ABSTRACT
Background
Alcohol-induced hangover constitutes a significant, yet understudied, global hazard and a large socio-economic burden. Old folk wisdoms such as “Beer before wine and you'll feel fine; wine before beer and you'll feel queer” exist in many languages. However, whether these concepts in fact reduce hangover severity is unclear.
Objectives
The aim of this study was to investigate the influence of the combination and order of beer and wine consumption on hangover intensity.
Methods
In this multiarm, parallel randomized controlled matched-triplet crossover open-label interventional trial, participants were matched into triplets and randomly assigned according to age, gender, body composition, alcohol drinking habits, and hangover frequency. Study group 1 consumed beer up to a breath alcohol concentration (BrAC) ≥0.05% and then wine to BrAC ≥0.11% (vice versa for study group 2). Control group subjects consumed either only beer or only wine. On a second intervention day (crossover) ≥1 wk later, study-group subjects were switched to the opposite drinking order. Control-group subjects who drank only beer on the first intervention received only wine on the second study day (and vice versa). Primary endpoint was hangover severity assessed by Acute Hangover Scale rating on the day following each intervention. Secondary endpoints were factors associated with hangover intensity.
Results
Ninety participants aged 19–40 y (mean age 23.9), 50% female, were included (study group 1 n = 31, study group 2 n = 31, controls n = 28). Neither type nor order of consumed alcoholic beverages significantly affected hangover intensity (P > 0.05). Multivariate regression analyses revealed perceived drunkenness and vomiting as the strongest predictors for hangover intensity.
Conclusions
Our findings dispel the traditional myths “Grape or grain but never the twain” and “Beer before wine and you'll feel fine; wine before beer and you'll feel queer” regarding moderate-to-severe alcohol intoxication, whereas subjective signs of progressive intoxication were confirmed as accurate predictors of hangover severity. This trial was prospectively registered at the Witten/Herdecke University Ethics Committee as 140/2016 and retrospectively registered at the German Clinical Trials Register as DRKS00015285.
Keywords: alcohol, hangover, veisalgia, beer, wine, headache, drunkenness, Kater
Introduction
Worldwide, excessive alcohol consumption is a major avoidable health risk (1–3). Besides the well-studied long-term sequelae, acute alcohol-induced hangover constitutes a significant, yet understudied, global hazard and a large burden to society (4, 5). Specifically, acute hangover-associated symptoms bring risk to daily tasks such as driving or operating heavy machinery (6). Socio-economic costs arise from reduced productivity, impaired professional performance [e.g., falling asleep at work (7)], workplace absenteeism, and academic underperformance (8).
Alcohol-induced hangover (also known as veisalgia) is characterized by a well-known symptom complex of unpleasant physical and mental symptoms that occur when elevated blood alcohol concentrations return to zero (9). Surprisingly, there are neither any sound pathophysiological hangover models nor any effective medical remedies (10). Instead, societies appear to rely on old folk aphorisms that exist in numerous languages and variations, e.g., “Beer before wine and you'll feel fine; wine before beer and you'll feel queer.” Similarly, the Germans say, “Wein auf Bier, das rat’ ich Dir—Bier auf Wein, das lass’ sein,” and the French say, “Bière sur vin est venin, vin sur bière est belle manière.” However, there are no currently available data to support or refute these sayings. To put an end to this uncertainty, we scientifically evaluated whether or not this time-honored wisdom truly reduces a hangover burden. We undertook a multiarm randomized controlled matched-triplet crossover open-label trial to test the effect of the order of beer and wine consumption on the next day's hangover severity.
Methods
Matched-triplet study design
A single-center multiarm parallel randomized controlled matched-triplet crossover open-label interventional trial was carried out over the course of the summer of 2017. Recruitment took place between September 2016 and June 2017. To enhance the statistical power, a matched-triplet study design was used. Triplets were generated, matching each 3 individuals of similar age, gender, weight, height, BMI, reported alcohol consumption rate, and hangover frequency, and then randomly assigned into 2 study groups and a control group using a balanced allocation ratio of 1:1:1. Two interventions were conducted in a crossover design with a washout period of ≥1 wk in between (Figure 1). Loss to follow-up in the study groups (but not in the control group, because the primary objective of this trial was to compare the 2 study groups) led to complete exclusion of the entire matched triplet across all study arms. Matching details for all 31 triplets are presented in Supplemental Table 1. Importantly, whereas female and male responses to alcohol consumption are known to be different (e.g., due to different amounts of gastric alcohol-metabolizing enzymes), this study was designed not primarily to compare gender-related differences but rather to determine the influence of the order in which beer and wine are consumed.
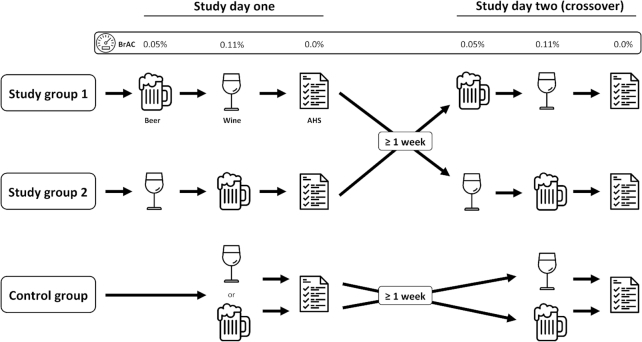
FIGURE 1.
Study design. On the first day, group 1 consumed beer until a breath alcohol concentration (BrAC) ≥0.05% was reached and afterwards wine until a BrAC ≥0.11% was reached. On day 2, they crossed over. Group 2 completed the opposite drinking regimen. Control-group subjects drank either only beer or only wine on day 1, with only the other drink on day 2. AHS ratings were recorded after the BrAC had returned to zero, on the day after each intervention. AHS, Acute Hangover Scale; BrAC, breath alcohol concentration.
Study setting
This study was conducted on the premises of Witten/Herdecke University between 1 July and 26 August 2017.
Study eligibility criteria
Volunteers were recruited from Witten/Herdecke University via an online survey, and 272 adults were screened for eligibility. Inclusion criteria were age between 18 and 60 y, a positive history of beer and wine consumption, good physical fitness, and the availability of 2 matching partners (to form a matched triplet). The study was approved by Witten/Herdecke University Ethics Committee and carried out in accordance with the Declaration of Helsinki's ethical principles for medical research involving human subjects. Written informed consent was obtained from each volunteer. Exclusion criteria were aversion to beer or wine (or both), a history of drug or alcohol abuse, complete alcohol abstinence or intolerance, Eastern Asian origin [due to common variants of genes coding for alcohol dehydrogenase (e.g., ADH1B, ADH1C) and acetaldehyde dehydrogenase (e.g., mitochondrial ALDH2 allele)], evidence of liver dysfunction (i.e., abnormal serum liver function tests), or history of any of the following: alcoholic liver disease, viral hepatitis, hepatocellular carcinoma, chronic pain, epilepsy, Wernicke encephalopathy, thiamine deficiency, Korsakov syndrome, gastritis, bariatric surgery, immunosuppression, or recent history of infection (i.e., respiratory, gastrointestinal, genitourinary, etc.). Further exclusion criteria were current pregnancy or breastfeeding, frequent use of pain medications, the use of medications known to interact with serum alcohol (i.e., via cytochrome 2E1, alcohol dehydrogenase, aldehyde dehydrogenase), e.g., antibiotics, opioids, nitrates, or antidepressant use.
Intervention
On study day 1, study group 1 consumed beer up to a breath alcohol concentration (BrAC) of ≥0.05% and subsequently drank wine to a BrAC of 0.11%, whereas participants in study group 2 consumed first wine and then beer to comparable BrAC limits. On study day 2 (≥1 wk later), each study group was switched to the opposite regimen. Control-group subjects consumed only beer or wine on study day 1 and switched to the other beverage on study day 2 (Figure 1).
All participants were asked to refrain from any alcohol consumption for 1 wk before each intervention. On each study day, the volunteers consumed food and water in usual amounts, as judged by each participant. Before each intervention, medical history was obtained, and all participants underwent a complete physical examination. Subsequently, all subjects received a standardized meal, as calculated according to their gender- and age-specific estimated mean energy requirements. Moreover, blood and urine test samples were acquired from all volunteers before alcohol consumption on the day of the intervention and on the day afterwards, when BrAC had returned to zero. All interventions took place under medical supervision.
The beer used in this study was a premium Pilsner lager recipe from 1847 by Carlsberg, with an alcohol content of 5%, served cold. We also used a 2015 Edelgräfler quality white wine (Chasselas blanc/Johanniter, Zähringer Winery; ECOVIN-, Bio-wine- and EU-Bio-certified, DE-ÖKO-039, A.P.-No 2,081,516), with an alcohol content of 11.1%, served cold at the same temperature as the beer.
Alcohol consumption could be terminated early according to the volunteer's personal preference or if safety concerns were raised (e.g., impaired level of consciousness, loss of orientation, impaired balance or gait, nystagmus, dysarthria, feeling unwell, nausea, tachycardia, neurologic symptoms, impairing psychomotor symptoms, prolonged reaction time, impaired protection reflexes, respiratory or cardiovascular abnormalities, illusionary misjudgment, etc.). BrAC was measured repetitively at 60-min intervals from start to termination of drinking and again on the following morning using an AlcoQuant 6020 + device from EnviteC/Honeywell. To minimize technical artifacts and standardize measurements, a 15-min nil-by-mouth interval was mandatory before each BrAC detection, as per the manufacturer's recommendation. To avoid any bias, measured BrAC levels were not disclosed to the participants during the study.
Participants were asked about their well-being at regular intervals and were asked to judge their perceived level of drunkenness on a scale between 0 and 10 at the end of each intervention. Upon completion of the intervention, all participants received an individualized amount of refrigerated drinking water (6 mL/kg body weight) to be consumed before going to sleep at the study site. Sleeping conditions (duration, room temperature) were similar for all participants, with all volunteers under medical supervision overnight.
Sample size
A priori, the study sample size was specified by a statistical power analysis primarily to compare the differences between the 2 study groups. In the absence of available pilot data, a pragmatic decision was taken; expecting a difference of 14% [=1 total Acute Hangover Scale (AHS) point per item] between the intervention arms and assuming a significance level of 5%, with a minimum statistical power of 80%, an effective sample size of 36 probands was targeted for each study group.
Randomization
Each matched triplet separately underwent stratified randomization according to a predetermined allocation ratio of 1:1:1 using a 6-sided dice. Moreover, control group subjects were further randomly assigned by the same means to drink either only beer or wine on study day 1 (and vice versa on study day 2). The “beverage of the day” was concealed at the time of study enrollment and only disclosed to the participants on the evening of the intervention by the respective research assistant under supervision.
Biostatistical analyses
To evaluate the primary hypothesis, a Welch 2-sample t-test was used at a 5% significance level. Data are described according to scale level, i.e., appropriate absolute and relative frequencies for categorical variables, medians, and quartiles (Tukey box plots) for continuous variables.
To analyze the primary aim of the study—the comparison of the alcohol-induced hangover severity subject to the order of beer and wine consumption—intraindividual differences were evaluated as indicated below (unpaired t-test):
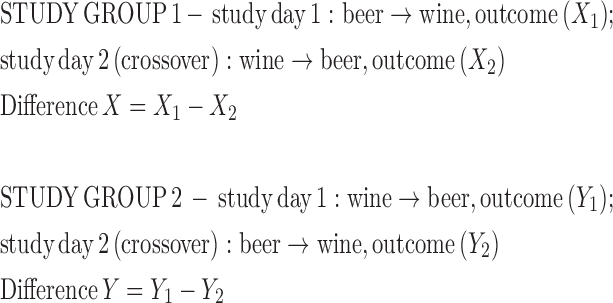 |
(1) |
Before the first intervention, we tested the potential carryover effect to ensure the washout phase (≥1 wk) was adequate.
A multivariate linear regression model was modified for the target variable AHS. Specifically, exploratory analyses were performed by means of multiple linear regression modeling/analysis of covariance (backward model selection via Akaike information criteria); results of the optimally fitted models were presented by means of unadjusted 95% CIs for respective regression coefficients.
Outcomes
The primary endpoint was the reported hangover severity on the day following each intervention. Hangover intensity was scored by (11) an 8-item compound score (including thirst, fatigue, headache, dizziness, nausea, stomach ache, tachycardia, and loss of appetite). Each item was rated between 0 and 7 by the study subject on the day following the intervention, once BrAC had returned to zero. A score of 56 corresponds to the worst imaginable hangover, 0 represents the absence of hangover symptoms. The secondary endpoints were factors associated with hangover intensity (e.g., demographics, laboratory parameters, etc.).
Results
Study population
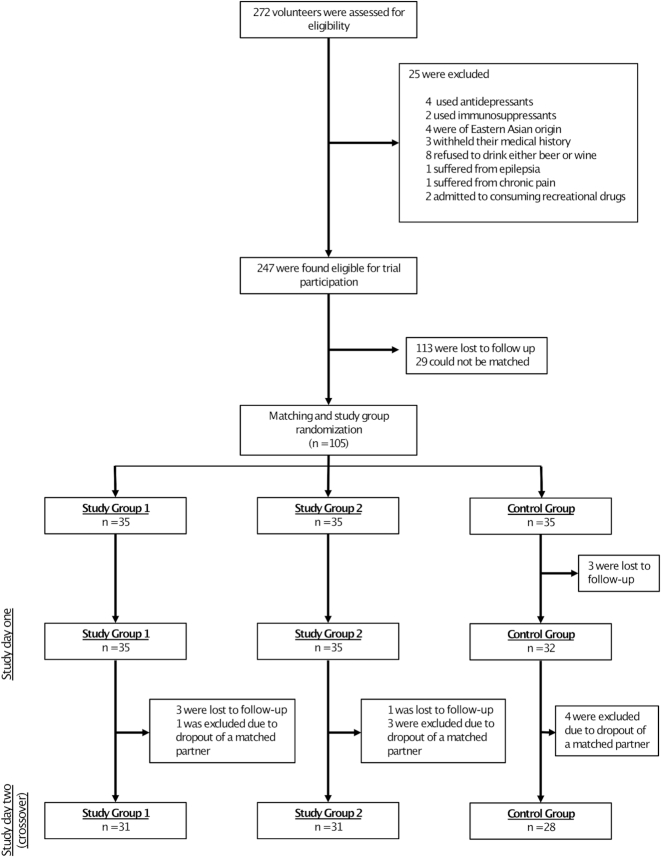
Enrollment and randomization details are shown in Figure 2. Out of the 272 volunteers that were assessed for eligibility, 247 were found eligible, and 105 could be matched/randomly assigned. Ninety completed the trial and underwent the per protocol analyses (study group 1: n = 31; study group 2: n = 31; control group: n = 28). The trial ended on 26 August 2017 as planned, after all intended data were collected. The difference in intended and final sample size is explained by the high loss to follow-up. The principal reason for loss to follow-up provided by dropped-out volunteers was a time overlap of the trial with academic exam periods and/or holidays.
FIGURE 2.
Enrollment and randomization.
Table 1 outlines the baseline demographics of the study population (mean ± SD). Participants were between 19 and 40 y old, and gender distribution was similar between the 3 groups. Body-composition indices such as BMI, weight, and height were comparable in all groups. The mean alcohol consumption rate was several times per month, and hangover frequency was reported to be between “rarely occurring” and “a few times per month” for all groups.
TABLE 1.
Baseline demographics of the study population
| Study group 1 (n = 31) | Study group 2 (n = 31) | Control group (n = 28) | P value | |
|---|---|---|---|---|
| Age, years | 23.9 ± 5.8 | 24.3 ± 5.3 | 23.6 ± 5.7 | NS |
| Female gender, % | 48.4 | 48.4 | 53.6 | NS |
| Body weight, kg | 71.2 ± 11.1 | 69.9 ± 10.9 | 69.8 ± 10.5 | NS |
| Height, cm | 178.4 ± 8.2 | 176.6 ± 8.7 | 176.1 ± 8.6 | NS |
| BMI, kg/m2 | 22.3 ± 2 | 22.3 ± 2 | 22.4 ± 1.9 | NS |
| Alcohol consumption rate1 | 2.8 ± 0.9 | 2.5 ± 0.9 | 2.8 ± 0.9 | NS |
| Hangover frequency1 | 1.3 ± 0.9 | 1.6 ± 1.1 | 1.2 ± 0.8 | NS |
10 = rarely, 1 = once monthly, 2 = more than once monthly and less than once weekly, 3 = once weekly, 4 = more than once weekly; data are presented as means ± SDs, P values calculated with ANOVA. NS, not significant (P > 0.05).
Alcohol-induced hangover intensity
Before analyzing the primary objective, the occurrence of a carryover effect was tested. As expected, AHS ratings added from both study groups for each participation were not significantly different between the 2 intervention days; in other words, no carryover effect was detected (P = 0.8).
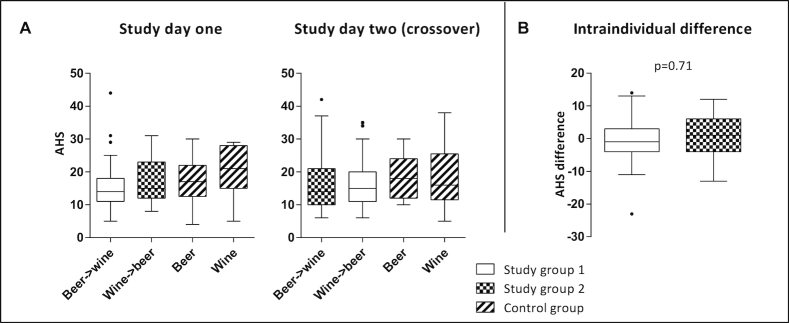
None of the 3 groups had a significantly different AHS rating with different orders of beverage (Figure 3A, P > 0.05). The comparison of intraindividual AHS rating difference X in study group 1 [–0.32 ± 7.63 (mean ± SD)] and difference Y in study group 2 (0.36 ± 6.82), i.e., the primary study endpoint, was not statistically significantly different (Figure 3B, P = 0.71). Individual AHS items and non-AHS alcohol-induced symptoms are presented in Supplemental Table 2. On study day 1, 7 of the 28 control-group subjects drank only beer, and 21 drank only wine (and vice versa for study day 2).
FIGURE 3.
Hangover severity in relation to alcohol consumption. (A) “Grape or grain but never the twain?”—AHS ratings (0 representing the absence of symptoms and 56 corresponding to the maximal hangover intensity) of all participants demonstrated for both intervention days according to type/order of consumed alcoholic beverages. (B) “Beer before wine and you'll feel fine; wine before beer and you'll feel queer?”—comparison of intraindividual differences of AHS ratings, according to the order in which beer and wine were consumed, depicted for the 2 study groups. Data are presented as medians and quartiles using Tukey box plots; to compare intraindividual differences of AHS ratings (B) Welch's two-sample t-test was used at a 5% significance level. AHS, Acute Hangover Scale.
To factor in the variation in peak BrAC (underlying the subsequent day's hangover), we calculated relative AHS—as the ratio of AHS and peak BrAC for each participant—and compared all groups (Supplemental Figure 1). Importantly, relative AHS results were found to be in line with “uncorrected” AHS ratings (Figure 3).
Trial progress specifics
Details regarding the interventional alcohol consumption are outlined in Table 2. The switch from wine to beer occurred earlier than with the opposite regimen. Control-group subjects who drank only wine reached the maximum BrAC considerably earlier than other (sub-) groups, but peak BrAC was similar in all groups.
TABLE 2.
Trial progress specifics1
| Study group 1 (n = 31) | Study group 2 (n = 31) | Control group (n = 29) | ||||
|---|---|---|---|---|---|---|
| Beer → wine | Wine → beer | Wine → beer | Beer → wine | Beer | Wine | |
| Beer consumption, L | 1.4 ± 0.3 | 1.3 ± 0.4 | 1.2 ± 0.4 | 1.3 ± 0.3 | 2.6 ± 0.7 | – |
| Wine consumption, L | 0.6 ± 0.2 | 0.6 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.2 | – | 1.2 ± 0.3 |
| Time to switch, h | 2.3 ± 0.6 | 1.8 ± 0.5 | 1.7 ± 0.5 | 2.3 ± 0.6 | – | – |
| BrAC at switch, % | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.06 ± 0.01 | – | – |
| Time to peak, h | 4.1 ± 0.7 | 4.6 ± 0.9 | 4.3 ± 1 | 4.3 ± 0.7 | 4.6 ± 0.9 | 3.6 ± 0.9 |
| Peak BrAC, % | 0.12 ± 0.01 | 0.12 ± 0.02 | 0.12 ± 0.01 | 0.13 ± 0.02 | 0.12 ± 0.01 | 0.13 ± 0.02 |
1Data are presented as means ± SDs. BrAC = breath alcohol concentration.
Factors associated with hangover intensity
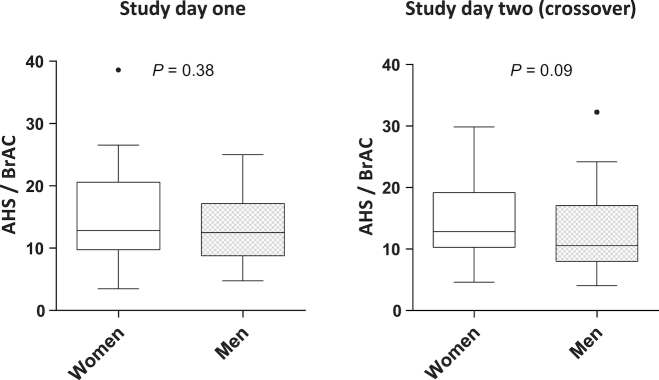
Secondary study endpoints were the evaluation of factors associated with hangover intensity. Interestingly, for both intervention days, women reported increased hangover severity, as compared with men (data not shown, P = 0.009). After adjusting for the variation in peak BrAC (relative AHS as the ratio of AHS and peak BrAC was compared separately for women and men), the same tendency of increased relative AHS ratings was found for female participants, without reaching statistical significance (Figure 4).
FIGURE 4.
Gender-specific comparison of relative AHS ratings (45 women, 45 men). Data are presented as medians and quartiles using Tukey box plots. AHS, Acute Hangover Scale; BrAC, breath alcohol concentration.
Although most participants had similar relative AHS ratings on both days, a subset showed an increased difference between the 2 interventions. With the aim to identify features that might predict a major difference in AHS as a result of the order of beverage, we performed a further cluster analysis. Volunteers with an AHS difference >8 (n = 20) and those with an AHS difference ≤8 (n = 70) were compared regarding both intervention days separately. No significant features were found in this subgroup analysis (data not shown).
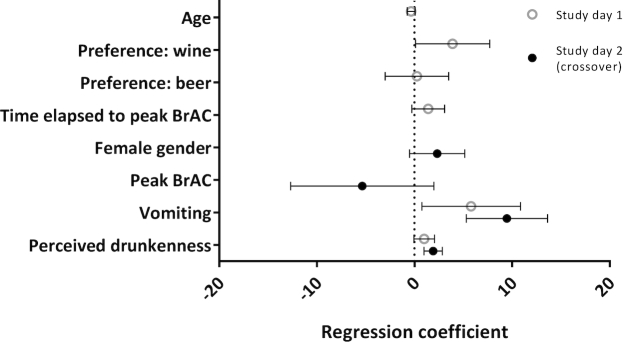
To determine the most important factors driving an increased hangover intensity, we modified a prespecified multivariate linear regression model for the target variable AHS including 89 participants for study day 1 and 88 for study day 2. For both intervention days separately, the occurrence of vomiting and perceived drunkenness was associated with increased AHS ratings (Figure 5).
FIGURE 5.
Multiple linear regression analysis. Analyses were performed separately for study day 1 (n = 89) and study day 2 (n = 88). The target variable was the subjects’ Acute Hangover Scale ratings reported on the day following each intervention. Data are presented as regression coefficients and 95% CIs. BrAC, breath alcohol concentration.
We undertook blood and urine tests before each intervention and on the following day (Supplemental Table 3). There was no significant difference in blood or urine test results between the 3 groups, either before or on the day following the intervention (P > 0.05). Multiple linear regression analyses performed individually for both the first intervention and the crossover study day did not yield any consistent strong predictive factors for increased hangover intensity (data not shown).
Adverse events
Vomiting occurred more often in the control group (wine only, n = 6; beer only, n = 5), than in the study groups (n = 2–4, Supplemental Table 2) and more women than men vomited both on study day 1 (5 compared with 4) and on study day 2 (8 compared with 4).
Discussion
In this randomized controlled multiarm matched-triplet crossover open-label interventional study, we were unable to confirm that the well-known folklore of drinking “beer before wine” purportedly results in a worse hangover than drinking “wine before beer.” Although this should rob tactical drinkers of the belief that they can reduce the aftereffects of a heavy night out by careful ordering of beverages, our findings suggest that “perceived drunkenness” and “vomiting” are useful predictors of misery in the morning after the night before. Furthermore, this is in line with the recent observation that no level of alcohol consumption improves health (12).
The fact that we did not find a direct correlation between maximal BrAC and hangover intensity should not be misinterpreted as an invitation to drink until the cows come home. Likely, this correlation overall does exist but is not directly apparent in the narrow range of peak alcohol levels studied here. Moreover, whereas the exact underlying etiology of alcohol-induced hangover remains unclear, the list of suggested causes is long and includes various mechanisms such as dehydration, proinflammatory cytokine perturbation, and endocrine and metabolic alterations (13, 14). These pathophysiological patterns are likely also to be influenced by ingredients other than the pure alcohol content of a given beverage. Congeners (substances that color and flavor drinks) in alcoholic beverages have been suggested as causative agents for the presence and severity of hangovers (13). This could be a valid explanation for why, at the same alcohol concentration, bourbon causes a more severe hangover than vodka (15). In addition, individual tolerance (e.g., due to genotype variation) and habituation to alcohol intake (altered degradative enzyme activity) are likely to play a key role for variations in hangover predisposition. Probably the most extreme example for this is the report of the surprising survival, with minimal hangover, after a serum ethanol concentration of 1.15% (16)—almost 10 times higher than peak values in our study population, and far exceeding reported lethal levels (17, 18). Interestingly, even though gender differences in alcohol metabolization have been described, we did not find a difference between females and males with regard to hangover severity in this study.
Although we chose a study design robust enough to provide a definitive result for this age-old folklore, we are aware of the study limitations. The exclusive usage of lager beer and white wine may limit generalizability, never mind assessing a recommendation from a time where both the beer and the wine are likely to have been very different. The lack of blinding can also be regarded as a study limitation. However, early efforts (a preliminary pretrial performed in search of optimal study methodology) made clear that effective blinding of beer or wine is not feasible, even in the least experienced drinker. Moreover, including a control group that received beer or wine without alcohol proved impossible, because real dissatisfaction and envy were reported by potential alcohol-free controls, because it became clear they might not be randomly assigned to the ever-so-happy booze-sipping study groups. We even noted surreptitious attempts to switch into the alcohol-consuming study groups during a pilot intervention, with underhand smuggling maneuvers, and a subsequent high loss to follow-up in the nonalcoholic control group. This is completely in line with findings from Rohsenow and Marlatt (19), who also found that at alcohol concentrations exceeding 0.08%, blinding is ineffective (because participants became aware of alcohol intoxication). In their consensus statement on best practice in alcohol hangover research, the Alcohol Hangover Research Group does suggest approaches to enhance blinding, such as the addition of mint as a strong flavor disguise and applying nose-clips to the participants (4). Both these measures were deemed inappropriate by the investigators of the present study: both out of respect for the brewers and vintners, and to prevent nasal trauma during the 5-h-long interventions.
In summary, our findings debunk the age-old myths “Grape or grain but never the twain” and “Beer before wine and you'll feel fine; wine before beer and you'll feel queer” with regard to moderate-to-severe alcohol intoxication. We further remind readers to take heed of red flags such as perceived drunkenness and vomiting to reduce hangover intensity. Finally, one should be mindful of the important benefits of a symptomatic hangover—a protective warning sign that will certainly have aided humans over the ages to modify future behavior, and hence pass on this evolutionary advantage to next generations. Cheers!
Supplementary Material
Acknowledgements
We thank Carlsberg for the beer donation; Frank Krummenauer for biostatistical input; Marc Baenkler, Parviz Ajmad-Nejad, and Beniam Ghebremedhin (HELIOS University Hospital Wuppertal) for support with laboratory investigations; Nicola M. Tomas and Andreas Jenke for their input regarding manuscript preparation; Rob Heuschkel and RRR for polishing up the wording; and Patrick Rebacz (www.visionom.de, Witten/Herdecke University), Good Ware, Webalys Freebies, and itim2101 (www.flaticon.com) for their help with the graphic/icon design for Figure 1.
The authors’ responsibilities were as follows—JK: helped to develop the protocol and obtain ethical consent, conducted the experiments, collected data, helped prepare the tables, and critically revised the final manuscript; BG: performed the biostatistical analyses and prepared the figures; SW: was involved in conducting the experiments and data collection, and critically reviewed the manuscript; KOH: conceived the study (project conception, development of overall research plan, and study oversight), wrote the initial protocol and ethical consent request, helped prepare the figures, interpreted the data, wrote the manuscript, and is the guarantor for the study; and all authors had access to the full dataset, take responsibility for the integrity of the data and the accuracy of the dataset, and gave final approval for the submission of this version for consideration of publication. The authors report no conflict of interest related to research presented in this article.
Notes
This study was supported by a beer donation from Carlsberg, which provided the beer free of charge to the investigators for the sole purpose of utilization in this study and had no role in the design, conduct, or analyses of the trial.
Supplemental Tables 1–3 and Supplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
A full data set is available from the corresponding author on reasonable request.
Abbreviations used: AHS, Acute Hangover Scale; BrAC, breath alcohol concentration.
References
- 1. Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373(9682):2223–33. [DOI] [PubMed] [Google Scholar]
- 2. GBD 2016 Alcohol Collaborators. Alcohol use and burden for 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392(10152):1015–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ma C, Bovet P, Yang L, Zhao M, Liang Y, Xi B. Alcohol use among young adolescents in low-income and middle-income countries: A population-based study. Lancet Child Adolesc Health. 2018;2(6):415–29. [DOI] [PubMed] [Google Scholar]
- 4. Verster JC, Stephens R, Penning R, Rohsenow D, McGeary J, Levy D, McKinney A, Finnigan F, Piasecki TM, Adan A et al.. The alcohol hangover research group consensus statement on best practice in alcohol hangover research. Curr Drug Abuse Rev. 2010;3(2):116–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Williams R, Alexander G, Armstrong I, Baker A, Bhala N, Camps-Walsh G, Cramp ME, de Lusignan S, Day N, Dhawan A et al.. Disease burden and costs from excess alcohol consumption, obesity, and viral hepatitis: Fourth report of the Lancet Standing Commission on Liver Disease in the UK. Lancet. 2018;391(10125):1097–107. [DOI] [PubMed] [Google Scholar]
- 6. Gunn C, Mackus M, Griffin C, Munafo MR, Adams S. A systematic review of the next-day effects of heavy alcohol consumption on cognitive performance. Addiction. 2018. doi:10.1111/add.14404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ames GM, Grube JW, Moore RS. The relationship of drinking and hangovers to workplace problems: An empirical study. J Stud Alcohol. 1997;58(1):37–47. [DOI] [PubMed] [Google Scholar]
- 8. Frone MR. Prevalence and distribution of alcohol use and impairment in the workplace: A US national survey. J Stud Alcohol. 2006;67(1):147–56. [DOI] [PubMed] [Google Scholar]
- 9. Woo YS, Yoon SJ, Lee HK, Lee CU, Chae JH, Lee CT, Kim DJ. Concentration changes of methanol in blood samples during an experimentally induced alcohol hangover state. Addict Biol. 2005;10(4):351–55. [DOI] [PubMed] [Google Scholar]
- 10. Pittler MH, Verster JC, Ernst E. Interventions for preventing or treating alcohol hangover: Systematic review of randomised controlled trials. BMJ. 2005;331(7531):1515–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rohsenow DJ, Howland J, Minsky SJ, Greece J, Almeida A, Roehrs TA. The Acute Hangover Scale: A new measure of immediate hangover symptoms. Addict Behav. 2007;32(6):1314–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Burton R, Sheron N. No level of alcohol consumption improves health. Lancet. 2018;392(10152):987–88. [DOI] [PubMed] [Google Scholar]
- 13. Verster JC. The alcohol hangover—A puzzling phenomenon. Alcohol Alcohol. 2008;43(2):124–26. [DOI] [PubMed] [Google Scholar]
- 14. Kim DJ, Kim W, Yoon SJ, Choi BM, Kim JS, Go HJ, Kim YK, Jeong J. Effects of alcohol hangover on cytokine production in healthy subjects. Alcohol. 2003;31(3):167–70. [DOI] [PubMed] [Google Scholar]
- 15. Rohsenow DJ, Howland J, Arnedt JT, Almeida AB, Greece J, Minsky S, Kempler CS, Sales S. Intoxication with bourbon versus vodka: Effects on hangover, sleep, and next-day neurocognitive performance in young adults. Alcohol Clin Exp Res. 2010;34(3):509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Johnson RA, Noll EC, Rodney WM. Survival after a serum ethanol concentration of 1 1/2%. Lancet. 1982;2(8312):1394. [DOI] [PubMed] [Google Scholar]
- 17. Heatley MK, Crane J. The blood alcohol concentration at post-mortem in 175 fatal cases of alcohol intoxication. Med Sci Law. 1990;30(2):101–5. [DOI] [PubMed] [Google Scholar]
- 18. Koch-Weser J, Sellers EM, Kalant H. Alcohol intoxication and withdrawal. N Engl J Med. 1976;294(14):757–62. [DOI] [PubMed] [Google Scholar]
- 19. Rohsenow DJ, Marlatt GA. The balanced placebo design: Methodological considerations. Addict Behav. 1981;6(2):107–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.