Abstract
Recessive congenital methemoglobinemia type II is a very rare autosomal recessive hematologic disorder due to NADH-cytochrome b5 reductase deficiency, usually caused by full-stop mutations or deletions. This disease classically presents with mild neonatal cyanosis, early onset severe progressive developmental delay, movement disorders, and progressive microcephaly. We report two siblings with recessive congenital methemoglobinemia type II whose evaluation revealed a novel p.Arg92Trp missense mutation of the CYB5R3 gene and a peculiar imaging finding of basal ganglia hypoplasia. Brain magnetic resonance imaging was performed at age 10 months in the older sibling and at age three months in the younger sibling. It revealed similar findings of bilateral small size of the lentiform and caudate nuclei and reduced frontotemporal brain volume. Our patient cases highlight that basal ganglia hypoplasia is an interesting clue to the very rare and frequently unsuspected diagnosis of recessive congenital methemoglobinemia type II, that may explain the associated movement disorders. The novel missense mutation is one of very few identified missense mutations known to cause severe type II recessive congenital methemoglobinemia.
Keywords: Methemoglobinemia, cyanosis, cytochrome b5 reductase, CYB5R3 gene, basal ganglia hypoplasia
Introduction
Recessive congenital methemoglobinemia (RCM) type II is a very rare autosomal recessive disorder due to NADH-cytochrome b5 reductase deficiency (NADH: Nicotinamide Adenine Dinucleotide). Cytochrome b5 reductase (previously called diaphorase) is encoded by the CYB5R3 gene (initially referred to as DIA1), located on chromosome 22. There are two forms of CYB5R3 enzymes; one that is membrane-bound, found on the endoplasmic reticulum and outer mitochondrial membranes, and a soluble form present in the cytosol of erythrocytes.1
CYB5R3 mutation can result in two clinical phenotypes. RCM type I manifests only with cyanosis and is usually caused by missense mutations that result in amino acid substitution,1,2 giving rise to enzymatically active but unstable proteins.1 Life expectancy is normal and there are no neurological symptoms.1 RCM type II is frequently caused by full stops or deletions,2 causing loss of protein expression or enzymatically inactive proteins,1 resulting in cyanosis and severe progressive neurological deterioration. Cyanosis is milder than in RCM type 1.
Methemoglobinemia should be suspected in cyanotic newborns with normal cardiac and pulmonary evaluation. A history of consanguinity and elevated methemoglobin level (above 1%) strongly suggest the diagnosis.3 The diagnosis of RCM type II is corroborated by measuring markedly reduced levels of cytochrome b5 reductase activity in erythrocytes and leukocytes,4,5 while in type I RCM the activity is reduced only in erythrocytes.5 Clinically, progressive microcephaly and early onset developmental delay at 3–4 months of age allow early differentiation of type II RCM from type I.4 The manifestations of RCM type II are quite consistent, including mild cyanosis, progressive microcephaly, severe encephalopathy, axial hypotonia, as well as dystonia with hyperkinetic movements. Growth retardation is frequent and exacerbated by feeding difficulties due to swallowing disturbances, and 88% of patients have strabismus.5
The neuroimaging findings of RCM type II have rarely been reported, are classically nonspecific, consisting of brain atrophy and delayed myelination,2–6 and of limited diagnostic contribution. We report two siblings with RCM II whose clinical presentation was quite suggestive of the diagnosis. Their evaluation revealed a de novo missense mutation of the CYB5R3 gene and a distinctive imaging finding of hypoplasia of the basal ganglia.
Case reports
Case 1
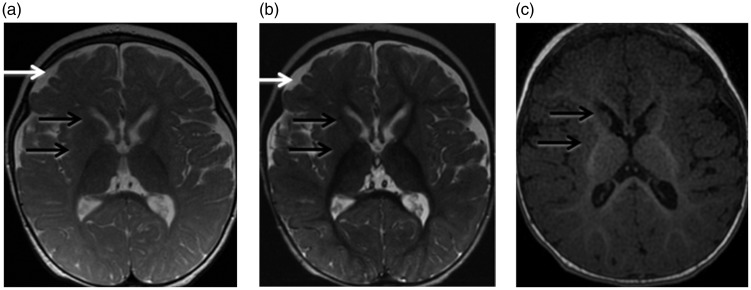
This patient was born full-term to first-degree Saudi cousins. Pregnancy and delivery were normal with an uncomplicated neonatal course. The mother noticed increased tone in the limbs, starting at age three months. The patient started having seizures at age six months, which were not controlled by multiple antiepileptic drugs. Brain magnetic resonance imaging (MRI) was performed at 10 months of age on a 1.5 Tesla machine (GE Medical Systems). The sequences obtained included sagittal and axial T1, axial volumetric T1, axial and coronal T2, axial inversion recovery and axial diffusion-weighted images. Single voxel magnetic resonance (MR) spectroscopy was obtained through the right basal ganglia, using TE (echo time) of 35 ms, 144 ms and 288 ms. The conventional MRI images and spectroscopy were interpreted by a neuroradiologist with nine years experience in pediatric and general neuroradiology (MN-J). The conventional MRI images (Figure 1) showed mild enlargement of the bilateral frontotemporal subarachnoid spaces compatible with mildly reduced frontotemporal brain volume. The bilateral caudate and lentiform nuclei were small in size. Myelination was age-appropriate when compared to age-matched controls.7 MR spectroscopy was also normal for age.8 Physical examination at age 22 months revealed a microcephalic patient with weight at 9.5 kg, below the third percentile, height at 79 cm at the third percentile and head circumference of 45 cm, below the third percentile (normative growth charts available at the Saudi Ministry of Health website).15 He had axial hypotonia with severe head lag. He could only produce cooing sounds. He had mildly increased appendicular tone with exaggerated deep tendon reflexes. An extensive metabolic and genetic workup revealed elevated venous methemoglobin level at 16.6%. Genetic analysis of whole blood cells revealed a homozygous p.Arg92Trp substitution of the CYB5R3 protein. A history of mild cyanosis during infancy was retrospectively elicited from the mother. The patient was started on coenzyme Q10 and ascorbic acid. He is currently three years old with treatment-resistant seizures and severe global developmental delay, spastic quadriplegia, axial hypotonia, hyperreflexia, and esotropia. He has difficulty swallowing due to severe oropharyngeal dysphagia with recurrent respiratory infections. He has occasional dystonic posturing, particularly of the upper extremities. No follow-up brain MRI imaging is available.
Figure 1.
Brain magnetic resonance imaging (MRI) in patient 1 at age 10 months. (a) Axial T2 (TR/TE 4733 ms/102 ms), (b) axial inversion recovery (TR/TE 6250 ms/45.7 ms), and (c) axial volumetric T1 (TR/TE 8.25 ms/3.11 ms) images. There is mild prominence of the bilateral frontal and temporal subarachnoid spaces indicating mild frontotemporal brain volume loss (white arrows). The basal ganglia are small in size (black arrows). Myelination is age adequate. TR: repetition time; TE: echo time.
Case 2
This patient is the younger sibling of patient 1, born full-term. Pregnancy and delivery were uncomplicated. At birth, he was noted to have mild cyanosis of the lips and tongue, with normal cardiac and respiratory evaluations. Blood tests in a local hospital showed elevated methemoglobin level at 18%. The patient was treated with weekly injections of intravenous methylene blue and daily ascorbic acid which improved his cyanosis and the methemoglobin level came down to 6.8%.
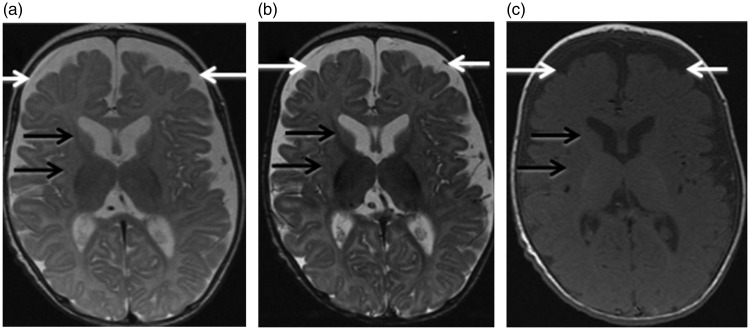
At eight weeks of age, the patient was referred to our institution. He had occasional fisting of the hands, posturing with flexion of the arms and increased tone with brisk reflexes. Head control was good. There were no clinical seizures. The patient was started on ascorbic acid and coenzyme Q-10. Brain MRI was performed at three months of age on a 1.5 Tesla machine (GE Medical Systems). The sequences obtained included sagittal and axial T1, axial and coronal T2, axial and coronal inversion recovery, axial fluid attenuated inversion recovery (FLAIR), axial T2 gradient echo, axial diffusion-weighted images as well as postcontrast axial and coronal T1-weighted images. Single voxel MR spectroscopy was obtained at the right basal ganglia, using TE of 35 ms, 144 ms and 288 ms. The conventional MRI images and spectroscopy were interpreted by the same neuroradiologist (MN-J) as for the older sibling. The conventional MRI images (Figure 2) showed enlargement of the bilateral frontotemporal subarachnoid spaces compatible with reduced frontotemporal brain volume. Small size and hypomyelination of the caudate and lentiform nuclei were also noted.7 MR spectroscopy was normal for age.8 On subsequent follow-up, the patient’s motor development was quite delayed with severe axial hypotonia and appendicular hypertonia. There were no abnormal movements or seizures. He was microcephalic with short stature. At age seven months, he underwent a partially-matched unrelated cord blood transplant which unfortunately failed. The postoperative course was complicated by severe gastroesophageal reflux, treated with Nissen fundoplication and gastric tube insertion at age nine months. Genetic testing showed that he had the same CYB5R3 mutation as his older brother. The patient is currently 19 months old with profound global delay, spasticity, and microcephaly. He is kept on G-tube feeding due to severe oropharyngeal dysphagia. No follow-up brain MRI imaging is available.
Figure 2.
Brain magnetic resonance imaging (MRI) in patient 2 at age three months. (a) Axial T2 (TR/TE 4350 ms/104 ms), (b) axial inversion recovery (TR/TE 5700 ms/45.8 ms) and (c) axial T1 (TR/TE 500 ms/9 ms) images. There is prominence of the bilateral frontal and temporal subarachnoid spaces indicating frontotemporal brain volume loss, more pronounced than in patient 1 (white arrows). The basal ganglia are small in size and poorly myelinated (black arrows). TR: Repetition Time; TE: Echo Time.
Discussion
There are very few reports of the neuroimaging findings in patients with RCM type II. The most commonly reported features are brain atrophy,2–6 commonly frontotemporal in distribution,2,3,5 delayed myelination,2–5 cerebellar atrophy,2,3 and a thin corpus callosum.4 Although hypoplasia of the basal ganglia would correlate with the dystonia and abnormal movements seen in RCM type II patients, there is only one reported case in the literature.2 Our patients had bilateral symmetrical basal ganglia hypoplasia, with associated hypomyelination in patient 2. Frontotemporal brain atrophy was also noted, similar to previously reported cases. Spectroscopy is typically normal, as in our patients.5
The pathogenesis of the progressive neurological deterioration which invariably occurs in RCM type II during the first year of age is not known. It was postulated to be secondary to the role played by the membrane-bound form of cytochrome b5-reductase in the desaturation and elongation of fatty acids and in cholesterol biosynthesis, hence leading to impaired myelination,9,10 which correlates with the commonly reported delayed myelination at imaging. Another contributing factor is a reduced capacity to regenerate ascorbic acid, which also accounts for non-neurological manifestations of the disease such as gum hypertrophy, delayed closure of the cranial sutures, and absent/underdeveloped teeth. Ascorbic acid is involved in numerous intracellular reactions in nervous and non-nervous system cells, and is, in particular, a cofactor of mono-oxygenases which play an important role in nervous and neuroendocrine tissues.6,11
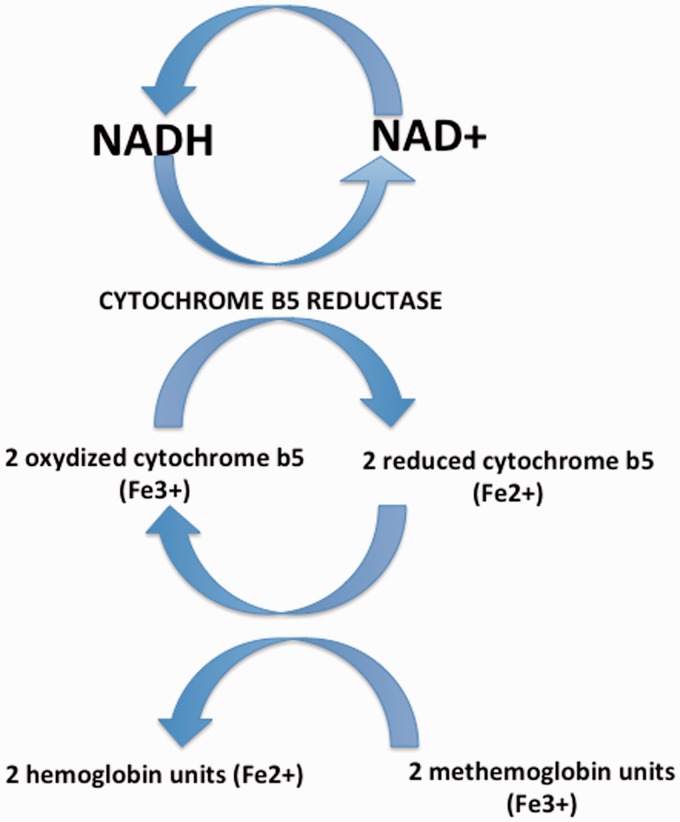
NADH-cytochrome b5 reductase is a flavoprotein that catalyzes the transfer of two electrons from NADH to two molecules of cytochrome b5.2 In erythrocytes, cytochrome b5 reductase conjointly catalyzes the transfer of these electrons to methemoglobin, then reduced to hemoglobin, which enables reduction of iron into the ferrous state and restores the oxygen-binding capacity of hemoglobin12 (Figure 3). The various mutations of the CYB5R3 gene identified to date have provided great insight into the clinical and biochemical differences between methemoglobinemia type I and type II. RCM type I is more often associated with missense mutations that result in amino acid substitution,1,2 situated in marginal segments of the enzyme, giving rise to enzymatically active but unstable proteins,1 with correlates with the benign clinical course of the disease. Conversely, RCM type II is frequently caused by full stops or deletions,2 located in consensus FAD- (Flavin Adenine Dinucleotide) or NADH-binding sites of the enzyme, causing loss of protein expression or enzymatically inactive proteins1 due to altered splicing, disruption of the active site of the enzyme, or premature truncation of the protein,5 which presumably accounts for the severe neurological impairment. The homozygous missense mutation identified in our patients p.Arg92Trp is one of a few identified homozygous missense mutations causing RCM type II.2 Other reported missense mutations include Lys111Met,5 Ser128Pro,13 Arg241Gly4 and Cys204Arg.14
Figure 3.
The metabolic pathway of cytochrome b5 reductase. Cytochrome b5 reductase catalyzes reduction of cytochrome b5 through transfer of electrons from NADH. Cytochrome b5 in turn catalyzes reduction of methemoglobin into hemoglobin. NADH: Nicotinamide Adenine Dinucleotide, reduced form; NAD+: Nicotinamide Adenine Dinucleotide, oxidized form.
RCM is treated with long-term ascorbic acid and riboflavin. A short course of methylene blue can be useful when the methemoglobin level is very high (above 20%) or the patient is severely symptomatic (lethargy, decreased level of consciousness, dyspnea). None of these treatments is proven to prevent the deterioration of neurological function.5
Recessive congenital methemoglobinemia has a 25% recurrence risk. This, along with the severity of the disease, are strong indications for prenatal diagnosis in all affected families.
Our patients highlight that basal ganglia hypoplasia is an interesting clue to the very rare and frequently unsuspected diagnosis of type II RCM, that may explain the associated movement disorders. Our findings also illustrate that missense mutations can rarely lead to severe type II RCM.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Dekker J, Eppink MH, van Zwieten R, et al. Seven new mutations in the nicotinamide adenine dinucleotide reduced-cytochrome b(5) reductase gene leading to methemoglobinemia type I. Blood 2001; 97: 1106–1114. [DOI] [PubMed] [Google Scholar]
- 2.Aalfs CM, Salieb-Beugelaar GB, Wanders RJ, et al. A case of methemoglobinemia type II due to NADH-cytochrome b5 reductase deficiency: Determination of the molecular basis. Hum Mutat 2000; 16: 18–22. [DOI] [PubMed] [Google Scholar]
- 3.Fusco C, Soncini G, Frattini D, et al. Cerebellar atrophy in a child with hereditary methemoglobinemia type II. Brain Dev 2011; 33: 357–360. [DOI] [PubMed] [Google Scholar]
- 4.Toelle SP, Boltshauser E, Mössner E, et al. Severe neurological impairment in hereditary methaemoglobinaemia type 2. Eur J Pediatr 2004; 163: 207–209. [DOI] [PubMed] [Google Scholar]
- 5.Ewenczyk C, Leroux A, Roubergue A, et al. Recessive hereditary methaemoglobinaemia, type II: Delineation of the clinical spectrum. Brain 2008; 131: 760–761. [DOI] [PubMed] [Google Scholar]
- 6.Shirabe K, Landi MT, Takeshita M, et al. A novel point mutation in a 3' splice site of the NADH-cytochrome b5 reductase gene results in immunologically undetectable enzyme and impaired NADH-dependent ascorbate regeneration in cultured fibroblasts of a patient with type II hereditary methemoglobinemia. Am J Hum Genet 1995; 57: 302–310. [PMC free article] [PubMed] [Google Scholar]
- 7.Branson HM. Normal myelination: A practical pictorial review. Neuroimaging Clin N Am 2013; 23: 183–195. [DOI] [PubMed] [Google Scholar]
- 8.Dezortova M, Hajek M. (1)H MR spectroscopy in pediatrics. Eur J Radiol 2008; 67: 240–249. [DOI] [PubMed] [Google Scholar]
- 9.Keyes SR, Cinti DL. Biochemical properties of cytochrome b5-dependent microsomal fatty acid elongation and identification of products. J Biol Chem 1980; 255: 11357–11364. [PubMed] [Google Scholar]
- 10.Hirono H. Lipids of myelin, white matter and gray matter in a case of generalized deficiency of cytochrome b5 reductase in congenital methemoglobinemia with mental retardation. Lipids 1980; 15: 272–275. [DOI] [PubMed] [Google Scholar]
- 11.Ito A, Hayashi S, Yoshida T. Participation of a cytochrome b5-like hemoprotein of outer mitochondrial membrane (OM cytochrome b) in NADH-semidehydroascorbic acid reductase activity of rat liver. Biochem Biophys Res Comm 1981; 101: 591–598. [DOI] [PubMed] [Google Scholar]
- 12.Cooper MS, Randall M, Rowell M, et al. Congenital methemoglobinemia type II–clinical improvement with short-term methylene blue treatment. Pediatr Blood Cancer 2016; 63: 558–560. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi Y, Fukumaki Y, Yubisui T, et al. Serine-proline replacement at residue 127 of NADH-cytochrome b5 reductase causes hereditary methemoglobinemia, generalized type. Blood 1990; 75: 1408–1413. [PubMed] [Google Scholar]
- 14.Maran J, Guan Y, Ou CN, et al. Heterogeneity of the molecular biology of methemoglobinemia: A study of eight consecutive patients. Haematologica 2005; 90: 687–689. [PubMed] [Google Scholar]
- 15.Saudi Ministry of Health. The Growth Charts for Saudi Children and Adolescents, https://www.moh.gov.sa/HealthAwareness/EducationalContent/BabyHealth/Documents/Intermediate%201%20Compatibility%20Mode.pdf (2009, accessed 26 December 2018).