Abstract
Introduction/background
Implantable cardiac devices are widely used in chronic heart failure (CHF) therapy. This review covers current CHF treatment with electronic cardiac devices, areas of discussion and emerging technologies.
Sources of data
A comprehensive search of available literature resources including Pubmed, MEDLINE and EMBASE was performed. National and international guidelines were accessed.
Areas of agreement
Excessive right ventricular pacing is detrimental to cardiac function. Cardiac resynchronization therapy is beneficial in specific individuals with CHF.
Areas of controversy
Implantable cardioverter defibrillators might not benefit all. Optimizing CRT delivery. Remote monitoring seems not to be of benefit in CHF.
Growing points
Device-based optimization.
Areas timely for developing research
Personalization of device therapy. Focussing implantable cardioverter defibrillator therapy. What to do at implantable cardioverter defibrillator box change?
Keywords: Chronic heart failure, pacemaker, CRT, ICD, optimization
Introduction
Chronic heart failure (CHF) is a common condition characterized by symptoms of breathlessness and fatigue in the presence of cardiac dysfunction, most frequently impairment of contraction of the left ventricle (left ventricular systolic dysfunction). The management of CHF due to left ventricular systolic dysfunction is well supported by evidence from clinical trials and includes angiotensin-converting enzyme inhibitors, beta-receptor antagonists, aldosterone receptor antagonists and newer agents such as ivabradine and neprolysin inhibitors. In addition, device therapy especially pacemaker therapy; principally implantable cardioverter defibrillators (ICD) and cardiac resynchronization therapy (CRT), has become a key part of the armamentarium used to control the condition.
Implantable electronic cardiac devices have revolutionized therapy within cardiology and are recommended in both national1 and international2,3 guidelines to treat bradycardia, tachy-arrhythmia and chronic heart failure secondary to left ventricular systolic dysfunction. The therapeutic use of cardiac pacing traditionally falls within the field of electrophysiology, but increasingly, heart failure physicians are taking the lead on implant decisions and the monitoring of CHF patients with these devices. Over time, through observational, preclinical and clinical studies, the pacemaker has developed from an externally powered device, to a fully implantable, automated device with battery longevity of more than 8 years capable of transmitting data wirelessly for remote follow-up. While early pacemakers were electronic metronomes, modern iterations have added complex hardware and software around that basic function to allow for extremely complex and sophisticated programmability.
Pacemakers are associated with improved quality of life for individuals with sick sinus syndrome3,4 and improved longevity and symptomatic benefit in patients with atrio-ventricular (AV) block.5 In patients with symptomatic CHF, a left ventricular ejection fraction (LVEF) <35% and QRS duration of >120 ms, CRT improves mortality, morbidity and quality of life.6 In certain subgroups ICDs can improve mortality over a finite period in those with/at risk of ventricular tachyarrhythmias.7
Here we provide an overview of current practice in device management with a focus on CHF, areas of controversy, emerging technology and areas ready for research.
Chronic heart failure and pacemakers
Despite proven mortality and quality of life benefits of pacemaker implantation,4,5 long-term pacing in the right ventricle may be detrimental to left ventricular function in some individuals. Pacing the heart using a lead situated in the right ventricular apex has been standard practice for many years, but it is now clear that right ventricular (RV) pacing should be limited where possible.8
Right VP for bradycardia a potential substrate for the development of left ventricular systolic dysfunction
The detrimental effects of right VP have been appreciated for almost 100 years.9 Wiggers observed right VP-induced adverse changes in LV intra-ventricular pressure curves in canine experiments, and preclinical experiments of rapid chronic right VP have been used for decades to induce heart failure,10 but it was in the late 1980s and early 1990s that the clinical association between persistent right VP and heart failure was documented.11 Observational cohort studies comparing atrial to VP confirmed an association with right VP and the increased incidence of CHF and atrial fibrillation.12,13 The disorganized ventricular activation through right VP,11,14 causes adverse cardiac structural, haemodynamic and neuro-hormonal changes and is associated with adverse clinical outcomes.12,14,15 In response, pacemaker manufacturers have developed algorithms designed to reduce unnecessary right VP.
In patients in whom right VP is unavoidable, alternative pacing sites of the right ventricular septum, right ventricular outflow tract and His bundle have been proposed as options to offset the potential negative effects of right ventricular apical pacing. However, although a meta-analysis published by Shimony et al.16 including 754 patients from 14 randomized studies, comparing right ventricular apical pacing vs. non-apical pacing suggested beneficial effects on LVEF in patients with impaired left ventricular function (LVEF ≤ 45%), there were no differences in any measure of quality of life, functional test (walk distance and peak oxygen uptake) and morbidity or mortality rates. Hence, in the absence of clinical benefit, septal and direct His bundle pacing are not widely used due to concerns around long-term lead function; since stability and sensing of intrinsic rhythm are less reliable when compared with right ventricular apical pacing.3
Pacing for chronic heart failure
Almost 50% of patients with CHF and left ventricular systolic dysfunction have ventricular conduction delays, such as left bundle branch block (LBBB),17 and the dyssynchronous contraction that occurs as a result creates a significant increase in myocardial work.18 The effect is particularly relevant in the presence of left ventricular systolic dysfunction, where dyssynchrony compounds the situation, worsening morbidity and mortality.19 An appreciation of this led to the concept that pacing might be able to improve conduction delay, reduce dyssynchrony and improve patient outcomes. Ground-breaking studies by Cazeau et al., Auricchio et al. and Kass et al.20–22 demonstrated the beneficial effects of multisite pacing in patients, leading to the development of CRT, credited as being the first method of improving cardiac function via the use of artificial electrical stimulation.23 Since then, larger randomized studies have proven that cardiac resynchronization therapy improves cardiac function, heart failure symptoms, quality of life and mortality and morbidity in specific individuals.24
CRT involves electrically stimulating the right and left ventricles simultaneously using a pacemaker lead positioned in the right ventricular apex and a pacemaker lead positioned on the left ventricular free wall via the coronary sinus.23 Although the treatment reduces morbidity and mortality from CHF,6 around 35% of implanted patients do not improve either symptoms or beneficial remodelling—frequently and unfortunately termed ‘non-response’. Although this is not different to response rates for medical therapy,25 the upfront approach in terms of cost and complications required for device therapy has led to a focus on de-selecting those less likely to ‘respond’ with considerable efforts to predict response prior to implant, all of which beyond the combination of left bundle branch block and impaired left ventricular function have been neutral.26 Although mechanical dyssynchrony in the absence of electrical dyssynchrony (broad QRS) does exist, all studies of CRT in this situation,27 have been at best neutral.28,29
CRT in patients with existing right ventricular pacemakers
Patients with permanent right ventricular pacemakers are frequently found to have left ventricular dysfunction and heart failure thought to be due to long-term dyssynchrony induced through right ventricular apical pacing on a background of increased risk. Internationally, ‘upgrades’ from right ventricular devices represent between 23% and 28% of all CRT implants,30 despite the fact that this approach has been tested only in small, mostly non-randomized studies. Three small randomized crossover trials,30–32 and one randomized, placebo controlled trial,33 have shown promising results with short-term (2–6 months) follow-up periods. All participants in these studies had conventional right VP indications for bradycardia (mainly AV block), CHF symptoms (NYHA class II–IV) and LVEF <50%. When compared with right VP, upgraded patients experienced fewer hospitalizations, improved left ventricular function and improved symptoms. Despite the lack of definitive data from large randomized control trials, there is accumulating observational evidence that patients with high volumes of right VP, reduced LVEF and CHF symptoms benefit from upgrade to CRT.
Implantable cardioverter defibrillators
Despite a significant reduction in mortality rates in high income countries,34 cardiovascular disease remains responsible for approximately 17 million deaths each year worldwide, of which around 25% are sudden cardiac deaths.34 In the past, around 40% of patients with CHF suffered sudden or unexplained death,35 usually assumed to be due to either brady or tachyarrhythmias. Antiarrhythmic medications do not reduce (and may worsen) overall mortality especially in the setting of CHF with reduced ejection fraction.36 Hence, following landmark trials published in 2002 and 2005 (Multicenter Automatic Defibrillator Implantation Trial II (MADIT-2) and Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT)) which both described the mortality benefit of ICD therapy in patients with left ventricular systolic dysfunction, primary prevention ICD implantation (in a patient with risk factors for but no proven ventricular arrhythmia) has become commonplace in patients with CHF due to left ventricular systolic dysfunction and is recommended in all major guidelines when optimal medical therapy has failed to increase the LVEF above 35%.36
However, new challenges are developing in ICD eligibility. Modern medical therapy and cardiac resynchronization therapy can significantly reduce the incidence of sudden death,37 while it is also becoming clear that in patients with more severe CHF and co-morbidities, arrhythmia forms a much smaller proportion of total mortality compared with the incidence of death due to deteriorating CHF.38 The appreciation of this in the immediate post-myocardial infarction setting has led to guidelines not recommending ICDs within 40 days of a myocardial infarction since a reduction in sudden death is offset by increases in heart failure death, demonstrating that treating arrhythmia in this setting converts only the mode of death.39 The same pattern is seen in patients with severe symptoms (New York Heart Association class IV heart failure) resistant to standard and unsuitable for advanced CHF therapies since mortality in these patients is overwhelmingly due to heart failure.36 Finally, ICD therapy is unlikely to provide any benefit in those with significant co-morbidities with an overall prognosis of less than 1 year.40 Controversy remains whether patients with an improvement of LVEF to >35% who have not required their device to deliver a shock should receive a replacement device when the original generator has depleted.41
ICDs should be considered in survivors of cardiac arrest, and in those with symptomatic sustained ventricular arrhythmia,36 although even in this situation, the patient should be counselled, and their quality of life, LVEF (survival benefit is unproven with LVEF >35%) and existing life-limiting co-morbidities should be considered.42
Recent data have suggested that the benefit of ICD implantation is greatest in those with CHF and ischaemic aetiology, rather than those with non-ischaemic aetiology, due to a greater risk of arrhythmia in those with ischaemic heart disease.43 Patients with CHF, left ventricular systolic dysfunction and a long QRS duration are also at increased risk, although these patients are also usually offered CRT.7,38 It is possible that the clinical and cost-effectiveness of ICDs in CHF patients without ischaemic heart disease, those with and without important co-morbidities and the incremental benefit in patients receiving CRT should be re-evaluated.
Subcutaneous implantable cardioverter defibrillators
By avoiding trans-venous leads and the intravascular complications associated with them, subcutaneous ICDs have some advantages over standard trans-venous systems and may be as effective at treating shockable ventricular arrhythmias.44 They can be used in patients with difficult or absent venous access, but patient selection is important due to their inability to treat brady-arrhythmia, provide CRT or anti-tachycardia pacing. More robust evidence from clinical trials, around the performance of these devices, is still required.45
Areas of controversy—CRT
What is response?
Despite the evidence around the success of CRT, only around 65% of patients ‘respond’ in terms of standard measures.46 Efforts to predict ‘response’ have been made, but no reliable feature has been found.26 Crucially, although ‘response’ however measured is associated with a better long-term outcome in both randomized and observational studies, it is unclear whether ‘non-responders’ do less well in terms of prognosis than those that do not receive an implant at all. This issue is impossible to explore in observational studies. The CARE-HF study steering committee and investigators group performed an analysis with the aim of developing a prognostic model, based on prospectively defined patient characteristics and treatment, on the trial primary outcome of death from any cause or unplanned hospitalization for a major cardiovascular event. They found that those with echocardiographic evidence of marked dyssynchrony and low systolic blood pressure gained superior benefit from implantation, however considerable benefits were found across the range of subjects enroled.47 What is clear is that non-LBBB conduction delay does not benefit from CRT, possibly due to differences in substrate. Straus et al. found that in those with LVEF ≤ 35%, undergoing ICD implantation, RBBB was associated with more scar on cardiac magnetic resonance than LBBB, while LBBB was more commonly associated with magnetic resonance-defined non-ischaemic aetiology.48
Improving response rates rather than de-selection
The alternative approach, possibly more acceptable than excluding potential non-responders prior to implant, is to improve ‘response’ rates by optimizing electrical therapy delivery. Two optimization technologies have recently emerged.
One option receiving much attention is multi-point pacing (MPP). The introduction of left ventricular leads with four electrodes capable of pacing from two sites at once, has allowed the opportunity to pace the lateral wall of the left ventricle from several points at once using a single lead. Although it seems logical that electrical activation beginning at several points on the left ventricular wall should improve coordination of the heart, there is no consistent evidence of increased response in terms of improved remodelling or composite scores of patient-related status. The largest study of multi-point pacing, randomly assigned 506 CRT patients to 6 months of MPP or standard programming. An equal number of patients improved as were worsened by MPP.49 Rather than improving response across the board, this suggests that careful patient selection might identify a subgroup of patients for whom MPP is of benefit and in whom one might choose to accept the additional battery drain in exchange for greater clinical effect, but there is little evidence that non-responders can be converted to responders in a general CRT population. On the other hand, since the technology leads to accelerated battery drain, with on average 1 year less longevity over the lifetime of the device, in patients with no appreciable benefit from MPP, it should be left de-activated sparing the device battery.
The other approach to improve post-implant response is to optimize atrio-ventricular (AV) timing—the timing by which ventricular electrical stimulation is offset following atrial activity—and ventricular-ventricular (VV) timing—the timing of stimulation between the two ventricular leads. A protocol where these variables are adjusted to optimize filling and cardiac output measured by echocardiography can improve left ventricular remodelling, although studies are small and short-term. In addition to the need for repeated scans of limited reproducibility, the major disadvantage of an imaging-based optimization is that resting haemodynamics might not reflect improved timings during exercise.50 It is doubtful whether optimization using non-invasive blood pressure assessment measured from the finger is of benefit on exercise capacity. The 400-patient British Randomized Controlled Trial of Atrioventricular (AV) and interventricular (VV) optimization (BRAVO) study completed in 2015 and the results have not yet been reported.51 A novel technology which uses a sensor located in the atrial lead that measures cardiac contractility during rest and exercise might be more reliable and logical. Cardiac contraction generates vibrations that transmit through the heart, the magnitude of these can measured by the atrial sensor, translated into a reproducible measure of cardiac contractility and used to optimize AV and VV timings at rest and exercise.52 In a landmark clinical trial, the use of this system improved response rates and over 24 months reduced all-cause hospitalization.53
Areas of controversy
Implantable cardioverter defibrillators for all?
We have described the two large trials that demonstrated the benefit of ICD over medical therapy in heart failure due to left ventricular systolic dysfunction. These were published before the widespread use of CRT. There has therefore long been controversy over whether a combined device including an ICD (known as CRT-D) is better than CRT alone (known as CRT-P) since this comparison has never been tested. This discussion, which is especially pertinent in patients with non-ischaemic cardiomyopathy in whom the sudden death risk is lower, has been heightened by recent trials. In 2016, Køber et al.54 randomized 1116 patients with non-ischaemic, symptomatic systolic heart failure (left ventricular ejection fraction ≤35%) to receive an ICD (n = 556), or usual care (no ICD) (n = 560). More than half (58%) received CRT in each group, with a primary outcome of all-cause mortality, and secondary outcomes of sudden cardiac death and cardiovascular death. There was no all-cause survival benefit in individuals receiving ICD although ICD implantation did reduce the risk of sudden cardiac death (by 50%), and younger patients in particular had a survival benefit associated with ICD implantation. There was no influence of concurrent CRT. These data are countered by a recently published meta-analysis of ICD mortality studies dating from 1996 to 2005 including 8567 subjects of whom 3128 did not have ischaemic heart disease, which suggests that the reduction in mortality from an ICD is the same in the non-ischaemic subgroup as in the patients with an ischaemic aetiology.55
Further information about sudden death in heart failure comes from Shen et al. who explored sudden death rates in a pooled analysis of 40 195 patients with heart failure with reduced ejection fractions (but without an ICD), enroled in 12 clinical trials from 1995 to 2014.56 They demonstrated a consistent reduction in sudden death over the period in question of 44% (P = 0.03) across the trials. The 90-day sudden death rate was 2.4% in the earliest trial and 1.0% in the most recent trial. Importantly, the rate of sudden death was no different in those with a recent heart failure diagnosis than those with ongoing heart failure.
The studies from Køber and Shen attribute their results to improved medical management of patients with heart failure, while the meta-analysis is limited to studies of patients recruited between 10 and 25 years ago. Nevertheless, these datasets will no doubt fuel intense debate amongst heart failure physicians, electrophysiologists and purchasers of healthcare around ICD implantation in CHF.
Cardiac resynchronization therapy for complete heart block
CHF or asymptomatic left ventricular systolic dysfunction is seen in up to 40% of patients with permanent pacemakers, and relates to right VP percentage and cardiovascular co-morbidities of the patient.57 Whether those at risk of exposure to high volumes of right VP should receive a CRT device at the time of the initial implant rather than a standard pacemaker is unknown. The BLOCK-HF study set out to examine this in 2003.58 Patients with an LVEF ≤ 50% and evidence of possible future high volumes of right VP were implanted with CRT devices and randomly allocated to biventricular pacing (n = 349) or right VP (n = 342). The primary endpoint was a composite of all-cause mortality, urgent heart failure care requiring intravenous diuretic therapy or increase in left ventricular end systolic volume index of >15% from baseline. The primary endpoint (driven by the remodelling component) was observed less frequently (53%) in the biventricular paced group than in the right VP group (64%); equating to a 26% lower relative risk in those randomized to biventricular pacing (HR 0.74 (0.60–0.90)). The BIOPACE study, presented at the European Society of Cardiology conference in 2014, but not yet published, also failed to show a difference in outcomes between these two approaches to heart rate support. Whether CRT is the treatment of choice for complete heart block is therefore unproven not least since an indication of ‘CHB’ does not closely predict high volumes of right VP,57 while the baseline risk factors predisposing to left ventricular dysfunction, its natural history and response to medical therapy are underexplored.
Remote management of heart failure using implantable devices
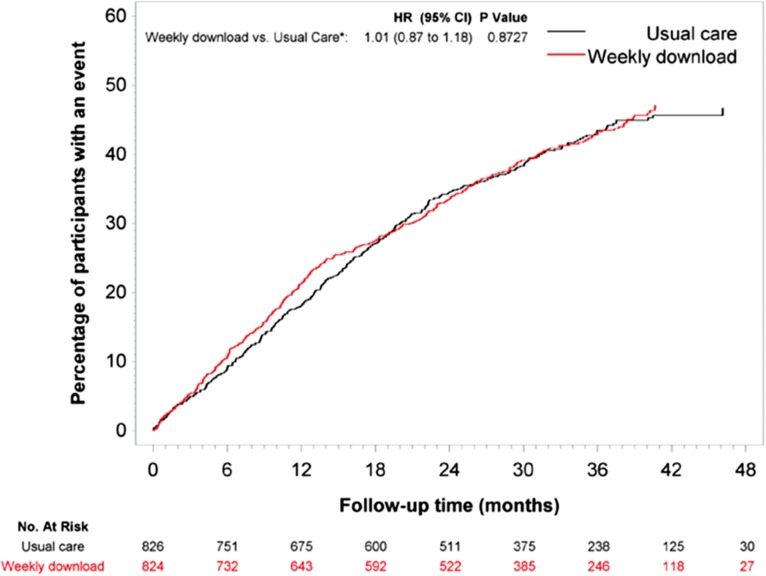
The majority of modern day devices implanted for heart failure have the ability to transmit diagnostic data remotely, offering the potential to adjust therapy remotely either by a central management service or by local services interpreting the data. Although widely accepted, some caution is required prior to adjusting current services in the hope that workloads will be reduced.59 As the largest study of remote monitoring using implantable devices, the Remote Monitoring of Heart Failure (REM-HF) study described no benefit of weekly home monitoring vs. usual care in 1650 patients (mean age 70 (range 23–98) years) with heart failure due to left ventricular systolic dysfunction on optimal medical therapy and a CRT or ICD device.60 The primary endpoint of death from any cause or unplanned hospitalization for cardiovascular reasons was not different after a mean follow-up time of 2.8 years between groups [HR 1.01; 95% CI: 0.87 to 1.18; P = 0.87] (Fig. 1). There were no significant differences between the two groups in any of the secondary endpoints or time to primary endpoint components.
Fig. 1.
Time-to-Event analysis for the primary endpoint of a composite of death from any cause and unplanned hospitalization for cardiovascular reasons, from the REM-HF study (with permission).
Areas timely for developing research
Personalization of programming
Cardiac devices offer many programmable settings, yet standard pacemakers and cardiac resynchronization devices are often implanted ‘out of the box’ with very little personalization.
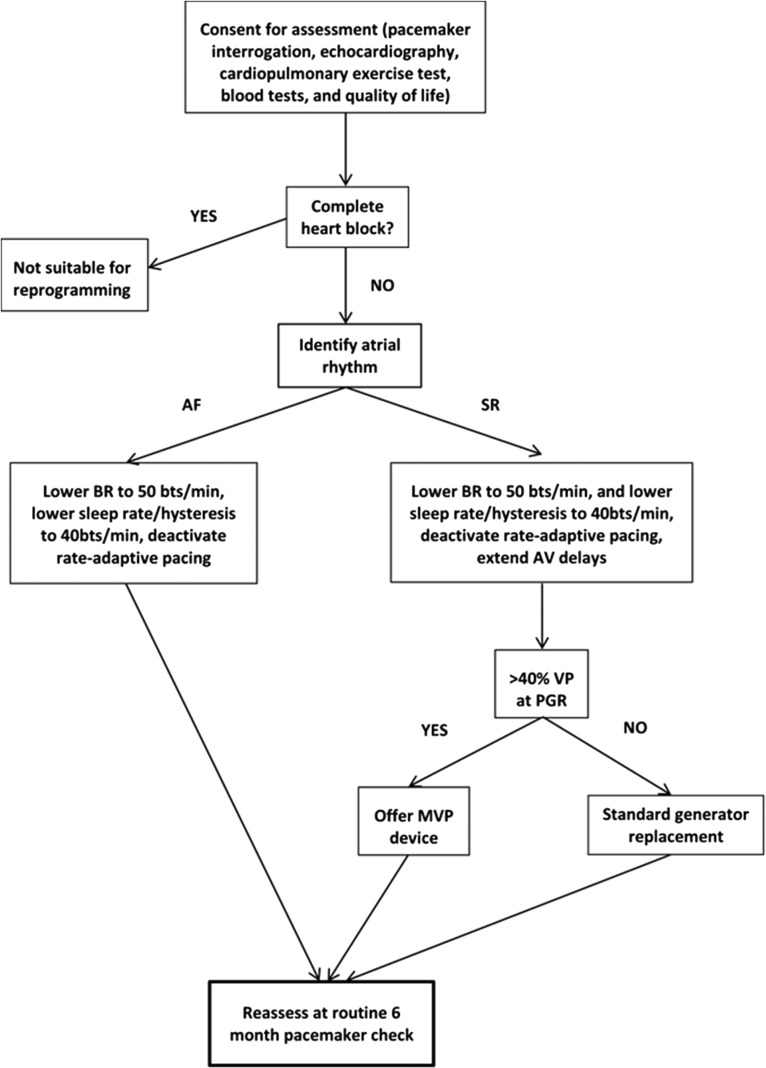
The benefits of personalization of right ventricular pacemakers to avoid unnecessary pacing is underestimated, with potential to lengthen battery longevity and avoid deteriorating left ventricular function. No independent clinical trials have been performed to explore these potential benefits, although we have previously described an approach that improved left ventricular function in patients with high amounts of right VP and left ventricular dysfunction (Fig. 2).8 A randomized controlled trial to test our approach in a larger group of patients is now underway. Careful programming to try to avoid right VP should be considered prior to upgrade in patients with right ventricular pacemakers, even those with AV block.
Fig. 2.
Leeds right VP optimization protocol (BR, base rate; PGR, pacemaker generator replacement; MVP, managed ventricular pacing, or other ventricular pacing avoidance algorithm).
Using echocardiography to define optimal CRT settings may also be of benefit but is not widely used. In addition, it is during physical exertion that symptoms occur in heart failure, yet most imaging functional assessments and optimization are performed at rest. Rather than aiming for de-selection of people currently eligible, optimization based on individual physiology, whether determined by a device-based measure or imaging should be the target to improve response and thereby cost-effectiveness. Bradycardia and a poor heart rate rise in response to exercise (known as chronotropic incompetence), whether iatrogenic or pathological, are common in CHF failure and little is known about how these affect exercise capacity. Using pacemaker algorithms to counter bradycardia in patients with CHF is not evidence based. Neither a minimal, or aggressive, increase in heart rate rise has been shown to improve exercise capacity in those with CHF and devices,61 but some degree of heart rate rise has been shown to improve outcomes,62 and exercise capacity.63 Since exercise intolerance is the cardinal feature of heart failure, it is appropriate that research that targets this continues and is focussed on individual physiology rather than assuming that standard programming is the best that we can offer (Fig. 3).
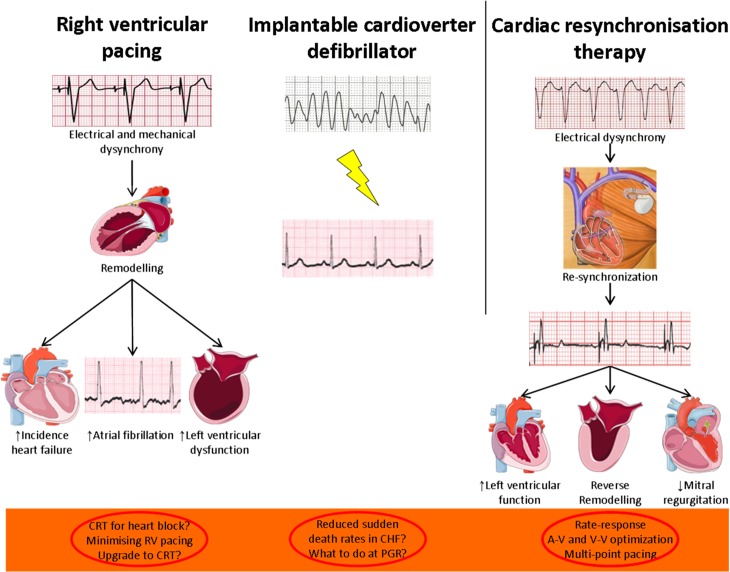
Fig. 3.
Implantable electronic devices in heart failure, and key therapeutic targets.
Do implantable cardioverter defibrillator guidelines in heart failure need re-examining?
Current implant guidance is largely based on MADIT-2 (2002) and SCD-HeFT (2005). Since these studies were completed, medical therapy has advanced and CRT is commonly offered, resulting in a decline in sudden cardiac death.56 Moreover, the most recent NICE guidance,64 omits diabetes mellitus as a risk factor for sudden cardiac death,65 despite this co-morbidity being a powerful predictor of increased risk in patients with heart failure,66 while including non-ischaemic cardiomyopathy which is associated with a low sudden cardiac death risk when optimally medically treated. It is possible that cardiac magnetic resonance scanning to identify the presence of scar in the myocardium might provide further risk stratification of patients with non-ischaemic cardiomyopathy, and a scar-guided approach to ICD implantation in people with a left ventricular ejection fraction >35% is currently being tested.51
Nevertheless, the current lack of clarity leads to the question of whether the impact of ICDs in heart failure need re-examining in the modern medical environment.
The implantable cardioverter defibrillator box change dilemma
Selection criteria for ICD implantation have been widely studied, but insufficient attention has been paid to the utility of generator replacements. Changes in the clinical circumstances including ongoing risks of arrhythmia and co-morbidities should influence decision making around replacement. Despite the critical nature of this decision for patients, their families and their physicians, there are no studies describing the risks and benefits of ICD generator replacement.67
Conclusions
Treatment advances have substantially improved outcomes in heart failure and implantable cardiac devices have played a critical role in this. Robust evidence shows that cardiac devices complement medical therapy to reduce mortality in chronic heart failure. A range of established and emerging device therapies have been reviewed in this article, along with some thoughts to provoke debate. CRT is an established and potent therapy for people with symptomatic heart failure and a broad QRS. Work in this area should be based on improving an already successful therapy by personalizing settings based on targeting individual physiology. ICDs are also a successful therapy for some, but not all, individuals with heart failure. Work in this area should focus on identifying who is at significant risk of sudden cardiac death and will therefore gain the most benefit from an ICD. The implantation of any cardiac device remains an invasive and potentially complex procedure with upfront cost and at a small but appreciable risk to the patient. Every effort should be made to ensure maximum benefit to individuals and society.
Conflict of interest statement
The authors have no potential conflicts of interest.
Funding
J.G. is funded by an National Institute of Health Research PhD fellowship award.
M.T.K. is a funded by a British Heart Foundation Professorship.
K.K.W. is funded by an NIHR Clinician Scientist fellowship award.
References
- 1. NICE Dual‑chamber pacemakers for symptomatic bradycardia due to sick sinus syndrome and/or atrioventricular block. 2005. [cited 2016 05 Jan]; Available from: http://www.nice.org.uk/guidance/TA88/history.
- 2. Brignole M, Auricchio A, Baron-Esquivias G, et al. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J 2013;34:2281–329. [DOI] [PubMed] [Google Scholar]
- 3. Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol 2008;51:1–62. [DOI] [PubMed] [Google Scholar]
- 4. Lamas GA, Orav EJ, Stambler BS, et al. Quality of life and clinical outcomes in elderly patients treated with ventricular pacing as compared with dual-chamber pacing. Pacemaker Selection in the Elderly Investigators. N Engl J Med 1998;338:1097–104. [DOI] [PubMed] [Google Scholar]
- 5. Shaw DB, Kekwick CA, Veale D, et al. Survival in second degree atrioventricular block. Br Heart J 1985;53:587–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005;352:1539–49. [DOI] [PubMed] [Google Scholar]
- 7. Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med 1996;335:1933–40. [DOI] [PubMed] [Google Scholar]
- 8. Gierula J, Jamil HA, Byrom R, et al. Pacing-associated left ventricular dysfunction? Think reprogramming first! Heart 2014;100:765–9. [DOI] [PubMed] [Google Scholar]
- 9. Wiggers CJ. The muscular reactions of the mammilian ventricles to artificial surface stimuli. Am J Physiol 1925;73:346–78. [Google Scholar]
- 10. Wilson JR, Douglas P, Hickey WF, et al. Experimental congestive heart failure produced by rapid ventricular pacing in the dog: cardiac effects. Circulation 1987;75:857–67. [DOI] [PubMed] [Google Scholar]
- 11. Thackray SD, Witte KK, Nikitin NP, et al. The prevalence of heart failure and asymptomatic left ventricular systolic dysfunction in a typical regional pacemaker population. Eur Heart J 2003;24:1143–52. [DOI] [PubMed] [Google Scholar]
- 12. Andersen HR, Nielsen JC, Thomsen PE, et al. Long-term follow-up of patients from a randomised trial of atrial versus ventricular pacing for sick-sinus syndrome. Lancet 1997;350:1210–6. [DOI] [PubMed] [Google Scholar]
- 13. Andersen HR, Thuesen L, Bagger JP, et al. Prospective randomised trial of atrial versus ventricular pacing in sick-sinus syndrome. Lancet 1994;344:1523–8. [DOI] [PubMed] [Google Scholar]
- 14. O'keefe JH, Jones PG, Thompson RC, et al. Effect of chronic right ventricular apical pacing on left ventricular function. Am J Cardiol 2005;95:771–3. [DOI] [PubMed] [Google Scholar]
- 15. Sweeney MO, Hellkamp AS, Ellenbogen KA, et al. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation 2003;107:2932–7. [DOI] [PubMed] [Google Scholar]
- 16. Shimony A, Eisenberg MJ, Filion KB, et al. Beneficial effects of right ventricular non-apical vs. apical pacing: a systematic review and meta-analysis of randomized-controlled trials. Europace 2012;14:81–91. [DOI] [PubMed] [Google Scholar]
- 17. Schuster I, Habib G, Jego C, et al. Diastolic asynchrony is more frequent than systolic asynchrony in dilated cardiomyopathy and is less improved by cardiac resynchronization therapy. J Am Coll Cardiol 2005;46:2250–7. [DOI] [PubMed] [Google Scholar]
- 18. Vernooy K, Verbeek XA, Peschar M, et al. Left bundle branch block induces ventricular remodelling and functional septal hypoperfusion. Eur Heart J 2005;26:91–8. [DOI] [PubMed] [Google Scholar]
- 19. Bader H, Garrigue S, Lafitte S, et al. Intra-left ventricular electromechanical asynchrony. A new independent predictor of severe cardiac events in heart failure patients. J Am Coll Cardiol 2004;43:248–56. [DOI] [PubMed] [Google Scholar]
- 20. Cazeau S, Ritter P, Bakdach S, et al. Four chamber pacing in dilated cardiomyopathy. Pacing Clin Electrophysiol 1994;17:1974–9. [DOI] [PubMed] [Google Scholar]
- 21. Auricchio A, Stellbrink C, Block M, et al. Effect of pacing chamber and atrioventricular delay on acute systolic function of paced patients with congestive heart failure. The Pacing Therapies for Congestive Heart Failure Study Group. The Guidant Congestive Heart Failure Research Group. Circulation 1999;99:2993–3001. [DOI] [PubMed] [Google Scholar]
- 22. Kass DA, Chen CH, Curry C, et al. Improved left ventricular mechanics from acute VDD pacing in patients with dilated cardiomyopathy and ventricular conduction delay. Circulation 1999;99:1567–73. [DOI] [PubMed] [Google Scholar]
- 23. Kirk JA, Kass DA. Electromechanical dyssynchrony and resynchronization of the failing heart. Circ Res 2013;113:765–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cleland JG, Abraham WT, Linde C, et al. An individual patient meta-analysis of five randomized trials assessing the effects of cardiac resynchronization therapy on morbidity and mortality in patients with symptomatic heart failure. Eur Heart J 2013;34:3547–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. The CONSENSUS Trial Study Group Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 1987;316:1429–35. [DOI] [PubMed] [Google Scholar]
- 26. Sung RK, Foster E. Assessment of systolic dyssynchrony for cardiac resynchronization therapy is not clinically useful. Circulation 2011;123:656–62. [DOI] [PubMed] [Google Scholar]
- 27. Yu CM, Chan YS, Zhang Q, et al. Benefits of cardiac resynchronization therapy for heart failure patients with narrow QRS complexes and coexisting systolic asynchrony by echocardiography. J Am Coll Cardiol 2006;48:2251–7. [DOI] [PubMed] [Google Scholar]
- 28. Beshai JF, Grimm RA, Nagueh SF, et al. Cardiac-resynchronization therapy in heart failure with narrow QRS complexes. N Engl J Med 2007;357:2461–71. [DOI] [PubMed] [Google Scholar]
- 29. Ruschitzka F, Abraham WT, Singh JP, et al. Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. N Engl J Med 2013;369:1395–405. [DOI] [PubMed] [Google Scholar]
- 30. Leclercq C. Upgrading from right ventricular pacing to biventricular pacing in pacemaker patients with chronic heart failure: heart failure. Heart 2008;94:102–7. [DOI] [PubMed] [Google Scholar]
- 31. Höijer CJ, Brandt C, Brandt J. Upgrade to biventricular pacing in patients with conventional pacemakers and heart failure: a double-blind, randomized crossover study. Europace 2006;8:51–5. [DOI] [PubMed] [Google Scholar]
- 32. van Geldorp IE, Vernooy K, Delhaas T, et al. Beneficial effects of biventricular pacing in chronically right ventricular paced patients with mild cardiomyopathy. Europace 2010;12:223–9. [DOI] [PubMed] [Google Scholar]
- 33. Gierula J, Cubbon RM, Jamil HA, et al. Cardiac resynchronization therapy in pacemaker-dependent patients with left ventricular dysfunction. Europace 2013;15:1609–14. [DOI] [PubMed] [Google Scholar]
- 34. Priori SG, Blomström-Lundqvist C, Mazzanti A, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2015;36:2793–867. [DOI] [PubMed] [Google Scholar]
- 35. Poole-Wilson PA, Uretsky BF, Thygesen K, et al. Mode of death in heart failure: findings from the ATLAS trial. Heart 2003;89:42–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 37. Cubbon RM, Gale CP, Kearney LC, et al. Changing characteristics and mode of death associated with chronic heart failure caused by left ventricular systolic dysfunction: a study across therapeutic eras. Circ Heart Fail 2011;4:396–403. [DOI] [PubMed] [Google Scholar]
- 38. Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005;352:225–37. [DOI] [PubMed] [Google Scholar]
- 39. Steinbeck G, Andresen D, Seidl K, et al. Defibrillator implantation early after myocardial infarction. N Engl J Med 2009;361:1427–36. [DOI] [PubMed] [Google Scholar]
- 40. Steinberg BA, Al-Khatib SM, Edwards R, et al. Outcomes of implantable cardioverter-defibrillator use in patients with comorbidities: results from a combined analysis of 4 randomized clinical trials. JACC Heart Fail 2014;2:623–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kini V, Soufi MK, Deo R, et al. Appropriateness of primary prevention implantable cardioverter-defibrillators at the time of generator replacement: are indications still met? J Am Coll Cardiol 2014;63:2388–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Connolly SJ, Hallstrom AP, Cappato R, et al. Meta-analysis of the implantable cardioverter defibrillator secondary prevention trials. AVID, CASH and CIDS studies. Antiarrhythmics vs Implantable Defibrillator study. Cardiac Arrest Study Hamburg. Canadian Implantable Defibrillator Study. Eur Heart J 2000;21:2071–8. [DOI] [PubMed] [Google Scholar]
- 43. Theuns D, Smith T, Hunink MG, et al. Effectiveness of prophylactic implantation of cardioverter-defibrillators without cardiac resynchronization therapy in patients with ischaemic or non-ischaemic heart disease: a systematic review and meta-analysis. Europace 2010;12:1564–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Aziz S, El-Chami AR, El-Chami MF. The subcutaneous defibrillator: a review of the literature. J Am Coll Cardiol 2014;63:1473–9. [DOI] [PubMed] [Google Scholar]
- 45. Burke MC, Gold MR, Knight BP, et al. Safety and efficacy of the totally subcutaneous implantable defibrillator: 2-year results from a pooled analysis of the IDE study and EFFORTLESS registry. J Am Coll Cardiol 2015;65:1605–15. [DOI] [PubMed] [Google Scholar]
- 46. Bax JJ, Gorcsan J 3rd. Echocardiography and noninvasive imaging in cardiac resynchronization therapy: results of the PROSPECT (Predictors of Response to Cardiac Resynchronization Therapy) study in perspective. J Am Coll Cardiol 2009;53:1933–43. [DOI] [PubMed] [Google Scholar]
- 47. Richardson M, Freemantle N, Calvert MJ, et al. Predictors and treatment response with cardiac resynchronization therapy in patients with heart failure characterized by dyssynchrony: a pre-defined analysis from the CARE-HF trial. Eur Heart J 2007;28:1827–34. [DOI] [PubMed] [Google Scholar]
- 48. Strauss DG, Loring Z, Selvester RH, et al. Right, but not left, bundle branch block is associated with large anteroseptal scar. J Am Coll Cardiol 2013;62:959–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. https://clinicaltrials.gov/ct2/show/NCT01786993.
- 50. Kosmala W, Marwick TH. Meta-analysis of effects of optimization of cardiac resynchronization therapy on left ventricular function, exercise capacity, and quality of life in patients with heart failure. Am J Cardiol 2014;113:988–94. [DOI] [PubMed] [Google Scholar]
- 51.Available from: https://clinicaltrials.gov/ct2/show/NCT01918215.
- 52. Rickards A, Bombardini T, Corbucci G, et al. An implantable intracardiac accelerometer for monitoring myocardial contractility. The Multicenter PEA Study Group. Pacing Clin Electrophysiol 1996;19:2066–71. [DOI] [PubMed] [Google Scholar]
- 53. Brugada J, Delnoy PP, Brachmann J, et al. Contractility sensor-guided optimization of cardiac resynchronization therapy: results from the RESPOND-CRT trial. Eur Heart J 2017;38:730–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Køber L, Thune JJ, Nielsen JC, et al. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med 2016;375:1221–30. [DOI] [PubMed] [Google Scholar]
- 55. Shun-Shin MJ, Zheng SL, Cole GD, et al. Implantable cardioverter defibrillators for primary prevention of death in left ventricular dysfunction with and without ischaemic heart disease: a meta-analysis of 8567 patients in the 11 trials. Eur Heart J 2017;38:1738–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shen L, Jhund PS, Petrie MC, et al. Declining risk of sudden death in heart failure. N Engl J Med 2017;377:41–51. [DOI] [PubMed] [Google Scholar]
- 57. Gierula J, Cubbon RM, Jamil HA, et al. Patients with long-term permanent pacemakers have a high prevalence of left ventricular dysfunction. J Cardiovasc Med (Hagerstown) 2015;16:743–50. [DOI] [PubMed] [Google Scholar]
- 58. Curtis AB, Worley SJ, Adamson PB, et al. Biventricular pacing for atrioventricular block and systolic dysfunction. N Engl J Med 2013;368:1585–93. [DOI] [PubMed] [Google Scholar]
- 59. Hindricks G, Taborsky M, Glikson M, et al. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): a randomised controlled trial. Lancet 2014;384:583–90. [DOI] [PubMed] [Google Scholar]
- 60. Morgan JM, Kitt S, Gill J, et al. Remote management of heart failure using implantable electronic devices. Eur Heart J 2017;38:2352–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jamil HA, Gierula J, Paton MF, et al. Chronotropic incompetence does not limit exercise capacity in chronic heart failure. J Am Coll Cardiol 2016;67:1885–96. [DOI] [PubMed] [Google Scholar]
- 62. Cubbon RM, Ruff N, Groves D, et al. Ambulatory heart rate range predicts mode-specific mortality and hospitalisation in chronic heart failure. Heart 2016;102:223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gierula J, Paton MP, Lowry JE, et al. Rate-response programming tailored to the force frequency relationship improves exercise tolerance in chronic heart failure. JACC Heart Fail 2017. [DOI] [PubMed]
- 64. Excellence, N.N.I.f.C Implantable cardioverter defibrillators and cardiac resynchronisation therapy for arrhythmias and heart failure 2014; Available from: https://www.nice.org.uk/guidance/ta314.
- 65. Cubbon RM, Witte KK, Kearney LC, et al. Performance of 2014 NICE defibrillator implantation guidelines in heart failure risk stratification. Heart 2016;102:735–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cubbon RM, Adams B, Rajwani A, et al. Diabetes mellitus is associated with adverse prognosis in chronic heart failure of ischaemic and non-ischaemic aetiology. Diab Vasc Dis Res 2013;10:330–6. [DOI] [PubMed] [Google Scholar]
- 67. Kramer DB, Zimetbaum AE, Buxton PJ. Time for a change—a new approach to ICD replacement. N Engl J Med 2012;366:291–3. [DOI] [PubMed] [Google Scholar]