Abstract
Background
Chronic heart failure (CHF) is associated with insulin resistance, indicating impairment in the control of energy metabolism. Insulin resistance in CHF relates to symptomatic status and independently predicts poor prognosis. We sought to determine whether insulin sensitivity is related to skeletal muscle strength in patients with CHF, taking into account muscle size and perfusion.
Methods
Quadriceps muscle size (square centimetre cross‐sectional area at mid‐femur level, computed tomography), isometric quadriceps muscle strength [absolute (in N) and strength per unit muscle area (N/cm2)], resting‐leg blood flow (plethysmography) and maximal oxygen consumption (treadmill exercise test) were measured in 33 patients with CHF (left ventricular ejection fraction 28 ± 3.2%, mean ± Standard Error of the mean (SEM)) and 20 healthy controls. Insulin sensitivity was assessed by intravenous glucose tolerance tests and minimal modelling analysis.
Results
Right quadriceps strength (−27.0%, P < 0.0001), strength per muscle area (−18.0%, P < 0.003) and insulin sensitivity (−64.2%, P < 0.001) were lower in patients with CHF. The correlation between insulin sensitivity and absolute muscle strength was significant in the CHF group (r = 0.54, P = 0.001) and borderline in controls (r = 0.47, P = 0.06). This association remained significant between insulin sensitivity and strength per muscle area (CHF: r = 0.52, P < 0.01; controls: r = 0.62, P < 0.05). In stepwise regression analyses in CHF, only insulin sensitivity emerged as a predictor of strength per unit area of muscle [standardized coefficient (SC) = 0.45, P = 0.006; diuretic dose, SC = −0.31, P = 0.051; R 2 = 0.37, P = 0.001], while age, left ventricular ejection fraction, maximal oxygen consumption, fasting glucose and insulin and blood flow were excluded. In controls, only insulin sensitivity remained in the final regression model (SC = 0.62, P = 0.004; R 2 = 0.39, P = 0.004).
Conclusions
The myofibril contractile function of the quadriceps, i.e. functional quality of skeletal muscle, is strongly related to insulin sensitivity in patients with CHF and in healthy controls, independently of muscle size. Therapies aimed at improving insulin sensitivity in patients with CHF may clarify whether this relationship is causal.
Keywords: Chronic heart failure, Skeletal muscle, Insulin sensitivity
Introduction
Muscle weakness is a common feature in chronic heart failure (CHF). This may be related to the frequently observed loss of muscle tissue,1 but increasing evidence has been accumulated suggesting that skeletal muscle strength in CHF is also realted to metabolic abnormalitites of skeletal muscle.2 These include disturbances in oxidative metabolism,3, 4 and reduced cellular availability of adenosine triposphate (ATP)5, 6 and free fatty acids.7 Both ATP and free fatty acids are important substrates for the energy generation needed for muscle contraction. Insulin plays a prominent role in the control of energy metabolism. We have previously shown that CHF is associated with insulin resistance8 independent of CHF aetiology. Impaired insulin sensitivity progresses in parallel with CHF symptomatic severity and has been shown to predict impaired prognosis in CHF independently of other prognostic markers.9
The tissue supply of ATP is closely linked to insulin action. Insulin resistance is associated with disturbances in glycolysis10 and the citric acid cycle,11 the principal generator of ATP. In addition, impaired balance of free fatty acids negatively relates to reduced glucose transporter 4 (GLUT4) transporter protein in CHF,12 which are known to contribute to impaired glucose utilization in myocardial and skeletal muscle tissue. In fact, reduced GLUT4 protein expression in myocardial and skeletal muscle tissue has been observed in CHF in parallel with disease severity.13
In view of the prominent involvement of insulin in the control of oxidative and non‐oxidative metabolism and the supply of energy substrates, we hypothesized that insulin sensitivity might be related to skeletal muscle strength in healthy subjects and patients with CHF. Because skeletal muscle underperfusion may induce insulin resistance14 and muscle weakness,15 we have also undertaken measurements of leg blood flow in patients with CHF.
Methods
Patient population and characteristics
The study groups consisted of 33 male patients with mild‐to‐severe CHF (age range 34–83 years) and 20 male healthy controls (range 32–68 years). CHF was secondary to coronary heart disease (n = 18) or idiopathic dilated cardiomyopathy (n = 15). All patients were treated in accordance with guideline recommendations in individually optimized treatment regimen. All patients had been in CHF for >3 months. All patients gave written informed consent, and the study was approved by the local ethics committee.
Insulin sensitivity
Insulin sensitivity was assessed by intravenous glucose tolerance testing (ivGTT) as previously described.7 A 3‐h ivGTT was performed under standardized conditions in a metabolic day ward starting in the morning between 8:00 and 9:00 a.m. following overnight fasting and after at least 20‐min supine rest. A glucose bolus (50% solution) was administered intravenously, at a dose of 0.5 g/kg body weight, and blood samples were obtained at 16 time points during 180 min. The insulin sensitivity index (SI) was calculated from the glucose and insulin dynamic profiles following the glucose challenge by minimal model approach according to Bergman.16 This method has been validated for the use of in CHF against the euglycemic clamp reference method17 and optimized as described previously.18 SI—the inverse to insulin resistance—is defined as the fraction of the glucose distribution space cleared per minute by insulin‐dependent glucose disposal relative to the concentration of insulin and is expressed in (105/min)/(pmol/L).
Muscle size
The cross‐sectional area of the quadriceps muscle was measured using ultrafast computed tomography (Imatron, San Francisco, CA, USA). Scans were performed in the supine position after at least 5‐min rest. A single 6‐mm slice was scanned transaxially at the mid‐femur level, at 12.5% of patient height above the knee joint.19 The cross‐sectional area was calculated in square centimetre by semi‐automatic generation of an outline of the area of interest.
Muscle strength
Isometric quadriceps muscle strength was measured as previously described.20 Patients were seated in a rigid frame, with the legs hanging freely. A strap attached the ankle to a pressure transducer. Strength was assessed from the recording (Multitrace 2, Lectromed, Jersey, Channel Islands), which also provided visual feedback for the subject. The loss of a superimposed 1‐Hz muscle twitch during the plateau of maximum force production indicated maximal contraction. The maximal quadriceps muscle strength was taken as the best of three voluntary contractions on each leg, with a rest period of at least 1 min in between (in Newton). Muscle strength was also expressed in Newton/quadriceps cross‐sectional area (N/cm2) to obtain a measure of myofibril contractile function, i.e. quality of skeletal muscle.
Leg blood flow
Mercury‐in‐silastic strain gauge venous occlusion plethysmography was employed.21 Patients were rested for 10 min in the supine position. A cuff was placed around the upper‐right thigh and connected to a rapid inflation pump (Hokanson, Bellevue, WA, USA). The strain gauge, placed at the largest part of the calf and connected to a plethysmograph (Hokanson EC4, Hokanson, Bellevue, WA, USA), was inflated to 40 mmHg. The highest flow of 3 to 5 measurements was taken as the resting‐leg blood flow (in mL/100 mL/min).
Statistical analysis
All results are presented as mean value ± SEM. The Student's t‐test was used to analyse differences between patients with CHF and controls. Interrelationships were assessed using simple linear regression (least square method), multivariate and stepwise regression analyses (SYSTAT, SYSTAT Inc., Evanston, IL, USA). A probability value of <0.05 was considered statistically significant. Because of skewed distribution, fasting insulin was logarithmically transformed, and insulin sensitivity square root transformed.
Results
In the CHF group, there were no significant differences in any of the variables between patients with CHF due to coronary heart disease and those with CHF due to dilated cardiomyopathy (data not shown).
Absolute strength
Absolute isometric quadriceps muscle strength (right leg: −27.0%, P < 0.0001; left leg: −35.0%, P < 0.001), strength per unit area of muscle (right leg: −18.0%, P < 0.003; left leg: −23.5%, P < 0.001) and SI (−64.2%, P < 0.001) were reduced in CHF patients, compared with healthy controls (Table 1). In univariate analysis, SI correlated with right (CHF: r = 0.54, P = 0.001; controls: r = 0.43, P = 0.06; all subjects: r = 0.61, P < 0.001) and left (CHF: 0.43, P = 0.009; controls: r = 0.20, non significant (NS); all subjects: r = 0.55, P < 0.001) absolute quadriceps muscle strength. In stepwise multivariate regression analysis, SI emerged as a predictor of absolute right quadriceps strength, independently of right quadriceps muscle size, in both CHF patients and controls [CHF: SI, standardised coefficient (SC) = 0.21, P = 0.026; muscle size, SC = 0.84, P = <0.001, joint R 2 = 0.79, P < 0.001; controls: SI, SC = 0.53, P = 0.008, muscle size, SC = 0.50, P = 0.013, R 2 = 0.50, P = 0.004], while age, left ventricular ejection fraction (LVEF), maximal oxygen consumption, fasting glucose and insulin, leg blood flow (only performed in the CHF group) and diuretic dose were excluded. Similar results were obtained for the left quadriceps muscle (data not shown).
Table 1.
Characteristics of the study group and control group
| CHF group | Controls | P‐valueb | |
|---|---|---|---|
| n = 33 | n = 20 | ||
| Age (years) | 63.1 (2.1) | 52.8 (2.6) | 0.003 |
| Body mass index (kg/m2) | 23.7 (0.6) | 26.4 (0.9) | 0.011 |
| Left ventricular ejection fraction (%) | 28.5 (3.2) | — | |
| peakVO2 (mL/kg/min) | 16.8 (1.1) | 36.7 (1.7) | <0.001 |
| NYHA class I/II/III/IV | 2/13/14/4 | — | |
| Diuretic dose (mg)a | 77.3 (9.7) | — | |
| Fasting glucose (mmol/L) | 5.9 (0.4) | 5.1 (0.1) | NS |
| Fasting insulin (pmol/L) | 57.8 (−5.8,6.5) | 35.6 (−5.2,6.3) | 0.051 |
| Insulin sensitivity (105/min/pmol/L) | 1.73 (−0.24,0.26) | 4.84 (−0.61,+0.65) | <0.001 |
| Right quadriceps muscle | |||
| Absolute strength (N) | 357.2 (21.4) | 489.4 (32.7) | 0.001 |
| Cross‐sectional area (cm2) | 55.6 (2.2) | 66.9 (3.0) | 0.003 |
| Strength per unit area of muscle (N/cm2) | 6.20 (0.20) | 7.56 (0.44) 0.003 | |
| Leg blood flow (mL/100 mL/min) | 2.86 (0.2) | — | |
Results are expressed as means ± SEM, except for fasting insulin and insulin sensitivity, which, because of skewed distributions, are expressed as mean and asymmetrical SEM. Fasting insulin was logarithmically transformed, and insulin sensitivity square‐root transformed.
CHF, chronic heart failure; peakVO2 = maximal oxygen consumption.
NYHA, New York Heart Association Class.
Diuretic dose is expressed as furosemide equivalent dose (1 mg of bumetanide = 40 mg of furosemide).
P‐values refer to the differences between the groups from t‐tests.
Strength per unit muscle
The results of the univariate correlation analyses for right quadriceps strength per unit muscle (N/cm2) are shown in Table 2. In CHF patients, SI (P = 0.002) and diuretic dose (P = 0.018) correlated with right quadriceps strength per unit area of muscle. In controls, right quadriceps strength per unit area of muscle correlated with SI (P = 0.004) (Figure 1), age (P < 0.001) and maximal oxygen consumption (P = 0.019). Left quadriceps strength per unit area of muscle also correlated with SI (CHF, P = 0.046; controls, P = 0.043) (data not shown). In stepwise regression analyses of the HF group, only SI entered as a significant predictor of right quadriceps strength per unit area of muscle (SC = 0.45, P = 0.006; diuretic dose, SC = −0.31, P = 0.051; R 2 = 0.37, P = 0.001), while age, LVEF, maximal oxygen consumption (peak VO2, treadmill spiroe‐ergometric testing), fasting glucose and insulin, and leg blood flow were excluded. In controls, only SI remained in the final stepwise regression models (SC = 0.62, P = 0.004; R 2 = 0.39, P = 0.004), while age, peak VO2 and fasting glucose and insulin failed to enter into final models (LVEF and blood flow not performed in controls).
Table 2.
Univariate Pearson correlations for right quadriceps muscle strength per unit quadriceps muscle (N/cm2)
| CHF group | Healthy controls | |
|---|---|---|
| (n = 33) | (n = 20) | |
| Age | −0.22 | −0.56* |
| Left ventricular ejection fraction | −0.01 | nd |
| Maximal oxygen consumption | 0.17 | 0.55* |
| Diuretic dose | −0.42* | −0.22 |
| Fasting glucose | −0.20 | −0.40 |
| Fasting insulin | −0.11 | −0.24 |
| Insulin sensitivity | 0.52** | 0.62* |
| Leg blood flow | −0.02 | nd |
CHF, chronic heart failure; nd, not done.
P < 0.05,
P < 0.01.
Figure 1.
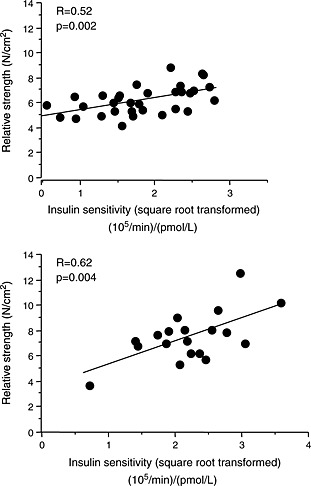
Scatterplot of insulin sensitivity against right quadriceps muscle strength per unit area of muscle in heart failure patients (upper panel) and in controls (lower panel).
In the CHF group, SI did not correlate with mean furosemide equivalent dose (r = −0.24, P = NS) or with resting‐leg blood flow (r = −0.23, P = NS).
Discussion
We have shown that in patients with CHF, there is a reduction in absolute quadriceps muscle strength. The novel finding from this study is that in CHF, skeletal muscle weakness is related to insulin resistance. In patients with CHF and in controls, the relationship between insulin sensitivity and absolute muscle strength is independent of muscle size, suggesting that muscle atrophy is not an important component of this relationship. The finding that reduced insulin sensitivity is the best predictor of reduced strength per unit quadriceps muscle, i.e. of impaired myofibril contractile function, raises the possibility that insulin sensitivity may be pathogenetically linked to skeletal muscle strength. Our finding of this relationship in healthy subjects with a wide range of insulin sensitivity supports this concept.
Although this cross‐sectional study provides no proof of causality, there are strong grounds for supposing that insulin resistance may be a cause of reduced myofibril contractile force production. Firstly, insulin resistance implies a reduction in the cellular uptake of glucose and therefore a reduced availability of the glycolytic intermediates needed for the ATP production in the citric acid cycle. Secondly, insulin resistance is associated with disturbances in skeletal muscle glycolytic and oxidative enzyme activity, as shown in the context of obesity9 and in non‐insulin‐dependent diabetes mellitus (NIDDM).10 NIDDM appears to involve an imbalance between non‐oxidative glycolysis and oxidative glycolysis. Moreover, there is evidence from human22 and animal studies23 in support of reduced activity in NIDDM of glyceraldehyde‐3‐phosphate dehydrogenase—the enzyme that catalyzes the oxidative step in glycolysis. Such glycolytic disturbances and reduced cellular uptake of glucose could lead to reductions in the flux of the citric acid cycle and therefore to a reduction in the ATP pool. The observation of decreases in oxidative enzyme capacity in patients with CHF is consistent with this concept.24
The effects of diuretic therapy on insulin sensitivity could reasonably contribute to our findings in the CHF group, and this is a potential limitation of our study. However, no significant correlations emerged between diuretic dose and insulin sensitivity, and diuretic dose failed to enter into multivariate models. Because hypoxia causes a reduction in ATP availability,25, 26 we might expect maximal oxygen consumption and measures of muscle perfusion to correlate with skeletal muscle strength. Skeletal muscle underperfusion could also account for insulin resistance.13 However, we did not observe a significant association between strength per unit muscle and either maximal oxygen consumption or resting‐leg blood flow. Moreover, insulin sensitivity was also strongly and independently related to strength per unit muscle in healthy controls, suggesting that a common pathophysiological link exists between these measures.
In conclusion, we have shown that in patients with CHF and healthy individuals, absolute quadriceps muscle strength is determined by quadriceps muscle size (quantity) and strength per unit quadriceps muscle (quality), both of which can be assessed non‐invasively. The principal determinant of quality of muscle was insulin sensitivity, as it seems to reflect an intrinsic disturbance of skeletal muscle. In the light of our findings, the effects on muscle weakness of insulin‐sensitizing agents in patients with CHF may warrant further investigation.
Doehner, W. , Turhan, G. , Leyva, F. , Rauchhaus, M. , Sandek, A. , Jankowska, E. A. , von Haehling, S. , and Anker, S. D. (2015), Skeletal muscle weakness is related to insulin resistance in patients with chronic heart failure. ESC Heart Failure, 2, 85–89. doi: 10.1002/ehf2.12035.
References
- 1. Mancini DM, Walter G, Reichek N, Lenkinski R, McCully KK, Mullen JL, Wilson JR. Contribution of skeletal muscle atrophy to exercise intolerance and altered muscle metabolism in heart failure. Circulation 1992; 85:1364–73. [DOI] [PubMed] [Google Scholar]
- 2. Doehner W, Frenneaux M, Anker SD. Metabolic impairment in heart failure: the myocardial and systemic perspective. J Am Coll Cardiol 2014; 64: 1388–400. [DOI] [PubMed] [Google Scholar]
- 3. Sullivan MJ, Green HJ, Cobb FR. Skeletal muscle biochemistry and histology in ambulatory patients with long‐term heart failure. Circulation 1990; 81:518–27. [DOI] [PubMed] [Google Scholar]
- 4. Brassard P, Maltais F, Noel M, Doyon JF, LeBlanc P, Allaire J, Simard C, Leblanc MH, Poirier P, Jobin J. Skeletal muscle endurance and muscle metabolism in patients with chronic heart failure. Can J Cardiol 2006; 22: 387–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Williams AD, Carey MF, Selig S, Hayes A, Krum H, Patterson J, Toia D, Hare DL. Circuit resistance training in chronic heart failure improves skeletal muscle mitochondrial ATP production rate‐‐a randomized controlled trial. J Card Fail 2007; 13: 79–85. [DOI] [PubMed] [Google Scholar]
- 6. Szentesi P, Bekedam MA, van Beek‐Harmsen BJ, van der Laarse WJ, Zaremba R, Boonstra A, Visser FC, Stienen GJ. Depression of force production and ATPase activity in different types of human skeletal muscle fibers from patients with chronic heart failure. J Appl Physiol 2005; 99: 2189–95. [DOI] [PubMed] [Google Scholar]
- 7. Näveri HK, Leinonen H, Kiilavuori K, Härkönen M. Skeletal muscle lactate accumulation and creatine phosphate depletion during heavy exercise in congestive heart failure. Cause of limited exercise capacity? Eur Heart J 1997; 18: 1937–45. [DOI] [PubMed] [Google Scholar]
- 8. Doehner W, Rauchhaus M, Godsland IF, Egerer K, Niebauer J, Sharma R, Cicoira M, Florea VG, Coats AJS, Anker SD. Insulin resistance in moderate chronic heart failure is related to hyperleptinaemia, but not to norepinephrine and TNF‐alpha. Int J Cardiol 2002; 83: 73–81. [DOI] [PubMed] [Google Scholar]
- 9. Doehner W, Rauchhaus M, Ponikowski P, Godsland IF, von Haehling S, Okonko DA, Leyva F, Proudler T, Coats AJ, Anker SD, Impaired insulin sensitivity as an independent risk factor for mortality in patients with stable chronic heart failure. J Am Coll Cardiol 2005; 46: 1019–26. [DOI] [PubMed] [Google Scholar]
- 10. Simoneau JA, Colberg SR, Leland Thaete F, Kelley DE. Skeletal muscle glycolytic and oxidative enzyme capacities are determinants of insulin sensitivity and muscle composition in obese women. FASEB 1995; 9:273–278. [PubMed] [Google Scholar]
- 11. Del Prato S, Bonadonna RC, Bonora E, Gulli G, Solini A, Shank M, DeFronzo RA. Characterisation of cellular defects of insulin action in type 2 (non‐insulin‐dependent) diabetes mellitus. J Clin Invest 1993; 91:484–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Murray AJ, Anderson RE, Watson GC, Radda GK, Clarke K. Uncoupling proteins in human heart. Lancet 2004; 364: 1786–8. [DOI] [PubMed] [Google Scholar]
- 13. Doehner W, Gathercole D, Cicoira M, Krack A, Coats AJ, Camici PG, Anker SD. Reduced glucose transporter GLUT4 in skeletal muscle predicts insulin resistance in non‐diabetic chronic heart failure patients independently of body composition. Int J Cardiol 2010; 138: 19–24. [DOI] [PubMed] [Google Scholar]
- 14. Lind L, Lithell H. Decreased peripheral blood flow in the pathogenesis of the metabolic syndrome comprising hypertension, hyperlipidemia, and hyperinsulinemia. Am Heart J 1993; 125: 1494–7. [DOI] [PubMed] [Google Scholar]
- 15. Wilson JR, Martin JL, Schwartz D, Ferraro N. Exercise tolerance in patients with chronic heart failure: role of impaired nutritive flow to skeletal muscle. Circulation 1984; 69: 1079–1087. [DOI] [PubMed] [Google Scholar]
- 16. Bergman RN, Ider YZ, Bowden CR, Cobelli C. Quantitative estimation of insulin sensitivity. Am J Physiol 1979; 236: E667–E677. [DOI] [PubMed] [Google Scholar]
- 17. Swan JW, Walton C, Godsland IF. Assessment of insulin sensitivity in man: a comparison of minimal model and euglycaemic clamp derived measures in health and heart failure. Clin Sci 1994; 86: 317–322. [DOI] [PubMed] [Google Scholar]
- 18. Doehner W, Anker SD, Godsland IF Optimising insulin sensitivity assessment using the modelling method in chronic heart failure. Horm Metab Res 2005; 37: 106–10. [DOI] [PubMed] [Google Scholar]
- 19. Jones DA, Round JM, Edwards RHT, Grindrod SR, Tofts PS. Size and composition of the calf and quadriceps in Duchenne muscular dystrophy. J Neurol Sci 1983; 60: 307–22. [DOI] [PubMed] [Google Scholar]
- 20. Volterrani M, Clark AL, Ludman PF, Swan JW, Adamopoulos S, Piepoli M, Coats AJ. Predictors of exercise capacity in chronic heart failure. Eur Heart J 1994; 15: 801–809. [DOI] [PubMed] [Google Scholar]
- 21. Doehner W, Bunck AC, Rauchhaus M, von Haehling S, Brunkhorst FM, Cicoira M, Tschope C, Ponikowski P, Claus RA, Anker SD. Secretory sphingomyelinase is upregulated in chronic heart failure: a second messenger system of immune activation relates to body composition, muscular functional capacity, and peripheral blood flow. Eur Heart J 2007; 28: 821–8. [DOI] [PubMed] [Google Scholar]
- 22. Cauchie P, Vertongen F, Bosson D, Dorchy H. Erythrocyte metabolic alterations in type I diabetes: relationship to metabolic control. Ann Biol Clin (Paris) 1992; 50: 9–13. [PubMed] [Google Scholar]
- 23. Ortmeyer HFK, Bodkin NL, Hansen BC. Relationship of skeletal muscle glucose‐6‐phosphate to glucose disposal rate and glycogen synthase activity in insulin‐resistant and non‐insulin resistant diabetic rhesus monkeys. Diabetologia 1994; 37: 127–33. [DOI] [PubMed] [Google Scholar]
- 24. Dei Cas A, Khan SS, Butler J, Mentz RJ, Bonow RO, Avogaro A, Tschoepe D, Doehner W, Greene SJ, Senni M, Gheorghiade M, Fonarow GC. Impact of diabetes on epidemiology, treatment, and outcomes of patients with heart failure. JACC Heart Fail 2015; 3: 136–145. [DOI] [PubMed] [Google Scholar]
- 25. Sutton JR, Toews CJ, Ward GR, Fox IH. Purine metabolism during strenuous muscular exercise in man. Metabolism 1980; 29: 254–260. [DOI] [PubMed] [Google Scholar]
- 26. Fox IH. Adenine triphosphate degradation in specific disease. J Lab Clin Med 1985; 106: 101–110. [PubMed] [Google Scholar]


