Abstract
Background and Objectives:
Pleural effusion is seen in around half of the cases of pulmonary embolism (PE). There are no data on the incidence of pleural effusion in cases of PE in the Kingdom of Bahrain. This study was done to determine the frequency and radiological features of pleural effusion in cases of acute PE and also to characterize the pleural fluid biochemistry and cell type in patients subjected to diagnostic thoracentesis.
Methods:
This was a retrospective, observational single-center study. All the data of patients subjected to computed tomography pulmonary angiography (CTPA) in suspected cases of acute PE over a 4-year period were analyzed.
Results:
A total of 1756 patients were subjected to CTPA from January 2013 to December 2016. A diagnosis of acute PE was made in 200 patients (11.4%). Pleural effusion was identified in 70 cases (35%). Majority of the effusions were small to moderate in size, bilateral, and associated with peripheral emboli. Consolidation, atelectasis, and ground glass attenuation were common associated findings on CTPA in these patients. Consolidation was more common in patients of PE associated with pleural effusion as compared to those with PE alone (62.85% and 33.8%, respectively, odds rato: 3.279 and 95% confidence interval: 1.798–6.091, P < 0.001). Diagnostic thoracentesis was done in 6 (8.6%) of the cases. All the patients had an exudative effusion with normal glucose values and neutrophil predominance.
Conclusion:
PE was associated with pleural effusion in around one-third of the patients in Bahrain. The effusions were mainly small and bilateral. The emboli in cases associated with pleural effusion were mostly peripheral. Consolidation was the parenchymal abnormality detected on CTPA which was significantly associated with the presence of pleural effusion. Most of the pleural effusions were not suitable for thoracentesis. In patients subjected to fluid analysis, the effusions were exudative, neutrophilic predominant, and associated with normal glucose levels.
KEY WORDS: Computed tomography pulmonary angiography, exudate, pleural effusion, pulmonary embolism
INTRODUCTION
Pulmonary embolism (PE) is an acute medical condition associated with a significant morbidity and mortality. It is the third common cardiovascular condition after coronary artery disease and stroke. Undiagnosed PE results in 30% mortality, while its correct identification and appropriate treatment bring down the mortality to <10%.[1] The exact incidence of PE is difficult to determine due to reasons varying from it being an incidental finding in a thoracic imaging, asymptomatic presentation, or it causing sudden death which may be easily missed. The annual incidence of venous thromboembolism in the US is estimated to be around 1–2/1000 of the population.[2] In Europe, the incidence of patients admitted with PE is increasing. In the Spanish study, the crude incidence increased from 20.44/100,000 inhabitants in 2002 to 32.69/100,000 in 2011.[3] In China, the annual incidence of PE was found to be 0.1% in 2003 and remained stable over the next 5 years.[4] In the neighboring Kingdom of Saudi Arabia, 25,000 people are affected with venous thromboembolism every year.[5] Little is known about the incidence of PE in the Kingdom of Bahrain. Less than a quarter to more than half of the patients with PE may have pleural effusion.[6] Interestingly, only 2% of nonmalignant effusions were found to be caused by PE, in an audit done for patients of pleural effusions referred to a tertiary care pleural disease facility in the UK.[7] This discrepancy may be explained by the following reasons: First, most of the effusions may be too small to warrant a thoracentesis; second, the possibility of PE as a cause of pleural effusion is not commonly entertained; and finally, in patients with moderate-to-high suspicion of PE, anticoagulation is immediately initiated while awaiting the confirmatory tests and thoracentesis in these situations may be associated with increased risk of hemorrhage.[8] The association of pleural effusion with PE in Bahrain is found in case reports.[9] To the best of our knowledge, there is no study reported from the Kingdom of Bahrain regarding the incidence of pleural effusion in patients with PE. The current study was conducted to describe the frequency and radiological features of pleural effusions in patients with acute PE. Pleural fluid characteristics with respect to biochemistry and cell type were determined in patients subjected to diagnostic thoracentesis.
METHODS
The study protocol was approved by the Secondary Health Care Research Sub-Committee at Salmaniya Medical Complex, an academic tertiary referral center, in the Kingdom of Bahrain. It is a 1200-bedded hospital with around 44,000 admissions annually. We retrospectively reviewed the medical records of computed tomography pulmonary angiography (CTPA) scan data of patients of suspected PE from January 2013 to December 2016 in our hospital.
The clinical details were accessed from the Medical Records Department of our hospital to acquire information on demographics, clinical profile, and relevant investigations in patients with confirmed cases of PE.
All the CTPA scans were interpreted by two experienced radiologists independently. The study protocol for the CTPA included performing the study on 128 slice CT system (Siemens Healthcare, Somatom Definition AS; Erlangen, Germany). A total volume of 100 ml of nonionic contrast (Omnipaque 300, GE Healthcare, United States) was delivered at a rate of 5 ml/s through the antecubital vein. Bolus tracking method was used to control the scan initiation. The scanning was performed on 5 mm (128 mm × 0.6 mm) collimation with a pitch of 0.8, and exposure factor determined by Siemens Care Dose 4D software. Reconstruction of images was done at 1 mm intervals.
The reports of CTPA scan were analyzed by the investigators. Cases of PE were determined from the scan data. Clots were identified as filling defects within the pulmonary vasculature. In the axial plane, these were determined by the polo mint sign, while in the longitudinal images of vessels, railway track sign was used to identify the clots.[10] PE was classified as central if the clots were seen in the main trunk of the pulmonary artery and/or in the right or left main pulmonary arteries up to the lobar branches. Peripheral emboli were diagnosed when the clots were seen exclusively in the segmental/subsegmental pulmonary arteries.[11] If a pleural effusion was present in patients with PE, it was classified as small, moderate, and large based on the anteroposterior quartile. Effusions occupying the first quartile were described as small, second quartiles as moderate, while those occupying the third or fourth quartiles were identified as large.[12]
Clinical presentation of patients with PE was classified into three groups: pulmonary infarction – if the patient had pleuritic pain or hemoptysis; isolated dyspnea – if the patient had dyspnea in the absence of pleuritic pain, hemoptysis, or circulatory collapse; circulatory collapse – if the patient had loss of consciousness or systolic BP <90 mmHg.[13]
In patients subjected to diagnostic thoracentesis, pleural fluid was classified as transudate or exudate, according to Light's criteria.[14] Glucose levels were assessed. Predominant white cell type in pleural fluid was also determined.
Statistical analysis
Demographic characteristics of patients were summarized according to measurement scales for patients of PE with and without pleural effusion. Age was expressed in terms of mean and standard deviation, while nominal variables were expressed in terms of numbers and percentage. The distribution of patients of PE with pleural effusion according to clinical presentation, effusion size, and laterality was obtained. The risk of different CTPA abnormalities in patients of PE with pleural effusion was determined in terms of odds ratio, and its statistical significance was obtained. The descriptive statistics such as mean and SD were also obtained for measurable fluid parameters for patients with pleural effusion. All the analyses were performed using SPSS version 20.0 (IBM Corp., Armonk, USA) and statistical significance was evaluated at 5% level.
RESULTS
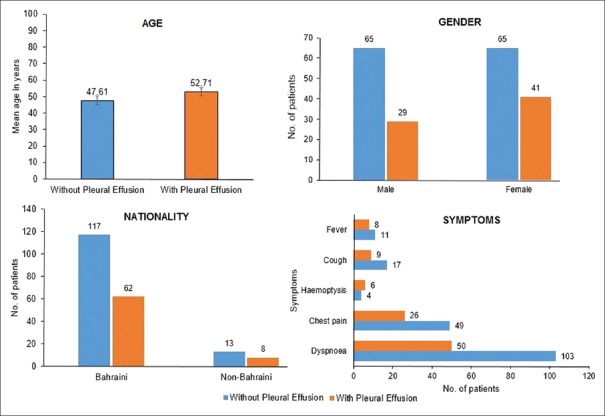
A total of 1756 patients were subjected to CTPA study over a period of 4 years from January 2013 to December 2016. CTPA study was done for evaluation of suspected PE in the Department of Radiology at our hospital. A diagnosis of PE was made in 200 patients (11.4%). Radiological appearance of pleural effusion was found in 70 patients (35%). As depicted in Figure 1, patients with PE and pleural effusion were slightly older than those with PE alone. Majority of the subjects were females (53%) and native Bahrainis accounted for around 90% of the study population. Symptoms were not helpful in providing clues to the presence of pleural effusion in patients with PE.
Figure 1.
Demographic and symptom distribution in patients of pulmonary embolism with and without pleural effusion
In patients of PE with pleural effusion, isolated dyspnea was the most common clinical presentation, followed by pulmonary infarction syndrome and circulatory collapse [Table 1]. Most of the effusions were small with a large amount of fluid seen in <5% of the cases [Table 2]. The binomial test revealed that the proportion of patients with bilateral pleural effusion (62.86%) was significantly higher than that of unilateral cases (37.14%) as indicated by P = 0.0414. Right-sided predilection was common in patients with unilateral pleural effusion [Table 3]. Majority of the patients with pleural effusion had clots in peripheral location that were significantly more than the central ones (P = 0.0414) [Table 4]. Consolidation, atelectasis, and ground glass attenuation were common associated findings on CTPA in these patients. Consolidation was more common in patients of PE associated with pleural effusion as compared to those with PE alone (62.85% and 33.8%, respectively, odds ratio: 3.279 and 95% confidence interval: 1.798–6.091, P < 0.001) [Figure 2]. The other CT findings of wedge-shaped opacities, atelectasis, ground glass attenuation, and linear opacities were not useful in identifying the presence of pleural effusion in patients with PE [Table 5].
Table 1.
Distribution of patients with pleural effusion in pulmonary embolism patients with respect to clinical presentation
| Clinical presentation, n (%) | |||
|---|---|---|---|
| Pulmonary infarction | Isolated dyspnea | Circulatory collapse | |
| Pleural effusion (70) | 24 (34.28) | 35 (50.00) | 11 (15.71) |
Table 2.
Size of pleural effusions in patients with pulmonary embolism and pleural effusion (n=70)
| Effusion size | n (%) |
|---|---|
| Small | 58 (82.86) |
| Moderate | 9 (12.86) |
| Large | 3 (4.28) |
| Total | 70 (100) |
Table 3.
Distribution of pleural effusion according to laterality (n=70)
| Unilateral | Bilateral | ||
|---|---|---|---|
| Left | Right | ||
| Pleural effusion, n (%) | 11 (15.71) | 15 (21.43) | 44 (62.86) |
P: 0.0414 using Binomial test
Table 4.
Location of pulmonary embolism in patients with pleural effusion (n=70)
| Location of emboli | n (%) |
|---|---|
| Central | 26 (37.14) |
| Peripheral | 44 (62.86) |
| Saddle | 0 |
P: 0.0414 using Binomial test
Figure 2.
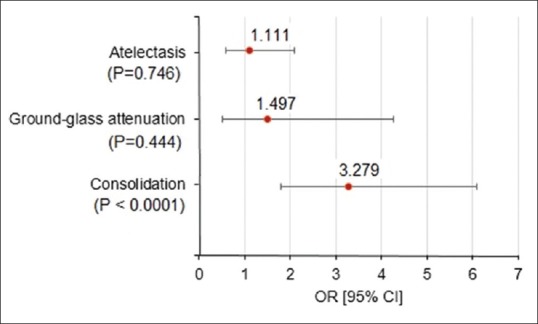
Forest plot showing odds ratios associated with different computed tomography abnormalities
Table 5.
Parenchymal computed tomography findings in pulmonary emboli patients with and without pleural effusion
| CT abnormalities, n (%) | PE without pleural effusion (n=130) | PE with pleural effusion (n=70) | OR (95% CI) | P |
|---|---|---|---|---|
| Wedge-shape opacity | 2 (1.54) | 1 (1.43) | 0.986 (0.031-12.382) | 0.951 (NS) |
| Atelectasis | 38 (29.23) | 22 (31.43) | 1.111 (0.584-2.084) | 0.746 (NS) |
| Linear opacity | 1 (0.76) | 1 (1.43) | 1.863 (0.047-73.383) | 0.582 (NS) |
| Ground-glass attenuation | 9 (6.92) | 7 (10.00) | 1.497 (0.504-4.272) | 0.444 (NS) |
| Consolidation | 44 (33.8) | 44 (62.85) | 3.279 (1.798-6.091) | < 0.001 (S) |
NS: Nonsignificant, S: Significant, CI: Confidence interval, OR: Odds ratio, CT: Computed tomography, PE: Pulmonary embolism
Pleural fluid analysis was available in 6 (8.6%) patients. All the patients had an exudative effusion with normal glucose values and neutrophil predominance [Table 6].
Table 6.
Descriptive statistics for different fluid parameters in patients with pleural effusion (n=6)
| Parameter | Descriptive statistics |
|---|---|
| Protein fluid (g/L), mean±SD (median) | 25.58±14.42 (25) |
| LDH fluid (u/L), mean±SD (median) | 539.17±481.29 (351) |
| Glucose fluid (mmol), mean±SD (median) | 5.38±3.92 (4.2) |
| Light’s criteria for exudate, n (%) | 6 (100) |
| Neutrophil (%), n (%) | 6 (100) |
LDH: Lactic dehydrogenase, SD: Standard deviation
DISCUSSION
The incidence of PE in a large cohort of patients clinically suspected to have PE in a tertiary health-care setting in the Kingdom of Bahrain was 11.4%. This figure agrees with the data reported in the literature where 9.4%–40% of patients subjected to CTPA are found to have PE.[15] The actual incidence of pleural effusion due to PE is not known because of difficulty in estimating the exact number of patients suffering from PE. In most of the series of pleural effusion subjected to thoracentesis, <5% of them are caused by PE.[8] Nearly 15% of patients of pleural effusion may remain undiagnosed despite pleurocentesis and pleural biopsy.[16] Most of these effusions may be caused by tuberculosis and malignancy. PE is an entity that needs to be considered in the workup of these cases. In a series of 27 cases of undiagnosed pleural effusion, a diagnosis could be established in 16 patients. PE was found in two cases on autopsy.[17] Thus, the actual number of pleural effusions related to PE may be more but are missed because they are not considered. The incidence of pleural effusions in PE is 19%–61%.[6] Pleural effusion was found in 23% of the patients with PE on chest radiography.[18] CT is more sensitive than chest radiography in detecting pleural effusion. In one study, CT detected 20 (32%) effusions that were not detected by the chest radiograph.[19] Interestingly, some studies showed transthoracic sonography outperforming CT in the detection of pleural effusion in patients with PE.[20] In our study, pleural effusion was found in 35% of the cases of PE. Pathogenesis of pleural effusion in PE is not completely understood. It is thought that inflammatory mediators released from the pulmonary thrombi cause an increase in capillary permeability. The resulting interstitial edema fluid finds its way into the pleural space causing pleural effusion. The other mechanism, though less likely, is the increase in systemic venous pressure caused by PE. The resultant increase in hydrostatic pressure in capillaries leads to accumulation of fluid in the pleural space.
Dyspnea is a common symptom occurring in more than three-fourth of the patients followed by pleuritic chest pain, hemoptysis, and cough.[21] Similar frequency of symptoms was seen in the present study. Symptom complexes recognized in PE are pulmonary infarction, isolated dyspnea, and circulatory collapse. In a study done by Stein, pleural effusion was seen in 56% of patients of PE presenting as pulmonary infarction syndrome, 26% presenting with isolated dyspnea, and none with circulatory collapse.[22] In another study, pleural effusion was seen in 27% of the patients with pulmonary infarction, 16% of patients with circulatory collapse, and 12% of patients with isolated dyspnea.[13] In the current study, isolated dyspnea was most commonly associated with pleural effusion followed by pulmonary infarction and circulatory collapse. This shows that there is no clear pattern of association between the presence of pleural effusion and clinical presentation of PE.
Pleural effusion associated with PE reaches their maximum size by the 3rd day. Any increase in size after this period or appearance of fluid on the contralateral side indicates recurrent showers of embolism, infection of the pleural space, or development of hemothorax as a complication of anticoagulant therapy. Most of the pleural effusions associated with PE are small and unilateral. Porcel et al., in their series of patients of PE with pleural effusion, found the effusions to be small in size (90% occupied less than one-third of the hemithorax on chest radiograph) and they were mainly unilateral (85%).[19] Yap et al. also found small-sized pleural effusion-associated PE.[23] Liu et al. conducted a study to see the incidence of pleural effusion in PE. They also found that majority of the effusions were small to moderate in size and were unilateral. Central pulmonary arteries were involved in almost two-third of these cases.[24] Depending on the modality of imaging used to identify the pleural effusion in patients with PE, 6.5%–15% on chest radiograph and 25%–43% on CT scan are distributed bilaterally.[6] In a few other studies, 46%–75% of the effusions were found to be bilateral.[19] The findings in our series were in concurrence with the above-mentioned data, in that, >90% of the effusions were small to moderate in size with almost two-thirds of the patients having bilateral effusion. Peripheral emboli were more commonly associated with pleural effusion. Most of the effusions associated with PE are free flowing. However, at times, they may become loculated. Reissig et al. found 12 of 39 patients with PE having loculated effusions.[20] In another study, one-fifth of pleural effusions associated with PE were loculated. These cases were associated with a delay in diagnosis of PE of almost 2 weeks from the onset of symptoms.[19] Interestingly, most of the loculations resolve with effective anticoagulant therapy.[25] Various types of parenchymal opacities are seen on CT in patients with PE. These include wedge-shaped opacities, consolidation, ground glass attenuation, atelectasis, and linear opacities. Liu et al. found these parenchymal opacities more commonly in PE associated with pleural effusion than those without it. Atelectasis, consolidation, and wedge-shaped opacities had a significant association with the presence of pleural effusion.[24] In other studies as well, parenchymal abnormalities such as wedge-shaped opacities were found in association with pleural effusion.[19] In our series, consolidation was significantly associated with the presence of pleural effusion in patients of PE.
Very few patients of pleural effusions associated with PE are subjected to thoracentesis.[24] Porcel et al. reported that thoracentesis was done in less than one-third of patients having pleural effusion.[19] In keeping with the reported data, the present series also showed that a small number (<10%) of the patients being subjected to thoracentesis. Earlier, there were reports that mentioned pleural fluid associated with PE could be either a transudate or an exudate.[6,8] Later, it was found that all the pleural effusions associated with PE were exudates.[19,25,26] Minority of patients may have low glucose levels in the pleural effusion.[19] The differential white cell count shows neutrophilic predominance in the majority of the cases while lymphocytic predominance may be encountered in few cases.[19,26] Rarely, a large number of eosinophils may be found in these effusions.[6] The findings in our patients agreed with these data, in that, all the effusions satisfied the Light's criteria for an exudate, had normal glucose values, and were neutrophilic predominant.
To the best of our knowledge, this is the first study to identify the incidence of pleural effusion in PE in the Middle East region. Due to retrospective observational design of our study, the expected limitations associated with this type of a study are applicable here. There were a very small number of patients subjected to thoracentesis and therefore the pleural fluid characteristics could be determined in fewer cases.
CONCLUSION
PE was associated with pleural effusion in around one-third of the patients in Bahrain. The effusions were mainly small and bilateral. The emboli in the cases associated with pleural effusion were mainly peripheral. Consolidation, atelectasis, and ground glass attenuation were the common associated parenchymal findings visualized on the CTPA. Of these, consolidation was significantly associated with the presence of pleural effusion. Most of the pleural effusions were not suitable for thoracentesis. In patients subjected to fluid analysis, the effusions were exudative, neutrophilic predominant, and associated with normal glucose levels. Therefore, when faced with a patient of small, exudative pleural effusion, and no obvious etiology, a diagnosis of PE should be entertained and investigated accordingly.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bĕlohlávek J, Dytrych V, Linhart A. Pulmonary embolism, part I: Epidemiology, risk factors and risk stratification, pathophysiology, clinical presentation, diagnosis and nonthrombotic pulmonary embolism. Exp Clin Cardiol. 2013;18:129–38. [PMC free article] [PubMed] [Google Scholar]
- 2.Beckman MG, Hooper WC, Critchley SE, Ortel TL. Venous thromboembolism: A public health concern. Am J Prev Med. 2010;38:S495–501. doi: 10.1016/j.amepre.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 3.de Miguel-Díez J, Jiménez-García R, Jiménez D, Monreal M, Guijarro R, Otero R, et al. Trends in hospital admissions for pulmonary embolism in spain from 2002 to 2011. Eur Respir J. 2014;44:942–50. doi: 10.1183/09031936.00194213. [DOI] [PubMed] [Google Scholar]
- 4.Yang Y, Liang L, Zhai Z, He H, Xie W, Peng X, et al. Pulmonary embolism incidence and fatality trends in Chinese hospitals from 1997 to 2008: A multicenter registration study. PLoS One. 2011;6:e26861. doi: 10.1371/journal.pone.0026861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Hameed F, Al-Dorzi HM, Shamy A, Qadi A, Bakhsh E, Aboelnazar E, et al. The Saudi clinical practice guideline for the diagnosis of the first deep venous thrombosis of the lower extremity. Ann Thorac Med. 2015;10:3–15. doi: 10.4103/1817-1737.146849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agarwal R, Singh N, Gupta D. Pleural effusions associated with pulmonary thromboembolism: A systematic review. Indian J Chest Dis Allied Sci. 2009;51:159–64. [Google Scholar]
- 7.Bintcliffe OJ, Lee GY, Rahman NM, Maskell NA. The management of benign non-infective pleural effusions. Eur Respir Rev. 2016;25:303–16. doi: 10.1183/16000617.0026-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Light RW. Pleural effusion in pulmonary embolism. Semin Respir Crit Care Med. 2010;31:716–22. doi: 10.1055/s-0030-1269832. [DOI] [PubMed] [Google Scholar]
- 9.Panjwani A, Zaid T. An interesting case of undiagnosed pleural effusion. Breathe (Sheff) 2017;13:e46–e52. doi: 10.1183/20734735.001117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wittram C, Maher MM, Yoo AJ, Kalra MK, Shepard JA, McLoud TC, et al. CT angiography of pulmonary embolism: Diagnostic criteria and causes of misdiagnosis. Radiographics. 2004;24:1219–38. doi: 10.1148/rg.245045008. [DOI] [PubMed] [Google Scholar]
- 11.Alonso Martinez JL, Anniccherico Sánchez FJ, Urbieta Echezarreta MA, García IV, Álvaro JR. Central versus peripheral pulmonary embolism: Analysis of the impact on the physiological parameters and long-term survival. N Am J Med Sci. 2016;8:134–42. doi: 10.4103/1947-2714.179128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moy MP, Levsky JM, Berko NS, Godelman A, Jain VR, Haramati LB, et al. A new, simple method for estimating pleural effusion size on CT scans. Chest. 2013;143:1054–9. doi: 10.1378/chest.12-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lobo JL, Zorrilla V, Aizpuru F, Uresandi F, Garcia-Bragado F, Conget F, et al. Clinical syndromes and clinical outcome in patients with pulmonary embolism: Findings from the RIETE registry. Chest. 2006;130:1817–22. doi: 10.1378/chest.130.6.1817. [DOI] [PubMed] [Google Scholar]
- 14.Light RW, Macgregor MI, Luchsinger PC, Ball WC., Jr Pleural effusions: The diagnostic separation of transudates and exudates. Ann Intern Med. 1972;77:507–13. doi: 10.7326/0003-4819-77-4-507. [DOI] [PubMed] [Google Scholar]
- 15.Herédia V, Ramalho M, Zapparoli M, Semelka RC. Incidence of pulmonary embolism and other chest findings in younger patients using multidetector computed tomography. Acta Radiol. 2010;51:402–6. doi: 10.3109/02841850903524439. [DOI] [PubMed] [Google Scholar]
- 16.Rahman NM, Chapman SJ, Davies RJ. Pleural effusion: A structured approach to care. Br Med Bull. 2004;72:31–47. doi: 10.1093/bmb/ldh040. [DOI] [PubMed] [Google Scholar]
- 17.Gunnels JJ. Perplexing pleural effusion. Chest. 1978;74:390–3. doi: 10.1378/chest.74.4.390. [DOI] [PubMed] [Google Scholar]
- 18.Elliott CG, Goldhaber SZ, Visani L, DeRosa M. Chest radiographs in acute pulmonary embolism. Results from the international cooperative pulmonary embolism registry. Chest. 2000;118:33–8. doi: 10.1378/chest.118.1.33. [DOI] [PubMed] [Google Scholar]
- 19.Porcel JM, Madroñero AB, Pardina M, Vives M, Esquerda A, Light RW, et al. Analysis of pleural effusions in acute pulmonary embolism: Radiological and pleural fluid data from 230 patients. Respirology. 2007;12:234–9. doi: 10.1111/j.1440-1843.2006.01026.x. [DOI] [PubMed] [Google Scholar]
- 20.Reissig A, Heyne JP, Kroegel C. Ancillary lung parenchymal findings at spiral CT scanning in pulmonary embolism. Relationship to chest sonography. Eur J Radiol. 2004;49:250–7. doi: 10.1016/S0720-048X(03)00141-4. [DOI] [PubMed] [Google Scholar]
- 21.Stein PD, Terrin ML, Hales CA, Palevsky HI, Saltzman HA, Thompson BT, et al. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest. 1991;100:598–603. doi: 10.1378/chest.100.3.598. [DOI] [PubMed] [Google Scholar]
- 22.Stein PD, Henry JW. Clinical characteristics of patients with acute pulmonary embolism stratified according to their presenting syndromes. Chest. 1997;112:974–9. doi: 10.1378/chest.112.4.974. [DOI] [PubMed] [Google Scholar]
- 23.Yap E, Anderson G, Donald J, Wong CA, Lee YC, Sivakumaran P, et al. Pleural effusion in patients with pulmonary embolism. Respirology. 2008;13:832–6. doi: 10.1111/j.1440-1843.2008.01345.x. [DOI] [PubMed] [Google Scholar]
- 24.Liu M, Cui A, Zhai ZG, Guo XJ, Li M, Teng LL, et al. Incidence of pleural effusion in patients with pulmonary embolism. Chin Med J (Engl) 2015;128:1032–6. doi: 10.4103/0366-6999.155073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erkan L, Fýndýk S, Uzun O, Atýcý AG, Light RW. A new radiologic appearance of pulmonary thromboembolism: Multiloculated pleural effusions. Chest. 2004;126:298–302. doi: 10.1378/chest.126.1.298. [DOI] [PubMed] [Google Scholar]
- 26.Romero Candeira S, Hernández Blasco L, Soler MJ, Muñoz A, Aranda I. Biochemical and cytologic characteristics of pleural effusions secondary to pulmonary embolism. Chest. 2002;121:465–9. doi: 10.1378/chest.121.2.465. [DOI] [PubMed] [Google Scholar]