Abstract
Background
The abuse of methamphetamine (METH) has become a public health problem worldwide. This type of new drug can not only lead to addiction but can also cause cognitive impairment. Currently, there is no effective treatment. Repetitive transcranial magnetic stimulation (rTMS) is a painless and non-invasive green physiotherapy which can be used in the clinical treatment of neuropsychiatric disorders such as depression, anxiety, schizophrenia, and Parkinson’s disease. However, whether it can be used to treat methamphetamine addiction is unclear.
Objective
To explore the effect of rTMS on the relapse behavior of methamphetamine addiction.
Methods
METH-induced rats conditioned place preference (CPP) model and rTMS technique were used in this study. Rats were given high frequency(10Hz) rTMS treatment for one day (experiment 1) or three days (experiment 2) after the extinction of CPP behavior. The reinstatement test was performed 24 hours after rTMS treatment and the effects of acute and chronic rTMS on the reinstatement of METH-induced CPP in rats were explored.
Results
Acute rTMS treatment (1 day) had a trend of an inhibitory effect on the relapse behavior of METH-induced CPP, but it was not statistically significant compared with the sham stimulation group (t=1.48, p=0.431). However, chronic rTMS treatment (3 days) had a significant inhibitory effect on the reinstatement of METH-induced CPP compared with the sham stimulation group (t=3.33, p=0.004).
Conclusion
Chronic rTMS treatment inhibited the relapse behavior of METH-induced CPP in rats.
Key words: methamphetamine, repetitive transcranial magnetic stimulation, conditioned place preference, reinstatement
Abstract
背景
甲基苯丙胺(Methamphetamine, METH)滥用 已成为全球性的公共卫生问题,这类新型毒品不仅 能够导致患者成瘾,还会造成认知功能损伤,目前 尚无有效的治疗方法。重复经颅磁刺激(Repetitive transcranial magnetic stimulation, rTMS)是一种无痛、 无创的绿色物理治疗方法,目前可用于临床治疗抑郁、 焦虑、精神分裂、帕金森等神经精神疾病,并获得较 好的疗效;但其对甲基苯丙胺成瘾的疗效与机制尚不 明确,有待进一步研究。
目的
探索rTMS 对甲基苯丙胺成瘾复吸行为的作用。
方法
本研究应用甲基苯丙胺诱导的条件性位置偏爱 模型(Conditioned Place Preference, CPP),结合rTMS 技 术,在条件性位置偏爱行为消退后,分别对大鼠进行 为期1 天(实验一)和3 天(实验二)的高频rTMS(10Hz) 刺激,并于刺激结束后24 小时进行复吸测试,探索急 慢性rTMS 对甲基苯丙胺诱导的大鼠CPP 复吸行为的 影响。
结果
急性rTMS(刺激1 天)对大鼠CPP 的复吸行为 具有一定的抑制作用,但与伪刺激组相比没有统计学 意义(t = 1.48, p=0.431);而慢性rTMS(刺激3 天)对 大鼠CPP 的复吸行为有明显的抑制作用,与假刺激组 相比,结果具有统计学意义(t =3.33, p=0.004)。
结论
rTMS 慢性刺激能够抑制甲基苯丙胺诱导的大鼠 CPP 的复吸行为。
关键词: 甲基苯丙胺, 重复经颅磁刺激, 条件性位置 偏爱, 复吸
1. Background
Methamphetamine (METH), belongs to the amphetamine-type stimulants (ATS) and is a type of addictive neuroactive substance.[1-2] Such psychoactive substance is addictive and have severe neurotoxicity,[3-4] bringing serious effects and economic burdens to the country, society, families and individuals, which also presents a new challenge to the medical community. Therefore, finding effective treatment is of utmost importance.
Repetitive transcranial magnetic stimulation (rTMS) is a neurostimulation and neuromodulation technique which forms an electric field in the brain based on the principle of electromagnetic induction. The magnetic field produced by rTMS can penetrate the skull into the cerebral cortex without attenuation and change the local electrical activity thus exerting a therapeutic effect.[5] Numerous studies have shown that rTMS can treat a variety of severe neuropsychiatric diseases, such as schizophrenia, depression, stroke, Parkinson’s disease, epilepsy, etc. The reason may be that rTMS can regulate cortical excitability, change synaptic structure and neuronal plasticity, regulate the release of neurotransmitters and promote nerve regeneration.[6-8]
Recently, some researchers have devoted themselves to study the therapeutic effects of rTMS on substance use disorder and progress has been made. Clinical studies have found that rTMS can effectively reduce the craving for heroin, nicotine, cocaine, and alcohol in individuals with addiction. For example, Shen and colleagues used high-frequency (10Hz) rTMS to stimulate the left DLPFC in heroin addicts and found that craving for heroin was decreased.[9] Pripfl and colleagues also applied 10Hz rTMS to stimulate the left DLPFC of nicotine addicts and the results show that rTMS reduced patients’ craving for nicotine.[10] Terraneo and his colleagues’ study found that rTMS (15Hz) could also alleviate the craving for cocaine in those with cocaine addiction.[11] And Mishra and colleagues found that rTMS (10 Hz) stimulating the right DLPFC reduced the craving for alcohol.[12] Meanwhile, using rTMS (20 Hz) to stimulate the frontal cortex of morphine withdrawn rats increased the release of dopamine in the nucleus accumbens.[13] However, Li and colleagues used low-frequency (1Hz) rTMS to stimulate the DLPFC of methamphetamine addicts and found that low-frequency stimulation increased craving for methamphetamine.[14] These studies suggest that high-frequency but not low-frequency rTMS may have a potential therapeutic effect on drug addiction.
Currently, there are few studies focusing on the effects of rTMS for methamphetamine addiction. Whether rTMS could be used to treat methamphetamine addiction remains unknown. Therefore, in this study, METH-induced rats conditioned place preference(CPP) model and rTMS technology were used to explore the effects of high-frequency rTMS on methamphetamine addiction, providing preliminary data for the clinical application of rTMS in the treatment of methamphetamine addiction in the future.
2. Materials and methods
2.1 Animals
Male Sprague Dawley rats weighing 250(100) g were used in this study. The total number was 70, of which 17 were used to establish METH-induced CPP reinstatement model (Con: n=6; METH 1: n=6; METH 2: n=5) 27 were used for Experiment 1 (SAL: n=7; METH + sham: n=10; METH + rTMS: n=10), and 26 were used for experiment 2 (SAL: n=7; METH + sham: n=9; METH + rTMS: n=10). They were provided by the Laboratory Animal Center, Chinese Academy of Sciences (Shanghai, China). All rats were housed in a homothermic environment (23 ± 2 ºC) with 50% air humidity, 12-hour light/dark cycle and free access to food and water. The experiments were in strict accordance with the National Institutes of Health, Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of the Chinese Academy of Sciences (Shanghai, China).
2.2 Drug reagents
Methamphetamine was provided by the China Academy of Military Medical Science.
2.3 Conditioned Place Preference reinstatement model
CPP laboratory equipment was purchased from the Shanghai Ji Liang Software and Instruments Co. (Shanghai, China). The CPP box consists of two striped compartments of the same size, 40cm(length)*40cm(width)*60cm(height), with a movable board (40cm × 60 cm) in the middle, allowing the rats free access to the two compartments. These two striped compartments are visible: one is composed of horizontal and black stripes, while the other one is vertical and black and white stripes.
The whole CPP experiment can be divided into three major stages: development, extinction and reinstatement.
We first established a conditioned place preference experiment in rats (development). During this the experiment was mainly divided into four phases: Habituation, Preconditioning Test, Conditioning and Post-conditioning/Post-training Test. During the habituation period, the middle board was closed, and the rats were adapted to the environment of the two compartments with 30 min for each, and the adaptive interval was 6 h. In the preconditioned test period, the middle board was open, and the rats were allowed to explore the whole box freely for 15 min, and the time spent in each compartment was recorded. The conditioning period lasted four days. The rats were divided into a saline group (Con) and CPP group (METH 1 and METH 2). According to the time recorded in the pre-conditioning period, the two compartments were marked as the preferred side and the non-preferred side for each rat of which the rat spent more time in was preferred one and the other was non-preferred. And the non-preferred side was the “drug-paired compartment”. In the CPP group, rats were intraperitoneally injected with saline (1 mg/kg) in the morning. Immediately after the injection, the rats were placed in the preferred side for 45min with the middle board closed. 6h later, they were injected intraperitoneally with METH (1 mg/kg) and immediately placed in the non-preferred compartment for 45 minutes. This was done for 4 consecutive days, therefore METH and saline injections were all performed 4 times totally. The saline group was injected with saline only. The last was the post-conditioning period. The procedure was the same as the preconditioning period in which the rats were allowed to move freely between the two compartments for 15 minutes, and the time spent in each compartment was recorded. CPP score represented the time spent in the non-preferred side during the testing period minus that during the preconditioning period.
After the successful establishment of the CPP model, rats were subjected to the extinction stage. The extinction method was similar to the conditioning period. Rats originally injected with METH were changed to saline injection (1 mg/kg) and placed in the non-preferred compartment for extinction training. After four consecutive days of training, an extinction test was performed.
Finally, after the extinction of rat preference behavior, a reinstatement test was performed. Rats were injected intraperitoneally with 0.5 mg/ml METH before the test in the CPP group.
Studies have shown that 1mg/kg of METH could establish a stable and lasting CPP model, [15-16] and the preference behavior can be successfully induced with 0.5mg/ml methamphetamine after the extinction of CPP.[17-18]
2.4 Experimental design
In order to explore the effects of acute and chronic rTMS on the reinstatement of METH-induced CPP in rats, we designed two experiments:
Experiment 1: To explore the effect of acute high-frequency (10Hz) rTMS on the reinstatement of METH-induced CPP in rats. Rats were randomly divided into three groups: (1) SAL group: saline control group; (2) METH + sham group (sham stimulation group), after the extinction of CPP, rats were given a one-day sham stimulation treatment; (3) METH + rTMS group (real stimulation group), the rats were given a one-day real stimulation treatment after the extinction of CPP. 24 hours after the stimulation, the reinstatement test was performed.
Experiment 2: To explore the effect of chronic high-frequency (10 Hz) rTMS on the reinstatement of CPP in rats. Rats were also randomly divided into three groups: SAL group, METH + sham group, and METH + rTMS group. After the extinction of CPP behavior, the METH + sham group was subjected to sham stimulation treatment for 3 days, while the METH + rTMS group was subjected to a period of 3 days of real stimulation. 24 hours after the stimulation, a reinstatement test was performed.
The stimulation parameters and methods are shown in the next section. The conditions of the two experiments are strictly identical, and the number, weight, saline control group, experimental procedure and environment of the experimental rats are all strictly consistent. That is to say, except the rTMS treatment days, the rest of the two experiments are performed under the same conditions.
2.5 Repetitive transcranial magnetic stimulus (rTMS) treatment
RTMS apparatus was purchased from Wuhan YIRD Medical Equipment Co., LTD (China). The inner diameter of the stimulate coil was 2.5 cm and the outer diameter was 5 cm. In the experiment, both acute and chronic rTMS stimulation(10Hz) were performed twice daily, with 500 stimuli comprised of 20 trains of stimuli and 25 pulses per train with a 15-second train-train interval each time in the morning and afternoon, and the intermediate interval was 6 hours. The total stimuli were 1000 per day. The stimulation intensity was 30% of the maximum output power, which was equal to 100% RMT. In the real stimulation group, the center of the coil was placed against the skull of the rats during stimulation; while the coil of the sham stimulation group was placed 8 cm above the head of the rats to ensure that the rats could feel the noise of the coil but would not receive the magnetic stimuli. During the stimulation period, rats were restrained by hand to limit their movement. In addition, in order to eliminate the stress caused by the stimulation noise and fixation method, we performed rTMS for 12 min each day before the experiment, and it lasted for 1 week to make the rats adapt to the rTMS procedure. During the experiment, neither real stimulation nor sham stimulation caused epilepsy or other obvious behavioral abnormalities in rats.
2.6 Data analysis
Statistical analysis was carried out using prism 6.0 software. Data were analyzed with two-way ANOVA followed by Bonfserroni post hoc test. Differences of p<0.05 were considered statistically significant. The results are presented as mean(SEM).
3. Results
3.1 0.5mg/ml methamphetamine can successfully induce the reinstatement of CPP in rats
The experimental protocol is shown in Table 1. Rats were randomly divided into 3 groups: Con (n=6), METH 1 (n=6) and METH 2 (n=5). And the treatment details of each group are shown in Fig. 2A.
Table 1.
The experimental protocol of rat conditioned place preference reinstatement model
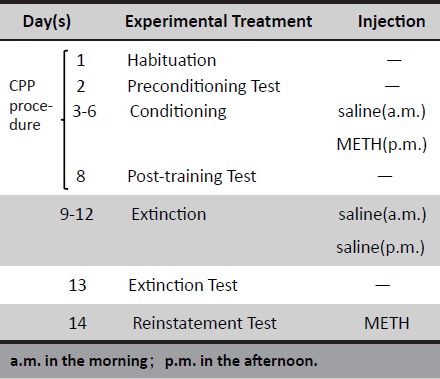 |
Figure 2.
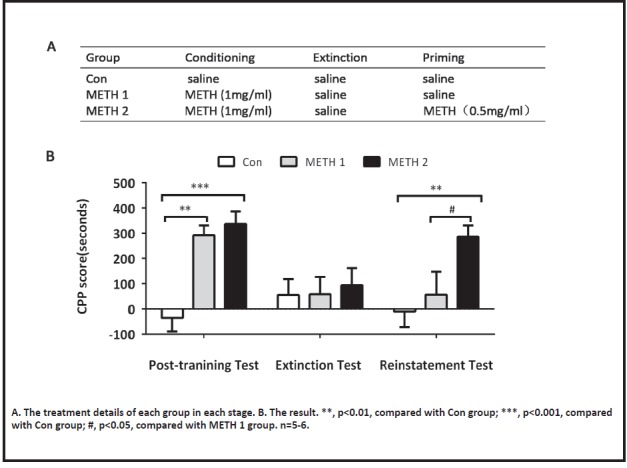
METH (0.5mg/ml) successfully induced the reinstatement behavior of CPP in rats
The CPP scores and statistical results of each group are shown in Table 2a and 2b. Our results showed that METH with a dose of 1 mg/ml could successfully establish a CPP model in rats. After the extinction of CPP, intraperitoneal injection of 0.5 mg/ml METH successfully induced the reinstatement of CPP (Fig. 2B), METH 2 vs. Con, t (42) = 3.31, p = 0.006; while intraperitoneal injection with saline could not induce the reinstatement of CPP, METH 1 vs. Con, t (42) = 0.79, p> 0.999.
Table 2a.
CPP reinstatement model, the CPP scores of each group between three tests
| Tests | Con (n=6) |
METH 1 (n=6) |
METH 2 (n=5) |
groups | times | groups*times | |||
|---|---|---|---|---|---|---|---|---|---|
| F(2,42) | p | F(2,42) | p | F(4,42) | p | ||||
| Post-training Test | -36.28 (52.73) |
292.9 (38.23) |
336.6 (49.95) |
10.55 | <0.001* | 3.28 | 0.047* | 3.16 | 0.023* |
| Extinction Test | 55.78 (62.50) |
58.67 (67.87) |
93.98 (67.81) |
||||||
| Reinstatement Test | -10.04 (62.09) |
57.47 (90.38) |
287.3 (43.54) |
||||||
*p<0.05 statistical significance; two-way ANOVA.
Table 2b.
CPP reinstatement model, the CPP scores of each group in each test
| Tests | METH 1 vs. Con | METH 2 vs. Con | METH 2 vs. METH 1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| t | df | p | t | df | p | t | df | p | |
| Post-training Test | 3.85 | 42 | 0.001* | 4.15 | 42 | <0.001* | 0.49 | 42 | >0.999 |
| Extinction Test | 0.03 | 42 | > 0.999 | 0.43 | 42 | > 0.999 | 0.39 | 42 | > 0.999 |
| Reinstatement Test | 0.79 | 42 | > 0.999 | 3.31 | 42 | 0.006* | 2.56 | 42 | 0.043* |
*p<0.05 statistical significance; Bonferroni post hoc test.
3.2 Acute rTMS treatment could not inhibit the reinstatement of CPP in rats
Table 3a and 3b are the CPP scores and statistical results of each group. The results showed that one-day rTMS treatment may inhibit the reinstatement behavior of addicted rats to a certain extent, but the difference was not statistically significant (Fig. 3C), METH + rTMS vs. METH +sham, t (72) = 1.48, p=0.431.
Table 3a.
Experiment 1, the CPP scores of each group between three tests
| Tests | SAL (n=7) |
METH+ sham (n=10) |
METH +rTMS (n=10) |
groups | Times | groups*times | |||
|---|---|---|---|---|---|---|---|---|---|
| F(2,72) | p | F(2,72) | p | F(4,72) | p | ||||
| Post-training Test(s) | -14.40 (61.24) |
229.1 (21.72) |
227.6 (18.47) |
18.45 | <0.001* | 7.40 | 0.001* | 4.51 | 0.003* |
| Extinction Test(s) | 15.12 (48.89) |
25.76 (34.90) |
24.44 (33.86) |
||||||
| Reinstatement Test(s) | -94.59 (75.82) |
240.2 (35.94) |
160.9 (38.35) |
||||||
*p<0.05 statistical significance; two-way ANOVA.
Table 3b.
Experiment 1, the CPP scores of each group in each test
| Tests | METH+sham vs. SAL | METH+rTMS vs. SAL | METH+rTMS vs. METH+sham | ||||||
|---|---|---|---|---|---|---|---|---|---|
| t | df | p | t | df | p | t | df | p | |
| Post-training Test | 4.12 | 72 | <0.001* | 4.10 | 72 | <0.001* | 0.03 | 72 | >0.999 |
| Extinction Test | 0.18 | 72 | > 0.999 | 0.16 | 72 | > 0.999 | 0.02 | 72 | > 0.999 |
| Reinstatement Test | 5.67 | 72 | < 0.001* | 4.33 | 72 | <0.001* | 1.48 | 72 | 0.43 |
*p<0.05 statistical significance; Bonferroni post hoc test.
Figure 3.
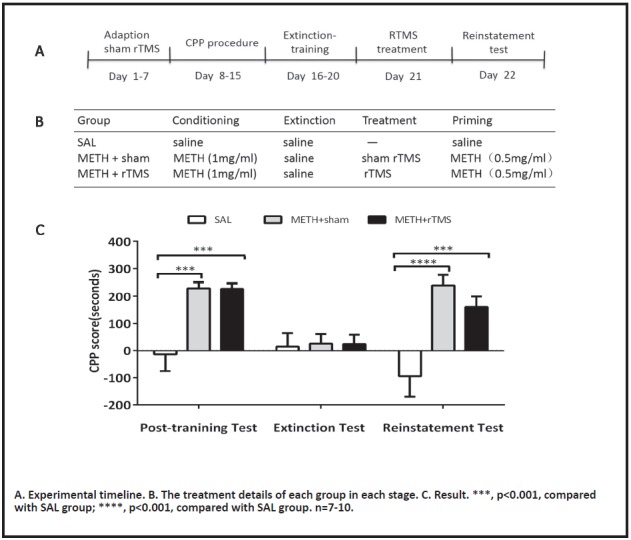
The effect of acute rTMS treatment on the reinstatement of METH-induced CPP
The results suggested that acute rTMS may have a certain inhibitory effect on the relapse behavior of addicted rats, but whether it is effective requires further exploration.
3.3 Chronic rTMS treatment significantly inhibited the reinstatement of METH-induced CPP in rats
The CPP scores and statistical results of each group are shown in Table 4a and 4b. The results showed that continuous administration of rTMS for 3 days after the extinction of CPP could inhibit the reinstatement behavior of CPP in rats (Fig. 4C): METH + rTMS vs. SAL, t (69) = 0.63, p > 0.999; METH + rTMS vs. METH + sham, t (69) =3.33, p=0.004.
Table 4a.
Experiment 2, the CPP scores of each group between three tests
| Tests | SAL (n=7) |
METH+ sham (n=9) |
METH +rTMS (n=10) |
groups | Times | groups*times | |||
|---|---|---|---|---|---|---|---|---|---|
| F(2,69) | p | F(2,69) | p | F(4,69) | p | ||||
| Post-training Test(s) | -25.34 (39.18) |
256.3 (15.46) |
223.0 (10.51) |
13.57 | <0.001* | 4.67 | 0.013* | 3.26 | 0.017* |
| Extinction Test(s) | 8.607 (54.87) |
68.66 (39.26) |
51.88 (30.19) |
||||||
| Reinstatement Test(s) | 6.489 (63.89) |
238.9 (51.09) |
45.88 (60.55) |
||||||
*p<0.05 statistical significance; two-way ANOVA.
Table 4b.
Experiment 2, the CPP scores of each group in each test
| Tests | METH + sham vs. SAL | METH + rTMS vs. SAL | METH + rTMS vs. METH + sham | ||||||
|---|---|---|---|---|---|---|---|---|---|
| t | df | p | t | df | p | t | df | p | |
| Post-training Test | 4.43 | 69 | <0.001* | 3.99 | 69 | <0.001* | 0.57 | 69 | >0.999 |
| Extinction Test | 0.94 | 69 | > 0.999 | 0.70 | 69 | > 0.999 | 0.29 | 69 | > 0.999 |
| Reinstatement Test | 3.65 | 69 | 0.002* | 0.63 | 69 | >0.999 | 3.33 | 69 | 0.004* |
*p<0.05 statistical significance. Bonferroni post hoc test
Figure 4.
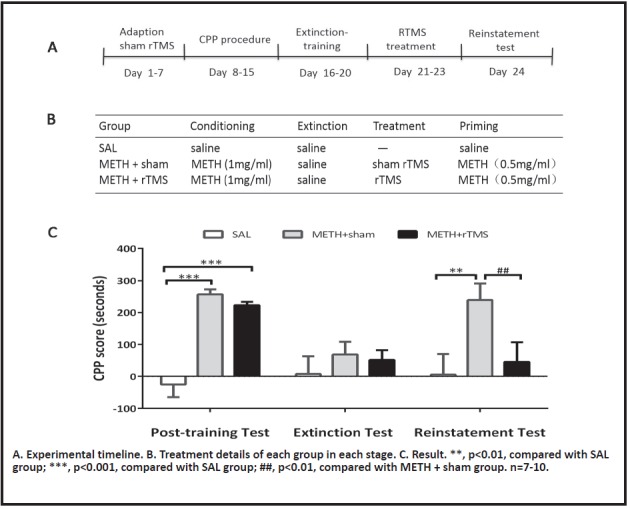
The effect of chronic rTMS treatment on the reinstatement of METH-induced CPP
The results indicated that chronic rTMS could inhibit the relapse behavior of methamphetamine-addicted rats.
4. Discussion
4.1 Main findings
Drug addiction is considered a pathological process of learning and memory currently. Addictive substances can alter the structure and function of the brain, making addicts sensitive to drug-related cues thus producing craving and inducing relapse behavior during the withdrawal period.[19]
Conditioned place preference experiment is a classical animal model to evaluate the mental dependence on drugs. It is essentially a learning and memory process based on Pavlovian classical conditioning.[20] The addiction model can imitate addiction in humans and includes three major stages: development, extinction and reinstatement. Addiction memory based on Pavlovian classical conditioning is quite valid, but the extinction memory is fragile, so relapse behavior could be easily induced once the animal is exposed to drug or drug-related cues even after the extinction of CPP,[21] as shown in Figure 2.
Numerous studies have shown that rTMS regulated synaptic plasticity, including long-time potentiation (LTP) and long-term depression (LTD). To the best of our knowledge, such effect of rTMS may be closely related to the stimulation frequency and stimulation intensity, as high-frequency rTMS induced LTP, while low-frequency induced LTD.[6,22] In this study, rats were subjected to acute or chronic high-frequency rTMS treatment after the extinction of CPP. As a result, it was found that chronic high-frequency rTMS inhibited the reinstatement behavior of addicted rats. The result may be due to the LTP effect induced by high-frequency stimulation which consolidated the extinction memory of rats and thus prevented relapse. Although the acute high-frequency rTMS failed to inhibit the relapse behavior of rats, there was a trend of inhibition effect compared with the sham stimulation group. In combination with Experiment 1 and Experiment 2, it was demonstrated that the inhibition effect of rTMS required sufficient magnetic stimuli.
4.2 Limitations
Firstly, our study only performed one reinstatement test. If possible, the test could be performed more times to examined the persistence of the rTMS effect. Secondly, the effect of acute and chronic rTMS on relapse behavior could have been tested in one experiment while it was divided into two experiments in our study due to limitations of the experimental condition and the fact that the number of rats used in one experiment was limited. Finally, the rTMS parameters in this study were determined by a number of previous studies. At present, the study only explored the effect of high-frequency stimulation on the reinstatement behavior of CPP, but the effects of other stimulation protocols, for example, low-frequency stimulation, need to be further explored.
4.3 Implications
This study explored the effects of acute and chronic rTMS on the reinstatement of methamphetamine-induced conditioned place preference and found that rTMS inhibited the relapse behavior of addiction, providing a preliminary theoretical foundation and basis for the clinical application of rTMS to treat drug addiction. Future studies can combine clinical studies to explore the effects and mechanisms of different rTMS patterns on drug addiction.
Figure 1.
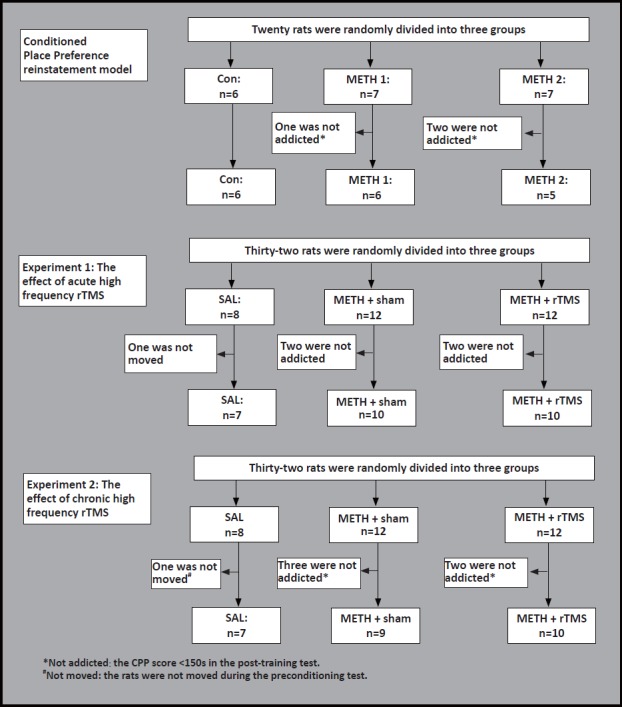
Flowchart of the study
Biography

Xueqing Wu, graduated from Nanchang University in 2015. Afterwards, she studied as a graduate student at the affiliated Mental Health Center of Shanghai Jiao Tong University School of Medicine until 2018. She is interested in the effects and mechanisms of treatment of rTMS for substance use disorder.
Footnotes
Funding statement
National Key R&D Program of China, PI, (2017YFC1310400), National Natural Science Foundation of China(U1502228), National Natural Science Foundation of China (81771436), Shanghai Municipal Health and Family Planning Commission (2014ZYJB0002), Program of Shanghai Academic Research Leader (17XD1403300), Shanghai Key Laboratory of Psychotic Disorders (13DZ2260500), key subject of Shanghai Municipal Health and Family Planning Commission(Psychiatry)(2017ZZ02021)
Conflict of interest statement
The authors declare they have no conflicts of interest.
Ethical approval
This study was approved by the Institutional Animal Care and Use Committee of the Chinese Academy of Sciences (Shanghai, China). The experiments were in strict accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals.
Authors’ contributions
Xueqing Wu designed and carried out the experiments and drafted the manuscript.
Yunyue Ju and Dongliang Jiao were involved in the experiment design and data analysis.
Min Zhao was responsible for the experiment design and manuscript revision.
All authors contributed to and have approved of the final manuscript.
References
- 1.Courtney KE, Ray LA. Methamphetamine: an update on epidemiology, pharmacology, clinical phenomenology, and treatment literature. Drug Alcohol Depend. 2014; 143: 11-21 10.1016/j.drugalcdep.2014.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glasner-Edwards S, Mooney LJ. Methamphetamine psychosis: epidemiology and management. CNS Drugs. 2014; 28(12): 1115-1126. 10.1007/s40263-014-0209-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.London ED, Kohno M, Morales AM, Ballard ME. Chronic methamphetamine abuse and corticostriatal deficits revealed by neuroimaging. Brain Res. 2015; 1628(Pt A): 174-185. 10.1016/j.brainres.2014.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halpin LE, Collins SA, Yamamoto BK. Neurotoxicity of methamphetamine and 3,4-methylenedioxymethamphetamine. Life Sci. 2014; 97(1): 37-44. 10.1016/j.lfs.2013.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety of TMSCG. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009; 120(12): 2008-2039. 10.1016/j.clinph.2009.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Houdayer E, Degardin A, Cassim F, Bocquillon P, Derambure P, Devanne H. The effects of low- and high-frequency repetitive TMS on the input/output properties of the human corticospinal pathway. Exp Brain Res. 2008; 187(2): 207-217. 10.1007/s00221-008-1294-z [DOI] [PubMed] [Google Scholar]
- 7.Gao F, Wang S, Guo Y, Wang J, Lou M, Wu J, et al. Protective effects of repetitive transcranial magnetic stimulation in a rat model of transient cerebral ischaemia: a microPET study. Eur J Nucl Med Mol Imaging. 2010; 37(5): 954-961. 10.1007/s00259-009-1342-3 [DOI] [PubMed] [Google Scholar]
- 8.Chervyakov AV, Chernyavsky AY, Sinitsyn DO, Piradov MA. Possible Mechanisms Underlying the Therapeutic Effects of Transcranial Magnetic Stimulation. Front Hum Neurosci. 2015; 9: 303 10.3389/fnhum.2015.00303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen Y, Cao X, Tan T, Shan C, Wang Y, Pan J, et al. 10-Hz Repetitive Transcranial Magnetic Stimulation of the Left Dorsolateral Prefrontal Cortex Reduces Heroin Cue Craving in Long-Term Addicts. Biol Psychiatry. 2016; 80(3): e13-e14. 10.1016/j.biopsych.2016.02.006 [DOI] [PubMed] [Google Scholar]
- 10.Pripfl J, Tomova L, Riecansky I, Lamm C. Transcranial magnetic stimulation of the left dorsolateral prefrontal cortex decreases cue-induced nicotine craving and EEG delta power. Brain Stimul. 2014; 7(2): 226-233. 10.1016/j.brs.2013.11.003 [DOI] [PubMed] [Google Scholar]
- 11.Terraneo A, Leggio L, Saladini M, Ermani M, Bonci A, Gallimberti L. Transcranial magnetic stimulation of dorsolateral prefrontal cortex reduces cocaine use: A pilot study. Eur Neuropsychopharmacol. 2016; 26(1): 37-44. 10.1016/j.euroneuro.2015.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mishra BR, Praharaj SK, Katshu MZ, Sarkar S, Nizamie SH. Comparison of anticraving efficacy of right and left repetitive transcranial magnetic stimulation in alcohol dependence: a randomized double-blind study. J Neuropsychiatry Clin Neurosci. 2015; 27(1): e54-e59. 10.1176/appi.neuropsych.13010013 [DOI] [PubMed] [Google Scholar]
- 13.Erhardt A, Sillaber I, Welt T, Muller MB, Singewald N, Keck ME. Repetitive transcranial magnetic stimulation increases the release of dopamine in the nucleus accumbens shell of morphine-sensitized rats during abstinence. Neuropsychopharmacology. 2004; 29(11): 2074-2080. 10.1038/sj.npp.1300493 [DOI] [PubMed] [Google Scholar]
- 14.Li X, Malcolm RJ, Huebner K, Hanlon CA, Taylor JJ, Brady KT, et al. Low frequency repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex transiently increases cue-induced craving for methamphetamine: a preliminary study. Drug Alcohol Depend. 2013; 133(2): 641-646. doi: 10.1016/j.drugalcdep.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrold AA, Shen F, Graham MP, Harper LK, Specio SE, Tedford CE, et al. Mirtazapine treatment after conditioning with methamphetamine alters subsequent expression of place preference. Drug Alcohol Depend. 2009; 99(1-3): 231-239. 10.1016/j.drugalcdep.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Jiao DL, Liu Y, Long JD, Du J, Ju YY, Zan GY, et al. Involvement of dorsal striatal alpha1-containing GABAA receptors in methamphetamine-associated rewarding memories. Neuroscience. 2016; 320: 230-238. 10.1016/j.neuroscience.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 17.Arezoomandan R, Moradi M, Attarzadeh-Yazdi G, Tomaz C, Haghparast A. Administration of activated glial condition medium in the nucleus accumbens extended extinction and intensified reinstatement of methamphetamine-induced conditioned place preference. Brain Res Bull. 2016; 125: 106-116 10.1016/j.brainresbull.2016.06.011 [DOI] [PubMed] [Google Scholar]
- 18.Berry JN, Neugebauer NM, Bardo MT. Reinstatement of methamphetamine conditioned place preference in nicotine-sensitized rats. Behav Brain Res. 2012; 235(2): 158-165. 10.1016/j.bbr.2012.07.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003; 168(1-2): 3-20. 10.1007/s00213-002-1224-x [DOI] [PubMed] [Google Scholar]
- 20.Huston JP, Silva MA, Topic B, Muller CP. What’s conditioned in conditioned place preference. Trends Pharmacol Sci. 2013; 34(3): 162-166. 10.1016/j.tips.2013.01.004 [DOI] [PubMed] [Google Scholar]
- 21.Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychol Bull. 1993; 114(1): 80-99 [DOI] [PubMed] [Google Scholar]
- 22.Hoogendam JM, Ramakers GM, Di Lazzaro V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul. 2010; 3(2): 95-118. 10.1016/j.brs.2009.10.005 [DOI] [PubMed] [Google Scholar]


