Abstract
Primary cilia are tiny microtubule-based signaling devices that regulate a variety of physiological functions, including metabolism and cell division. Defects in primary cilia lead to a myriad of diseases in humans such as obesity and cancers. In the mature brain, both neurons and astrocytes contain a single primary cilium. Although neuronal primary cilia are not directly involved in synaptic communication, their pathophysiological impacts on obesity and mental disorders are well recognized. In contrast, research on astrocytic primary cilia lags far behind. Currently, little is known about their functions and molecular pathways in the mature brain. Unlike neurons, postnatal astrocytes retain the capacity of cell division and can become reactive and proliferate in response to various brain insults such as epilepsy, ischemia, traumatic brain injury, and neurodegenerative β-amyloid plaques. Since primary cilia derive from the mother centrioles, astrocyte proliferation must occur in coordination with the dismantling and ciliogenesis of astrocyte cilia. In this regard, the functions, signal pathways, and structural dynamics of neuronal and astrocytic primary cilia are fundamentally different. Here we discuss and compare the current understanding of neuronal and astrocytic primary cilia.
Keywords: Primary Cilia, Astrocytes, Type 3 Adenylyl Cyclase (AC3), ARL13B, Sonic Hedgehog
Graphical Abstract
Introduction
The primary cilium is a centriole-derived, membrane-ensheathed process present in most mammalian cells [1]. It relies on a highly conserved intraflagellar transport (IFT) system for trafficking select protein cargo into and out of the ciliary compartment [2]. There is a diffusion barrier at the base of the primary cilium, which restricts the free diffusion of unselected molecules into the compartment [3]. Hence, the microenvironment in the primary cilium is insulated from the main cytosolic compartment. Moreover, the volume of the primary cilium is very tiny compared to other cellular compartments, but it has a high ratio of plasma membrane to cytosol and can enrich a high density of membrane receptors. Second messengers such as cAMP can easily reach high concentrations in the narrow ciliary compartment. Given this, cilia are exquisitely sensitive to extracellular signals, including nutrients, neuropeptides, morphogens, and hormones [1, 4, 5]. As such, primary cilia are considered cellular “antennae” to detect extracellular signals [1, 4, 5].
The primary cilium emanates from the basal body residing underneath the plasma membrane, which is a special form of the mother centriole [6]. It maintains stability during interphase of the cell cycle, but dismantling the primary cilium is a prerequisite for the progression of mitosis [7]. Accordingly, primary cilia regulate cell division [7], development [4, 8, 9], and tissue regeneration [10]. Vigorous research in the past two decades has collected a great deal of evidence supporting the significance of primary cilia in both physiology and pathology [11, 12]. It is well recognized that malfunction of primary cilia causes numerous disorders, including polycystic kidney disease, obesity, cognitive impairment, developmental disorders [8], and certain cancers [11, 13, 14], which are collectively termed “ciliopathies” [11].
The brain is mainly comprised of neurons and glial cells. Glial cells in the brain include astrocytes, ependymal cells, microglia, and oligodendrocytes. It is known that neurons and astrocytes possess a single, non-motile primary cilium [8]. Ependymal cells, which line the ventricles of the brain and aid in the circulation of cerebrospinal fluid, have multiple motile cilia. Microglia, the resident macrophages in the central nervous system (CNS), do not display primary cilia [15] (see Figure 1). Primary cilia are observable in young oligodendrocyte precursor cells, but are lost as these cells differentiate [16]. Putatively, mature oligodendrocytes may also have primary cilia [17], but no direct evidence has been shown thus far. Additionally, adult neural stem cells (or astrocyte-like type 1 radial glial cells) in the subgranular zone (SGZ) in the dentate gyrus of the hippocampus and likely the subventricular zone (SVZ) of the lateral ventricles [18] possess primary cilia, which are essential for Sonic hedgehog (Shh) signaling and adult neurogenesis [19–21].
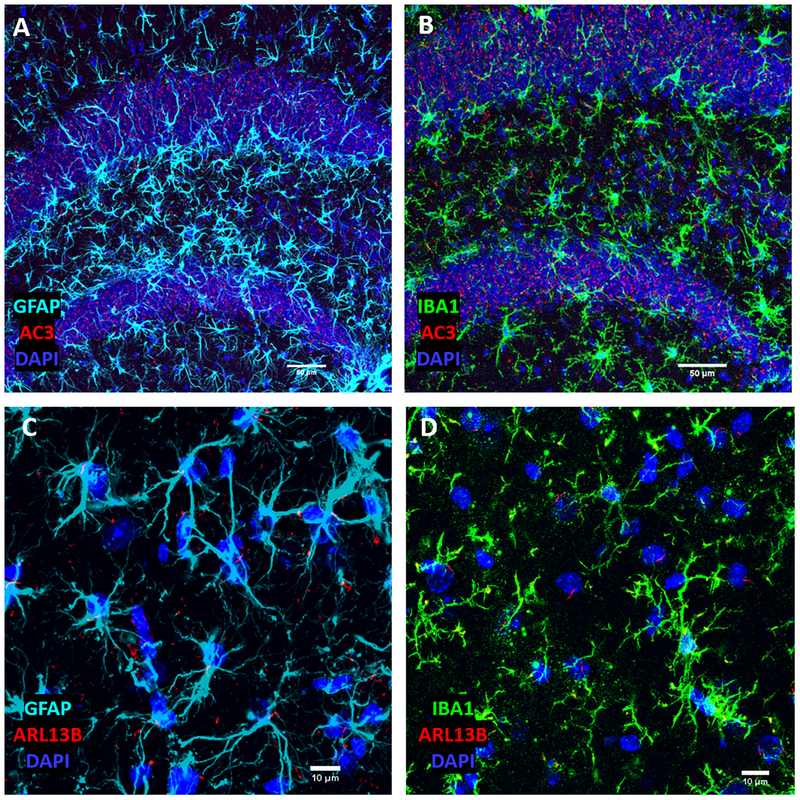
Figure 1.
Neuronal and astrocytic primary cilia are marked by AC3 and ARL13B, respectively, while microglia do not posess primary cilia. (A) AC3 is highly expressed in neuronal primary cilia, but not well expressed GFAP-labeled astrocyte cilia. (B) IBA1-marked microglia lack AC3-stained primary cilia. (C) ARL13B is highly expressed in astrocyte primary cilia. (D) IBA1-marked microglia lack ARL13B-stained primary cilia. All images were taken from the mouse hippocampus.
Astrocytes comprise the largest number of glia in the mature brain and maintain a great level of heterogeneity in function and morphology [22, 23]. Astrocytes perform a wide variety of functions, including regulation of synaptic function by recycling neurotransmitters [22], supplying neurons with neurotransmitter precursors [23], and regulating synaptic pruning by marking neuronal junctions for degradation via complement proteins [24]. In the event of neuropathologies, astrocytes are altered phenotypically to become reactive. When this occurs, these cells act to repair the blood brain barrier [25], support neuronal survival, and restore homeostasis within the brain via creation of a glial scar followed by metabolite and chemical control [26].
While astrocytes and neurons derive from the same origin (neuroepithelial cells and radial glial cells) in the early stage of neurodevelopment [27], research on astrocytic cilia in the mature brain lags far behind that of neuronal cilia. Neuronal primary cilia in the adult brain have been the most extensively studied compared to other cilia [4, 8, 17, 28, 29]. They have been found to regulate metabolism [13, 30, 31], mood state [32], and cognitive function [33, 34]. To date, very little, except for a role in adult neurogenesis, is known regarding the function and molecular pathways of astrocytic primary cilia in the mature brain. Nevertheless, as postnatal astrocytes maintain the ability to proliferate throughout life [25, 26, 35–37], astrocyte cell division should occur in coordination with the resorption and ciliogenesis of primary cilia. Herein represents a fundamental difference between neuronal and astrocyte cilia. Neuronal primary cilia in the brain have been well described in several elegant review articles [4, 17, 28, 38]. Here in this review, we aim to describe some fundamentals related to primary cilia and compare neuronal and astrocytic primary cilia in the adult brain (Summarized in Table 1).
Table 1.
Comparision of Neuronal and Astrocytic Primary Cilia in the Mature Brain
| Features | Neurons | Astrocytes |
|---|---|---|
| Origination | Ectoderm [119] Neuroepithelial Cells and Radial Glial Cells [27] |
Ectoderm [119] Neuroepithelial Cells and Radial Glial Cells[27] |
| Excitability | Excitable | Non-excitable |
| Connectivity | Highly connected via synapses | Not wired to one another via chemical synapses; albeit gap junctions found [120, 121] |
| Differentiation | Terminally differentiate upon maturation | Regional differentiation & become reactive in response to insults |
| Proliferative Capacity | Lose mitotic ability in maturation [52] | Maintain proliferative capacity throughout life [26] |
| Primary Cilia Markers | AC3 (Figure 1), SSTR3, 5-HT6, & MCHR1 [15] | Arl13B (Figure 1) [15] |
| AC3 | Highly expressed in neuronal primary cilia [45] | Faintly expressed in immature astrocyte cilia, few in adult astrocyte cilia [45] |
| ARL13B | Faintly expressed in immature neurons, not prominent in mature neurons [45] | Highly expressed in astrocyte primary cilia in the mature brain [45] (Figure 1) |
| Cilia Length (Hippocampus) | 5.0–5.91 μm (AC3-positive) [15] | 2.8–3.2 μm (ARL13B-positive) [15] |
| Shh Components | Smoothened [17], Patched1 [63], GPR161 [64,56] and Gli transcription factors [63] detected in primary cilia in the neural tube. Presence in primary cilia of mature neurons not shown. | Smoothened and Patched1 detected in primary cilia of astrocytes in the postnatal brain [122] |
| Structural Dynamics | Relatively stable | Subject to dynamic change during astrocyte proliferation |
| Ciliogenesis | Research confined to embryonic neuronal ciliogenesis; Lack of de novo ciliogenesis in mature neurons |
5 known stages of ciliogenesis in adult astrocytes [53, 54] |
| Ciliary Disease Implications | Obesity, cognitive impairment & mental disorders [89] | Astrocytoma/glioblastoma [53, 54] |
Cilia Markers and Transgenic Mouse Strains to Label Primary Cilia with Fluorescent Proteins or Calcium Sensors
The necessity for visualizing primary cilia and detecting ciliary molecules has grown as studies on primary cilia have rapidly expanded. To date, primary cilia in the brain can be visualized in immunofluorescence staining using commercial antibodies to target type 3 adenylyl cyclase (AC3), type 3 somatostatin receptor (SSTR3), ADP-ribosylation factor-like 13B (ARL13B) [15], type 6 serotonin receptor (5-HT6) [39], and melanin-concentrating hormone receptor 1 (MCHR1) [40]. However, it has been recognized that these cilia markers are not ubiquitously expressed, and their expression levels can vary. For example, 5-HT6 has a focal expression and is enriched in neuronal primary cilia in the striatum region [39]. MCHR1 expression is detected in neuronal primary cilia in the hippocampus, amygdala, piriform cortex, and hypothalamus [40, 41]. In a comparison of primary cilia markers, Sipos et al. showed regional differences in expression of AC3, ARL13B, and SSTR3 [15]. A high level of AC3 expression in the primary cilia of mature neurons was detected. In contrast, AC3 is only faintly expressed in a small number of glial fibrillary acidic protein (GFAP)-positive astrocytes and absent in adenomatous polyposis coli (APC)-positive oligodendrocytes and ionizing calcium-binding adaptor molecule 1 (IBA1)-labeled microglia [15] (confirmed by our observations, see Figure 1). SSTR3 was found to be expressed in mature neuronal primary cilia [15]. Several studies have demonstrated that SSTR3 is co-expressed with AC3 in neuronal primary cilia in the hippocampus [34] and the olfactory bulb [42]. It is noteworthy that AC3 is also distributed to other tissues and is highly expressed in olfactory sensory cilia, primary cilia of kidney epithelial cells [43], and primary cilia of brown and white adipose tissue [44]. Generally, AC3 is a commonly accepted neuronal primary cilia marker protein and ARL13B antibody is commonly used to label astrocytic primary cilia (Figure 1), but neither is universally localized in primary cilia. A study focusing on visualization of astrocytic primary cilia in mice showed differential expression based on age [45]. This study detected a high expression of AC3 in the primary cilia of both astrocytes and neurons ten days after birth, but by postnatal day 56, neuronal primary cilia highly expressed AC3 and astrocytic primary cilia showed high levels of ARL13B expression [45].
Utilizing cilia-specific proteins, strains of mice with fluorescent proteins restricted to primary cilia have been generated. These strains have proven effective in visualizing cilia or detecting calcium levels. For example, O’Connor et al. generated an inducible cilia-GFP model of mice to enable direct visualization primary cilia [46]. This strain linked GFP with the ciliary protein SSTR3. In observations of deeply anesthetized adult mice, primary cilia were found to be expressed in numerous cells within the mature brain, including neurons and in choroid plexus epithelial cells [46]. In 2016, Delling et al. developed an imaging tool to study calcium signaling in response to mechanical force. They engineered a strain of mouse with ARL13B marked with mCherry and genetically encoded calcium indicator 1.2 localized in primary cilia. This tool allows for detection of calcium influx into ciliary compartment responding to mechanical stimuli. They discovered that calcium signaling is not responsible for the mechano-sensation of renal cilia in both embryonic and adult murine cilia models [47]. Additionally, in 2015, Bangs et al. conducted a study using a strain of mice that had both primary cilia and centrosomes labeled with mCherry and GFP, respectively. This model, ARL13B-mCerry;Centrin2-GFP, allowed for visualization of primary cilia and centrioles throughout embryonic development. Using that strain, primary cilia were found to arise from epiblast cells at E6, and cells arising from the visceral endoderm and trophoectoderm maintained centrosomes, but lack cilia through development [48]. The ARL13B-pmCerry;Centrin2-GFP strain marks primary cilia of many epiblast-derived cells including neuroepithelial cells in the neural tube very clearly [48]. However, we observed that although ARL13-mCherry also labels astrocytes very well, but not well on neurons, in the mature brain. Thus, ARL13B is not a ubiquitous ciliary maker in mature. Presently, research tools to visualize primary cilia or detect ciliary molecules are far from adequate. Consequently, there remains the need to develop novel tools (or mouse strains) to detect key messenger molecules in cilia such as cAMP, as the explorations of primary cilia grow.
Ciliogenesis
Ciliogenesis is the process by which the microtubule-based cilium arises from the basal body within the cell [49]. Ciliogenesis is generally inhibited in actively dividing cells [50]. Initiation of ciliogenesis requires that a cell exit its mitotic cell cycle to allow the centriole to dock at the plasma membrane by fusing with a ciliary vesicle [49]. At this point, IFT begins to transport protein complexes in anterograde and retrograde directions to promote the growth of the cilium [51]. Due to the terminally differentiating nature of neurons and the consequential loss of mitotic ability [52], there is little need for neuronal primary cilia to destabilize and retract into the soma. This results in the absence of de novo ciliogenesis in mature neurons. Thus, studies exploring neuronal ciliogenesis are primarily limited to the early embryonic development stage or in cultured cells.
The ciliogenesis of astrocytes occurs in the embryonic development stage as well as in the mature brain. Human astrocytes have five stages of ciliogenesis [53, 54]. The first stage can be identified by the localization and fusion of vesicles with the distal end of the basal body. During the second stage, the non-motile 9+0 axoneme begins to arise from the basal body. This structure continues to grow in the third stage and fuses with the plasma membrane. At this point, a ciliary bud can be detected on the surface of the cell. The fourth stage consists of continued growth and extension of the cilium past the surface of the cell due to IFT. In the fifth and final stage of astrocyte ciliogenesis, a fully formed primary cilium can be observed on the surface of the cell [54, 55]. Once fully mature, astrocyte-like neural precursors display a primary cilium with a length significantly shorter than that of a neuronal primary cilium [56] (see table 1). Moser et al. also explored the potential effects of disruption of the mammalian processing body, namely the GW/P body, on ciliogenesis of human astrocytes [57]. Greater than half of transcriptional silencing of mRNA occurs within GW/P bodies. The early stages of ciliogenesis were observed in astrocytes studied in this experiment, but GW/P small interfering RNA-transfected cells failed to display a matured cilium that extended properly out of the soma. This study demonstrates that inhibition of GW/P body components and the RNAi microprocessor disrupts ciliogenesis of astrocytes [57]. To date, there is limited understanding of the structural dynamics of astrocyte cilia. We postulate that elucidating the molecular mechanisms of ciliogenesis will help unravel the contributions of astrocyte cilia to astrocyte reactivity and pathology in the mature brain.
Primary Cilia are Required for Sonic Hedgehog Signal Transduction in Vertebrate Cells
Shh signal transduction is essential for proper embryonic development and morphogenesis of vertebrates. The components within this pathway are required for patterning and organization of the notochord, floorplate, and cells within the zone of polarizing activity [58]. This patterning is needed for embryonic organization of tissue that is required not simply for symmetry, but also appropriate organogenesis. Hence, the Shh pathway is necessary for proper CNS development. Conceivably, malfunction of Shh pathway is also implicated in certain forms of cancer [59]. Shh mediates cell signaling and also regulates cell survival. The best-known components in the Shh pathway are the Shh ligand, Smoothened (Smo), and Patched1 (Ptch1) [59]. Binding of Shh to Ptch1 leads to translocation of Smo from the plasma membrane into primary ciliary compartment and activation of the Shh pathway, promoting the Gli transcription factors to translocate to the nucleus, where they can stimulate cell proliferation and encourage cell survival [60].
In vertebrate cells, primary cilia constitute key modulators for Shh transduction, which is essential for embryonic development of neural cells [61]. In the developing notochord, neural progenitor cells are guided in proliferation and differentiation by the Shh signaling [61]. Many key components of the Shh pathway localize in the primary cilium and can shift regionally upon receipt of the Shh ligand [62]. Patched1 localizes in the primary cilium in the absence of Shh and regulates the activity of Smoothened [63]. Suppressor of Fused, a downstream regulator of this pathway, localizes in the tip of the primary cilia of cells in the neural tube [61]. The G-protein-coupled receptor GPR161 is expressed on the primary cilium in the neural tube and can suppress Shh signaling and inhibit the growth of medulloblastomas [64].
Extensive evidence has underscored the importance of primary cilia in Shh signaling. Transgenic mice lacking cilia in astrocyte-like neural precursors have abnormal development and disruption in Shh signaling [56]. Disturbance of ARL13B in primary cilia can result in lessened Shh signaling in mouse medulloblastoma cultures [65]. Ablation of ciliary genes or Smoothened results in interrupted development of radial astrocytes (the postnatal progenitors in the dentate gyrus), while constitutive expression of these genes results in an enlargement of the dentate gyrus [66]. SAG, a Shh agonist, can act in a protective fashion via ciliary signaling for astrocytes under starving conditions [67]. However, most research focuses on the aspect of neurodevelopment or neurogenesis (and adult neurogenesis). To date, there are few reports directly addressing the Shh pathway in neuronal and astrocytic primary cilia in the mature brain. This is possibly because the Shh pathway in neuronal primary cilia is more prominent in neurodevelopment, not so much in adulthood, and astrocyte cilia in the mature brain is generally under-studied.
Potential Function of Astrocytic Primary Cilia in the Mature Brain
Many studies have emphasized the importance of primary cilia in cell division [4, 8, 9]. Evidence is emerging to associate astrocyte cilia with brain pathophysiology, particularly brain tumorigenesis. As primary cilia must first destabilize and retract into the cell to allow for the movement of the centriole and cell division, inability to stabilize the primary cilium can be related with abnormal proliferation of cells. Indeed, dysfunction of the primary cilium is recognized in the development of some types of tumors [12, 64, 68]. For example, glioblastomas, brain tumors arising from abnormal proliferation of astrocytes, are highly related to loss of or malformed primary cilia [68]. In an experiment characterizing the tissue of seven human glioblastoma samples from mature brains [53], defects in the early stages of ciliogenesis of astrocytes have been identified. These abnormalities included absent vesicle pairs, non-mature axonemes, and other morphological differences that impeded ciliary maturation and normal function. Lisophosphatidic acid receptor 1 (LPAR1), a G-protein coupled receptor, localizes on the primary cilia of astrocytes, but when the primary cilium is absent, LPAR1 transitions into the plasma membrane. This regionalization is accompanied by an increase of association with Gα12 and Gαq [68], which have been reported to be associated with cancer proliferation [69]. Consequently, glioblastoma proliferation is enhanced [68].
Remarkably, adult neurogenesis is well recognized as occurring in the mature brain [18]. Generation of new neurons arises from adult neural stem cells in the subventricular zone and dentate gyrus, rather than from neurons [18]. Adult neural stem cells exhibit glial nature [27] and astrocyte-like adult neural stem cells are maintained in the subgranular layer (SGL) of the dentate gyrus (DG) and in the subventricular zone (SGZ) in the mammalian brain, and they give rise to new neurons in the adult mammalian brain throughout life [18, 70]. The primary cilium and Shh signaling of adult neural stem cells were found to be required for adult neurogenesis and regulate the proliferation of progenitors in the adult hippocampus, implicating in learning and memory [19]. Moreover, the dendritic refinement and synaptic integration of adult-born neurons in the dentate gyrus’s subgranular zone was reported to be modulated by primary cilia [71].
In contrast to neurons, which terminally differentiate and lose proliferative capacity upon maturation, astrocytes throughout the brain maintain the capacity of cell division following differentiation and new astrocytes are continuously generated during postnatal development. Astrocytes have the ability to become reactive and a proportion of reactive astrocytes proliferate in response to various neuropathological conditions such as ischemia, traumatic injury, and epilepsy, as well as neurodegenerative amyloid plaques [25, 35, 72, 73]. During the process of astrocyte proliferation, astrocyte primary cilia need to be resorbed to liberate the centriole and allow the centrosomes to form the mitotic spindle. Thus, astrocyte cilia are conceivably subjected to a dynamic change in conjunction with astrocyte proliferation. However, to date, how astrocytic primary cilia modulate astrocyte reactivity in vivo and how astrocytic primary cilia dynamically change their structure in accordance with astrocyte proliferation remain to be elusive. We postulate that investigation into molecular fundamentals of astrocyte cilia in the context of reactive astrogliosis will advance our understanding of how the “antenna” of astrocytes senses pathological milieu and how astrocyte cilia regulate the initiation and termination of astrocytes.
ARL13B, a Ciliary Protein Essential for Cilia Structure, is Implicated in Joubert Syndrome and Developmental Abnormalities
ADP-ribosylation factor-like 13B (ARL13B) is a member of the monomeric small GTPase superfamily [74, 75] and selectively expressed in many primary cilia including astrocytic primary cilia [45]. ARL13B is required for ciliogenesis in certain organs [76]. Studies have shown that ARL13B modulates ciliary protein trafficking and cilia length [62, 77], and supports connectivity between neurons [78]. ARL13B is required for radial glial polarity, disruption of which causes abnormal formation of the cerebral cortex [79]. Notably, ARL13B also regulates ciliary targeting of inositol polyphosphate-5-phosphatase E (INPP5E) [80]. INPP5E is a ciliary protein which prevents actin polymerization in primary cilia [81]. Prior to ciliary destabilization, INPP5E is depleted in cilia, allowing for the re-localization of phosphatidylinositol 4,5-bisphosphate into the primary cilium, which triggers actin polymerization and cilia decapitation, and consequently drives the cell cycle [81].
Joubert syndrome is an autosomal recessive ciliopathy featured by the “molar tooth sign” [82] and characterized by dysfunction in different viscera, eyes, digits, and the brain [74, 83]. ARL13B mutations cause Joubert syndrome, which was first identified in two families [74, 84]. Joubert syndrome patients demonstrate cerebral disorder, mental retardation and developmental delay as well as other variable common-shared ciliopathy manifestations such as cystic kidney, blindness, and polydactyly. Many mutations in ARL13B have been identified in Joubert syndrome patients, all having impeded neural development to some degree. A homozygous missense variant c.[223G>A] (p.(Gly75Arg) identified in the ARL13B gene cause intellectual disability, ataxia, ocular defects, and epilepsy in Jourbert syndrome patients [85]. Interestingly, ARL13B-c.[223G>A] (p.(Gly75Arg) displayed a marked loss of ARL13B guanine nucleotide-exchange factor activity, with retention of its GTPase activities [85]. A crystal structure of Chlamydomonas rheinhardtii ARL13B shows that R79Q as well as R200C, two missense ARL13B mutations identified in Joubert syndrome patients, are involved in stabilizing important intramolecular interactions [86].
ARL13B also plays a critical role in supporting the activity of Shh signaling and acts to regulate the trafficking of its signaling components into the primary cilium [87]. It can induce cell proliferation and survival [87]. Given the localization of Shh components to primary cilia [16, 62], disruption of ARL13B results in abrogated Shh signaling [62]. Consequently, ablation of ARL13B leads to medulloblastoma formation, due to the correlated deletion of cilia and Shh components [65].
AC3, a Key Enzyme Mediating the cAMP Signaling in Neuronal Cilia, is Associated with Obesity and Psychiatric Diseases
Neuronal primary cilia are commonly fortified with G protein-coupled receptors (GPCRs) [40] including Smoothened, SSTR3, 5-HT6, MCHR1, and many others [88]. Some types of GPCRs, such as Smoothened, do not continuously reside in primary cilia and can transition into and out of the cilium [88]. Interestingly, at this time all ciliary GPCRs are found to be either Gαs- or Gαi-protein coupled receptors, which depend on adenylyl cyclases (ACs) to generate cAMP and transduce a signal into the cell. AC3 is widely recognized as a key enzyme mediating the cAMP signaling in neuronal cilia [89]. AC3 was first established as an obligate protein in olfactory cilia mediating olfactory signal transduction in the main olfactory epithelium [90–92]. In 2007, Bishop et al. reported that AC3 predominantly localizes to neuronal primary cilia throughout the mature mouse brain [93], expanding the AC3’s fame originally as the olfactory adenylyl cyclase to a well-known neuronal cilia marker in the CNS.
The most prominent function of AC3 in the CNS is to regulate metabolism [94]. Many lines of evidence support the pathological role of AC3 in obesity. First, numerous human genetic analyses have clearly defined ADCY3 as a gene associated with obesity [95–101]. Second, obesity is also one of the most common symptoms for ciliopathies including Bardet-Biedl Syndrome [9, 102], and Joubert Syndrome [83]. Third, both conventional and tamoxifen-induced conditional AC3 KO mice exhibit adult-onset obesity [44, 103]. Conversely, a gain-of-function mutation of AC3 in mice can protect the animals from diet-induced obesity [104]. Hence, AC3 plays a critical role in regulating energy balance, and defects in the ciliary cAMP pathway lead to obesity [89, 94]. This is in line with the evidence demonstrating that neuronal primary cilia play a critical role in regulating energy balance. Specifically, ablations of many ciliary proteins including KIF3a [105], Bbip10 [106], IFT88 [107], Tubby [108], BBS1, and BBS4 [107, 109] all lead to obesity in mouse models [89].
However, it is not well understood how cAMP in neuronal primary cilia regulates energy balance. It has been postulated that AC3 functionally couples to melanocortin 4 receptor (MC4R) in the hypothalamus [89, 103], because activation of adenylyl cyclase by alpha-melanocyte stimulating hormone (α-MSH) downstream in the leptin pathway is required for the anorectic activity of leptin[110, 111]. Siljee at al. have recently shown that MC4R co-localizes with ADCY3 at the primary cilia of a subset of neurons of the paraventricular nucleus (PVN) of the hypothalamus [31]. They also discovered that obesity-associated MC4R mutations impair ciliary localization and that suppression of cAMP production using GPR88, a constitutively active version of the cilia-specific Gαi-protein-coupled receptor [112], at the primary cilia in these neurons increases body weight. The findings of Siljee et al. suggest that MC4R may couple to AC3 and positively regulate cAMP generation in neuronal primary cilia in a subset of neurons in the PVN, and impaired cAMP signaling in the primary cilia of MC4R-positive neurons lead to obesity [31]. However, further research is warranted to clearly address how leptin, α-MSH, MC4R and ciliary cAMP signaling in different sublocations of the hypothalamus hook up to regulate feeding and energy balance.
Another pathological role of AC3 relates to mental disorders. A genome-wide association study based on over five thousand patients with major depressive disorder (MDD) and healthy subjects identified AC3 (ADCY3) as a top-ranked gene for MDD [113]. In animal model, we discovered that constitutive AC3 knockout (KO) mice exhibit strong depression-like phenotypes in several behavioral assays [32]. Disturbances of sleep including alterations in sleep architecture and increased REM sleep are one of the core symptoms associated with MDD [114]. Our sleep analysis based on electroencephalogram-electromyogram showed that AC3 KO mice have altered sleep patterns characterized by an increased percentage of rapid eye movement sleep [32]. Moreover, MDD also is associated with neuronal atrophy [115]. We further found that basal synaptic activity at CA3-CA1 synapses was significantly lower in AC3 KO mice, and they also exhibited attenuated long-term potentiation as well as deficits in spatial navigation [32]. Conditional knockout mice with AC3 ablated in the mature brain also exhibit depression-like phenotypes [32]. In addition to MDD, human genetic studies have also associated AC3 with autism [116, 117] and intellectual disability [118], Although the underlying molecular mechanisms remain to be elucidated. In summary, AC3 in the mature brain mediates olfactory signal transduction, regulates energy balance and mood state, and contributes to psychiatric diseases.
Conclusion
Neurons are terminally differentiated excitable cells that lose mitotic ability in maturity [52]. Neuronal primary cilia are relatively stable and apparently lack de novo ciliogenesis. Moreover, no synaptic structures, ionotropic glutamate receptors, or GABAA receptors have been identified in neuronal primary cilia thus far. But many types of GPCRs have been found in neuronal primary cilia [88] (Table 1). Therefore, neuronal primary cilia mostly depend on these metabotropic receptors and downstream effector proteins to send a signal to regulate neuronal activity [89]. Hence, AC3 represents a key enzyme to mediate the cAMP signaling in neuronal primary cilia in the mature brain and regulate energy balance, mood state, and probably cognitive function.
In contrast, astrocytes are non-excitable cells and do not electrically wire to one another via chemical synapses. They are not terminally differentiated cells and maintain proliferative ability throughout life (Table 1). Presently, little is known about the function, signaling pathways, and structural dynamics of astrocytic primary cilia in the mature brain, although astrocytes fulfill a wide range of functions including providing trophic support, maintaining homeostasis, and protecting neurons from acute insults or brain injury [36]. Since astrocytes can proliferate under certain pathological conditions [26], astrocytic primary cilia are not static but subject to dynamic changes. Hence, it is not surprising that ARL13B, a protein regulating cilia protein trafficking, Shh pathway and cell division, prevails over AC3 in astrocytic cilia as a protein marker.
In summary, the function, molecular markers, signaling pathways, and structural dynamics of neuronal primary cilia and astrocytic cilia are fundamentally distinct (Table 1). Conceivably, ciliary GPCR- and AC3-mediated cAMP signaling in neurons provide excellent targets to design therapeutics to combat obesity, depression and cognitive disorders. In the long term, research on astrocytic primary cilia will provide useful clues to intervene in astrocyte-proliferation and reactive astrogliosis to combat various neuropathologies such as ischemia and brain injury.
Acknowledgements:
We thank the members of the Chen Laboratory for critical review of the manuscript. This work is supported by National Institutes of Health Grants MH105746, AG054729 and GM113131 to X.C.; a Cole Neuroscience and Behavior Faculty Research Award to X.C.; and UNH Summer TA Research Fellowship (STAF) to A.S.
Footnotes
Declarations of Interest: None
References:
- 1.Singla V, and Reiter JF (2006). The primary cilium as the cell’s antenna: signaling at a sensory organelle. Science 313, 629–633. [DOI] [PubMed] [Google Scholar]
- 2.Rosenbaum JL, and Witman GB (2002). Intraflagellar transport. Nat Rev Mol Cell Biol 3, 813–825. [DOI] [PubMed] [Google Scholar]
- 3.Endicott SJ, and Brueckner M (2018). NUP98 Sets the Size-Exclusion Diffusion Limit through the Ciliary Base. Curr Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JH, and Gleeson JG (2010). The role of primary cilia in neuronal function. Neurobiol Dis 38, 167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goetz SC, and Anderson KV (2010). The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet 11, 331–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nigg EA, and Stearns T (2011). The centrosome cycle: Centriole biogenesis, duplication and inherent asymmetries. Nat Cell Biol 13, 1154–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan J, and Snell W (2007). The primary cilium: keeper of the key to cell division. Cell 129, 1255–1257. [DOI] [PubMed] [Google Scholar]
- 8.Guemez-Gamboa A, Coufal NG, and Gleeson JG (2014). Primary cilia in the developing and mature brain. Neuron 82, 511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valente EM, Rosti RO, Gibbs E, and Gleeson JG (2014). Primary cilia in neurodevelopmental disorders. Nat Rev Neurol 10, 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson PK (2012). TTBK2 kinase: linking primary cilia and cerebellar ataxias. Cell 151, 697–699. [DOI] [PubMed] [Google Scholar]
- 11.Braun DA, and Hildebrandt F (2017). Ciliopathies. Cold Spring Harb Perspect Biol 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Youn YH, and Han YG (2018). Primary Cilia in Brain Development and Diseases. Am J Pathol 188, 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green JA, and Mykytyn K (2010). Neuronal ciliary signaling in homeostasis and disease. Cell Mol Life Sci 67, 3287–3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marley A, and von Zastrow M (2012). A simple cell-based assay reveals that diverse neuropsychiatric risk genes converge on primary cilia. PLoS One 7, e46647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sipos E, Komoly S, and Acs P (2018). Quantitative Comparison of Primary Cilia Marker Expression and Length in the Mouse Brain. J Mol Neurosci. [DOI] [PubMed] [Google Scholar]
- 16.Falcon-Urrutia P, Carrasco CM, Lois P, Palma V, and Roth AD (2015). Shh Signaling through the Primary Cilium Modulates Rat Oligodendrocyte Differentiation. PLoS One 10, e0133567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Louvi A, and Grove EA (2011). Cilia in the CNS: the quiet organelle claims center stage. Neuron 69, 1046–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ming GL, and Song H (2011). Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70, 687–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amador-Arjona A, Elliott J, Miller A, Ginbey A, Pazour GJ, Enikolopov G, Roberts AJ, and Terskikh AV (2011). Primary cilia regulate proliferation of amplifying progenitors in adult hippocampus: implications for learning and memory. J Neurosci 31, 9933–9944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han YG, Spassky N, Romaguera-Ros M, Garcia-Verdugo JM, Aguilar A, Schneider-Maunoury S, and Alvarez-Buylla A (2008). Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci 11, 277–284. [DOI] [PubMed] [Google Scholar]
- 21.Tong CK, Han YG, Shah JK, Obernier K, Guinto CD, and Alvarez-Buylla A (2014). Primary cilia are required in a unique subpopulation of neural progenitors. Proc Natl Acad Sci U S A 111, 12438–12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zuchero JB, and Barres BA (2015). Glia in mammalian development and disease. Development 142, 3805–3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verkhratsky A, and Nedergaard M (2018). Physiology of Astroglia. Physiol Rev 98, 239–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stephan AH, Barres BA, and Stevens B (2012). The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci 35, 369–389. [DOI] [PubMed] [Google Scholar]
- 25.Liddelow SA, and Barres BA (2017). Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 46, 957–967. [DOI] [PubMed] [Google Scholar]
- 26.Sofroniew MV, and Vinters HV (2010). Astrocytes: biology and pathology. Acta Neuropathol 119, 7–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kriegstein A, and Alvarez-Buylla A (2009). The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci 32, 149–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lepanto P, Badano JL, and Zolessi FR (2016). Neuron’s little helper: The role of primary cilia in neurogenesis. Neurogenesis (Austin) 3, e1253363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JE, and Gleeson JG (2011). Cilia in the nervous system: linking cilia function and neurodevelopmental disorders. Curr Opin Neurol 24, 98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song DK, Choi JH, and Kim MS (2018). Primary Cilia as a Signaling Platform for Control of Energy Metabolism. Diabetes Metab J 42, 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siljee JE, Wang Y, Bernard AA, Ersoy BA, Zhang S, Marley A, Von Zastrow M, Reiter JF, and Vaisse C (2018). Subcellular localization of MC4R with ADCY3 at neuronal primary cilia underlies a common pathway for genetic predisposition to obesity. Nat Genet 50, 180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen X, Luo J, Leng Y, Yang Y, Zweifel LS, Palmiter RD, and Storm DR (2016). Ablation of Type III Adenylyl Cyclase in Mice Causes Reduced Neuronal Activity, Altered Sleep Pattern, and Depression-like Phenotypes. Biol Psychiatry 80, 836–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berbari NF, Malarkey EB, Yazdi SM, McNair AD, Kippe JM, Croyle MJ, Kraft TW, and Yoder BK (2014). Hippocampal and cortical primary cilia are required for aversive memory in mice. PLoS One 9, e106576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Z, Phan T, and Storm DR (2011). The type 3 adenylyl cyclase is required for novel object learning and extinction of contextual memory: role of cAMP signaling in primary cilia. J Neurosci 31, 5557–5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liddelow S, and Barres B (2015). SnapShot: Astrocytes in Health and Disease. Cell 162, 1170–1170 e1171. [DOI] [PubMed] [Google Scholar]
- 36.Molofsky AV, Krencik R, Ullian EM, Tsai HH, Deneen B, Richardson WD, Barres BA, and Rowitch DH (2012). Astrocytes and disease: a neurodevelopmental perspective. Genes Dev 26, 891–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pekny M, and Pekna M (2016). Reactive gliosis in the pathogenesis of CNS diseases. Biochim Biophys Acta 1862, 483–491. [DOI] [PubMed] [Google Scholar]
- 38.Sarkisian MR, and Guadiana SM (2015). Influences of primary cilia on cortical morphogenesis and neuronal subtype maturation. Neuroscientist 21, 136–151. [DOI] [PubMed] [Google Scholar]
- 39.Brodsky M, Lesiak AJ, Croicu A, Cohenca N, Sullivan JM, and Neumaier JF (2017). 5-HT6 receptor blockade regulates primary cilia morphology in striatal neurons. Brain Res 1660, 10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Green JA, Gu C, and Mykytyn K (2012). Heteromerization of ciliary G protein-coupled receptors in the mouse brain. PLoS One 7, e46304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun X, Haley J, Bulgakov OV, Cai X, McGinnis J, and Li T (2012). Tubby is required for trafficking G protein-coupled receptors to neuronal cilia. Cilia 1, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luo J, Chen X, Pan YW, Lu S, Xia Z, and Storm DR (2015). The type 3 adenylyl cyclase is required for the survival and maturation of newly generated granule cells in the olfactory bulb. PLoS One 10, e0122057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pluznick JL, Zou DJ, Zhang X, Yan Q, Rodriguez-Gil DJ, Eisner C, Wells E, Greer CA, Wang T, Firestein S, et al. (2009). Functional expression of the olfactory signaling system in the kidney. Proc Natl Acad Sci U S A 106, 2059–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Z, Li V, Chan GC, Phan T, Nudelman AS, Xia Z, and Storm DR (2009). Adult type 3 adenylyl cyclase-deficient mice are obese. PLoS One 4, e6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kasahara K, Miyoshi K, Murakami S, Miyazaki I, and Asanuma M (2014). Visualization of astrocytic primary cilia in the mouse brain by immunofluorescent analysis using the cilia marker Arl13b. Acta Med Okayama 68, 317–322. [DOI] [PubMed] [Google Scholar]
- 46.O’Connor AK, Malarkey EB, Berbari NF, Croyle MJ, Haycraft CJ, Bell PD, Hohenstein P, Kesterson RA, and Yoder BK (2013). An inducible CiliaGFP mouse model for in vivo visualization and analysis of cilia in live tissue. Cilia 2, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Delling M, Indzhykulian AA, Liu X, Li Y, Xie T, Corey DP, and Clapham DE (2016). Primary cilia are not calcium-responsive mechanosensors. Nature 531, 656–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bangs FK, Schrode N, Hadjantonakis AK, and Anderson KV (2015). Lineage specificity of primary cilia in the mouse embryo. Nat Cell Biol 17, 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ishikawa H, and Marshall WF (2011). Ciliogenesis: building the cell’s antenna. Nat Rev Mol Cell Biol 12, 222–234. [DOI] [PubMed] [Google Scholar]
- 50.Kasahara K, Aoki H, Kiyono T, Wang S, Kagiwada H, Yuge M, Tanaka T, Nishimura Y, Mizoguchi A, Goshima N, et al. (2018). EGF receptor kinase suppresses ciliogenesis through activation of USP8 deubiquitinase. Nat Commun 9, 758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Avasthi P, and Marshall WF (2012). Stages of ciliogenesis and regulation of ciliary length. Differentiation 83, S30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Myster DL, and Duronio RJ (2000). To differentiate or not to differentiate? Curr Biol 10, R302–304. [DOI] [PubMed] [Google Scholar]
- 53.Moser JJ, Fritzler MJ, and Rattner JB (2014). Ultrastructural characterization of primary cilia in pathologically characterized human glioblastoma multiforme (GBM) tumors. BMC Clin Pathol 14, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moser JJ, Fritzler MJ, and Rattner JB (2009). Primary ciliogenesis defects are associated with human astrocytoma/glioblastoma cells. BMC Cancer 9, 448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ou Y, Ruan Y, Cheng M, Moser JJ, Rattner JB, and van der Hoorn FA (2009). Adenylate cyclase regulates elongation of mammalian primary cilia. Exp Cell Res 315, 2802–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Breunig JJ, Sarkisian MR, Arellano JI, Morozov YM, Ayoub AE, Sojitra S, Wang B, Flavell RA, Rakic P, and Town T (2008). Primary cilia regulate hippocampal neurogenesis by mediating sonic hedgehog signaling. Proc Natl Acad Sci U S A 105, 13127–13132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moser JJ, Fritzler MJ, and Rattner JB (2011). Repression of GW/P body components and the RNAi microprocessor impacts primary ciliogenesis in human astrocytes. BMC Cell Biol 12, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ingham PW, and McMahon AP (2001). Hedgehog signaling in animal development: paradigms and principles. Genes Dev 15, 3059–3087. [DOI] [PubMed] [Google Scholar]
- 59.Carballo GB, Honorato JR, de Lopes GPF, and Spohr T (2018). A highlight on Sonic hedgehog pathway. Cell Commun Signal 16, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rimkus TK, Carpenter RL, Qasem S, Chan M, and Lo HW (2016). Targeting the Sonic Hedgehog Signaling Pathway: Review of Smoothened and GLI Inhibitors. Cancers (Basel) 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Z, Shen L, Law K, Zhang Z, Liu X, Hua H, Li S, Huang H, Yue S, Hui CC, et al. (2017). Suppressor of Fused Chaperones Gli Proteins To Generate Transcriptional Responses to Sonic Hedgehog Signaling. Mol Cell Biol 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Larkins CE, Aviles GD, East MP, Kahn RA, and Caspary T (2011). Arl13b regulates ciliogenesis and the dynamic localization of Shh signaling proteins. Mol Biol Cell 22, 4694–4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rohatgi R, Milenkovic L, and Scott MP (2007). Patched1 regulates hedgehog signaling at the primary cilium. Science 317, 372–376. [DOI] [PubMed] [Google Scholar]
- 64.Shimada IS, Hwang SH, Somatilaka BN, Wang X, Skowron P, Kim J, Kim M, Shelton JM, Rajaram V, Xuan Z, et al. (2018). Basal Suppression of the Sonic Hedgehog Pathway by the G-Protein-Coupled Receptor Gpr161 Restricts Medulloblastoma Pathogenesis. Cell Rep 22, 1169–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bay SN, Long AB, and Caspary T (2018). Disruption of the ciliary GTPase Arl13b suppresses Sonic hedgehog overactivation and inhibits medulloblastoma formation. Proc Natl Acad Sci U S A 115, 1570–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Han YG, and Alvarez-Buylla A (2010). Role of primary cilia in brain development and cancer. Curr Opin Neurobiol 20, 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yoshimura K, Kawate T, and Takeda S (2011). Signaling through the primary cilium affects glial cell survival under a stressed environment. Glia 59, 333–344. [DOI] [PubMed] [Google Scholar]
- 68.Loskutov YV, Griffin CL, Marinak KM, Bobko A, Margaryan NV, Geldenhuys WJ, Sarkaria JN, and Pugacheva EN (2018). LPA signaling is regulated through the primary cilium: a novel target in glioblastoma. Oncogene 37, 1457–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Park J, Jang JH, Oh S, Kim M, Shin C, Jeong M, Heo K, Park JB, Kim SR, and Oh YS (2018). LPA-induced migration of ovarian cancer cells requires activation of ERM proteins via LPA1 and LPA2. Cell Signal 44, 138–147. [DOI] [PubMed] [Google Scholar]
- 70.Seri B, Garcia-Verdugo JM, McEwen BS, and Alvarez-Buylla A (2001). Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci 21, 7153–7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kumamoto N, Gu Y, Wang J, Janoschka S, Takemaru K, Levine J, and Ge S (2012). A role for primary cilia in glutamatergic synaptic integration of adult-born neurons. Nat Neurosci 15, 399–405, S391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Frost GR, and Li YM (2017). The role of astrocytes in amyloid production and Alzheimer’s disease. Open Biol 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khakh BS, and Sofroniew MV (2015). Diversity of astrocyte functions and phenotypes in neural circuits. Nat Neurosci 18, 942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cantagrel V, Silhavy JL, Bielas SL, Swistun D, Marsh SE, Bertrand JY, Audollent S, Attie-Bitach T, Holden KR, Dobyns WB, et al. (2008). Mutations in the cilia gene ARL13B lead to the classical form of Joubert syndrome. Am J Hum Genet 83, 170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wennerberg K, Rossman KL, and Der CJ (2005). The Ras superfamily at a glance. J Cell Sci 118, 843–846. [DOI] [PubMed] [Google Scholar]
- 76.Seixas C, Choi SY, Polgar N, Umberger NL, East MP, Zuo X, Moreiras H, Ghossoub R, Benmerah A, Kahn RA, et al. (2016). Arl13b and the exocyst interact synergistically in ciliogenesis. Mol Biol Cell 27, 308–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mariani LE, Bijlsma MF, Ivanova AI, Suciu SK, Kahn RA, and Caspary T (2016). Arl13b regulates Shh signaling from both inside and outside the cilium. Mol Biol Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Higginbotham H, Eom TY, Mariani LE, Bachleda A, Hirt J, Gukassyan V, Cusack CL, Lai C, Caspary T, and Anton ES (2012). Arl13b in primary cilia regulates the migration and placement of interneurons in the developing cerebral cortex. Dev Cell 23, 925–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Higginbotham H, Guo J, Yokota Y, Umberger NL, Su CY, Li J, Verma N, Hirt J, Ghukasyan V, Caspary T, et al. (2013). Arl13b-regulated cilia activities are essential for polarized radial glial scaffold formation. Nat Neurosci 16, 1000–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Humbert MC, Weihbrecht K, Searby CC, Li Y, Pope RM, Sheffield VC, and Seo S (2012). ARL13B, PDE6D, and CEP164 form a functional network for INPP5E ciliary targeting. Proc Natl Acad Sci U S A 109, 19691–19696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Phua SC, Chiba S, Suzuki M, Su E, Roberson EC, Pusapati GV, Setou M, Rohatgi R, Reiter JF, Ikegami K, et al. (2017). Dynamic Remodeling of Membrane Composition Drives Cell Cycle through Primary Cilia Excision. Cell 168, 264–279 e215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Doherty D (2009). Joubert syndrome: insights into brain development, cilium biology, and complex disease. Semin Pediatr Neurol 16, 143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thomas S, Cantagrel V, Mariani L, Serre V, Lee JE, Elkhartoufi N, de Lonlay P, Desguerre I, Munnich A, Boddaert N, et al. (2015). Identification of a novel ARL13B variant in a Joubert syndrome-affected patient with retinal impairment and obesity. Eur J Hum Genet 23, 621–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Parisi MA (2009). Clinical and molecular features of Joubert syndrome and related disorders. Am J Med Genet C Semin Med Genet 151C, 326–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rafiullah R, Long AB, Ivanova AA, Ali H, Berkel S, Mustafa G, Paramasivam N, Schlesner M, Wiemann S, Wade RC, et al. (2017). A novel homozygous ARL13B variant in patients with Joubert syndrome impairs its guanine nucleotide-exchange factor activity. Eur J Hum Genet 25, 1324–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Miertzschke M, Koerner C, Spoerner M, and Wittinghofer A (2014). Structural insights into the small G-protein Arl13B and implications for Joubert syndrome. Biochem J 457, 301–311. [DOI] [PubMed] [Google Scholar]
- 87.Shao J, Xu L, Chen L, Lu Q, Xie X, Shi W, Xiong H, Shi C, Huang X, Mei J, et al. (2017). Arl13b Promotes Gastric Tumorigenesis by Regulating Smo Trafficking and Activation of the Hedgehog Signaling Pathway. Cancer Res 77, 4000–4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schou KB, Pedersen LB, and Christensen ST (2015). Ins and outs of GPCR signaling in primary cilia. EMBO Rep 16, 1099–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Qiu L, LeBel RP, Storm DR, and Chen X (2016). Type 3 adenylyl cyclase: a key enzyme mediating the cAMP signaling in neuronal cilia. Int J Physiol Pathophysiol Pharmacol 8, 95–108. [PMC free article] [PubMed] [Google Scholar]
- 90.Challis RC, Tian H, Wang J, He J, Jiang J, Chen X, Yin W, Connelly T, Ma L, Yu CR, et al. (2015). An Olfactory Cilia Pattern in the Mammalian Nose Ensures High Sensitivity to Odors. Curr Biol 25, 2503–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen X, Xia Z, and Storm DR (2012). Stimulation of electro-olfactogram responses in the main olfactory epithelia by airflow depends on the type 3 adenylyl cyclase. J Neurosci 32, 15769–15778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wong ST, Trinh K, Hacker B, Chan GC, Lowe G, Gaggar A, Xia Z, Gold GH, and Storm DR (2000). Disruption of the type III adenylyl cyclase gene leads to peripheral and behavioral anosmia in transgenic mice. Neuron 27, 487–497. [DOI] [PubMed] [Google Scholar]
- 93.Bishop GA, Berbari NF, Lewis J, and Mykytyn K (2007). Type III adenylyl cyclase localizes to primary cilia throughout the adult mouse brain. J Comp Neurol 505, 562–571. [DOI] [PubMed] [Google Scholar]
- 94.Wu L, Shen C, Seed Ahmed M, Ostenson CG, and Gu HF (2016). Adenylate cyclase 3: a new target for anti-obesity drug development. Obes Rev 17, 907–914. [DOI] [PubMed] [Google Scholar]
- 95.Nordman S, Abulaiti A, Hilding A, Langberg EC, Humphreys K, Ostenson CG, Efendic S, and Gu HF (2008). Genetic variation of the adenylyl cyclase 3 (AC3) locus and its influence on type 2 diabetes and obesity susceptibility in Swedish men. Int J Obes (Lond) 32, 407–412. [DOI] [PubMed] [Google Scholar]
- 96.Wen W, Cho YS, Zheng W, Dorajoo R, Kato N, Qi L, Chen CH, Delahanty RJ, Okada Y, Tabara Y, et al. (2012). Meta-analysis identifies common variants associated with body mass index in east Asians. Nat Genet 44, 307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Warrington NM, Howe LD, Paternoster L, Kaakinen M, Herrala S, Huikari V, Wu YY, Kemp JP, Timpson NJ, St Pourcain B, et al. (2015). A genome-wide association study of body mass index across early life and childhood. Int J Epidemiol 44, 700–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Felix JF, Bradfield JP, Monnereau C, van der Valk RJ, Stergiakouli E, Chesi A, Gaillard R, Feenstra B, Thiering E, Kreiner-Moller E, et al. (2015). Genome-wide association analysis identifies three new susceptibility loci for childhood body mass index. Hum Mol Genet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cousminer DL, Berry DJ, Timpson NJ, Ang W, Thiering E, Byrne EM, Taal HR, Huikari V, Bradfield JP, Kerkhof M, et al. (2013). Genome-wide association and longitudinal analyses reveal genetic loci linking pubertal height growth, pubertal timing and childhood adiposity. Hum Mol Genet 22, 2735–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stergiakouli E, Gaillard R, Tavare JM, Balthasar N, Loos RJ, Taal HR, Evans DM, Rivadeneira F, St Pourcain B, Uitterlinden AG, et al. (2014). Genome-wide association study of height-adjusted BMI in childhood identifies functional variant in ADCY3. Obesity (Silver Spring) 22, 2252–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Volkov P, Olsson AH, Gillberg L, Jorgensen SW, Brons C, Eriksson KF, Groop L, Jansson PA, Nilsson E, Ronn T, et al. (2016). A Genome-Wide mQTL Analysis in Human Adipose Tissue Identifies Genetic Variants Associated with DNA Methylation, Gene Expression and Metabolic Traits. PLoS One 11, e0157776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fliegauf M, Benzing T, and Omran H (2007). When cilia go bad: cilia defects and ciliopathies. Nat Rev Mol Cell Biol 8, 880–893. [DOI] [PubMed] [Google Scholar]
- 103.Cao H, Chen X, Yang Y, and Storm DR (2016). Disruption of type 3 adenylyl cyclase expression in the hypothalamus leads to obesity. Integr Obes Diabetes 2, 225–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pitman JL, Wheeler MC, Lloyd DJ, Walker JR, Glynne RJ, and Gekakis N (2014). A gain-of-function mutation in adenylate cyclase 3 protects mice from diet-induced obesity. PLoS One 9, e110226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Davenport JR, Watts AJ, Roper VC, Croyle MJ, van Groen T, Wyss JM, Nagy TR, Kesterson RA, and Yoder BK (2007). Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr Biol 17, 1586–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Loktev AV, and Jackson PK (2013). Neuropeptide Y Family Receptors Traffic via the Bardet-Biedl Syndrome Pathway to Signal in Neuronal Primary Cilia. Cell Rep. [DOI] [PubMed] [Google Scholar]
- 107.Berbari NF, Pasek RC, Malarkey EB, Yazdi SM, McNair AD, Lewis WR, Nagy TR, Kesterson RA, and Yoder BK (2013). Leptin resistance is a secondary consequence of the obesity in ciliopathy mutant mice. Proc Natl Acad Sci U S A 110, 7796–7801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mukhopadhyay S, and Jackson PK (2013). Cilia, tubby mice, and obesity. Cilia 2, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mok CA, Heon E, and Zhen M (2010). Ciliary dysfunction and obesity. Clin Genet 77, 18–27. [DOI] [PubMed] [Google Scholar]
- 110.Klok MD, Jakobsdottir S, and Drent ML (2007). The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obes Rev 8, 21–34. [DOI] [PubMed] [Google Scholar]
- 111.Oswal A, and Yeo G (2010). Leptin and the control of body weight: a review of its diverse central targets, signaling mechanisms, and role in the pathogenesis of obesity. Obesity (Silver Spring) 18, 221–229. [DOI] [PubMed] [Google Scholar]
- 112.Alavi MS, Shamsizadeh A, Azhdari-Zarmehri H, and Roohbakhsh A (2018). Orphan G protein-coupled receptors: The role in CNS disorders. Biomed Pharmacother 98, 222–232. [DOI] [PubMed] [Google Scholar]
- 113.Wray NR, Pergadia ML, Blackwood DH, Penninx BW, Gordon SD, Nyholt DR, Ripke S, MacIntyre DJ, McGhee KA, Maclean AW, et al. (2012). Genome-wide association study of major depressive disorder: new results, meta-analysis, and lessons learned. Mol Psychiatry 17, 36–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pillai V, Kalmbach DA, and Ciesla JA (2011). A meta-analysis of electroencephalographic sleep in depression: evidence for genetic biomarkers. Biol Psychiatry 70, 912–919. [DOI] [PubMed] [Google Scholar]
- 115.Sapolsky RM (2001). Depression, antidepressants, and the shrinking hippocampus. Proc Natl Acad Sci U S A 98, 12320–12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Skafidas E, Testa R, Zantomio D, Chana G, Everall IP, and Pantelis C (2014). Predicting the diagnosis of autism spectrum disorder using gene pathway analysis. Mol Psychiatry 19, 504–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.C.Y. RK, Merico D, Bookman M, L.H. J, Thiruvahindrapuram B, Patel RV, Whitney J, Deflaux N, Bingham J, Wang Z, et al. (2017). Whole genome sequencing resource identifies 18 new candidate genes for autism spectrum disorder. Nat Neurosci 20, 602–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Saeed S, Bonnefond A, Tamanini F, Mirza MU, Manzoor J, Janjua QM, Din SM, Gaitan J, Milochau A, Durand E, et al. (2018). Loss-of-function mutations in ADCY3 cause monogenic severe obesity. Nat Genet 50, 175–179. [DOI] [PubMed] [Google Scholar]
- 119.Weiner LP (2008). Definitions and criteria for stem cells. Methods Mol Biol 438, 3–8. [DOI] [PubMed] [Google Scholar]
- 120.Bennett MV, Contreras JE, Bukauskas FF, and Saez JC (2003). New roles for astrocytes: gap junction hemichannels have something to communicate. Trends in neurosciences 26, 610–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Porter JT, and McCarthy KD (1997). Astrocytic neurotransmitter receptors in situ and in vivo. Progress in neurobiology 51, 439–455. [DOI] [PubMed] [Google Scholar]
- 122.Di Pietro C, Marazziti D, La Sala G, Abbaszadeh Z, Golini E, Matteoni R, and Tocchini-Valentini GP (2017). Primary Cilia in the Murine Cerebellum and in Mutant Models of Medulloblastoma. Cell Mol Neurobiol 37, 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]