Abstract
Aims
Our aim is to describe the clinical characteristics and management of patients hospitalized with acute heart failure (HHF) and ambulatory patients with chronic heart failure (CHF) in Egypt and compare them with heart failure (HF) patients from other countries in the European Society of Cardiology‐Heart Failure (ESC‐HF) registry.
Methods and results
The ESC‐HF Long‐term Registry is a prospective, multi‐centre, observational study of patients presenting to cardiology centres in member countries of the ESC. From April 2011 to February 2014, a total of 2145 patients with HF were recruited from 20 centres all over Egypt. Of these patients, 1475 (68.8%) were hospitalized with HHF, while 670 (31.2%) had CHF. Less than one‐third (32.1%) of all patients were females. HHF patients {median age of 61 years [interquartile range (IQR), 53–69]} were older than CHF patients [median age of 57 years (IQR,46‐64)]; P < 0.0001. They had more diabetes mellitus (45.4% vs. 31.8%; P < 0.0001). Left ventricular ejection fraction > 45% was present in 22% of HHF vs. 25.6% of CHF (P = 0.17). Atrial fibrillation existed in about a quarter of all patients (24.5%). Ischaemic heart disease was the main cause of HF in Egyptian patients. All‐cause in‐hospital mortality was 5%. Egyptian patients presented at a much earlier age than in other regions in the registry. They had more diabetes mellitus. Atrial fibrillation prevalence was remarkably lower. Other co‐morbidities (renal dysfunction, stroke, and peripheral arterial disease) occurred less frequently.
Conclusion
Patients in the Egyptian cohort exhibited distinct features from HF patients in other countries in the ESC‐HF Long‐term Registry.
Keywords: Egypt, Heart failure, Registry, Demographic features, Co‐morbidities
Introduction
Heart failure (HF) is a major and growing public health problem worldwide given the ageing of the population and the success in prolonging the survival of those with coronary events.1 Heart failure has become the leading cause of hospitalization in persons older than 65 years of age. Reported death rates appear excessive both during and after hospitalization, and high re‐admission rates reveal the failure of admission to result in effective long‐term care.2
Most of large registries and surveys have been performed in North America and Europe, and limited data are available for Middle East countries that have different ethnic and cultural backgrounds. Apart from a small number of single‐centre studies,3, 4 there are limited descriptive data about HF patients in Egypt. Data on the clinical characteristics of patients, physician practice, and treatment patterns as well as the impact of management on outcomes during admission for HF remain incomplete and inconsistent. Lack of representative data and the bleak picture of morbidity and mortality associated with admission for HF underscore the need for a national representative registry database.
Methods
Study design
The European Society of Cardiology‐Heart Failure (ESC‐HF) Long‐term Registry is a prospective, multi‐centre, observational study of patients presenting to 211 cardiology centres of 21 European and Mediterranean countries which are members of the ESC. The ESC‐HF Long‐term Registry study design has been described in detail in a recent publication in the European Journal of Heart Failure.5 Egypt, as a member country of the ESC willingly participated in this registry as there is no available national database concerning this serious disease. Twenty centres, representing diverse geographic regions of Egypt (Mediterranean coast, Nile delta, Cairo, Upper Egypt and Suez Canal region), voluntarily participated in this registry. Site selection was aimed to target a sample of hospitals of different levels of complexity from which patients were recruited, focusing on capturing a broad spectrum of cardiology and HF specialty units regularly following outpatients with HF and admitting patients with acute, pre‐existing, or new onset HF in order to build‐up a network of centres representative of Egyptian reality. Nine participating centres were university hospitals. Seven centres had neither catheterization laboratories nor cardiac surgery facilities. Outpatients' visits were performed according to the usual practice of the participating centres.
The aim of this registry was to describe the demographic and clinical characteristics of outpatients with chronic HF (CHF) seen in the clinics and inpatients admitted with acute HF (HHF) who were being taken care of by the participating centres. Specific attention was focused on clinically relevant co‐morbidities, which frequently were associated with HF and impact patient outcomes. We aimed to describe the diagnostic and therapeutic approaches undertaken in the routine practice of physicians in following outpatients with CHF or during the hospital phase for HHF and to assess the in‐hospital outcomes of patients with HF. We also performed a comparative descriptive analysis of HF patients recruited from Egypt and those HF patients enrolled from other countries participating in the same registry. The aim of such analysis was to look for differences in demographic and clinical characteristics between the Egyptian HF cohort and other populations in this registry. The EURObservational Research Programme (EORP) department at the European Heart House co‐ordinated the project operationally, provided support to the participating centres, and guarded the methodological aspects of the survey. Moreover, study sites were monitored on a random basis by an audit, named by the Executive Committee, who checked compliance with the protocol and reviewed consecutiveness and quality of data. The database was set up at the European Heart House according to the requirements defined by the appointed Executive Committee, with the support of the EORP department.
Patient population
This included all outpatients with HF seen at the clinics and those admitted for acute, pre‐existing, or new onset HF in participating centres during the enrolment period. To facilitate consecutive enrolment, patients were enrolled in the registry on a 1‐day‐per‐week basis and followed up for at least once a year. In the latter phase of the ESC‐HF Long‐term Registry (autumn 2013), the 1‐day‐per‐week policy was changed to 5 days per season, as recommended by the steering committee of the registry. So, for CHF, every outpatient with CHF diagnosed, according to the clinical judgement of participating centres' responsible cardiologist, was enrolled in the registry. Acute heart failure was defined as either new‐onset HF or decompensation of chronic established HF with symptoms sufficient to warrant hospitalization. Registry participation did not require any alteration of treatment or hospital care, and entry of data was not contingent on the use of any therapeutic agent or treatment regimen. There were no specific exclusion criteria, with the exception of age that should be over 18 years. Data were collected in the period from April 2011 to February 2014. The survey was approved by each local Institutional Review Board according to the rules of each participating centre. No data were collected before detailed information was given to the patient and a signed informed consent was obtained.
Statistical analysis
Continuous variables were reported as median and interquartile range (IQR). Categorical variables were reported as percentages and compared using the χ 2 test. Continuous variables were compared by the Mann–Whitney U‐test. Kruskal–Wallis test was used when more than two groups were compared. A P‐value of < 0.05 was considered statistically significant. All tests were two‐sided. Analyses were performed using the R program software.6
Results
Baseline characteristics
From April 2011 to February 2014, 2145 patients with HF were recruited from all participating centres. Of these patients, 1475 (68.8%) were patients hospitalized for acute heart failure (HHF), while 670 (31.2%) were outpatients with CHF seen and followed up in outpatient clinics.
Table 1 shows the demographic–clinical characteristics of patients enrolled in Egypt. Hospitalized patients were older than outpatients and they were more often males. Co‐morbidities were more frequently observed in hospitalized patients. Obesity was more prevalent in hospitalized patients. Patients with HF and preserved ejection fraction (>45%) comprised 22% of HHF vs. 25.6% of CHF, P = 0.170. Atrial fibrillation existed in 24.3% of hospitalized vs. 24.8% of outpatients, P = 0.870. Ischemic heart disease was the dominant cause of HF in 68.1% of hospitalized vs. 41% of outpatients; P < 0.0001.
Table 1.
Baseline characteristics
| HHF (n = 1475) | CHF (n = 670) | P‐value | |
|---|---|---|---|
| Demographics | |||
| Age (years), median (IQR) | 61 (53–69) | 57 (46–64) | <0.0001 |
| Age ≥ 70 years, % | 22.9 | 12.4 | <0.0001 |
| Females, % | 30.4 | 35.8 | 0.010 |
| BMI (kg/m2), median (IQR) | 29.4 (26.5–33.2) | 27.7 (24.2–31.2) | <0.0001 |
| BMI ≥ 30 kg/m2, % | 46.9 | 33.2 | <0.0001 |
| Smoker (current/ever), % | 61.0 | 51.8 | <0.0003 |
| Female smokers, % | 5.0 | 6.0 | 0.441 |
| Initial symptoms and evaluation | |||
| NYHA class III/IV | 92.3 | 30.9 | <0.0001 |
| SBP (mmHg), median (IQR) | 130 (110–150) | 120(110–133) | <0.0001 |
| HR (bpm), median (IQR) | 100 (90–114) | 90 (80–100) | <0.0001 |
| EF (%), median (IQR) | 36 (30–45)a | 40 (30–46)b | 0.020 |
| EF > 45% | 22.0 | 25.6 | 0.170 |
| Atrial fibrillation % | 24.3 | 24.8 | 0.870 |
| Haemoglobin gm/dL median (IQR) | 12 (11–13) | 11 (10–12) | <0.0001 |
| Haemoglobin ≤ 12 g/dL, % | 42.3 | 62.9 | <0.0001 |
| Medical history | |||
| Prior HF without previous hospitalization, % | 43.4 | 34.5 | <0.001 |
| MI | 67.6 | 41.6 | <0.0001 |
| Diabetes mellitus, % | 45.4 | 31.8 | <0.0001 |
| Hypertension, % | 43.5 | 40.8 | 0.250 |
| Renal dysfunction, % | 17.6 | 13.4 | 0.020 |
| COPD, % | 14.8 | 13.3 | 0.400 |
| Prior stroke/TIA, % | 7.7 | 5.1 | 0.030 |
| PAD, % | 5.3 | 7.5 | 0.060 |
| Hepatic dysfunction, % | 9.2 | 5.4 | 0.004 |
| Primary aetiology | |||
| Ischaemic | 68.1 | 41.0 | <0.0001 |
| DCM | 15.5 | 24.6 | |
| Valvular | 7.7 | 17.5 | |
| Hypertension | 3.7 | 9.7 | |
| Other | 5.0 | 7.2 |
BMI, body mass index; CHF, chronic heart failure patients; COPD, chronic obstructive pulmonary disease; DCM, dilated cardiomyopathy; EF, ejection fraction; HHF, hospitalized heart failure patients; HR, heart rate; IQR,interquartile range; NYHA, New York Heart Association; MI, myocardial infarction; PAD, peripheral arterial disease; SBP, systolic blood pressure; TIA, transient ischemic attack.
Availale for 1076 patients.
Available in 391 patients
Hospital presentation and events and procedures during hospitalization
Table 2 shows that acute decompensated HF was the most common presentation (54.3%) followed by HF associated with acute coronary syndromes (20.5%). Electrocardiography, chest roentgenography, and echocardiography were frequently performed (97.3%, 83.7%, and 76.3%, respectively). More sophisticated techniques like cardiac computed tomography and right heart catheterization were rarely used. Devices were greatly underutilized in hospitalized patients (1.5%). Median hospital length of stay was 4 days (IQR, 3–5). The all‐cause in‐hospital mortality was 5%.
Table 2.
Hospital presentation, events, and procedures during hospitalization
| (n = 1475) | |
|---|---|
| Hospital presentation | |
| ACS/HF, % | 20.5 |
| Decompensated HF, % | 54.3 |
| Cardiogenic shock, % | 3.2 |
| Pulmonary edema, % | 12.9 |
| Hypertensive HF, % | 4.5 |
| Right HF, % | 4.5 |
| ECG, % | 97.3 |
| Echo, % | 76.3 |
| CXR, % | 83.7 |
| Cardiac CT, % | 0.3 |
| RHC, % | 0.3 |
| DC‐cardioversion, % | 1.9 |
| PM, % | 0.9 |
| CRT‐D, % | 0.1 |
| CRT‐P, % | 0.3 |
| ICD, % | 0.1 |
| LOS (days), median (IQR) | 4(3–5) |
| Mortality, % | 5.0 |
ACS, acute coronary syndrome; CRT, cardiac resynchronization therapy; CT, computed tomography; CXR, chest X‐ray; D, defibrillator; ECG, electrocardiogram; HF, heart failure; ICD, implantable cardioverter defibrillator; LOS, length of stay; P, programmed; PM, permanent pacemaker; RHC, right heart catheterization.
Pharmacologic treatment at hospital discharge and in outpatients
Table 3 shows that oral treatments recommended by guidelines [ACE/ARBs, beta blockers (BBs) and mineraloreceptor antagonists (MRAs)] were well prescribed in both types of patients. Diuretics were more commonly used during hospitalization, whereas MRA's were quite frequently used in outpatients. Digitalis was used in a high percentage of outpatients (47%). Ivabradine was used in 20.4% of outpatients.
Table 3.
Oral medications at hospital discharge and in outpatients with chronic heart failure
| HHF | CHF | P‐value | |
|---|---|---|---|
| ACE/ARBs, % | 85.8 | 89.8 | <0.0001 |
| Beta blockers, % | 65.8 | 67.0 | 0.252 |
| MRAs, % | 68.2 | 86.4 | <0.0001 |
| Diuretics, % | 93.0 | 84.9 | <0.0001 |
| Digitalis, % | 36.1 | 47.0 | <0.0001 |
| Statins, % | 71.5 | 50.9 | <0.0001 |
| Anti‐platelets, % | 79.7 | 58.2 | <0.0001 |
| Nitrates, % | 51.7 | 41.0 | <0.0001 |
| CCBs, % | 8.4 | 5.4 | 0.017 |
| Anticoagulants, % | 30.7 | 30.0 | 0.962 |
| Amiodarone, % | 10.7 | 10.8 | 0.868 |
| Ivabradine, % | 6.4 | 20.4 | <0.0001 |
ACE, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blocker; CCBs: calcium channel blockers; CHF, chronic heart failure patients; HHF, hospitalized heart failure patients; MRAs, mineraloreceptor antagonists.
Comparison between heart failure patients in Egypt and other member countries of the ESC participating in the registry (other regions)
Table 4 shows that patients were hospitalized with acute HF in Egypt at a much earlier age. Patients ≥ 70 years old accounted for 22.9% of all HHF patients in Egypt vs. 59.6% in other regions (P < 0.0001). Acute heart failure patients in Egypt had a higher BMI and were more obese. Women were less frequent in the Egyptian hospitalized cohort. Ejection fraction > 45% was more prevalent in HHF patients in other regions than in Egypt (35.7% vs. 22%, P < 0.0001). Atrial fibrillation occurred in 24.3% of HHF patients in Egypt vs. 48.4% of patients in other regions, P < 0.0001 (Figure 1). Diabetes mellitus was more prevalent in HHF patients in Egypt, whereas hypertension occurred less frequently. Co‐morbidities (renal dysfunction, COPD, prior stroke, and peripheral arterial disease) occurred more frequently in HHF patients in other regions (Figure 2). A higher percentage of HHF patients in Egypt had underlying ischaemic aetiology. Devices were much less frequently utilized among Egyptian patients. In‐hospital mortality was close to that seen in other regions (5% vs. 4.7%, P = 0.670).
Table 4.
Comparison between hospitalized patients with heart failure in Egypt and other countries participating in ESC‐HF long‐term registry (other regions)
| Egypt (n = 1475) | Other regions (n = 6131) | P‐value | |
|---|---|---|---|
| Age (years), median (IQR) | 61 (53–69) | 73 (63–80) | <0.0001 |
| Age ≥ 70 years, % | 22.9 | 59.6 | <0.0001 |
| SBP (mmHg), median (IQR) | 130(110–150) | 130(112–150) | 0.650 |
| BMI (kg/m2), median (IQR) | 29.4 (26.5–33.2) | 27.2 (24.4–30.5) | <0.0001 |
| BMI ≥ 30, % | 46.9 | 28.5 | <0.0001 |
| Females, % | 30.4 | 39.2 | <0.0001 |
| EF (%), median (IQR) | 36 (30–45) | 40 (30–54)a | 0.0035 |
| EF > 45%, % | 22.0 | 35.7 | <0.0001 |
| Atrial fibrillation % | 24.3 | 48.4 | <0.0001 |
| Diabetes mellitus, % | 45.4 | 36.9 | <0.0001 |
| Hypertension, % | 43.5 | 70.8 | <0.0001 |
| Renal dysfunction, % | 17.6 | 27.6 | <0.0001 |
| Hepatic dysfunction, % | 9.2 | 7.4 | 0.030 |
| COPD, % | 14.8 | 21.1 | <0.0001 |
| Prior stroke/TIA, % | 7.7 | 13.4 | <0.0001 |
| PAD, % | 5.3 | 16.7 | <0.0001 |
| Smoker (current/ever), % | 61.0 | 47.0 | <0.0001 |
| Haemoglobin ≤ 12 g/dL, % | 42.3 | 39.3 | 0.080 |
| Primary aetiology | <0.0001 | ||
| Ischaemic, % | 68.1 | 52.8 | |
| Hypertension, % | 3.7 | 9.8 | |
| DCM, % | 15.5 | 12.8 | |
| Valvular | 7.7 | 13.2 | |
| Other | 5.0 | 10.9 | |
| Hospital presentation | <0.0001 | ||
| ACS/HF, % | 20.5 | 12.5 | |
| Cardiogenic shock, % | 3.2 | 2.9 | |
| Decompensated HF, % | 54.3 | 63.2 | |
| Pulmonary edema, % | 12.9 | 13.1 | |
| Hypertensive HF, % | 4.5 | 5.3 | |
| Right HF, % | 4.5 | 3.0 | |
| In‐hospital mortality, % | 5.0 | 4.6 | 0.670 |
| Devices, % | 1.5 | 18.6 | <0.0001 |
| Medications, % | |||
| ACE/ARBs, % | 85.5 | 74.8 | <0.0001 |
| Beta blockers, % | 65.8 | 76.2 | <0.0001 |
| MRAs, % | 68.2 | 50.8 | <0.0001 |
| Diuretics, % | 93.0 | 90.3 | 0.0018 |
| Digitalis, % | 36.1 | 22.1 | <0.0001 |
ACE, ACE inhibitors; ACS, acute coronary syndrome; ARB, angiotensin receptor blocker; BMI, body mass index; COPD, chronic obstructive pulmonary disease, DCM, dilated cardiomyopathy; EF, ejection fraction; HF, heart failure; MRA, mineraloreceptor blocker; PAD, peripheral arterial disease; SBP, systolic blood pressure; TIA, transient ischemic attack.
Available for 3709 patients.
Figure 1.
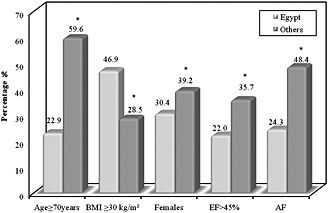
Comparison between hospitalized heart failure patients in Egypt and other regions in the registry: baseline characteristics. *P‐value < 0.0001 for all parameters. BMI, body mass index; EF, ejection fraction; AF, atrial fibrillation.
Figure 2.
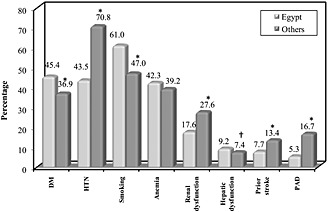
Comparison between hospitalized heart failure patients in Egypt and other regions in the registry: cardiovascular risk factors and co‐morbidities. *P‐value < 0.0001; †P‐value = 0.030. DM, diabetes mellitus; HTN, hypertension; PAD, peripheral arterial disease.
Table 5 compares between CHF patients from Egypt and other regions. Egyptian patients still presented at a relatively younger age. Women were more frequently represented in the Egyptian outpatient cohort. Median left ventricular EF was higher in Egyptian patients, but an EF > 45% was not significantly different between both groups. Atrial fibrillation prevalence was still far less frequent in Egyptian patients than in other regions (24.8% vs. 38%; P < 0.0001). A haemoglobin < 12.0 g/dL was remarkably higher in Egyptian CHF patients. Valvular heart disease contributed more to HF in the Egyptian CHF patients than in other regions. Devices for treatment of HF were still largely underutilized in the Egyptian cohort.
Table 5.
Comparison between CHF patients in Egypt and other countries participating in ESC‐HF long‐term registry (other regions)
| Egypt (n = 670) | Other regions (n = 9625) | P‐value | |
|---|---|---|---|
| Age (years), median (IQR) | 57 (46–64) | 67 (58–76) | <0.0001 |
| Age ≥ 70 years, % | 12.4 | 41.9 | <0.0001 |
| SBP (mmHg), median (IQR) | 120 (110–133) | 121 (110–138) | <0.0001 |
| BMI (kg/m2), median (IQR) | 27.7 (24.2–31.2) | 27.5 (24.6–30.9) | 0.450 |
| BMI ≥ 30 kg/m2, % | 33.2 | 30.6 | 0.160 |
| Females, % | 35.8 | 28.3 | <0.0001 |
| EF (%), median (IQR) | 40 (30–46) | 35 (27–45) | <0.0001 |
| EF > 45%, % | 25.6a | 22.9b | 0.230 |
| Atrial fibrillation, % | 24.8 | 38.0 | <0.0001 |
| Diabetes mellitus, % | 31.8 | 31.1 | 0.0012 |
| Hypertension, % | 40.8 | 60.5 | <0.0001 |
| Renal dysfunction, % | 13.4 | 18.5 | 0.0014 |
| Hepatic dysfunction, % | 5.4 | 3.2 | 0.0035 |
| COPD, % | 13.3 | 14.2 | 0.550 |
| Prior stroke/TIA, % | 5.1 | 9.7 | <0.0001 |
| PAD, % | 7.5 | 12.4 | 0.0002 |
| Smokers (current/ever), % | 51.8 | 51.5 | <0.0001 |
| Haemoglobin ≤ 12.0 g/dL, % | 62.9 | 19.1 | <0.0001 |
| Primary aetiology | <0.0001 | ||
| Ischaemic, % | 41.0 | 43.7 | |
| DCM, % | 24.6 | 28.9 | |
| Hypertension, % | 9.7 | 7.9 | |
| Valvular, % | 17.5 | 8.0 | |
| Other, % | 7.1 | 11.5 | |
| Devices, % | 2.2 | 35.8 | <0.0001 |
| ACE/ARBs, % | 89.8 | 88.6 | 0.370 |
| Beta blockers, % | 67.0 | 89.5 | <0.0001 |
| MRAs, % | 86.4 | 56.5 | <0.0001 |
| Diuretics, % | 78.7 | 89.3 | <0.0001 |
| Digitalis, % | 47.0 | 21.0 | <0.0001 |
ACE inhibitors; ACS, acute coronary syndrome; ARB, angiotensin receptor blocker; BMI, body mass index; COPD, chronic obstructive pulmonary disease, DCM, dilated cardiomyopathy; EF, ejection fraction; HF, heart failure; ACE, MRA, mineraloreceptor blocker; PAD, peripheral arterial disease; SBP, systolic blood pressure; TIA, transient ischemic attack.
Available for 391 patients.
Available for 8759 patients.
Discussion
This is the first national large‐scale prospective multi‐centre registry to study HF patients in Egypt. Here, we report the demographics, overall clinical presentation, primary aetiology, co‐morbidities, management, and in‐hospital mortality of this cohort of patients. The participating centres represent diverse geographic regions of the country. The diversity of hospitals (university, non‐university, and community) reflects the actual practice and management of HF in this country. Furthermore, we carried out a detailed descriptive comparative analysis between HF cohort in Egypt and other ESC member countries participating in the same registry.
Patients' baseline characteristics
The median age of presentation of our patients was much earlier than the other countries participating in this registry.5 Those patients who were ≥ 70 years of age comprised a minority in our population, whereas they were the majority in the rest of the registry. The relatively younger age of our patients is again confirmed when compared with other Western HF populations from the Acute Decompensated Heart Failure National Registry7 and Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure8 registries. On the other hand, the age of the Egyptian HF patients was very close to Saudi HF patients from the recent HEARTS registry.9 This might be explained in part by the observation that patients in this region of the world suffer from myocardial infarction at a relatively younger age.10 Ischemic heart disease was the primary aetiology of HF in the majority of our patients.
Fewer women were hospitalized with HF in our cohort compared with the other regions in this registry and with those reported by Masoudi et al. 11 Women were less afflicted with ischemic heart disease than men in the ACCESS registry.10 Obesity among our hospitalized HF patients was a salient feature. Little less than half of our hospitalized patients had a body mass index ≥ 30 kg/m2. This is significantly higher than other regions in the registry. El‐Zanaty and Way12 reported in a survey carried out in Egypt in 2008 that obesity in women increased directly with age, from a level of 10% among women aged 15–19 years to 65% or more among women in the 45–59 years age groups. Obesity increased in Egyptian women by 1.67 kg/m2 per decade.
Less than one quarter of our hospitalized patients had HF with preserved ejection fraction (HFpEF > 45%). This was confirmed in a previous Egyptian cohort by Ibrahim.3 This prevalence is significantly lower than the other regions participating in this registry (35.7%) and definitely lower than that of 47% reported by Owan et al. 13 The prevalence of HFpEF was 50.4% in the ADHERE database as reported by Yancy et al. among 52 187 HF hospital admissions.14 The Framingham Study investigators15 reported increased systolic blood pressure, atrial fibrillation, and female sex as predictors of HFpEF. Besides, HFpEF is mostly a disease of the elderly.16 All these factors were less frequent in the Egyptian cohort. This might explain why HF with reduced ejection fraction was the more dominant clinical presentation in our patients.
Atrial fibrillation prevalence was intriguingly low in our population (24.3%) compared with other regions in the registry (48.4%), almost half the prevalence rate. This large difference may be attributed to the younger age of the Egyptian cohort and lower prevalence of hypertension. In the Saudi HEARTS registry,9 17.2% of patients had atrial fibrillation. The prevalence of atrial fibrillation in patients with HF varies from <10% to 50%, depending on in part upon the severity of HF and New York Heart Association class.17
Ischaemic heart disease was the principal primary aetiology in our HF cohort. Mokdad et al.18 reported that ischaemic heart disease was the top cause of death in the Arab world in 2010, contributing to 14.4% of deaths, whereas, in 1990, it was ranked second. It was also the leading cause of disability‐adjusted life years in middle‐income countries in the region (including Egypt) in 2010 for male individuals. Valvular heart disease (particularly rheumatic) was not a major contributor to HF in our hospitalized cohort. Rheumatic fever and rheumatic heart disease which were quite prevalent in Egypt during the last century have been declining, probably due to better socio‐economic structure and health care in the community.19
Cardiovascular risk factors and co‐morbidities
The prevalence of diabetes mellitus was higher in our HHF cohort (44.8%) compared with other regions (35.5%) and other Western populations (30%)20 but definitely lower than that reported in the Saudi HEARTS database (64.1%).9 Egypt ranked ninth in the top 10 countries of number of people with diabetes (20–79 years old) in 2013 with a national prevalence rate of 15.5%.21 On the other hand, hypertension prevalence was lower in our cohort compared with other regions and HEARTS database. Renal dysfunction, prior stroke, and peripheral arterial disease were more prevalent in other regions in the registry than in the Egyptian cohort. This might be related to the older age and higher prevalence of hypertension in that group. The high prevalence rate of smoking among our male HF patients is consistent with the WHO global status report on non‐communicable diseases22 which showed an age‐adjusted prevalence of daily tobacco smoking in Egypt in adults aged 15 years or older of 37.2% in men and 0.6% in women.
Management and outcome
Devices (CRT‐P, CRT‐D, implantable cardivertor/defibrillators and pacemakers) were largely underutilized in our patients. This might be related to lack of knowledge of indications in recent guidelines or to socio‐economic issues or both. Pharmacologic treatment at hospital discharge was satisfactory, and medications with class I level of evidence A (ACE/ARBs, BB's, MRAs) were properly prescribed. Digoxin was likely overprescribed (36.1%) in HHF patients. Using data from a large heart failure registry, Hussain et al.23 reported a decline in the use of digoxin significantly from 31.4% in 2001 to 23.5% in late 2004 after the DIG trial.24 Digoxin is a class IIb, B in recent ESC guidelines for HF.25 Length of hospital stay was really short in Egyptian patients (median of 4 days). This is largely due to shortage in beds. Once the patient is decongested and feels better, it is the duty of the caring physician to get him out of hospital for a faster turnover of beds. It is also the wish of patients to continue their treatment at home where they feel more comfortable amidst their families. In‐hospital mortality was 5% in our cohort, which was not significantly different from the rest of the registry. However, in light of the younger age presentation of our patients, this would be considered relatively increased.
Chronic heart failure cohort
Ambulatory patients with CHF represented less than one‐third of the total number of patients recruited in the Egyptian cohort. The majority of HF patients in Egypt present to hospital when they are extremely ill. So, they are usually managed as inpatients. Moreover, few hospitals have dedicated outpatient HF clinics.
Major differences between hospitalized patients from Egypt and other regions were maintained in the outpatient setting of CHF patients, namely, younger age at presentation (even earlier than hospitalized patients), lower prevalence of atrial fibrillation, and lower prevalence of co‐morbidities (except for diabetes mellitus, hepatic dysfunction, and anaemia). Prevalence of anaemia (haemoglobin < 12.0 g/dL) was remarkably high in CHF patients. This needs further study of causes of anaemia in Egyptian HF patients and in Egyptian population as a whole. El‐Sahn et al.26 reported an overall prevalence of anaemia of 46.6% among adolescents in Egypt. Another study27 estimated prevalence of anaemia of 49.6% among clients of family planning clinics. Women were more represented in the Egyptian CHF cohort in contrast to hospitalized patients and to CHF patients from other regions. Even though ischemic heart disease maintained its position as the dominant cause of HF among CHF patients, valvular heart disease (mostly rheumatic) emerged as a significant contributor to HF in this population. This may be related to the younger age of ambulatory patients with heart failure, and that patients with chronic valvular heart disease are usually managed on outpatient basis. The rate of prescription of beta blockers was lower, whereas that of MRAs was higher in the Egyptian cohort with CHF vs. their peers in the registry. Continuous medical education of physicians and general practitioners and raising awareness of the guidelines should lead to more frequent use of beta blockers. The wide availability of a low‐priced single combination pill in the Egyptian market containing both furosemide and spironolactone may explain the more frequent prescription of MRAs in our patients.
Limitations
There are several limitations of this registry. First, the diagnosis of HF was made by each centre's practicing physician and was not validated centrally. Second, patients enrolled in the registry did not include those patients with HF admitted to other facilities in the hospital. Third, brain natriuretic peptide (BNP) testing was not included in the diagnosis of HF, because it was performed in a minority of our patients. Moreover, we did not record re‐admission rates in our patients.
Conclusions
In conclusion, this is the first national registry of HF in Egypt which included patients admitted for treatment of CHF and ambulatory patients with CHF. Results showed that HF patients in Egypt had demographic and clinical features which were distinctly different from other countries participating in the registry. They presented at a much younger age; women were less represented, and obesity was more prevalent in the hospitalized cohort. The majority of our patients had HF with reduced ejection fraction. Of the cardiovascular risk factors, diabetes mellitus and smoking were more prevalent in Egyptian patients; co‐morbidities were less frequent. Ischaemic heart disease was the dominant primary aetiology in Egyptian patients with HF. Prevalence of atrial fibrillation was remarkably lower than in the rest of the registry. Devices for treatment of HF were largely underutilized in the Egyptian cohort. In‐hospital mortality was similar to other regions in the registry. These data highlight the value of national registries in exploring the dimensions of a worldwide epidemic from a national perspective and imply that primary prevention programmes are urgently needed on a nationwide basis.
Acknowledgements
I would like to express my sincere gratitude to all investigators and sub‐investigators who contributed to this work. I would like to thank Renato Urso from the pharmacology unit, ‘Giorgio Segre’, University of Siena, Italy, and Cecile Laroche from the EORP team for their tireless efforts in performing and reviewing the statistical analysis of this work.
Funding
This survey was funded by the ESC. Each participating national cardiology society was given a grant of €10 000 to help with the organizational needs of national network implementation. The following companies supported the EURObservational Research Programme: Gold‐level support: Abbot Vascular, Bayer Pharma, BMS/Pfizer, Boehringer Ingelheim International, Daiichi Sankyo Europe, Menarini International, Novartis Pharma, Laboratoires Servier. Silver‐level support: Amgen. Bronze‐level support: Boston Scientific International, MSD/Merck & Co, Sanofi‐Aventis Group. Special thanks is due to Laboratoires Servier, Egypt, for their sincere support during the whole period of the survey in Egypt.
Conflict of Interest
None declared.
Appendix 1.
Collaborators
Alexandria: Judi Rizk, Rasha Bayazid, Basma Abdel Kader. Cairo: Kareem Said, Sara Halawa. National Heart Institute: Rania Nabil, Mohamed Abou Eleinein. Zagazig: Mohamed Gouda. International Cardiac Centre: Mohamed Abdel Hamid. Tanta University: Mohamed Bayoumi. Damanhour: Ayman Omran. Menoufeya University: Ahmed Soliman. Assiut University: Ali Tohamy, Shaimaa Khedr. Dar ElFouad: Mohamed Rizk. Bani Suef: Khalid Hussein, Alzahraa Esmat, Sylvia Maher. Ismailya: Omar Saleh, Paula Shohdy. Ain Shams University: Mohamed Abdel Kader, Basem Enani Saeid. Benha University: Hager Hussein.
Hassanein, M. , Abdelhamid, M. , Ibrahim, B. , Elshazly, A. , Aboleineen, M. W. , Sobhy, H. , Nasr, G. , Elmesseiry, F. , Abdelmoniem, A. , Ashmawy, M. , Farag, N. , Youssef, A. , Elbahry, A. , Elrakshy, Y. , Sobhy, M. , Khairy Abdel Dayem, T. M. , Ebeid, H. , Reda, A. , Boshra, H. , Saleh, A. , and Maggioni, A. P. (2015) Clinical characteristics and management of hospitalized and ambulatory patients with heart failure–results from ESC heart failure long‐term registry–Egyptian cohort. ESC Heart Failure, 2: 159–167. doi: 10.1002/ehf2.12046.
References
- 1. Lloyd‐Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespieet C. Heart disease and stroke statistics 2010 update: a report from the American Heart Association. Circulation 2010; 121: e46–e215. [DOI] [PubMed] [Google Scholar]
- 2. Krumholz HM, Parent EM, Tu N, Vaccarino V, Wang Y, Radford,MJ , Hennen J. Readmission after hospitalization for congestive heart failure among Medicare beneficiaries. Arch Intern Med 1997; 157: 99–104. [PubMed] [Google Scholar]
- 3. Ibrahim BS.The frequency of systolic versus diastolic heart failure in an Egyptian cohort. Euro J Heart Fail 2003; 5: 41–5. [DOI] [PubMed] [Google Scholar]
- 4. Abdelhamid M, Said K, Rizk H. Experience in a specialized heart failure unit in a tertiary care hospital in Egypt: demographic characteristics and in‐hospital outcome of patients with severe heart failure. Eur J Heart Fail suppl 2003; 2(suppS1): 78–79. [Google Scholar]
- 5. Maggioni AP, Anker SD, Dahlstrom U, Filippatos G, Ponikowski P, Zannad F, Amir O, Chioncel O, Crespo Leiro M, Drozdz J, Erglis A, Fazlibegovic E, Fonseca C, Fruhwald F, Gatzov P, Goncalvesova E, Hassanein M, Hradec J, Kavoliuniene A, Lainscak M, Logeart D, Merkely B, Metra M, Persson H, Seferovic P, Temizhan A, Tousoulis D, Tavazzi L on behalf of the Heart Failure Association of the ESC (HFA) . Are hospitalized or ambulatory patients with heart failure treated in accordance with European Society of Cardiology guidelines? Evidence from 12440 patients of the ESC Heart Failure Long‐Term Registry. Euro J Heart Fail 2013; 15: 1173–1184. [DOI] [PubMed] [Google Scholar]
- 6. R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. http://www.R‐project.org/. [Google Scholar]
- 7. Adams KF Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP. ADHERE Scientific Advisory Committee and Investigators. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J 2005; 149: 209–216. [DOI] [PubMed] [Google Scholar]
- 8. Abraham WT, Fonarow GC, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, O'Connor CM, Sun JL, Yancy CW, Young JB. OPTIMIZE‐HF Investigators and Coordinators. Predictors of in hospital mortality in patients hospitalized for heart failure: insights from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE‐HF). J Am Coll Cardiol 2008; 52: 347–356. [DOI] [PubMed] [Google Scholar]
- 9. AlHabib KF, Elasfar AA, Alfaleh H, Kashour T, Hersi A, AlBackr H, Alshaer F, AlNemer K, Hussein GA, Mimish L, Almasood A, AlHabeeb W, AlGhamdi S, Alsharari M, Chakra E, Malik A, Soomro R, Ghabashi A, Al‐Murayeh M, AhAbuosa A. Clinical features, management, and short and long‐term outcomes of patients with acute decompensated heart failure: phase I results of the HEARTS database. Euro J Heart Fail 2014; 16: 461–69. [DOI] [PubMed] [Google Scholar]
- 10. The ACCESS Investigators . Management of acute coronary syndromes in developing countries: acute coronary events—a multinational survey of current management strategies. Am Heart J 2011; 162: 852–859.e22. [DOI] [PubMed] [Google Scholar]
- 11. Masoudi FA, Havranek PE, Smith G, Fish RH, Steiner JF, Ordin DL, Krumholz HM. Gender, age, and heart failure with preserved left ventricular systolic function. J Am Coll Cardiol 2003; 41: 217–223. [DOI] [PubMed] [Google Scholar]
- 12. El‐Zanaty F, Way A. Egypt Demographic and Health Survey 2008. Cairo, Egypt: Ministry of Health, El‐Zanaty and Associates, and Macro International; 2009. [Google Scholar]
- 13. Owan TE, Hodge DO, Herges RM, Jacobson SJ, Roger VL, Redfield MM. Trends in the prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006; 355: 251–9. [DOI] [PubMed] [Google Scholar]
- 14. Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow G. For the ADHERE scientific advisory. Clinical presentation, management, and in‐hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function. J Am Coll Cardiol 2006; 47: 76–84. [DOI] [PubMed] [Google Scholar]
- 15. Lee DS.,Gona P, Vasan RS, Larson MG, Benjamin EJ, Wang TJ, Tu JV, Levy D. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the Framingham Heart Study of the National Heart, Lung and Blood Institute. Circulation 2009; 119: 3070–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kitzman W, Gardin JM, Gottdiener JS, Arnold A, Boineau R, Aurigemma G, Marino EK, Lyles M, Cushman M, Enright PL. Importance of heart failure with preserved systolic function in patients > or = 65 years of age. CHS research group. Cardiovascular Health Study. Am J Cardiol 2001; 87: 413–9. [DOI] [PubMed] [Google Scholar]
- 17. Maisel WH, Stevenson LW. Atrial fibrillation in heart failure: epidemiology, pathophysiology and rationale for therapy. Am J Cardiol 2003; 91: 2D–8D. [DOI] [PubMed] [Google Scholar]
- 18. Mokdad AH, Jaber S, Abdel Aziz MI, AlBuhairan F, AlGhaithi A, AlHamad NM, Al‐Hooti SN, Al‐Jasari A, AlMazroa MA, AlQasmi AM, Alsowaidi S, Asad M, Atkinson C, Badawi A, Bakfalouni T, Barkia A, Biryukov S, El Bcheraoui C, Daoud F, Forouzanfar MH, Gonzalez‐Medina D, Hamadeh RR, Hsairi M, Hussein SS, Karam N, Khalifa SEAH, AKhoja TAM, Lami F, Leach‐Kemon K, Memish ZA, Mokdad AA, Naghavi M, Nasher J, Qasem MBH, Shuaib M, Al Thani AAM, Al Thani MH, Zamakhshary M, Lopez AD, Murray CJL. The state of health in the Arab world, 1990‐2010: an analysis of the burden of diseases, injuries and risk factors. Lancet 2014; 383: 309–20. [DOI] [PubMed] [Google Scholar]
- 19. Sourour KA. Rheumatic heart disease in Egypt: gloomy past and promising future. EHJ 2014; 66: 139–142. [Google Scholar]
- 20. Cohen Solal A, Beauvais F, Logeart D. Heart failure and diabetes mellitus: epidemiology and management of an alarming association. J Card Fail 2008; 14: 615–25. [DOI] [PubMed] [Google Scholar]
- 21. International Diabetes Federation . IDF Diabetes Atlas, 6th edn Brussels, Belgium: International Diabetes Federation; 2013. http://www.idf.org/diabetesatlas [Google Scholar]
- 22. WHO . Global Status Report on Non‐communicable Diseases, 2010. Geneva: World Health Organization; 2011. [Google Scholar]
- 23. Hussain Z, Swindle J, Hauptman PJ. Digoxin use and digoxin toxicity in the post‐DIG trial era. J Card Fail 2006; 12: 343–6. [DOI] [PubMed] [Google Scholar]
- 24. The Digitalis Investigation Group . The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med 1997; 336: 525–533. [DOI] [PubMed] [Google Scholar]
- 25. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck‐Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2012; 14: 803–869. [DOI] [PubMed] [Google Scholar]
- 26. El‐Sahn F, Sallam S, Mandil A, Galal O. Anaemia among Egyptian adolescents: prevalence and determinants. East Mediterr Health J 2000; 6: 1017–1025. [PubMed] [Google Scholar]
- 27. Hassan EO, El‐Hussein M, El‐Nahal N. The prevalence of anemia among clients of family planning clinics in Egypt. Contraception 1999; 60: 93–9. [DOI] [PubMed] [Google Scholar]


