Abstract
Spontaneous internal carotid artery dissection occurs in patients of all ages, rarely presenting with hypoglossal nerve palsy. The characteristic imaging findings of internal carotid artery dissection and tongue denervation are reviewed in four patients. Recognition of internal carotid artery dissection is critical for appropriate treatment and to minimise the risk of thromboembolic–ischaemic complications. Radiologists must be aware of the radiological appearance of hypoglossal nerve palsy and maintain a high index of suspicion for internal carotid artery dissection when this finding is present.
Keywords: Internal carotid artery dissection, hypoglossal nerve palsy, vascular imaging, MRI, CT
Introduction
Internal carotid artery dissection (ICAD) is an important cause of ischaemic stroke among young and middle-aged patients, accounting for up to 25% of all cases in patients under the age of 45 years.1,2 Most cases are spontaneous or are related to trauma, both minor (e.g. sneezing, coughing, neck manipulation) and major (e.g. motor vehicle accidents). Additional aetiologies include, but are not limited to, arterial hypertension, fibromuscular dysplasia, autosomal dominant polycystic kidney disease, osteogenesis imperfecta, internal carotid artery (ICA) redundancy, oestrogen–progesterone therapy, infectious diseases and connective tissue disorders.3,4 In most cases, the extracranial ICA is affected, but rarely the intracranial ICA may be involved.4
ICAD often presents with headache, ipsilateral facial pain, neck pain, ipsilateral oculosympathetic palsy (Horner’s syndrome) and/or cerebral ischaemic symptoms (from transient ischaemic attack and amaurosis fugax to dense hemiplegia). Less common manifestations of ICAD include bruits, dysgeusia and cranial nerve palsies.2,4–6 One large series of 190 patients demonstrated that cranial nerve palsy was present in 12% of patients with extracranial ICAD.7 Lower cranial nerve palsies (IX to XII) were found in 5% of patients, with the hypoglossal nerve (CNXII) invariably involved.7,8 The hypoglossal nerve was the only cranial nerve involved in only three patients in this cohort (1.5%). Symptoms of CNXII palsy include dysarthria and impaired tongue movement and swallowing. While hypoglossal nerve palsy is a rare complication of ICAD, it may be the only presenting manifestation of this diagnosis.
The imaging appearance of CNXII denervation is characteristic. In the acute and early subacute setting, the involved hemi-tongue may be oedematous and swollen with geographical T1 hypointensity and T2 hyperintensity on magnetic resonance imaging (MRI), and there is often enhancement. In late subacute and chronic tongue denervation, the base of the tongue protrudes into the oropharyngeal lumen, potentially mimicking a mass. In the chronic phase, volume loss and fatty infiltration of the involved hemitongue are distinctive. Although skull base lesions are the most common cause of hypoglossal nerve palsy, vascular causes should be considered, especially in younger and middle-aged patients in whom mass lesions are less common.
Here we review the imaging findings in four cases of ICAD presenting with CNXII palsy. Although hypoglossal nerve palsy is rare in this setting, it may be the only presenting manifestation of ICAD. ICAD is not uncommon and characteristic non-invasive imaging findings usually make the diagnosis straightforward when it is clinically suspected. As such, radiologists must be aware of the radiological findings of CNXII palsy, and have a high index of suspicion for ICAD in this setting so that prompt diagnosis is made and appropriate therapy is initiated.
Cases
Four patients with ICAD were reviewed (Table 1). Three patients had spontaneous dissection, while one had a traumatic ICAD secondary to a bicycle accident. All patients presented with similar complaints related to their tongue, including difficulty and/or inability to move the tongue, tongue numbness, tongue swelling, difficulty chewing, inability to make a food bolus, difficulty swallowing liquids and solids and difficulty speaking. Additional complaints not related to the tongue included transient unilateral neck pain, headache and blurred vision. On physical examination, similar findings related to the tongue were noted in all patients, including unilateral tongue atrophy, tongue deviation to one side and unilateral tongue weakness and/or paralysis.
Table 1.
Patient demographics.
| Patient | Age | Sex | Side of ICAD | Aetiology |
|---|---|---|---|---|
| 1 | 52 | F | R | Spontaneous |
| 2 | 46 | M | L | Spontaneous |
| 3 | 38 | M | L | Spontaneous |
| 4 | 47 | M | L | Trauma (bicycle accident) |
ICAD: internal carotid artery dissection.
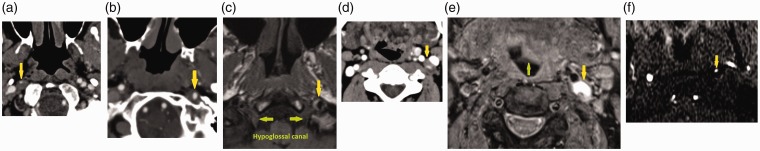
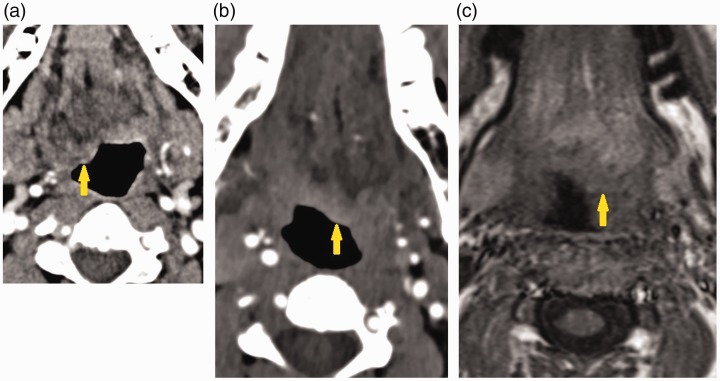
Imaging findings of ICAD were similar across all patients. Computed tomography angiogram (CTA) of the neck in patient one obtained 14 days after the onset of symptoms showed a long segmental area of prominent thickening of the right ICA wall and greater than 80% luminal narrowing consistent with a ICAD (Figure 1(a)). Magnetic resonance angiography (MRA) of the neck showed focal irregularity and significant narrowing of the distal cervical right ICA due to peripheral T1 hyperintensity around the ICA on fat suppressed images typical of mural thrombus. In patient two, CTA of the neck showed mild irregularity and focal narrowing of the distal cervical left ICA at the skull base compatible with a focal dissection with mural haematoma (Figure 1(b)). MRI of the brain demonstrated a short segmental distal left cervical ICAD at the skull base adjacent to the hypoglossal canal (Figure 1(c)). In patient three, CTA of the neck was ordered and showed occlusive dissection of the left ICA from the level of the carotid bulb to the petrous carotid artery (Figure 1(d)). There was irregular narrowing of the right ICA lumen just distal to the carotid bulb consistent with known right ICAD. On MRA of the head and neck, there was abrupt tapering and occlusion of the left ICA approximately 2 cm distal to its origin, with reconstitution of the distal supraclinoid ICA segment. There was associated crescentic T1 and T2 hyperintensity in the left cervical ICA extending from 2 cm above its origin to the skull base (Figure 1(e)). Intracranially, there was absence of flow-related enhancement on time-of-flight MRA in the petrous, cavernous and proximal supraclinoid portions of the left ICA; however, these segments showed contrast enhancement on a delayed phase of dynamic contrast MRA. In patient four, three-dimensional time-of-flight neck MRA demonstrated lack of the normal flow in the left ICA at the C1–C2 level (Figure 1(f)) and T1-weighted MRI demonstrated focal abnormal signal within the left ICA at this level. After contrast, the dissection was seen as a linear intraluminal defect.
Figure 1.
(a) Neck computed tomography angiogram (CTA) in patient one shows dissection of the right internal carotid artery (ICA) with wall non-enhancing intramural haematoma and 80% luminal narrowing (yellow arrow). (b) Neck CTA in patient two shows dissection of the left ICA with wall non-enhancing intramural haematoma and 50% luminal narrowing (yellow arrow). (c) T1-weighted fat-suppressed magnetic resonance imaging (MRI) in patient two shows crescentic hyperintense mural haematoma surrounding the left ICA with mild luminal narrowing (yellow arrow). This is in close proximity to the hypoglossal canal and exiting hypoglossal nerve (green arrows). (d) Neck CTA in patient three shows dissection of the left ICA with wall non-enhancing intramural haematoma and 75% luminal narrowing (yellow arrow). (e) T1-weighted fat-suppressed MRI in patient three shows crescentic intramural haematoma surrounding the left ICA (yellow arrow). There is also left tongue protrusion into the oral cavity (green arrow). (f) Patient four neck magnetic resonance angiography showing circumferential narrowing of the left ICA (yellow arrow) in the region of the left hypoglossal canal, in keeping with left ICA dissection.
Imaging findings of hypoglossal nerve palsy were also similar across all patients. All four patients demonstrated asymmetry of the tongue base, with bulbous protrusion of the tongue base into the oropharynx, with fatty replacement suggestive of denervation of the hemitongue, on the right in patient one (computed tomography (CT) scan: Figure 2(a)), on the left in patient two (CT scan: Figure 2(b); MRI: Figure 2(c)), on the left in patient three (MRI; Figure 1(e)) and on the left in patient four.
Figure 2.
(a) Neck computed tomography angiogram (CTA) in patient one shows bulbous protrusion of the right hemitongue into the oropharynx (arrow). (b) Neck CTA in patient two neck protrusion of the left hemitongue into the oropharynx consistent with tongue paralysis (arrow). (c) T1-weighted fat-suppressed magnetic resonance imaging in patient two shows protrusion of the left hemitongue into the oropharynx (arrow).
Once the diagnosis of ICAD was made, all patients were started on aspirin, with two also put on clopidogrel and one on enoxaparin. For patient one, 2 weeks following the initial diagnosis she had increased tongue function, including the ability to place the tongue against her hard palate and great improvement with swallowing. For patient two, clinical examination 3 years following diagnosis revealed no persisting CNXII deficit – a full recovery. For patient three, clinical examination 2 months after diagnosis was significant for mild left tongue atrophy and leftward tongue deviation, but on subsequent follow-up visits over the following 2 years, there was only mild persisting leftward tongue deviation. For patient four, clinical examination 2 months after diagnosis was significant for mild left tongue atrophy and leftward tongue deviation, which persisted on follow-up over 2 years.
Discussion
ICAD may present with a spectrum of symptoms including rare hypoglossal nerve palsy. We presented four patients with extracranial ICAD and imaging findings of CNXII palsy. Imaging findings of ICAD are characteristic, while the findings of hypoglossal nerve paresis are frequently more subtle but should direct the radiologist to closer evaluation of the ICA and skull base.
The pathophysiology of ICAD lies in a compromise of the structural integrity of the arterial wall due to a tear in the tunica intima and secondary intramural haematoma formation often leading to stenosis, occlusion and intraluminal thrombus. The dissection may separate the tunica media from the tunica adventitia resulting in aneurysmal dilatation of the affected vessel.2 Most ICAD occurs spontaneously and may be due to minor subclinical trauma (e.g. sneezing, coughing, vomiting, neck manipulation). Additional aetiologies include, but are not limited to, arterial hypertension, fibromuscular dysplasia, autosomal dominant polycystic kidney disease, osteogenesis imperfecta, ICA redundancy, oestrogen–progesterone therapy, infectious diseases, or connective tissue disorders.3,4 In most cases, the extracranial ICA is affected, but rarely the intracranial ICA is involved.4
The characteristic imaging appearance of extracranial ICAD, mural haematoma with associated luminal narrowing, usually makes this diagnosis straightforward. When the tunica media and tunica adventitia become separated, a pseudoaneurysm may develop at the site of intimal tear. On MRI, the characteristic appearance is seen on fat-suppressed T1-weighted images as crescentic T1 high signal within the vessel wall (mural haematoma), with varying degrees of associated luminal narrowing.
The hypoglossal nerve is a motor nerve controlling intrinsic and extrinsic tongue muscles and infrahyoid strap muscles by the ansa cervicalis. Identifying the cause of CNXII nerve palsy requires understanding the course of the nerve and typical pathologies along its course, of which ICAD is only one. The inferolateral precentral gyrus cortical centre for tongue movement sends fibres to the contralateral medullary hypoglossal nucleus. The medullary segment of CNXII travels anterolaterally from the hypoglossal nuclei, exiting at the preolivary sulcus. This segment is most commonly affected by vascular lesions, such as aneurysms, as well as demyelinating lesions and neoplasms. The cisternal segment travels in the premedullary cistern posterolateral to the vertebral artery, making it susceptible to vascular lesions and neoplasia, as well as inflammatory pannus from rheumatoid arthritis involving the dens. CNXII then enters the hypoglossal canal of the occipital bone, which demarcates the skull base segment. Neoplasms are the main cause of hypoglossal nerve dysfunction at this level, most commonly osseous metastases, direct spread of nasopharyngeal carcinoma and benign neoplasms such as nerve sheath and glomus tumours. The extracranial course of CNXII has two named segments. The first is the carotid segment which extends from the hypoglossal canal through the carotid space at the level of the nasopharynx, passing between the internal jugular vein and the internal carotid artery. It is at this point where CNXII is vulnerable to injury from carotid artery dissection or pseudoaneurysm. Two mechanisms have been proposed to explain lower cranial nerve involvement in the setting of ICAD: (a) compression or stretching of nerves below the jugular foramen by an expanded or aneurysmal ICA and (b) impairment of the blood supply to the lower cranial nerve due to mechanical, embolic, or haemodynamic factors.7 However, adenopathy and soft tissue neoplasms are the most common causes of injury to the hypoglossal nerve in the carotid segment. At the level of the mandibular angle, CNXII curves anteroinferiorly, running along the surface of the hyoglossus muscle, defining the sublingual segment, where it continues to innervate tongue musculature. This final segment is susceptible to injury from floor of mouth neoplasms, infections and inflammation. More inferiorly, CNXII joins with the ansa cervicalis which is made up of fibres from the first three cervical nerves, which course through the carotid space with CNXII, innervating the infrahyoid strap muscles.
The imaging findings of hypoglossal nerve injury vary with the amount of time elapsed between initial nerve injury and the time of imaging. In the acute and early subacute denervation, the involved hemitongue may be swollen with oedema and enhancement. In late subacute and early chronic tongue denervation the base of tongue begins to protrude into the oropharyngeal lumen which may mimic a mass. In the chronic phase, there is characteristic fat infiltration and volume loss of the involved hemitongue. When these findings are identified, a search for the underlying cause must be initiated along the full course of CNXII.
Spontaneous ICAD occurs in patients of all ages, with or without predisposing risk factors. Characteristic non-invasive imaging findings usually make the diagnosis straightforward when the diagnosis is clinically suspected and appropriate vascular imaging is performed. While hypoglossal nerve palsy is a rare manifestation of ICAD, it may be the only clinical sign at presentation. To facilitate prompt and appropriate therapy for ICAD, radiologists must be aware of the imaging findings of hypoglossal nerve palsy, maintain a high index of suspicion for ICAD as a potential underlying aetiology, and recommend vascular imaging in such cases.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Bogousslavsky J, Pierre P. Ischemic stroke in patients under 45 years. Neurol Clin 1992; 10: 113–124. [PubMed] [Google Scholar]
- 2.Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med 2001; 344: 898–906. [DOI] [PubMed] [Google Scholar]
- 3.Campos-Herrera CR, Scaff M, Yamamoto FI, et al. Spontaneous cervical artery dissection: an update on clinical and diagnostic aspects. Arq Neuropsiquiatr 2008; 66: 922–927. [DOI] [PubMed] [Google Scholar]
- 4.Fusco MR, Harrigan MR. Cerebrovascular dissections – a review part I: spontaneous dissections. Neurosurgery 2011; 68: 242–257. [DOI] [PubMed] [Google Scholar]
- 5.Mokri B, Sundt TM, Houser OW, et al. Spontaneous dissection of the cervcal internal carotid artery. Ann Neurol 1986; 19: 126–138. [DOI] [PubMed] [Google Scholar]
- 6.Zetterling M, Carlström C, Konrad P. Internal carotid artery dissection. Acta Neurol Scand 2000; 101: 1–7. [DOI] [PubMed] [Google Scholar]
- 7.Mokri B, Silbert PL, Schievink WI, et al. Cranial nerve palsy in spontaneous dissection of the extracranial carotid artery. Neurology 1996; 46: 356–359. [DOI] [PubMed] [Google Scholar]
- 8.Olzowy B, Lorenzl S, Guerkov R. Bilateral and unilateral internal carotid artery dissection causing isolated hypoglossal nerve palsy: a case report and review of the literature. Eur Arch Otorhinolaryngol 2006; 263: 390–393. [DOI] [PubMed] [Google Scholar]