Abstract
Background
Most observations of non-intubated anesthesia under spontaneous breathing are small-cohort, non-homogeneous surgery types and lack an intubation control. We therefore retrospectively compared the perioperative conditions and postoperative recovery of non-intubated video-assisted thoracoscopic surgery (NIVATS group) and intubated VATS (IVATS group) with a propensity score-matching analysis.
Material/Methods
We case-matched 119 patients in the NIVATS group with patients in the IVATS group by a propensity score-matched analysis. All of them underwent lobectomy.
Results
In the NIVATS group, operative and anesthesia times were significantly shorter (P<0.01). NIVATS showed a faster and more stable recovery in the PACU, postoperative awaking and post-anesthesia care unit (PACU) stay times was shorter (P<0.01), and use of sedatives and analgesics was lower (P<0.05). The incidence of pulmonary exudation, atelectasis, and pleural effusion were higher (P<0.05). Although intraoperative SpO2 was lower and PETCO2 was higher in the NIVATS group (P<0.01), postoperative PaCO2 and SaO2 in both groups were similar (P>0.05). Postoperative counts of leukocytes and neutrophils and hemoglobin levels also had no difference between the 2 groups (P>0.05).
Conclusions
NIVATS has a more rapid and stable recovery in the PACU, and has no significant influence on oxygenation, but is more likely to cause postoperative radiologic complications.
MeSH Keywords: Intubation, Intratracheal; Postanesthesia Nursing; Thoracic Surgery, Video-Assisted
Background
Thoracic anesthesia under spontaneous ventilation is not a recent innovation. Awake thoracic surgery was first proposed to be performed under thoracic epidural anesthesia in 1950 by Buckingham [1]. A few years later, Vischnevski performed major lung resections and even esophagectomies under a multistep local analgesia [2].
Non-intubated video-assisted thoracoscopic surgery (VATS) gradually gained popularity in the past 2 decades. In 1998, Mukaida first reported thoracoscopic surgery for pneumothorax under local and epidural anesthesia [3]. The successful debut of non-intubated anesthesia with spontaneous breathing for VATS wedge resection of lung masses was reported in 2004 [4]. Non-intubated anesthesia with spontaneous breathing has been widely performed in a variety of VATS procedures, such as lung biopsies [5], mediastinal tumors [6], bullectomy [3,7], metastatic tumors [8], empyema thoracis [9], emphysematous bulla [7], wedge resections [10], segmentectomy [11], lobectomies [12,13], and even lung-volume reduction surgery was safe and feasible using awake VATS technique, with similar or even better results [14]. All of these studies yielded encouraging results.
However, small sample size, use of a variety of procedures, and the absence of standard VATS control under intubation make these studies less convincing [3,8,13,15,16]. In addition, few of these studies observed the pulmonary gas exchange during procedures and most ignored the early recovery changes in the PACU, which reduce the credibility of the results. Therefore, we performed a more delicately designed study to shed light on the issue and obtain a more well-established theory.
Non-intubated VATS had been used at our institute for various diagnostic and therapeutic procedures since 2011 [17,18], and this provided a basis for our study. The aim of this study was to retrospectively compare the perioperative conditions of non-intubated VATS and intubated VATS with large-cohort design using a propensity score-matching analysis. Our primary hypothesis was that non-intubated VATS is superior in PACU, and the secondary hypothesis was that non-intubated VATS has no significant influence on pulmonary gas exchange during the operation.
Material and Methods
Study design
The study design was reviewed and approved by the First Affiliated Hospital of Guangzhou Medical University Research Ethics Committee. All patients provided informed consent and the data were collected from the institutional medical records database.
All cases dated up to December 2011 received intubated anesthesia, whereas those dated after December 2011 received non-intubated anesthesia. From January 2012 onwards, patients were able to choose the anesthesia type (NIVATS or IVATS anesthesia) and gave their consent after the procedures of the 2 anesthesia types were explained in detail. In fact, 983 patients underwent VATS with double-lumen tube intubation anesthesia (IVATS group) from January 1, 2010 to December 30, 2011 and 798 patients underwent VATS with non-intubation spontaneous breathing anesthesia (NIVATS group) from January 1, 2012 to December 30, 2014. There were no major changes in the approach of surgery during this period. In order to counterbalance the discrepancies between the 2 groups, propensity score-matching analysis was used to minimize the selection bias between the 2 groups. Two comparable patient groups who underwent lobectomy (n=119 for each group) were identified using this method. The baseline characteristic of the propensity score-matched pairs is listed in Tables 1 and 2.
Table 1.
Preoperative patients’ characteristic of VATS lobectomy after matching.
| Variable | IVATS group (n=119) | NIVATS group (n=119) | P value |
|---|---|---|---|
| Age (years) | 55.34±13.83 | 56.98±11.05 | 0.311 |
|
| |||
| Gender (M/F) | 68/51 | 69/50 | 0.896 |
|
| |||
| Body mass index (Kg/m2) | 22.40±2.85 | 22.51±2.57 | 0.756 |
|
| |||
| ASA physical status class (N. [%]) | 0.623 | ||
| I | 95 (79.8) | 98 (82.4) | |
| II | 23 (19.3) | 20 (16.8) | |
| III | 1 (0.8) | 1 (0.8) | |
|
| |||
| Comorbidity (N. [%]) | 0.290 | ||
| Cardiovascular diseases | 9 (7.6) | 8 (6.7) | |
| Diabetes | 4 (3.4) | 6 (5.0) | |
| Pulmonary diseases | 7 (5.9) | 2 (1.7) | |
| Prior Thoracic Surgery | 4 (3.4) | 1 (0.8) | |
| None | 95 (79.8) | 102 (85.7) | |
|
| |||
| LVEF | 71.40±6.43 (n=92) | 71.68±5.90 (n=107) | 0.749 |
|
| |||
| Cardiac Risk index (N. [%]) | 0.562 | ||
| 1 point | 117 (98.3) | 118 (99.2) | |
| 2 points | 2 (1.7) | 1 (0.8) | |
|
| |||
| Preoperative hospital stay (d) | 9 (7, 13) | 8 (5, 12) | 0.934 |
|
| |||
| Preoperative Leukocyte (×109) | 6.86±1.96 | 6.46±1.83 | 0.104 |
|
| |||
| Preoperative Neutrophil (%) | 59.43±11.92 | 59.36±9.50 | 0.960 |
|
| |||
| Preoperative Hemoglobin (g) | 134.08±15.10 | 132.77±14.239 | 0.494 |
|
| |||
| Pulmonary function tests (%) | |||
| FVC% predicted | 97.70±17.04 (n=82) | 96.56±17.35 (n=110) | 0.649 |
| FEV1% predicted | 90.74±20.74 (n=82) | 90.96±16.64 (n=110) | 0.935 |
| FEV1/FVC | 92.40±19.60 (n=82) | 97.61±10.45 (n=110) | 0.019 |
Table 2.
Intraoperative variables.
| Variables | IVATS group (n=119) | NIVATS group (n=119) | P value |
|---|---|---|---|
| Pathologic diagnosis (N. [%]) | 0.000 | ||
| Benign/malignant | 37 (31.1)/82 (68.9) | 10 (8.4)/109 (91.6) | 0.000 |
| Primary/metastatic | 80 (97.6)/2 (2.4) | 106 (97.2)/3 (2.8) | 0.487 |
|
| |||
| Surgical location (N. [%]) | 1.000 | ||
| Left/right lung | 44 (37.0)/75 (63.0) | 44 (37.0)/75 (63.0) | |
|
| |||
| The number of lobes involved in surgery (N. [%]) | 0.270 | ||
| Upper lobe | 50 (42.0) | 57 (47.9) | |
| Middle lobe | 7 (5.9) | 7 (5.9) | |
| Lower lobe | 53 (44.5) | 50 (42.0) | |
| Two lobes | 9 (7.6) | 5 (4.2) | |
|
| |||
| Operative time (min) | 217.64±59.71 | 175.63±55.67 | 0.000 |
|
| |||
| Anesthesia time (min) | 301.87±63.69 | 250.63±55.67 | 0.000 |
|
| |||
| Blood loss (ml) | 100 (50, 300) | 50 (30, 120) | 0.000 |
|
| |||
| The amount of liquid infusion (ml) | 1822.29±536.64 | 2105.04±520.24 | 0.000 |
|
| |||
| Urine volume (ml) | 700 (488, 1000) | 850 (700, 1000) | 0.002 |
|
| |||
| The lowest intraoperative SpO2 (%) | 99.10±1.75 | 97.12±3.19 | 0.000 |
|
| |||
| The highest intraoperative PETCO2 (mmHg) | 37.93±3.50 | 48.02±8.90 | 0.000 |
Anesthesia time began from the first electronically monitored observation in the operation room to until the time the patient was transferred to PACU. Operative time was comprised between skin incision and completion of skin closure.
Eligibility criteria for NIVATS group
Exclusion criteria were: younger than age 18 years, American Society of Anesthesiologists (ASA) physical status IV or higher, morbidly obese, bleeding disorders, sleep apnea, compromised coagulation, unfavorable airway or spinal anatomy, preoperative decompensated heart diseases, expected dense or extensive pleural adhesions, noncompliance to the procedure, and patient refusal [13,14].
Anesthesia
Non-intubation VATS group
Anesthetic techniques were performed as described previously [18]. Patients received thoracic epidural anesthesia, or LMA Classic (Tele flex, Sweetmeat, Ireland) insertion combined with intrathoracic vagal blockade and intercostal nerve blockade. An epidural catheter was inserted at the T6/T7 or T8/T9 thoracic interspace. We administered 2% lidocaine 2 mL as a testing dose and 0.375–0.5% ropivacaine was used to attain a sensory block between the T2 and T12 dermatomes. Mask- and nasopharyngeal airway-assisted ventilation was provided with an oxygen flow of 3–5 L/min. Sedation was initiated by intravenous infusion of remifentanil and propofol. LMA was inserted after anesthetic induction, allowing spontaneous ventilation.
At the end of procedure, the collapsed lung was re-expanded with positive pressure through mask ventilation or negative-pressure suction through the chest tube. Intravenous drugs were stopped immediately and the epidural catheter was removed. Patients were transferred to the PACU, then to the ward or ICU according to the evaluation of their preoperative cardio-pulmonary function and intraoperative conditions.
Intubation VATS group
In the IVATS group, a Mallinckrodt double-lumen endobronchial tube (DLT, Medtronic, Minneapolis, MN) was inserted after induction with propofol, sufentanil, and neuromuscular blockade. Proper tube position was confirmed by flexible fiberoptic bronchoscopy. Protective ventilation strategy was commenced with tidal volumes 5–6 mL/kg and positive end-expiratory pressure 5 cmH2O, with peak pressure of under 30 cmH2O. Patients were normally extubated in the PACU or occasionally remained intubated, then were transferred to the ICU or ward.
Surgical procedure
The procedures in NIVATS and IVATS group were similar. Thoracoscopic lobectomy was performed using 3-port VATS, as described by McKenna [19]. One 22Ch chest drainage tube was inserted at the end of the procedure.
In the NIVATS group, 1% lidocaine was used as an intercostal block. Lung collapse was achieved by iatrogenic pneumothorax in the NIVATS group and by DLT one-lung ventilation in the IVATS group. Cough reflex was effectively suppressed by vagus nerve block or 2% lidocaine spraying on the surface of the lung under direct thoracoscopic vision.
Criteria for discharge from the PACU were standardized, including stable clinical conditions with an oxygen saturation of 90% or above at rest; effective control of nausea, vomiting, and pain; and Alderete score >9. Postoperative chest radiograph, leukocyte, neutrophil, hemoglobin, and arterial blood gas analyses were tested immediately after the operation.
Statistical analysis
All statistical analyses were performed using JMP, version 9, for Windows (JMP, Cary, NC). The missing data of BMI and preoperative leukocyte, neutrophil, and hemoglobin values were replaced by series mean. A propensity score-matching analysis was used to minimize the selection bias between the NIVATS and IVATS groups. The propensity score-matching was conducted using R 3.4.0 [20].
Continuous data were presented as the mean ± standard deviations for normal distribution, or as median (lower and upper quartiles) for skewness distribution. Dichotomous data were presented as numbers (%). The differences between the 2 groups were analyzed by the independent samples t test for continuous data and the Mann-Whitney U test was used for dichotomous data and skewed distributed data. The preoperative and postoperative counts of leukocytes, neutrophils, and hemoglobin levels were analyzed by paired t test and analysis of covariance (ANCOVA). The comparisons of drugs used in the PACU and chest radiograph results between the 2 groups were analyzed by Binomial test. P<0.05 was considered to be statistically significant.
Results
The significant skewed distributed data were preoperative hospital days, blood loss and urine volume. Patient characteristics had no difference between the 2 groups after matching, except for FEV1/FVC (P=0.019), but it was still in normal range (Table 1).
In the NIVATS group, the number of cases of malignant disease was higher, the operation and anesthesia time was significantly shorter (P<0.01), blood loss was lower, but urine volume was higher (P<0.05), and the lowest SpO2 was lower but the highest PETCO2 was higher (P<0.01) (Table 2).
The NIVATS group had a faster and more stable recovery in the PACU. The postoperative awaking time and PACU stay time were shorter (P<0.01). Although there was no difference in total drugs usage in the PACU between the 2 groups (P>0.05), the use of sedatives, analgesics, and urapidil were lower in the NIVATS group (P<0.05) (Table 3).
Table 3.
Early recovery in Post-Anesthesia Care Unit.
| Variables | IVATS group (n=119) | NIVATS group (n=119) | P value |
|---|---|---|---|
| Extubation time (min) | 30.92±9.07 (n=119) (Double-lumen tube) | 20.38±6.28 (n=13) (Laryngeal mask) | 0.000 |
|
| |||
| Post-operative awaking time (min) | 46.89±10.54 | 21.48±6.74 | 0.000 |
|
| |||
| Vital signs before leaving PACU | |||
| HR (bpm) | 81.77±10.96 | 79.71±10.44 | 0.139 |
| MAP (mmHg) | 81.63±12.29 | 78.51±12.35 | 0.052 |
| SpO2 (%) | 99.13±0.82 | 99.24±0.83 | 0.310 |
|
| |||
| Drugs usage in PACU (N. [%]) | |||
| Neostigmine + atropine | 82 (68.9) | 0 (0.0) | 0.142 |
| Sedatives (midazolam, propofol) | 27 (22.7) | 14 (11.8) | 0.000 |
| Analgesics (tramadol, opioids, NSAIDs) | 47 (39.5) | 24 (20.2) | 0.002 |
| Naloxone | 2 (1.7) | 3 (2.5) | 0.000 |
| Flumazenil | 0 (0.0) | 4 (3.4) | 0.330 |
| Urapidil | 17 (14.3) | 6 (5.0) | 0.001 |
|
| |||
| Staying in PACU time (min) | 61.60±10.27 | 37.24±7.27 | 0.000 |
Staying in PACU time was defined as the time from arrival in the PACU until the patient was discharged to the ward.
The chest radiograph results were significantly different between the 2 groups (P<0.05), and the incidences of pulmonary exudation, atelectasis, and pleural effusion were higher in the NIVATS group (P<0.05) (Table 4).
Table 4.
Postoperative chest radiography results.
| Variables | IVATS group (n=119) | NIVATS group (n=119) | P value |
|---|---|---|---|
| Chest radiograph results (N. [%]) | 0.009 | ||
| Normal | 95 (79.8) | 78 (65.5) | 0.000 |
| Pulmonary exudation | 11 (9.2) | 18 (15.1) | 0.025 |
| Atelectasis | 3 (2.5) | 19 (16.0) | 0.000 |
| Pleural effusion | 2 (1.7) | 0 | 0.000 |
| Pneumonitis | 3 (2.5) | 2 (1.7) | 0.426 |
| Two or more kinds of results | 5 (4.2) | 2 (1.7) | 0.119 |
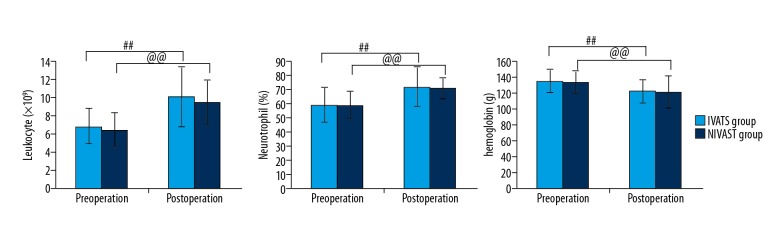
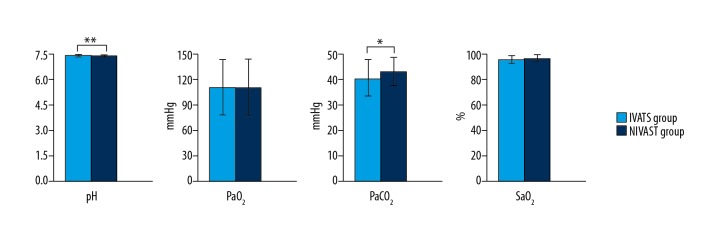
Counts of leukocyte, neutrophils, and hemoglobin had no difference before and after the operation in each group (P>0.05). Postoperative counts of leukocyte and neutrophils and hemoglobin levels were higher than preoperative values in both groups (P<0.01) (Figure 1). There was no significant difference in postoperative PaO2 and SaO2 between the 2 groups (P>0.05), but PaCO2 was higher and pH was lower in the NIVATS group (P<0.05) (Figure 2).
Figure 1.
Changes in routine blood cells analysis of IVATS group and NIVATS group before and after the operation. # P values shown are for the comparison of pre-operation and post-operation in IVATS group. @vP values shown are for the comparison of pre-operation and post-operation in NIVATS group.
Figure 2.
Changes in postoperative blood gas values in IVATS group and NIVATS group. * P values shown are for the comparison between IVATS group and NIVATS group after the operation.
Discussion
This was a retrospective study comparing the specific peri-operative conditions of NIVATS under spontaneous breathing with those of IVATS under one-lung ventilation. Our results suggest that NIVATS has a faster and more stable recovery in the PACU, which has not been reported before. NIVATS had no significant influence on oxygenation during the operation but resulted in more atelectasis or exudation in postoperative chest radiograph results.
Klijian reported a large-sample observation of 293 patients under awake VATS [12], but the author merely compared his results with others’ reports and did not list the results of the control studies. The studies of Chen and Hung also failed to provide comparable control groups, which made the results less credible [10,21–23]. The heterogeneity in global operation room stay time and analytical power resulted from the heterogeneous surgery types, and the small cohort design make Deng’s study less convincing [24]. The pitfall of Tommaso’s study design on talc pleurodesis VATS performed under local anesthesia is the narrow time span of selecting control patients, which could result in a selection bias [25].
NIVATS is performed more often in malignant disease, but the composition ratio of primary and metastatic diseases was the same in our 2 groups. Non-intubation anesthesia requires more concentrated, careful, and delicate performance by the surgeon, who must complete the operation as soon as possible to minimize complications, consequently shortening the operative time, which was also confirmed in other reports [13,17]. In addition, omitting intubation and intraoperative fiberoptic bronchoscopy for tube positioning simplify the anesthesia procedures, minimize interference with the operation, and greatly shorten the anesthesia time. The operating time and anesthesia time in our study were longer than in other reports. We assume that this was due in part to relatively older data, which was recorded nearly 4–7 years ago. At that time, backward surgical techniques and equipment might limit the speed of the operation. The changes of SpO2 and PETCO2 during the operation have not been reported in previous large-scale studies of patients. In our study, although the intraoperative SpO2 was lower and PETCO2 was much higher than those in IVATS, the similar postoperative PaO2 and SaO2 of the 2 groups suggest that NIVATS had little influence on oxygenation during the operation.
Enhance Recovery after Surgery (ERAS) has been proposed and applied in many other surgeries [26,27]. Our results suggest that application of non-intubated VATS may evolve into an ERAS program in many thoracic surgeries. In the PACU, patients in the NIVATS group had a faster and more stable recovery, and fewer patients received sedatives, analgesics, and urapidil, possibly due to the use of epidural anesthesia in 97% of patients. NIVATS excludes tracheal intubation, one-lung ventilation, and muscle paralysis; therefore, intraoperative spontaneous ventilation is preserved and unnecessary deep general anesthesia is avoided, so a much shorter surgery and anesthesia time becomes possible. Furthermore, patients in the NIVATS group regained consciousness earlier and most of them had stable hemodynamics. The mechanism of iatrogenic pneumothorax via small intercostal incision VATS under spontaneous breathing is more physiological than that via one-lung ventilation, and results in less lung inflammation and stress [28]. Consequently, there is faster and improved early recovery and outcome in the PACU.
We found that the proportions of atelectasis, pulmonary exudation, and pleural effusion were larger in the NIVATS group, while Klijian and Tacconi argued that spontaneous breathing reduced the incidence of atelectasis and air leakage [12,29]. The surgery type may primarily account for this discrepancy. We mainly focused on lobectomy, but Klijian’s research included non-pulmonary surgeries such as pleural biopsy, heart fenestration, and pleurodesis [12]. Therefore, their studies found a low incidence of atelectasis and earlier removal of chest tubes. In addition, using the mask or laryngeal mask to inflate the lung in NIVATS may also contribute to higher incidence of atelectasis. Lung expansion is not as sufficient as that in intubation, in which the lung is directly inflated through an endotracheal-tube. Furthermore, secondary infection caused by atelectasis might result in exudation. All these factors can increase the incidence of postoperative atelectasis and exudation. More chest radiography complications can lead to delayed chest tube extraction. To avoid atelectasis, surgeons tried to re-expand the collapsed lung with negative-pressure suction through the chest tube at the end of the procedure, in the PACU, and in the ward.
As this is a retrospective study, several limitations need to be mentioned. First, this is a single-center study with inevitable selection bias. We attempted to offset the selection bias through propensity-matching. Second, it is difficult to judge whether the epidural anesthesia or the spontaneous breathing can improve recovery, but we are sure that spontaneous breathing under epidural anesthesia improves the recovery more than intubation does. Third, the fact that the 2 groups had surgery in different time periods may have affected the results. Other factors, such as general improvement in the use of VATS and improved postoperative care over time may have contributed to the difference in outcomes between NIVATS and IVATS. However, we matched the basic patient characteristics and observed the same type of operation in a single center to reduce the influence. Moreover, similar studies should avoid the period when both techniques are being used, as this is a transition period, and there could be a bias in play whereby more complex patients received the older approach while the less sick patients getting the newer approach. Fourth, we just retrospectively recorded the analgesic usage in the PACU, while the comparison of the pain scores in recovery was ignored, which limited our ability to assess postoperative pain. We hope to improve this observation in prospective clinical trials.
Conclusions
NIVATS results in a faster and more stable recovery with less analgesic use in the PACU. NIVATS has no significant influence on oxygenation during the operation but resulted in more atelectasis in postoperative chest radiography, which should be promptly dealt with after the operation. A further evaluation in multi-center, prospective, and large-cohort clinical trial is required on different effects of specific anesthesia methods.
Acknowledgments
We are grateful for advice from Professor John Laffey on how to collect the medical data. We also thank Dr Xiaotao Zhen and Dr Chongyang Duan for help with imaging diagnosis and statistics.
Footnotes
Source of support: Departmental sources
Conflicts of interest
None.
References
- 1.Buckingham WW, Beatty AJ, Brasher CA, Ottosen P. An analysis of 607 surgical procedures done under epidural anesthesia. Mo Med. 1950;47:485–87. [PubMed] [Google Scholar]
- 2.Petrovsky BV. Role of local anesthesia according to Vischnevsky in thoracic surgery. Anesth Analg. 1952;9:75–79. [PubMed] [Google Scholar]
- 3.Mukaida T, Andou A, Date H, et al. Thoracoscopic operation for secondary pneumothorax under local and epidural anesthesia in high-risk patients. Ann Thorac Surg. 1998;65:924–26. doi: 10.1016/s0003-4975(98)00108-8. [DOI] [PubMed] [Google Scholar]
- 4.Pompeo E, Mineo D, Rogliani P, et al. Feasibility and results of awake thoracoscopic resection of solitary pulmonary nodules. Ann Thorac Surg. 2004;78:1761–68. doi: 10.1016/j.athoracsur.2004.05.083. [DOI] [PubMed] [Google Scholar]
- 5.Pompeo E, Rogliani P, Cristino B, et al. Awake thoracoscopic biopsy of interstitial lung disease. Ann Thorac Surg. 2013;95:445–52. doi: 10.1016/j.athoracsur.2012.10.043. [DOI] [PubMed] [Google Scholar]
- 6.Pompeo E, Tacconi F, Mineo TC. Awake video-assisted thoracoscopic biopsy in complex anterior mediastinal masses. Thorac Surg Clin. 2010;20:225–33. doi: 10.1016/j.thorsurg.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Pompeo E, Tacconi F, Frasca L, Mineo TC. Awake thoracoscopic bullaplasty. Eur J Cardiothorac Surg. 2011;39:1012–17. doi: 10.1016/j.ejcts.2010.09.029. [DOI] [PubMed] [Google Scholar]
- 8.Pompeo E, Mineo TC. Awake pulmonary metastasectomy. J Thorac Cardiovasc Surg. 2007;133:960–66. doi: 10.1016/j.jtcvs.2006.09.078. [DOI] [PubMed] [Google Scholar]
- 9.Tacconi F, Pompeo E, Fabbi E, Mineo TC. Awake video-assisted pleural decortication for empyema thoracis. Eur J Cardiothorac Surg. 2010;37:594–601. doi: 10.1016/j.ejcts.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Chen KC, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic lung resection: A 3-year experience with 285 cases in a single institution. J Thorac Dis. 2012;4:347–51. doi: 10.3978/j.issn.2072-1439.2012.08.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hung MH, Hsu HH, Chen KC, et al. Nonintubated thoracoscopic anatomical segmentectomy for lung tumors. Ann Thorac Surg. 2013;96:1209–15. doi: 10.1016/j.athoracsur.2013.05.065. [DOI] [PubMed] [Google Scholar]
- 12.Klijian AS, Gibbs M, Andonian NT. AVATS: Awake Video Assisted Thoracic Surgery – extended series report. J Cardiothorac Surg. 2014;9:149. doi: 10.1186/s13019-014-0149-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen JS, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic lobectomy for lung cancer. Ann Surg. 2011;254:1038–43. doi: 10.1097/SLA.0b013e31822ed19b. [DOI] [PubMed] [Google Scholar]
- 14.Pompeo E, Rogliani P, Tacconi F, et al. Randomized comparison of awake nonresectional versus nonawake resectional lung volume reduction surgery. J Thorac Cardiovasc Surg. 2012;143:47–54. 54.e1. doi: 10.1016/j.jtcvs.2011.09.050. [DOI] [PubMed] [Google Scholar]
- 15.Irons JF, Miles LF, Joshi KR. Intubated versus nonintubated general anesthesia or video-assisted thoracoscopic surgery – a case control study. J Cardiothorac Vasc Anesth. 2016;1:1–6. doi: 10.1053/j.jvca.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Tseng YD, Cheng YJ, Hung MH, et al. Nonintubated needlescopic videoassisted thoracic surgery for management of peripheral lung nodules. Ann Thorac Surg. 2012;93:1049–54. doi: 10.1016/j.athoracsur.2012.01.062. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Cui F, He J. Non-intubated video-assisted thoracoscopic surgery anatomical resections: A new perspective for treatment of lung cancer. Ann Transl Med. 2015;3:102–8. doi: 10.3978/j.issn.2305-5839.2015.04.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong Q, Liang L, Li Y, et al. Anesthesia with nontracheal intubation in thoracic surgery. J Thorac Dis. 2012;4:126–30. doi: 10.3978/j.issn.2072-1439.2012.03.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKenna RJ., Jr Lobectomy by video-assisted thoracic surgery with mediastinal node sampling for lung cancer. J Thorac Cardiovasc Surg. 1994;107:879–82. [PubMed] [Google Scholar]
- 20.R R Develeopment Core Team. A language and environment for statistical computing. Foundation for Statistical Computing; Vienna, Austria: 2017. URL https://www.R-project.org/ [Google Scholar]
- 21.Chen KC, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic surgery using regional anesthesia and vagal block and targeted sedation. J Thorac Dis. 2014;6:31–36. doi: 10.3978/j.issn.2072-1439.2014.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hung MH, Chan KC, Liu YJ, et al. Nonintubated thoracoscopic lobectomy for lung cancer using epidural anesthesia and intercostal blockade: A retrospective cohort study of 238 cases. Medicine. 2015;94:e727. doi: 10.1097/MD.0000000000000727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hung MH, Hsu HH, Chan KC, et al. Non-intubated thoracoscopic surgery using internal intercostal nerve block, vagal block and targeted sedation. Eur J Cardiothoracic Surg. 2014;46:620–25. doi: 10.1093/ejcts/ezu054. [DOI] [PubMed] [Google Scholar]
- 24.Deng HY, Zhu ZJ, Wang YC, et al. Non-intubated video-assisted thoracoscopic surgery under loco-regional anaesthesia for thoracic surgery: A meta-analysis. Interact Cardiovasc Thorac Surg. 2016;23:31–40. doi: 10.1093/icvts/ivw055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mineo TC, Sellitri F, Tacconi F, Ambroqi V. Quality of life and outcomes after nonintubated versus intubated videothoracoscopic pleurodesis for malignant pleural effusion: Comparison by a case-matched study. J Palliat Med. 2014;17:761–68. doi: 10.1089/jpm.2013.0617. [DOI] [PubMed] [Google Scholar]
- 26.Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: A review. JAMA Surg. 2017;152:292–98. doi: 10.1001/jamasurg.2016.4952. [DOI] [PubMed] [Google Scholar]
- 27.Isik A, Okan I, Firat D, et al. A new prognostic strategy for gastric carcinoma: Albumin level and metastatic lymph node ratio. Minerva Chirurgica. 2014;69:147–53. [PubMed] [Google Scholar]
- 28.Gonzalez-Rivas D, Bonome C, Fieira E, et al. Non-intubated video-assisted thoracoscopic lung resections: the future of thoracic surgery? Eur J Cardiothorac Surg. 2016;49:721–31. doi: 10.1093/ejcts/ezv136. [DOI] [PubMed] [Google Scholar]
- 29.Tacconi F, Pompeo E, Mineo TC. Duration of air leak is reduced after awake nonresectional lung volume reduction surgery. Eur J Cardiothorac Surg. 2009;35:822–28. doi: 10.1016/j.ejcts.2009.01.010. [DOI] [PubMed] [Google Scholar]