes
Conflict of Interest: KJC receives partial salary support from grants funded by the NIH to UW that are related to the topics of this manuscript. CO is a board member for Research to Prevent Blindness (Chicago, IL) and holds a patent for, “Method and Apparatus for the Detection of Impaired Dark Adaptation.” JP is a PI for NIH Grants R01DC014281 and R21DC015884 and is paid honoraria by NIH for study section grant reviewing. GR received funding from the NIA that included some summer salary. GR received an honorarium for speaking and serving on study section. AS receives grant funding from the NIH and the Hearing Industry Research Consortium. KY receives grant funding from the NIA. FRL reports grants from the MIH and Eleanor Schwartz Charitable Foundation. FRL is a consultant for Amplifon and Cochlear Ltd.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article.
Supplemental Appendix. Report summarizing the presentations and recommendations from the AGS/NIA U13 Conference: Sensory Impairment and Cognitive Decline.
REFERENCES
- 1.McArdle JJ, Fisher GG, Kadlec KM. Latent variable analysis of age trends of cognition in the Health Retirement Study, 1992–2004. Psychol Aging 2007; 22:525–545. [DOI] [PubMed] [Google Scholar]
- 2.Petersen RC, Smith GE, Waring SC et al. Mild cognitive impairment: Clinical characterization and outcome. Arch Neurol 1999;56:303–308. [DOI] [PubMed] [Google Scholar]
- 3.Salthouse TA. Continuity of cognitive change across adulthood. Psychon Bull Rev 2016;23:932–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schaie KA. Intellectual Development in Adulthood: The Seattle Longitudinal Study, 1st Ed. New York: Cambridge University Press, 1996. [Google Scholar]
- 5.Alzheimer’s Association and Centers for Disease Control and Prevention The Healthy Brain Initiative: The Public Health Road Map for State and National Partnerships, 2013–2018. Chicago, IL: Alzheimer’s Association, 2013. [Google Scholar]
- 6.Petersen RC, Doody R, Kurz A et al. Current concepts in mild cognitive impairment. Arch Neurol 2001;58:1985–1992. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell AJ, Shiri-Feshki M. Temporal trends in the long term risk of progression of mild cognitive impairment: A pooled analysis. J Neurol Neurosurg Psychiatry 2008;79:1386–1389. [DOI] [PubMed] [Google Scholar]
- 8.Farias ST, Cahn-Weiner DA, Harvey DJ et al. Longitudinal changes in memory and executive functioning are associated with longitudinal change in instrumental activities of daily living in older adults. Clin Neuropsychol 2009;23:446–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McEwen BS. Effects of adverse experiences for brain structure and function. Biol Psychiatry 2000;48:721–731. [DOI] [PubMed] [Google Scholar]
- 10.Sandi C Stress and cognition. Wiley Interdiscip Rev Cogn Sci 2013;4: 245–261. [DOI] [PubMed] [Google Scholar]
- 11.Lupien SJ, McEwen BS, Gunnar MR et al. Effects of stress throughout the lifespan on the brain, behavior and cognition. Nat Rev Neurosci 2009;10:434–445. [DOI] [PubMed] [Google Scholar]
- 12.Magariños AM, McEwen BS, Flugge G et al. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci 1996;16:3534–3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lupien SJ, Lepage M. Stress, memory, and the hippocampus: Can’t live with it, can’t live without it. Behav Brain Res 2001;127:137–158. [DOI] [PubMed] [Google Scholar]
- 14.Lazarus RS, Folkman S. Stress, Appraisal, and Coping, 1st Ed. New York: Springer Publishing Company, 1984. [Google Scholar]
- 15.McEwen BS. The brain is the central organ of stress and adaptation. Neuro-image 2009;47:911–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sands JD. The relationship of stressful life events to intellectual functioning in women over 65. Int J Aging Hum Dev 1981;14:11–22. [DOI] [PubMed] [Google Scholar]
- 17.Munoz E, Sliwinski MJ, Scott SB et al. Global perceived stress predicts cognitive change among older adults. Psychol Aging 2015;30:487–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aggarwal NT, Wilson RS, Beck TL, et al. Perceived stress and change in cognitive function among adults aged 65 and older. Psychosom Med 2013;76: 80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, Peng Y, Ma X et al. Conscientiousness moderates the relationship between perceived stress and depressive symptoms among U.S. Chinese older adults. J Gerontol A Biol Sci Med Sci 2017;72A:S108–S112. [DOI] [PubMed] [Google Scholar]
- 20.Mui AC, Kang SY. Acculturation stress and depression among Asian immigrant elders. Soc Work 2006;51:243–255. [DOI] [PubMed] [Google Scholar]
- 21.Wei M, Shi J, Li T et al. Diagnostic accuracy of the Chinese version of the trail-making test for screening cognitive impairment. Am J Geriatr Psychiatry 2018;66: 92–99. [DOI] [PubMed] [Google Scholar]
- 22.Chow TW, Ross L, Fox P et al. Utilization of Alzheimer’s disease community resources by Asian-Americans in California. Int J Geriatr Psychiatry 2010; 15:838–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Still CN, Jackson KL, Brandes DA et al. Distribution of major dementias by race and sex in South Carolina. J S C Med Assoc 1990;86:453–456. [PubMed] [Google Scholar]
- 24.Zhang M, Simon MA, Dong X. The prevalence of perceived stress among U.S. Chinese older adults. AIMS Med Sci 2014;1:40–56. [Google Scholar]
- 25.Stawski RS, Sliwinski MJ, Almeida DM et al. Reported exposure and emotional reactivity to daily stressors: The role of adult-age and global perceived stress. Psychol Aging 2008;23:52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang ES, Dong XQ. A battery of tests for assessing cognitive function in U.S. Chinese older adults: Findings from the PINE study. J Gerontol A Biol Sci Med Sci 2014;69A(Suppl 2):S23–S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong X, Wong E, Simon MA. Study design and implementation of the PINE Study. J Aging Health 2014;26:1085–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simon M, Chang E, Rajan K et al. Demographic characteristics of U.S. Chinese older adults in the Greater Chicago area: Assessing the representativeness of the PINE Study. J Aging Health 2014;26:1100–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Albert M, Smith LA, Scherr PA et al. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer’s disease. Int J Neurosci 1991;57:167–178. [DOI] [PubMed] [Google Scholar]
- 30.Smith A Symbol Digit Modalities Test, 1st Ed. Los Angeles, CA: Western Psychological Services, 1982. [Google Scholar]
- 31.Wechsler D WMS-R: Wechsler Memory Scale—Revised: Manual. San Antonio: Harcourt Brace Jovanovich, 1987. [Google Scholar]
- 32.Yeung A, Fung F, Yu SC et al. Validation of the patient health questionnaire for depression screening among Chinese Americans. Compr Psychiatry 2008; 49:211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics 1982;38:963–974. [PubMed] [Google Scholar]
- 34.Chen Y, Wang J, Liang Y et al. Perceived stress and cognitive functions among Chinese older adults: The moderating role of health status. Gerontol Geriatr Med 2018;4:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li LW, Ding D, Wu B et al. Change of cognitive function in U.S Chinese older adults: A population-based study. J Gerontol A Biol Sci Med Sci 2017; 72A:5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Folkman S, Lazarus RS, Primley S et al. Age differences in stress and coping processes. Psychol Aging 1987;2:171–184. [DOI] [PubMed] [Google Scholar]
- 37.Ge S, Bei B, Bailey DE et al. Social support, social strain, and cognitive function among community-dwelling U.S. Chinese older adults. J Gerontol A Biol Sci Med Sci 2017;72A:16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dong XQ. Addressing health and well-being of U.S. Chinese older adults through community-based participatory research: Introduction to the PINE Study. J Gerontol A Biol Sci Med Sci 2014;69A(Suppl 2):1–6. [DOI] [PubMed] [Google Scholar]
- 39.Wu B, Chi I, Plassman BL et al. Depressive symptoms and health problems among Chinese immigrant elders in the U.S. and Chinese elders in China. Aging Ment Health 2010;14:695–704. [DOI] [PubMed] [Google Scholar]
- 40.Zhong BL, Chen SL, Conwell Y. Effects of transient versus chronic loneliness on cognitive function in older adults: Findings from the Chinese Longitudinal Healthy Longevity Survey. Am J Geriatr Psychiatry 2016;24: 389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.