Abstract
Background
Patients with attention deficit/hyperactivity disorder (ADHD) often experience comorbid conditions, such as autism spectrum disorder (ASD) and Tourette syndrome (TS). Although pharmacotherapies are effective for treating ADHD, they are likely to elicit a variety of adverse effects. It is, thus, important to select an effective and well-tolerated pharmacotherapeutic treatment for patients with ADHD/ASD comorbid with TS.
Case presentation
We report the case of a 10-year-old boy with ADHD/ASD comorbid with TS who was treated with guanfacine (GUAN). He experienced several side effects of atomoxetine (ATX) and methylphenidate (MPH) before being treated with GUAN. In the presented case, symptoms of ADHD as well as tic symptoms were improved by treatment with GUAN.
Conclusion
GUAN might be effective and well tolerated in the treatment of patients with ADHD/ASD comorbid with TS who experience side effects of ATX and MPH.
Keywords: Guanfacine, Atomoxetine, Methylphenidate, Attention deficit/hyperactivity disorder, Autism spectrum disorder, Tourette syndrome
Background
Attention deficit/hyperactivity disorder (ADHD), which affects 5%–10% of school-aged children [1], is characterized by inattention, hyperactivity, impulsivity, and abnormalities in cognitive processes [2]. Recently, in the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5), the term “pervasive developmental disorders” (PDD) has been changed to “autism spectrum disorder” (ASD), and the coexistence of ADHD and ASD (ADHD/ASD) is now recognized. In a 2001 survey, 83% of children with PDD were also diagnosed with ADHD [3]. Similarly, in Japan, 67.9% of high-functioning children with PDD meet the diagnostic criteria for ADHD [4]. Also, many patients with ADHD suffer from comorbidities such as Tourette syndrome (TS) [5]. ADHD is mainly treated with non-pharmacological interventions and pharmacotherapy [6]. Pharmacotherapy, in particular, is important for the treatment of moderate to severe symptoms of ADHD in children and adolescents, including, for example, psychostimulant methylphenidate (MPH) and the selective norepinephrine (NE) reuptake inhibitor atomoxetine (ATX) [7]. MPH acts as an indirect dopamine (DA) agonist, inhibiting DA reuptake by occupying the DA transporter [8] and thus increasing DA in the striatum and the prefrontal cortex (PFC) [9]. In contrast, although ATX is a selective NE reuptake inhibitor, it also inhibits DA reuptake in the PFC. It has been found that NE transporters are relatively abundant compared with DA transporters in the PFC [10, 11]. In addition, it has been shown that DA is taken up non-selectively as well as co-released by NE transporters in the PFC [12–14]. The NE transporter has similar affinities for NE [15] and DA, and DA that is released extracellularly may diffuse transsynaptically to the NE transporters [13]. While this does not increase DA in the striatum, it increases both DA and NE in the PFC [9]. These findings suggest that both types of medication increase DA in the PFC. Approximately, 10%–30% of children and adolescents with ADHD are unresponsive to psychostimulant medications or experience adverse effects such as exacerbation of comorbid psychiatric disorders (tic symptoms), loss of appetite, insomnia, anxiety, and sympathomimetic cardiovascular side effects [16, 17]. Moreover, previous research has found that 18% of children with ASD/ADHD discontinued treatment due to adverse effects, particularly irritability, compared to only 1.4% of children with only ADHD [18].
TS, on the other hand, is characterized by the presence of multiple involuntary motor and vocal tics. TS is treated with pharmacological medications such as D2-DA receptor antagonists combined with psychological behavior therapy [19]. Importantly, pharmacological treatments for ADHD and TS have the opposite effect in the PFC.
Guanfacine (GUAN) was approved for children and adolescents (6–17 years) in Japan in May 2017. Orally administered GUAN is rapidly and completely absorbed, with maximum plasma concentrations occurring 1–4 h after administration [20]. The total clearance of GUAN from human plasma equals 11–22 Lh, and the drug is mainly metabolized by the liver, with 24–37% of GUAN from human plasma excreted unchanged by the kidneys [20]. The pharmacokinetics of GUAN are essentially identical in erythrocytes and the plasma. At an average hematocrit value of 32%, the distribution of GUAN is 60% in the plasma and 40% in erythrocytes [21]. It directly stimulates postsynaptic α2A-adrenergic receptors in the PFC to enhance noradrenaline neurotransmission [22], thereby strengthening the cortical network [23]. A beneficial effect of GUAN on ADHD core symptoms in children and adolescents with ADHD has been shown, without modulation of DA in the PFC [24].
To the best of our knowledge, this is the first case report that assesses the use of GUAN in the treatment of a patient with ADHD/ASD comorbid with TS. This case corroborates the effectiveness of GUAN as a viable alternative to other pharmacological treatments for patients with ADHD/ASD comorbid with TS.
The symptoms of the current case and the severity of ADHD were assessed using the Japanese version of the ADHD Rating Scale IV (ADHD-RS-IV-J) of the ADHD-RS-IV “home version”, which is an 18-item scale that is reliable and easy to administer. In addition, the tic severity of the current case was assessed using the Yale Global Tic Severity Scale (YGTSS), in which a higher YGTSS score is associated with higher tic symptom severity. This scale yields three summary scores: total motor (0–25), total phonic (0–25), and total tic (sum of motor and phonic) scores. The YGTSS also contains an impairment scale (0–50), which evaluates the global level of functional impairment arising from tics.
Case presentation
The 10-year-old boy described here (Full Intelligence Quotient [FIQ] = 112, Verbal Intelligence Quotient [VIQ] = 106, Performance Intelligence Quotient [PIQ] = 117) had been diagnosed with a developmental delay in head control, speech, and language by a paediatrician when he was 1 year and 6 months old. When he entered kindergarten, he often played by himself and did not make friends because of his communication problems. After entering the local elementary school, at the age of 6, he began to show hyperactivity and impulsivity. In addition, he displayed symptoms of motor and vocal tics. He was assessed at a local clinic, and diagnosed with ADHD/ASD comorbid with TS. Although he initially continued to take risperidone (0.5 mg/day), side effects such as headache and anxiety led him to discontinue the treatment. When he was 9 years old, worsened impulsivity led him to behave violently toward his mother. He, therefore, began treatment, at a local clinic, with atomoxetine (ATX) (30 mg/day). However, he discontinued the medication as he experienced worsening irritability. Although he was prescribed MPH (18 mg/day) after discontinuing the treatment with ATX, he also discontinued taking MPH, because his motor and vocal tic symptoms were exacerbated. As these symptoms continued, he was referred to our hospital at 10 years of age, with an ADHD-RS-IV-J score of 23 and a YGTSS score of 29.
According to his father, he had only few friends because he had so little interest in making friends in school. The teachers often reported problems to his parents, such as when he showed physical aggression toward his friends or ran away from school during the lesson. He often got angry when the timing of activities deviated from his usual schedule. He was, therefore, diagnosed with ADHD/ASD/TS according to the criteria specified in the DSM-5.
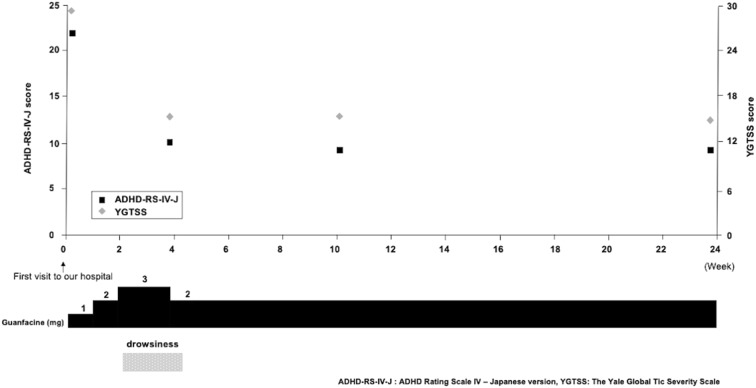
The patient was continuously prescribed GUAN at a dose starting at 1 mg/day and increasing to 3 mg/day. However, the 3-mg dose led to drowsiness (ADHD-RS-IV-J score of 10, YGTSS score of 15), and was, therefore, reduced again to 2 mg/day. At the decreased dose, he continued to take GUAN without side effects (ADHD-RS-IV-J score of 9, YGTSS score of 15), while there was no clear difference in effect between the 2-mg/day and the 3-mg/day doses. Importantly, his ADHD-related symptoms, such as irritability, hyperactivity, and inattention, as well as his tic symptoms, gradually improved. On the other hand, GUAN had no effect on ASD symptoms in this case. The patient was able to continue taking GUAN for 6 more months (ADHD-RS-IV-J score of 9, YGTSS score of 15) (Fig. 1).
Fig. 1.
Course of the ADHD-RS-IV-J score and YGTSS score
Discussion
To the best of our knowledge, the case presented here represents original evidence for the effectiveness of GUAN monotherapy and shows that it is well tolerated and improves ADHD symptoms and tic symptoms in a patient with ADHD/ASD comorbid with TS.
Psychostimulant and non-psychostimulant medications, such as MPH and ATX, are currently the first-choice medication for children and adolescents with ADHD [7]. Psychostimulants, therefore, are tried for the treatment of many patients with ADHD, and approximately 70% of children and adolescents with ADHD respond to a single stimulant [25]. However, previous reports showed that psychostimulants could cause exacerbation of tic symptoms in patients with ADHD comorbid with TS [26]. A pathogenic hypothesis of tics associates them with increased DA activity in the basal ganglia [27], while dysfunction in dopaminergic fronto-striatal neuronal networks causes dysfunction of inhibitory executive functions or cognitive control [28]. Psychostimulants are blocking the DA transporters in the synapse and increase extracellular DA levels in the brain [29]. In consequence, they are effective for patients with ADHD but could exacerbate tic symptoms. In contrast to MPH, GUAN stimulates post-synaptic α2A-adrenergic receptors and enhances noradrenaline neurotransmission [22, 30, 31]. Several studies have shown reliable effects of GUAN, as monotherapy or as adjunctive therapy with stimulants, in the treatment of children and adolescents with ADHD [24, 32]. Interestingly, α2 adrenergic receptor agonists, such as clonidine, are effective for patients with TS [33]. We, therefore, suggest that GUAN is effective as well as clonidine, for both patients with ADHD and those with TS. Furthermore, previous studies have revealed that GUAN has beneficial effects in the treatment of patients with ADHD comorbid with TS [34, 35].
The present study shows that GUAN improved tic as well as ADHD symptoms in a patient with ADHD/ASD comorbid with TS, although MPH caused an exacerbation of tic symptoms. This case indicates that treatment with GUAN is effective and well tolerated in patients with ADHD/ASD comorbid with TS. However, GUAN led to drowsiness in the current case. A previous study showed that the most common side effects are somnolence (30.4%) and sedation (13.3%) [36], as the α2-adrenergic receptor agonist leads to high peak plasma concentrations associated with these side effects [37, 38]. The side effects were, however, improved by decreasing the GUAN dose.
No previous study has shown that GUAN influences ASD symptoms, and our case likewise showed no effect on ASD symptoms. GUAN might, thus, be an alternative treatment for some patients who experience side effects of ATX and MPH, without exacerbating other psychiatric symptoms.
The main limitation of our study is that it involves only one case, and we should, therefore, be careful when drawing conclusions. Future studies are, therefore, needed to continuously assess the effectiveness of GUAN in treating patients with ADHD comorbid with ASD and TS.
Conclusion
This case indicates that GUAN might be an efficacious and well-tolerated treatment for ADHD and tic symptoms in patients with ADHD/ASD comorbid with TS, because pharmacological medication tends to cause different side effects in patients with ADHD comorbid with ASD than in those with only ADHD or ASD. Further studies with larger sample sizes are needed to validate the apparent efficacy, safety, and tolerability of medical treatment with GUAN.
Authors’ contributions
KO collected data and wrote the first draft of the manuscript. KY, JI, and TK supervised the project, were critically involved in its design, and assisted in editing the final manuscript. All authors read and approved the final manuscript.
Acknowledgements
We thank the patient and his family for their cooperation.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Not applicable.
Consent for publication
The patient and the parent of the patient gave written informed consent before the publication of this case report.
Ethics approval and consent to participate
Not applicable.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not for profit sectors.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- ADHD
attention deficit/hyperactivity disorder
- ADHD-RS-IV-J
ADHD Rating Scale IV-Japanese version
- ASD
autism spectrum disorder
- ATX
atomoxetine
- FIQ
full intelligence quotient
- GUAN
guanfacine
- MPH
methylphenidate
- OCD
obsessive–compulsive disorder
- ODD
oppositional defiant disorder
- PDD
pervasive developmental disorders
- PIQ
performance intelligence quotient
- PO
perceptual organization
- PS
process speed
- TS
Tourette syndrome
- VC
verbal comprehension
- VIQ
verbal intelligence quotient
- YGTSS
Yale Global Tic Severity Scale
References
- 1.Scahill L, Schwab-Stone M. Epidemiology of ADHD in school-age children. Child Adolesc Psychiatr Clin. 2000;9(3):541–555. [PubMed] [Google Scholar]
- 2.Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet. 2005;366(9481):237–248. doi: 10.1016/S0140-6736(05)66915-2. [DOI] [PubMed] [Google Scholar]
- 3.Frazier JA, Biederman J, Bellordre CA, Garfield SB, Geller DA, Coffey BJ, et al. Should the diagnosis of attention-deficit/hyperactivity disorder be considered in children with pervasive developmental disorder? J Atten Disord. 2001;4(4):203–211. [Google Scholar]
- 4.Yoshida Y, Uchiyama T. The clinical necessity for assessing Attention Deficit/Hyperactivity Disorder (AD/HD) symptoms in children with high-functioning pervasive developmental disorder (PDD) Eur Child Adolesc Psychiatry. 2004;13(5):307–314. doi: 10.1007/s00787-004-0391-1. [DOI] [PubMed] [Google Scholar]
- 5.Jensen CM, Steinhausen HC. Comorbid mental disorders in children and adolescents with attention-deficit/hyperactivity disorder in a large nationwide study. Atten Defic Hyperact Disord. 2015;7(1):27–38. doi: 10.1007/s12402-014-0142-1. [DOI] [PubMed] [Google Scholar]
- 6.Taylor E, Dopfner M, Sergeant J, Asherson P, Banaschewski T, Buitelaar J, et al. European clinical guidelines for hyperkinetic disorder—first upgrade. Eur Child Adolesc Psychiatry. 2004;13(Suppl 1):I7–I30. doi: 10.1007/s00787-004-1002-x. [DOI] [PubMed] [Google Scholar]
- 7.National Collaborating Centre for Mental H. National Institute for Health and Clinical Excellence: Guidance. In: Attention Deficit Hyperactivity Disorder: Diagnosis and Management of ADHD in Children, Young People and Adults. edn. Leicester (UK): British Psychological Society (UK) The British Psychological Society & The Royal College of Psychiatrists. 2009. [PubMed]
- 8.Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Logan J, Ding YS, et al. Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. Am J Psychiatry. 1998;155(10):1325–1331. doi: 10.1176/ajp.155.10.1325. [DOI] [PubMed] [Google Scholar]
- 9.Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27(5):699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- 10.Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci. 1998;18(7):2697–2708. doi: 10.1523/JNEUROSCI.18-07-02697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coulter CL, Happe HK, Bergman DA, Murrin LC. Localization and quantification of the dopamine transporter: comparison of [3H]WIN 35,428 and [125I]RTI-55. Brain Res. 1995;690(2):217–224. doi: 10.1016/0006-8993(95)00614-v. [DOI] [PubMed] [Google Scholar]
- 12.Tanda G, Pontieri FE, Frau R, Di Chiara G. Contribution of blockade of the noradrenaline carrier to the increase of extracellular dopamine in the rat prefrontal cortex by amphetamine and cocaine. Eur J Neurosci. 1997;9(10):2077–2085. doi: 10.1111/j.1460-9568.1997.tb01375.x. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto BK, Novotney S. Regulation of extracellular dopamine by the norepinephrine transporter. J Neurochem. 1998;71(1):274–280. doi: 10.1046/j.1471-4159.1998.71010274.x. [DOI] [PubMed] [Google Scholar]
- 14.Devoto P, Flore G, Pani L, Gessa GL. Evidence for co-release of noradrenaline and dopamine from noradrenergic neurons in the cerebral cortex. Mol Psychiatry. 2001;6(6):657–664. doi: 10.1038/sj.mp.4000904. [DOI] [PubMed] [Google Scholar]
- 15.Raiteri M, Del Carmine R, Bertollini A, Levi G. Effect of sympathomimetic amines on the synaptosomal transport of noradrenaline, dopamine and 5-hydroxytryptamine. Eur J Pharmacol. 1977;41(2):133–143. doi: 10.1016/0014-2999(77)90202-3. [DOI] [PubMed] [Google Scholar]
- 16.Wigal SB. Efficacy and safety limitations of attention-deficit hyperactivity disorder pharmacotherapy in children and adults. CNS Drugs. 2009;23(Suppl 1):21–31. doi: 10.2165/00023210-200923000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Dalsgaard S, Kvist AP, Leckman JF, Nielsen HS, Simonsen M. Cardiovascular safety of stimulants in children with attention-deficit/hyperactivity disorder: a nationwide prospective cohort study. J Child Adolesc Psychopharmacol. 2014;24(6):302–310. doi: 10.1089/cap.2014.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Autism P. Randomized, controlled, crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Arch Gen Psychiatry. 2005;62(11):1266–1274. doi: 10.1001/archpsyc.62.11.1266. [DOI] [PubMed] [Google Scholar]
- 19.Dooley JM. Tic disorders in childhood. Semin Pediatr Neurol. 2006;13(4):231–242. doi: 10.1016/j.spen.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Sorkin EM, Heel RC. Guanfacine A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in the treatment of hypertension. Drugs. 1986;31(4):301–336. doi: 10.2165/00003495-198631040-00003. [DOI] [PubMed] [Google Scholar]
- 21.Kiechel JR. Pharmacokinetics and metabolism of guanfacine in man: a review. Br J Clin Pharmacol. 1980;10(Suppl 1):25s–32s. doi: 10.1111/j.1365-2125.1980.tb04901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawaura K, Karasawa J, Chaki S, Hikichi H. Stimulation of postsynapse adrenergic alpha2A receptor improves attention/cognition performance in an animal model of attention deficit hyperactivity disorder. Behav Brain Res. 2014;270:349–356. doi: 10.1016/j.bbr.2014.05.044. [DOI] [PubMed] [Google Scholar]
- 23.Wang M, Ramos BP, Paspalas CD, Shu Y, Simen A, Duque A, et al. Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129(2):397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 24.Hervas A, Huss M, Johnson M, McNicholas F, van Stralen J, Sreckovic S, et al. Efficacy and safety of extended-release guanfacine hydrochloride in children and adolescents with attention-deficit/hyperactivity disorder: a randomized, controlled, phase III trial. Eur Neuropsychopharmacol. 2014;24(12):1861–1872. doi: 10.1016/j.euroneuro.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 25.Spencer T, Biederman J, Wilens T, Harding M, O’Donnell D, Griffin S. Pharmacotherapy of attention-deficit hyperactivity disorder across the life cycle. J Am Acad Child Adolesc Psychiatry. 1996;35(4):409–432. doi: 10.1097/00004583-199604000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Lowe TL, Cohen DJ, Detlor J, Kremenitzer MW, Shaywitz BA. Stimulant medications precipitate Tourette’s syndrome. JAMA. 1982;247(8):1168–1169. [PubMed] [Google Scholar]
- 27.Albin RL. Neurobiology of basal ganglia and Tourette syndrome: striatal and dopamine function. Adv Neurol. 2006;99:99–106. [PubMed] [Google Scholar]
- 28.Sonuga-Barke EJ. Causal models of attention-deficit/hyperactivity disorder: from common simple deficits to multiple developmental pathways. Biol Psychiatry. 2005;57(11):1231–1238. doi: 10.1016/j.biopsych.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Volkow ND, Wang GJ, Tomasi D, Kollins SH, Wigal TL, Newcorn JH, et al. Methylphenidate-elicited dopamine increases in ventral striatum are associated with long-term symptom improvement in adults with attention deficit hyperactivity disorder. J Neurosci. 2012;32(3):841–849. doi: 10.1523/JNEUROSCI.4461-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arnsten AF, Scahill L, Findling RL. alpha2-Adrenergic receptor agonists for the treatment of attention-deficit/hyperactivity disorder: emerging concepts from new data. J Child Adolesc Psychopharmacol. 2007;17(4):393–406. doi: 10.1089/cap.2006.0098. [DOI] [PubMed] [Google Scholar]
- 31.Franowicz JS, Kessler LE, Borja CM, Kobilka BK, Limbird LE, Arnsten AF. Mutation of the alpha2A-adrenoceptor impairs working memory performance and annuls cognitive enhancement by guanfacine. J Neurosci. 2002;22(19):8771–8777. doi: 10.1523/JNEUROSCI.22-19-08771.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newcorn JH, Stein MA, Childress AC, Youcha S, White C, Enright G, et al. Randomized, double-blind trial of guanfacine extended release in children with attention-deficit/hyperactivity disorder: morning or evening administration. J Am Acad Child Adolesc Psychiatry. 2013;52(9):921–930. doi: 10.1016/j.jaac.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Goetz CG, Tanner CM, Wilson RS, Carroll VS, Como PG, Shannon KM. Clonidine and Gilles de la Tourette’s syndrome: double-blind study using objective rating methods. Ann Neurol. 1987;21(3):307–310. doi: 10.1002/ana.410210313. [DOI] [PubMed] [Google Scholar]
- 34.Bloch MH, Panza KE, Landeros-Weisenberger A, Leckman JF. Meta-analysis: treatment of attention-deficit/hyperactivity disorder in children with comorbid tic disorders. J Am Acad Child Adolesc Psychiatry. 2009;48(9):884–893. doi: 10.1097/CHI.0b013e3181b26e9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scahill L, Chappell PB, Kim YS, Schultz RT, Katsovich L, Shepherd E, et al. A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am J Psychiatry. 2001;158(7):1067–1074. doi: 10.1176/appi.ajp.158.7.1067. [DOI] [PubMed] [Google Scholar]
- 36.Biederman J, Melmed RD, Patel A, McBurnett K, Donahue J, Lyne A. Long-term, open-label extension study of guanfacine extended release in children and adolescents with ADHD. CNS Spectr. 2008;13(12):1047–1055. doi: 10.1017/s1092852900017107. [DOI] [PubMed] [Google Scholar]
- 37.Gizer IR, Ficks C, Waldman ID. Candidate gene studies of ADHD: a meta-analytic review. Hum Genet. 2009;126(1):51–90. doi: 10.1007/s00439-009-0694-x. [DOI] [PubMed] [Google Scholar]
- 38.Sallee FR. The role of alpha2-adrenergic agonists in attention-deficit/hyperactivity disorder. Postgrad Med. 2010;122(5):78–87. doi: 10.3810/pgm.2010.09.2204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.