Abstract

H-BiVO4–x:Mo was successfully deposited on microwire-structured silicon substrates, using indium tin oxide (ITO) as an interlayer and BiOI prepared by electrodeposition as precursor. Electrodeposition of BiOI, induced by the electrochemical reduction of p-benzoquinone, appeared to proceed through three stages, being nucleation of particles at the base and bottom of the microwire arrays, followed by rapid (homogeneous) growth, and termination by increasing interfacial resistances. Variations in charge density and morphology as a function of spacing of the microwires are explained by (a) variations in mass transfer limitations, most likely associated with the electrochemical reduction of p-benzoquinone, and (b) inhomogeneity in ITO deposition. Unexpectedly, H-BiVO4–x:Mo on microwire substrates (4 μm radius, 4 to 20 μm spacing, and 5 to 16 μm length) underperformed compared to H-BiVO4–x:Mo on flat surfaces in photocatalytic tests employing sulfite (SO32–) oxidation in a KPi buffer solution at pH 7.0. While we cannot exclude optical effects, or differences in material properties on the nanoscale, we predominantly attribute this to detrimental diffusion limitations of the redox species within the internal volume of the microwire arrays, in agreement with existing literature and the observations regarding the electrodeposition of BiOI. Our results may assist in developing high-efficiency PEC devices.
Keywords: PEC devices, BiOI, Silicon geometry, BiVO4, Performance, Sulfite oxidation
Short abstract
We discuss the effect of the Si architecture on the performance of H-BiVO4:Mo in the oxidation of sulfite to sulfate, providing indications of applicability in solar-to-fuel devices.
Introduction
Bismuth vanadate (BiVO4), an n-type semiconductor, is one of the most promising photoanode materials for photoelectrochemical oxidation of water. It is stable in a pH range between 3 and 11,1 environmentally benign, widely available, nontoxic, inexpensive, and is commonly used in industry as a yellow pigment. The valence band edge of the monoclinic phase is located at 2.8 V vs NHE2 and is thus sufficiently positive to oxidize water at a potential of 1.23 V vs NHE. The conduction band edge is located at 0.3 V vs NHE,2 which is near the H2 evolution potential of 0 V vs NHE. Thus, the photocurrent onset potential is relatively low, leading to high photocurrents in comparison to other photoanode materials with more positive conduction band edges.3 The indirect bandgap of 2.4 to 2.5 eV2,4 allows to absorb up to 11% of the solar spectrum, which equals a theoretically maximum photocurrent of 7.5 mA/cm2 and a solar to hydrogen efficiency of 9% when illuminated with an AM 1.5 spectrum.5
Further efficiency improvement can be achieved by stacking BiVO4 on a lower band gap absorber, such as silicon.6,7 However, the BiVO4 minority-carrier diffusion length of 70 nm8 is below the required thickness for optimal optical light absorption. This means that the layers in BiVO4 photoanodes cannot be made sufficiently thick to absorb all of the incident photons with energies above 2.5 eV. When the layer becomes too thick, the photogenerated charges will not be able to reach the electrolyte to perform the desired oxidation reaction.
Several routes have been described in literature to overcome this drawback. Most of them focus on improvement of charge transport by doping with for instance molybdenum,9−11 tungsten5,12 or nitrogen,13 or by the creation of oxygen vacancies by annealing in hydrogen atmosphere.14,15 Others have created BiVO4 layers with a high internal surface area. This was achieved by developing a procedure to create nanoporous BiVO4 from electrodeposited BiOI sheets. This method of preparation suppressed bulk carrier recombination without additional doping.3,16 Furthermore, others have created WO3/BiVO4:W core/shell heterostructures which combine the merits of the two semiconductors, i.e., the excellent charge transport characteristics of WO3 and the good light absorption capability of BiVO4.17−22
A further alternative to improve the activity of BiVO4 photoanodes is to deposit BiVO4 on structured conductive substrates which provide a high internal surface area such as arrays of nano- or microwires. The BiVO4 layers are thin, providing short minority carrier diffusion lengths, and thus reducing electron and hole recombination.23 Additionally, these systems have light trapping properties and thus lead to a much higher absorption of incident photons with energies above the semiconductor bandgap.24−27
Many methods have been developed to deposit BiVO4 on substrates, such as spray pyrolysis,28,29 chemical bath deposition,30 chemical vapor deposition,31 pulsed laser deposition,32 hydrothermal33 or direct34 and indirect3,16,35 electrodeposition. However, the method has to be compatible with (i) deposition on three-dimensional structures, (ii) introduction of dopants, (iii) a high surface area of nanoporous BiVO4, and (iv) favorable pricing and scalability.
In this study, we evaluate the performance of systems combining all positive effects and opportunities for improvement of BiVO4: we deposit nanoporous, molybdenum-doped, and hydrogen-annealed H-BiVO4–x:Mo on p-type silicon microwire arrays (with an intermediate indium tin oxide (ITO) layer) using a two-step synthesis procedure developed by Choi et al.3,16 The p-doped Si used here was not modified to create p/n-junctions, and was merely used as a conductive, easy to structure substrate in the present study. First, the electrodeposition of BiOI was studied with a focus on the influence of microwire spacing and length on deposition rate. The BiOI deposition process is triggered by a local pH change which allows to analyze the formation of pH gradients in-between the microwire structures through the observation of gradients in BiOI thickness. Then, the transformation to H-BiVO4–x:Mo was evaluated, analyzing the effect of doping and annealing of BiOI-derived BiVO4 on flat Si surfaces. Finally, the effect of Si architecture on the performance of H-BiVO4–x:Mo was evaluated, including arrays of microwires of variable length and spacing.
Materials and methods
Fabrication of Silicon Microwire Arrays
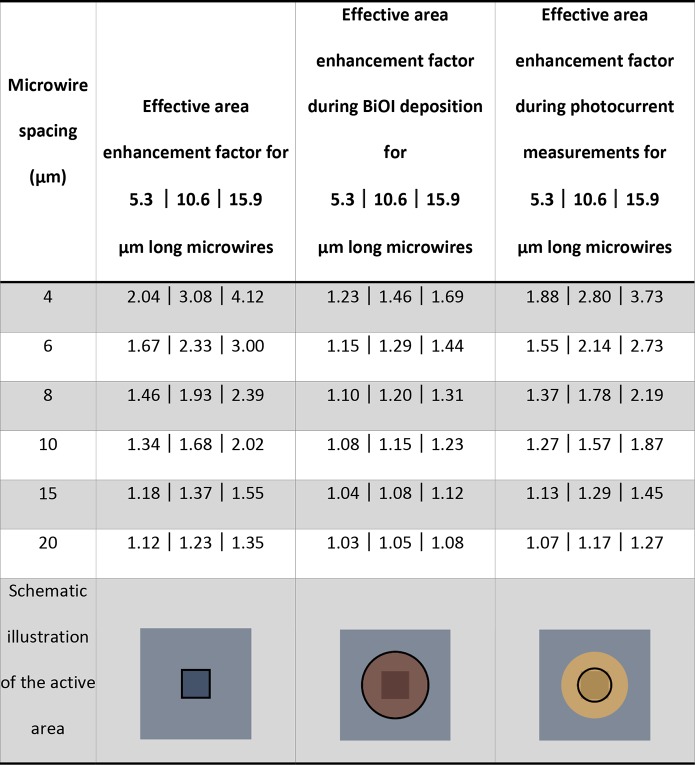
Figure 1 schematically shows the fabrication process of BiVO4–x:Mo-coated silicon microwire arrays. Highly boron-doped, p-type silicon substrates (⟨100⟩-oriented, resistivity 0.010–0.025 Ωcm, 100 mm diameter, 525 μm thickness, single-side polished, Okmetic Finland) were cleaned by immersion in 100% nitric acid (HNO3) (2 × 5 min) and in 69% nitric acid (10 min), followed by quick dump rinsing in demineralized (DI) water, immersion in a 1% aqueous hydrofluoric (HF) acid for at least 1 min to remove the formed oxide shell, and another quick dump rinsing cycle. After spin-drying of the wafers, areas with an array of microwires (specifications: diameter 4 μm, spacing 4–20 μm, hexagonally stacked, 0.5 cm × 0.5 cm cell size on a specimen of 2 cm × 2 cm) were defined in photoresist (Olin 907-17), and postbaked for 10 min at 120 °C after exposure and development. The photoresist acted as a mask layer during deep reactive ion etching (CF-chemistry, SPTS Pegasus). The height of the wires was determined by the etch duration, which was set to 60, 120 or 180 s, resulting in microwire lengths of approximately 5.3, 10.6 and 15.9 μm. The enhancement factors between projected and effective surface area for a complete and partially microwire structured area are shown in Table 1.
Figure 1.
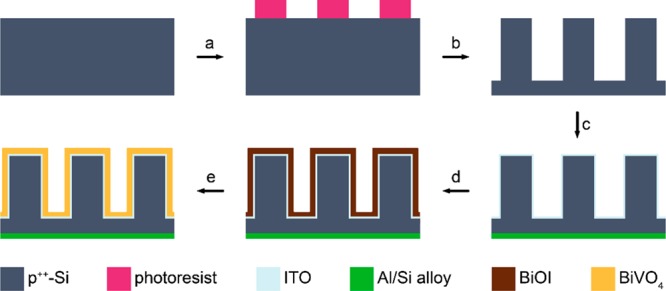
Schematic illustration of the integration of H-BiVO4–x:Mo on silicon microwire arrays as a photoanode. (a) Definition of microwire arrays by standard UV lithography. (b) Deep reactive ion etching (DRIE) and removal of photoresist. (c) HF etching of silicon oxide and sputtering of aluminum/silicon alloy (99% Al, 1% Si) on the backside and ITO on the frontside. (d) Electrochemical deposition of BiOI from a KI, Bi(NO3)3 and p-benzoquinone in water/ethanol solution at −0.1 V vs Ag/AgCl (3 M NaCl). (e) Formation of H-BiVO4–x:Mo by dropcasting VO(acac)2 and Na2MoO4 in DMSO, evaporation of DMSO at 100 °C, calcination at 450 °C in air, soaking in 1 M KOH and reduction in a 5% H2/95% Ar atmosphere at 300 °C for 0.5 h.
Table 1. Total Surface Area Enhancement by Microwires of 4 μm Diameter and Sample Area Enhancement Containing 22.1% Microwire-Structured Area During BiOI Deposition and 85.7% Microwire-Structured Area during Photocurrent Measurementsa.
The active area is illustrated inside the black frame in the schematic illustrations. The centered square is a microwire-structured area with a projected surface area of 0.25 cm2.
Sputtering of Indium Tin Oxide (ITO)
The wafers were treated with oxygen plasma for 20 min followed by C4F8 cleaning for 1 min to remove fluorocarbon residues (GIGAbatch 360P), and immersed in 100% nitric acid (HNO3) (2 × 5 min) and consecutively in 69% nitric acid (10 min), followed by a quick dump rinse in DI water to remove any residue from the etching process, immersion in 1% aqueous HF for at least 1 min to remove the silicon oxide shell, washing by quick dump rinsing in DI water, and spin-drying. Subsequently, the backside was sputter-coated with a 1 μm thick aluminum/silicon alloy (99% Al, 1% Si) (Oxford PL 400, 7000 W) to create a low resistance Ohmic contact. Thereafter, the frontside was coated with ITO in a home-built reactive magnetron sputtering system (TCOater) in direct current (DC) mode, using a 90 wt % In2O3–10 wt % SnO2 (99.99%) target of 4.00 in. diameter. A DC power of 100 W was applied. The distance between the target and the substrate was kept at 44 mm, and the substrate was rotated at 5 rpm during the whole deposition process. The reactor chamber was pumped down to a base pressure of 5.0 × 10–7 mbar prior to sputtering, and a mix of 40 sccm Ar and 1 sccm O2 (99.5%) was introduced as reactive gas and an Ar (99.99%) flow of 20 sccm as sputtering gas. The process pressure was 5.0 × 10–7 mbar and the substrate temperature during deposition was maintained at 20 °C. Before ITO was sputtered on the wafer, presputtering was carried out under the same conditions for 1 min with a shutter covering the substrate. The deposition times were either 5 or 20 min and the ITO deposition rate varied from 7 nm/min in the center to 6 nm/min at the edge on the horizontal part of the wafer. ITO thicknesses were determined by SEM.
Electrodeposition of BiOI
The electrodeposition procedure of BiOI and the following transformation to BiVO4 was derived from publications of Choi et al.3,16 A 0.4 M potassium iodide (KI) and 0.04 M bismuth(III) nitrate (Bi(NO3)3) solution was prepared by dissolving KI (≥99.0%, Sigma-Aldrich) in high-purity water (Millipore, Milli-Q, R = 18.2 MΩcm), adjusting the pH to 1.7 with nitric acid (HNO3, 70%, ACS reagent, Sigma-Aldrich) and adding Bi(NO3)3·5H2O (≥98.0%, Sigma-Aldrich). To this solution, a solution of 0.23 M p-benzoquinone (≥98%, Sigma-Aldrich) in ethanol (99.8%, AA Chemie B.V.) was added in a ratio of 2:5 (ethanol:aqueous) and stirred vigorously for 1 h. The deposition was performed potentiostatically at −0.1 V vs a Ag/AgCl (3 M NaCl, BioLogic RE-1B) reference electrode in a custom-made Teflon-based reactor with a volume of about 8 mL and a platinum mesh counter electrode. A potentiostat (PAR, VersaStat 3) served as a power source. The active silicon/ITO surface area was 1.13 cm2 as defined by the O-ring, and the deposition times were 30, 60, 120, or 300 s. This area covered 0.25 cm2 of the structured, and 0.88 cm2 of a flat surface (see drawing in Table 1). All deposition experiments were performed at room temperature, and the reactor was open to the environment. The samples were prepared in two batches, and each sample in each batch was processed consecutively. The data reported in the results paragraph are based on samples from the same batch.
Synthesis of H-BiVO4–x:Mo
A solution (25 μL) of dimethyl sulfoxide (DMSO, ≥99.5%, Sigma-Aldrich) containing 0.4 M vanadyl acetylacetonate (VO(acac)2, 98%, Sigma-Aldrich) with or without 0.004 M sodium molybdate (Na2MoO4, ≥99%, Sigma-Aldrich) was added on top of the BiOI-coated substrate and dried in a preheated drying oven at 100 °C for 60 min. Samples were calcined immediately at 450 °C for 2 h (at a heating rate of 2 °C/min). Afterward, the excess of vanadium(III) oxide (V2O3) was removed by placing 500 μL of an aqueous (Milli-Q) 1 M KOH (99.99%, Sigma-Aldrich) solution on top of the sample for 30 min, rinsing with Milli-Q water, and drying with compressed air, and repeating this whole procedure. Subsequently, the samples were annealed in a tube furnace at 300 °C for 0.5 h at a heating rate of 10 °C/min in a 5% H2/95% Ar atmosphere at a flow of 500 sccm. The microwire-structured samples were prepared in two batches, and all samples in each batch were processed simultaneously. The data reported in the results paragraph are based on samples from the same batch.
Photoelectrochemical Measurements
Photoelectrochemical measurements of individual electrodes were performed in a custom-made Teflon-based reactor with a volume of about 8 mL which was open to the environment. Illumination occurred through a quartz glass window and 25 mm electrolyte (front-side illumination), and the incident beam was perpendicular to the specimen surface. A solar simulator (Newport) with a 300 W xenon lamp and an air mass (AM) 1.5 global filter was used as light source. The intensity of the simulated sunlight was adjusted to 1 sun (100 mW/cm2) with a standard reference silicon solar cell. The J–V photocurrent data were obtained using a standard three-electrode setup with a platinum mesh counter electrode and a Ag/AgCl (3 M NaCl, BASi MF 2052) reference electrode at room temperature. The reference electrode has a potential (EAg/AgClo) of +0.209 V with respect to the standard hydrogen electrode (SHE), which was confirmed with a new Ag/AgCl (3 M NaCl, BASi MF 2052) master reference electrode before measurements. All voltages reported were calculated versus the reversible hydrogen electrode (RHE) using the following eq 1:
| 1 |
An air-saturated 1 M sodium sulfite (Na2SO3) in 0.5 M potassium phosphate (KPi) buffer solution with a pH of 7.0 served as the electrolyte. The potential was swept with a scan rate of 10 mV/s from 0 to 2.0 V vs RHE first in dark and then under illumination. A potentiostat (PAR, VersaStat 4) served as a power source. The active surface area was 0.28 cm2 as determined by the O-ring. This area covered 0.24 cm2 of the structured and 0.04 cm2 of flat surface (see drawing in Table 1). These values were incorporated in the determination of the effective surface area calculation. The samples were prepared in two batches, and all samples in each batch were processed consecutively. The data reported in the results paragraph are based on samples from the same batch.
Scanning Electron Microscopy (SEM)
High-resolution scanning electron microscopy (HR SEM) images were taken with a FEI Sirion FEG SEM with a Through the Lens Detector (TLD), operated at acceleration voltages of 10 kV. The imaged areas are random, and somewhere around the center of the sample.
X-ray Powder Diffraction (XRD)
X-ray diffraction patterns were determined with a Bruker D2 PHASER XRD using Cu Kα radiation at an acceleration voltage of 30 kV.
Raman Spectroscopy
Raman spectra were collected at ambient conditions by using a Bruker SENTERRA Raman Spectrometer equipped with a 532 nm laser and a cooled CCD detector. Measurements were performed at 10 mW laser power intensity and integration times of 2.0 s.
Results and discussion
Electrodeposition of BiOI
Hexagonally stacked microwires of 5.3, 10.6, and 15.9 μm length, 4 μm diameter and 4, 6, 8, 10, 15, and 20 μm spacing were prepared on areas of 0.5 cm × 0.5 cm in the center of 2 cm × 2 cm cells on highly boron-doped (∼2 × 1018 cm–3–∼8 × 1018 cm–3) silicon substrates. High boron doping was chosen because of its high conductivity and thus low series resistance, the possibility to use silicon with p/n junctions of which the surface would be highly p-type doped, and the creation of a low resistance ohmic contact to sputtered ITO. Such an ITO interlayer has been shown to enhance anodic photocurrent densities of WO3 on p-Si,36 which can also be expected for H-BiVO4–x:Mo on p-Si.
The first step in H-BiVO4–x:Mo preparation is the electrodeposition of BiOI from a solution of KI, Bi(NO3)3 and p-benzoquinone in aqueous ethanol.16 Following the literature procedure, a pH increase near the p-Si surface is induced by the electrochemical reduction of p-benzoquinone to hydroquinone (eq 2), which in turn promotes the deposition of BiOI.3
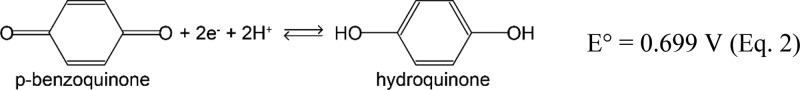 |
2 |
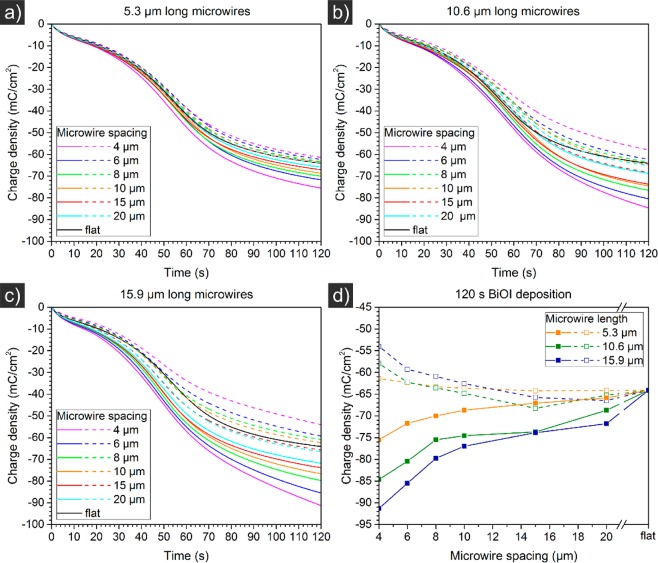
Figure 2a–c shows the charge densities passed during the BiOI growth on a 0.25 cm2 structured area containing pillared Si, as a function of deposition time. The curves show the current densities as per projected surface area (solid lines), and were recalculated to values per effective surface area, considering the length and spacing of the pillars (dashed lines). The values are negative to represent the direction of electron flow toward the working electrode. All curves, including the curves for flat Si (recorded for reference), show an S-shaped profile. Initially some nuclei of BiOI are formed, which presumably grow in quantity and size with time. The formation of these nuclei generates additional surface area for deposition, explaining the increase in charge accumulation, most obvious after approximately 20 s of deposition. After 60 s, the pillars are likely fully covered by a film of BiOI, which leads to an increase of the resistance, resulting in the decelerating accumulation of additional charge density in time.
Figure 2.
Transferred charge densities during electrochemical deposition of BiOI from a solution of KI, Bi(NO3)3 and p-benzoquinone in water/ethanol at −0.1 V vs Ag/AgCl (3 M NaCl) on flat and pillared p-type doped silicon substrates, the latter with 5.3 μm (a), 10.6 μm (b) and 15.9 μm (c) long hexagonally arranged microwire arrays of 4 μm diameter and various spacing. (d) Direct comparison of the effect of microwire spacing on charge density after 120 s deposition time, demonstrating increasing charge density (more negative) per effective surface area (dashed lines) as a function of increasing microwire spacing. The projected sample area was 1.13 cm2 as defined by the O-ring which contained microwires in a 0.25 cm2 cell.
At all microwire lengths, and in particular for the longer lengths (Figure 2b,c), the passed charge densities were larger on structured samples than on flat ones. When corrected for the increased surface area generated by the pillared structure (per effective surface area), however, the charge density was generally lower than obtained for flat surfaces.
Differences as a function of microwire spacing are illustrated in Figure 2d. Although the charge densities per projected surface area (solid lines) increased (became more negative) as a function of decreasing microwire spacing, these values decreased (became less negative) per effective surface area. This suggests, on average, less reactant is available for deposition per surface area, if the microwire spacing is small. This should result in thinner films for small microwire spacings.
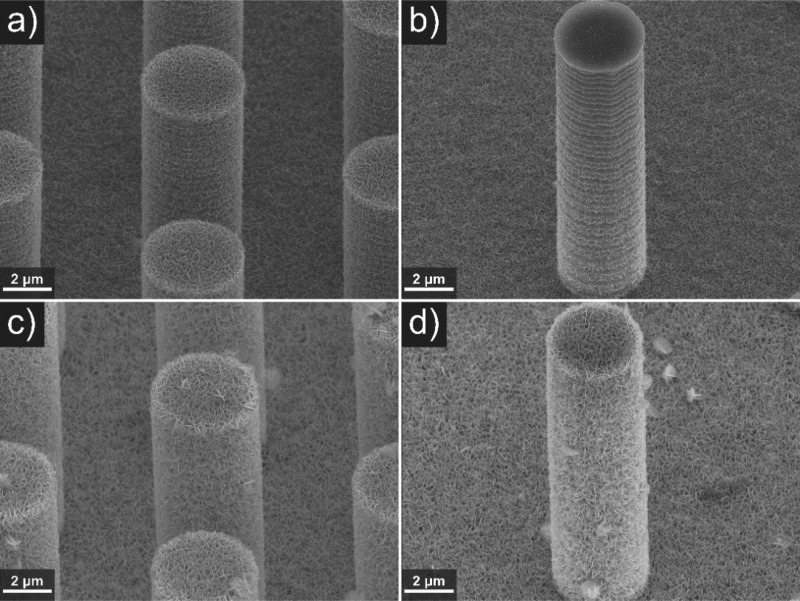
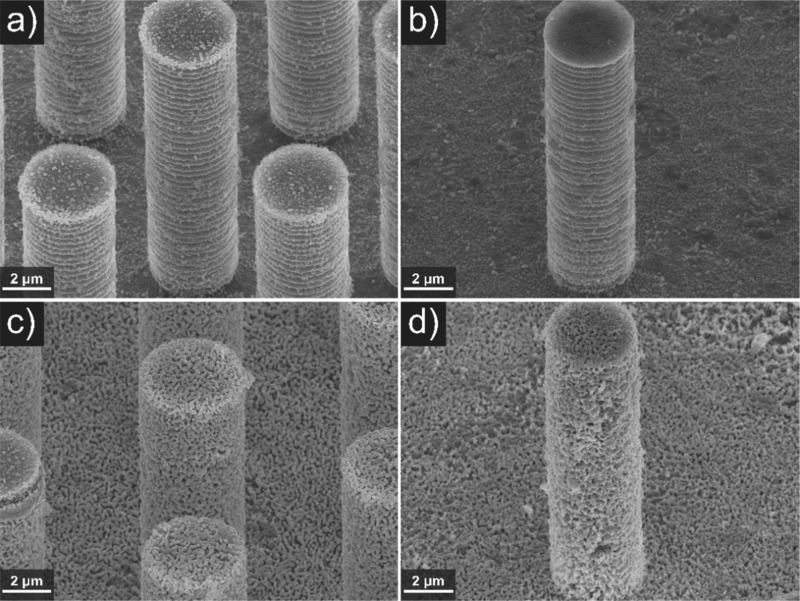
To visually inspect this assumption, Figure 3 shows selected SEM images of microwire arrays with 6 and 15 μm spacing and 15.9 μm length after BiOI deposition. SEM images of all samples coated with BiOI are shown in Figures S1, S2 and S3.
Figure 3.
SEM images (45° tilted) of electrodeposited BiOI for 30 s (a, b) and 120 s (c, d) on 15.9 μm long hexagonally arranged p-type doped silicon microwire arrays of 4 μm diameter and 6 μm (a, c) or 15 μm (b, d) spacing, with ITO sputtered for 5 min.
It can be seen that the BiOI growth is feasible on three-dimensional surfaces and that the microwire surface is covered completely with BiOI after a deposition time of 120 s (Figure 3c,d). In agreement with Figure 2d (the larger charge density), the film appears somewhat thicker for the sample with the (wider) 15 μm spacing.
After deposition times of 30 s, i.e., at the onset of the rapid growth stage (Figure 2), the samples were either completely (Figure 3a, 6 μm spacing) or partially covered (Figure 3b, 15 μm spacing) with a thin layer of BiOI. BiOI apparently starts to grow gradually from the bottom to the top. Several explanations are possible for the phenomena observed in Figures 2 and 3. The growth from the bottom to the top in the first 30 s, is explained by the pH increase being locally the highest at the base of the microwire arrays, because there protons and p-benzoquinone are consumed not only by electrochemical conversion on the surface of the pillar but also on the exposed base, likely inducing a pH gradient. Other reported results strengthen this hypothesis. In earlier work,37 we have observed an inversed gradient of electrodeposited Pt particles from a solution containing H2PtCl6 on an array of microwires (with 7 μm length, 4 μm diameter and 2 μm spacing). There, the deposited Pt particle density was also higher at the bottom than the top. We have tentatively attributed this to diffusion limitation of the precursor in between the microwire arrays and have verified this with a control experiment in which we performed the deposition under the same conditions on microwire arrays of 16 μm spacing. We have found visibly significantly less inhomogeneity. Shaner et al. have electrodeposited WO3 from a tungstic peroxy-acid solution on ITO-coated silicon microwires of 40 to 70 μm length, ∼2 μm diameter and 4 μm spacing in a square arrangement.38 Also, they have found a gradient in WO3 thickness over the microwire length for which the WO3 thickness decreased from the top to the bottom due to a quicker depletion of the peroxo-tungstic acid at the bottom compared to the top.
We exclude that a voltage drop along the microwires caused the gradient in BiOI during its deposition. Accounting for the highest wafer resistivity of 0.025 Ωcm, 15.9 μm length and 4 μm diameter of the microwire and a highest current density during BiOI deposition of 3 mA/cm2, the maximal potential drop can be calculated to be 1.1 × 10–7 V.
When the rate of deposition increases after 30 s, diffusion-limited mass transport of species involved in eq 2 (p-benzoquinone, hydroquinone) into and/or out of the intramicrowire space, appears to control the deposition process, which is apparently more dominant for low microwire spacings.
Another potential cause for disproportionality in charge density during BiOI deposition and its inhomogeneous growth is that the sputtered ITO layer is too thin and does not cover the sides of the microwires completely, in particular when present at low spacing. Using our recipe, a ∼30 nm film formed on a flat substrate during 5 min of sputtering. However, if ITO is truly inhomogeneously distributed, then we would expect BiOI growth to be similar on the bottom between the microwires and on the microwire tops, which is not evident. Nevertheless, we have sputtered a 4-fold amount of ITO (20 min sputtering time, ∼120 nm thickness on a flat substrate) on the samples in a second batch of production of microwires. We have constructed microwire arrays with spacings of 6 to 15 μm, kept the microwire length constant at 10.6 μm, and deposited BiOI for 30 to 300 s. Figure S4 shows the charge densities passed during the BiOI growth on these samples which had an active surface area of 1.13 cm2 including a 0.25 cm2 structured area. Also with a thicker ITO layer, BiOI deposited well on microwire-structured surfaces (Figure S5), while more charge passed through the system when thicker ITO layers were sputtered. We speculate that this is due to a lower resistance of the thicker ITO layer, which might originate from a different relative oxygen content in ITO or a higher crystallinity. We note that a less pronounced trend in effect of smaller microwire spacing on increasing charge density is observed in Figure S4 compared to that shown in Figure 2, while a wider spread in absolute values of charge density was obtained when comparing variable BiOI deposition times. A direct control experiment in Figure S4 in which we deposited BiOI for 120 s on microwires with an ITO coating sputtered for 5 or 20 min confirmed this. Thus, some effect of ITO uniformity, besides the advocated mass transfer limitation of quinone or hydroquinone, cannot be excluded.
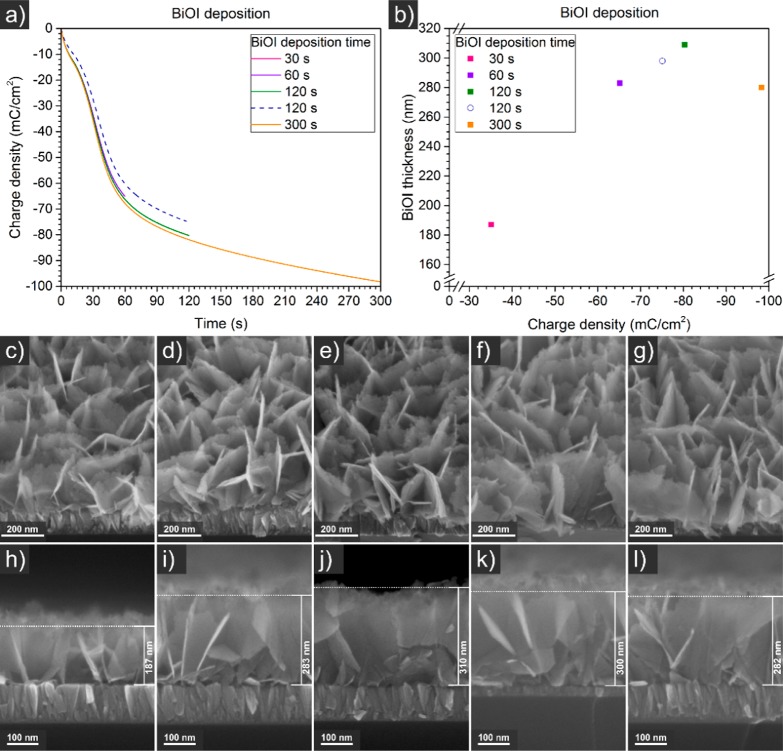
On flat Si substrates, the BiOI growth rate appeared linear over the passed charge density between a deposition time of 30 and 120 s (Figure 4). At a higher deposition time of 300 s, the BiOI thickness decreased. This is due to a decreasing current density over time, evident from Figure 4a, which became too low to sustain a significant pH gradient between the substrate surface and the bulk solution. Apparently, BiOI starts to redissolve back into solution when the pH near the surface becomes too low.
Figure 4.
Cumulative transferred charge densities (a, b), 45° tilted (c–g) and cross sectional (k, l) SEM images of BiOI electrochemically deposited from a solution of KI, Bi(NO3)3 and p-benzoquinone in water/ethanol at −0.1 V vs Ag/AgCl (3 M NaCl) on flat p-type doped silicon with ITO sputtered for 5 min (a dotted line, b circle, f, k) and 20 min (a solid lines, b squares, c, d, e, g, h, i, j, l). The projected sample area was 1.13 cm2 as defined by the O-ring.
In summary, the current density profiles and SEM images have demonstrated the following:
Growth is initiated (in the first 30 s) by nucleation at the base of the microwire arrays and bottom of the microwires, explained by the most effective increase in pH at these locations.
A gradient in pH causes a decreasing gradient in deposition rate from bottom to top (within the first 30 s).
Rapid growth after 30 s is likely limited by ineffective transport of p-benzoquinone to or from the intra-microwire space, leading to lower charge density per effective surface area for microwires with the lowest spacing.
Growth is self-limiting due to enhanced interfacial resistance induced by the BiOI film.
Film thicknesses range from 200 to 300 nm, depending on the applied deposition time.
Doping and Hydrogen Annealing to Transfer BiOI into H-BiVO4–x:Mo
Figure 5 shows selected SEM images of microwire arrays with spacings of 6 and 15 μm and a length of 15.9 μm, after transformation of BiOI (deposition times of 30 and 120 s) into H-BiVO4–x:Mo. SEM images of all prepared samples of 5.3, 10.6 and 15.9 μm length and 4, 6, 8, 10, 15 and 20 μm spacing are shown in Figure S6, S7 and S8. The H-BiVO4–x:Mo coating was nanoporous and covered the three-dimensional microwire surfaces when BiOI was deposited for 120 s, but showed defects in the form of cracks (Figure 5c,d). These defects are attributed to crystallization during the DMSO evaporation process. Microwires onto which BiOI was deposited for only 30 s and thus were not completely covered were also incompletely covered with H-BiVO4–x:Mo (Figure 5a,b). Similar results were achieved when H-BiVO4–x:Mo was prepared on microwires sputtered with ITO for 20 min and BiOI deposition times were 30, 60, 120 and 300 s (Figure S9). Also here, a complete H-BiVO4–x:Mo coating was only obtained at BiOI deposition times greater than 30 s.
Figure 5.
SEM images (45° tilted) of H-BiVO4–x:Mo prepared from BiOI electrodeposited for 30 s (a, b) and 120 s (c, d) on 15.9 μm long hexagonally arranged p-type doped silicon microwire arrays of 4 μm diameter and 6 μm (a, c) or 15 μm (b, d) spacing with ITO sputtered for 5 min.
To study the effects of Mo addition and hydrogen annealing, reference samples were prepared in which the transformation of BiOI to BiVO4 was performed without Mo addition and in which BiVO4:Mo was not annealed in hydrogen atmosphere. X-ray diffractometry (XRD) and Raman spectroscopy revealed that nanoporous doped and undoped BiVO4 were highly crystalline and of purely the monoclinic scheelite phase (Figure S10).39,40 No detectable diffraction peak shifts were observed in the XRD due to Mo doping or annealing in hydrogen atmosphere which is in agreement with earlier reports.11,14,15,41,42
The Raman spectra (Figure S10) show characteristic VO4 tetrahedron vibrational modes of monoclinic BiVO4 at around 211, 328, 367, 712 and 826 cm–1. The major band at 826 cm–1 represents the Ag mode of the symmetric V–O stretching.39 A shift of this vibrational mode to lower frequency of 822 cm–1 and the appearance of an additional peak at 876 cm–1 confirmed the Mo doping in BiVO4 in these samples. The redshift of the Ag mode frequency in BiVO4:Mo and H-BiVO4–x:Mo is interpreted as an increase of the bond lengths in the tetrahedrons by a substitution of Mo for V, and the peak at 876 cm–1 is due to Mo–O–Mo stretching.43,44
The exact doping level of Mo in our experiments is not known but it is assumed to be below 6.0%. Mo doping levels higher than 6.0% should lead to a noticeable change in the XRD pattern due to a structural change from monoclinic to tetragonal phase or precipitation of V2O5.41,45,46
Photoelectrical and Photoelectrochemical Characterization of Flat Substrates
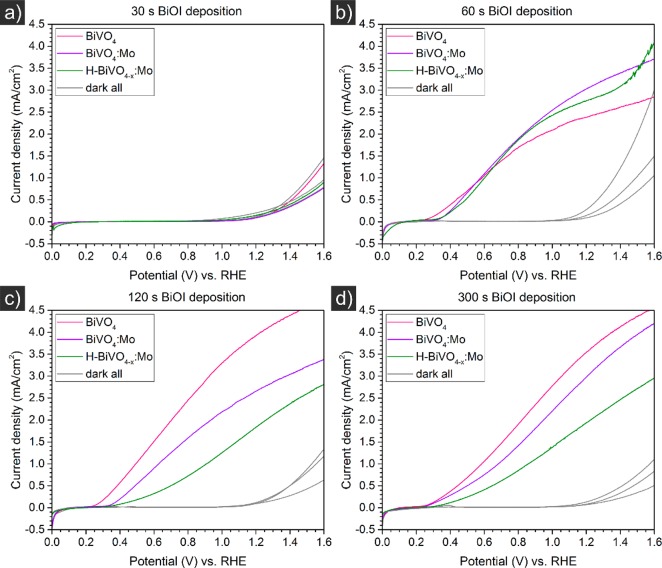
We initially studied the dependence of doping and annealing in hydrogen on the photocurrent properties. In our photocurrent measurements of BiVO4, BiVO4:Mo and H-BiVO4–x:Mo prepared from BiOI deposited for 30 to 300 s (Figure 6), we observed a slight improvement due to Mo doping and hydrogen annealing only for samples prepared at a BiOI deposition time of 60 s. At BiOI deposition times of 120 and 300 s, undoped, unannealed BiVO4 showed the highest photocurrents, followed by BiVO4:Mo and then H-BiVO4–x:Mo.
Figure 6.
Linear potential sweeps at a scan rate of 10 mV/s for BiVO4, BiVO4:Mo and H-BiVO4–x:Mo obtained from BiOI deposited for 30 s (a), 60 s (b), 120 s (c) and 300 s (d) on flat p-type silicon in a solution of 1 M sodium sulfite (Na2SO3) and 0.5 M potassium phosphate (KPi) buffer at a pH of 7.0 under simulated AM 1.5 solar illumination.
The reason for the decrease in photocurrent due to Mo doping and hydrogen annealing in these samples, contrary to a positive effect of these procedures reported in the literature,41,46 is not known. We assume that this is, in the case of Mo doping, due to a different preparation procedure, i.e. reactive cosputtering, and a different electrodeposition route, in comparison to literature.41,46 As for the hydrogen annealing, the used temperature in the present study might have been too high. While the applied temperature of 300 °C is below the value of 320 °C reported by Cooper et al. to induce lower photocurrents of BiVO4 when annealed in pure hydrogen atmosphere due to the formation of metallic species by reduction of BiVO4,15 Gan et al. have reported the optimum to be at 250 °C in a 5% H2/95% Ar atmosphere.14 Gan et al.14 found that the photocurrent density of H-BiVO4–x in a solution of 1 M sodium sulfite in 0.1 M borate buffer at pH 9 was substantially higher than that of BiVO4. They have attributed this to an increase in oxygen vacancies and introduction of hydrogen impurities through hydrogenation. These oxygen vacancies improve photoelectrochemical performance by improving the electrical conductivity, which enhances the charge transfer properties of H-BiVO4–x. Cooper et al. in contrast have argued that the improvement of photocurrent of postsynthetically annealed BiVO4 in 100% hydrogen atmosphere is not due to the introduction of oxygen vacancies, but rather to incorporation of hydrogen donors.15 The authors found that annealing in H2 at temperatures up to 290 °C led to near-complete elimination of majority carrier transport limitations.15
Performance of Flat vs Microwired Samples Containing H-BiVO4–x:Mo
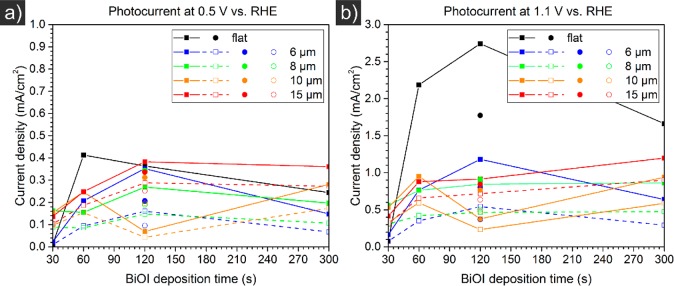
Photocurrent measurements were performed by linear potential sweeps from 0 to 2.0 V vs RHE at a scan rate of 10 mV/s, first in dark and then under simulated AM 1.5 solar illumination. The scans are shown in the Supporting Information. The onset potential occurred at ∼1.10 V vs RHE in the absence, and at ∼0.35 V vs RHE in the presence of illumination. Photocurrents obtained at 0.5 and 1.1 V vs RHE are shown as a function of BiOI deposition time and microwire spacing in Figure 7. The choice of photocurrent comparisons at 0.5 and 1.1 V is arbitrary and serves only to represent a low and a high bias region.
Figure 7.
Photocurrent densities vs microwire spacing (a, b) and BiOI deposition time (c, d) as a function of microwire spacing at 0.5 V (a, c) and 1.1 V (b, d) for projected (filled symbols, solid lines) and effective (empty symbols, dashed lines) sample areas of H-BiVO4–x:Mo-coated Si microwire arrays, of different lengths (a, b) and of 10.6 μm length (c, d) with sputtered ITO (20 min (squares), 5 min (circles)). Data is obtained from linear potential sweeps presented in Figure S11.
First, Figure 7 shows that deposition of BiOI for more than 60 s has little effect on the photocurrent density, independent of the microwire spacing. Furthermore, both in the low bias region at 0.5 V vs RHE and at 1.1 V vs RHE, microwire samples underperform flat Si surfaces. Finally, a larger microwire spacing is beneficial for the obtained current density.
Several reasons are plausible for this behavior. As discussed in the BiOI deposition section above, the ITO layer thickness on the microwires might have been too thin or the grown H-BiVO4–x:Mo layers might have been closer to the optimal range on the flat samples than on the microwires. However, the linear potential sweeps shown in Figure S11 and the comparison of photoactivities at 0.5 and 1.1 V vs RHE in Figure 7 show that the underperformance of microwire-structured samples in comparison to flat samples is not due to ITO and H-BiVO4–x:Mo layer thicknesses, since the flat samples also show a better performance than the microwire-structured samples for thicker ITO and H-BiVO4–x:Mo layers. Therefore, we focus here on possible optical effects of the microwire arrays, and possible diffusion limitations present in the internal volume of the microwire array, to address the underperformance of the system containing silicon microwires. Furthermore, differences in the concentration and type of surface defect sites or specific catalytic sites (edges, steps, etc.) for structured versus planar semiconductor geometries will be addressed.
It has been frequently reported that structuring of the silicon surface with microwires enhances the photovoltaic performance due to enhanced light absorption and a larger junction area.25,47,48 Indeed, Chakthranont et al. obtained higher photocurrents in BiVO4-induced sulfite oxidation on microwire-structured samples than on flat equivalents.49 Chakthranont et al. deposited BiVO4 on so-called black silicon which shows a very high light absorption and is composed of needle-shaped (tapered) nanowires, in their case ∼3 μm length, ∼200 nm average diameter and ∼300 nm spacing. Our results appear to be in agreement with Boettcher et al., who prepared similarly sized microwire arrays with a radial n+/p-junction, deposited platinum on the tops and tested them for photocatalytic hydrogen evolution.50 They found that the efficiency of silicon microwire photocathodes was 5.8% while that of planar control samples was 9.6%. In another publication, Boettcher et al. tested energy conversion efficiencies of p-type microwires for the reduction of methyl viologen (MV2+).51 Again, they observed that flat surfaces outperformed microwires, demonstrating energy conversion efficiencies of 3.6% for structured and 12.9% for flat substrates, respectively. The authors argued that this is due to minimized optical absorption of the perpendicularly oriented illumination beam to the Si microwire arrays. Indeed, by tilting the substrate, the external quantum yield increased from ∼0.2 to close to 0.7 at 60°. Also, others have observed similar angle-dependent photocatalytic behavior on microwires.52 On the basis of our results, we cannot determine whether microwires result in favorable, as shown by Elbersen et al.,47,48 Kelzenberg et al.25 or Chakthranont et al.,49 or unfavorable optical characteristics.47,48
Mass transfer effects have also been reported in the literature, which are in line with our observations and discussions on the electrochemical BiOI deposition. For instance, Xiang et al. studied the mass transport of 50 mM/5.0 mM cobaltocenium+/cobaltocene0 redox species in CH3CN – 1.0 M LiClO4 electrolyte in-between p-type microwire arrays of 3 μm diameter, ∼100 μm length and 4 μm spacing in a square lattice.53 They found that fill factors were low and that the obtainable photocurrent densities were limited to 17.6 mA/cm2 even under high illumination intensities due to mass transport limitations of the redox species within the internal volume of the silicon microwire arrays.53 Their simulations with COMSOL Multiphysics showed that the solution-phase reagent concentration decreased to zero over the lower ∼70% of the wire length relative to the bulk concentration above the microwire, implying that more than two-thirds of the microwire length only contributed to light absorption but not to the redox process.53,54 In agreement with our observations, Vijselaar et al. found that photovoltaic current densities of silicon microwires with a radial p/n-junction increase with increasing microwire spacing.55 These results strengthen our hypothesis of diffusion limitation of redox species in the internal volume of the microwire array, which leads to an overall underperformance of the microwire structures compared to flat samples. To justify the results of Chakthranont et al., a positive light trapping effect of their nanowires (rather than microwires) might have overcompensated the negative diffusion effects, while the smaller dimensions of the wires also lead to a significantly shorter ion diffusion path length.
Finally, we would like to point out that changing the geometry of Si from flat to Si micropillars might lead to differences in the nanoscale morphology of the BiOI (which could arise from the observed different growth process and/or the increased roughness at the sidewalls of the micropillars compared to the flat Si substrates). This could be transferred onto the (again nanoscale) morphology of the BiVO4, and that way could contribute to a change in the intrinsic catalytic activity of the material. We see no clear signs of morphology changes in the deposited materials, yet a detailed assessment of the effect of structured defects, as e.g. provided for MoS2 by Jaramillo and co-workers56 using in situ corrosion by combined illumination and cyclic voltammetry, should be performed to further assess the effects of defects on the performance comparison of flat p-doped Si and structured architectures. Whether or not it matters depends on the specific system at hand, as also illustrated for TiO2/BiVO4 nanowire heterostructures used as photoanode.57
Conclusions
In conclusion, molybdenum-doped and hydrogen-annealed H-BiVO4–x:Mo in different thicknesses was successfully deposited on ITO-coated, highly doped p-type silicon microwire arrays of 4 μm diameter, 4 to 20 μm spacing and 5 to 16 μm length, using BiOI as precursor. Deposition of BiOI appears to occur in three stages, i.e. nucleation of particles at the base and bottom of the wires, followed by rapid (homogeneous) growth, and termination by increasing interfacial resistances.
We encountered several unexpected effects that hampered the efficiency of our devices. After conversion of BiOI to BiVO4, the influence of Mo doping and hydrogen annealing in combination with different preparation methods was negative, as they decreased the photocatalytic performance of our H-BiVO4–x:Mo films. Since this is contrary to literature, this requires further investigation. Observed photocurrents for the oxidation of sodium sulfite (Na2SO3) on microwire-structured samples were mainly lower than on flat equivalents. While the presence of positive or negative optical effects cannot be excluded (photocatalytic activity tests at various tilt angles would be required), our results support the hypothesis that ion diffusion in the internal volume of the microwires dominates the lower performance of microwire-structured devices compared to flat samples. This is in agreement with the electrochemical data of BiOI deposition, and some existing literature. Overall, the results indicate that micro/nanostructuring of electrode surfaces can have different, counteracting effects on the various aspects of photoelectrical and photoelectrochemical processes, on the micro-, as well as nanolevel. These effects need to be unraveled for optimized designs of future PEC devices.
Acknowledgments
This work was supported by The Netherlands Organization for Scientific Research (NWO-CW Vici Grant 700.58.443 to J.H., and FOM Project 13CO12-2).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acssuschemeng.8b05756.
Additional images of Si architectures containing BiOI and BiVO4 layers, time-dependent charge-density curves, XRD patterns and Raman spectra of the synthesized surfaces, linear potential sweeps of the devices in oxidation of sulfite (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Abdi F. F.; Firet N.; Dabirian A.; van de Krol R. Spray-deposited Co-Pi Catalyzed BiVO4: a low-cost route towards highly efficient photoanodes. MRS Online Proc. Libr. 2012, 1446, 1446. 10.1557/opl.2012.811. [DOI] [Google Scholar]
- Jiang H.-q.; Endo H.; Natori H.; Nagai M.; Kobayashi K. Fabrication and efficient photocatalytic degradation of methylene blue over CuO/BiVO4 composite under visible-light irradiation. Mater. Res. Bull. 2009, 44, 700. 10.1016/j.materresbull.2008.06.007. [DOI] [Google Scholar]
- McDonald K. J.; Choi K.-S. A new electrochemical synthesis route for a BiOI electrode and its conversion to a highly efficient porous BiVO4 photoanode for solar water oxidation. Energy Environ. Sci. 2012, 5, 8553. 10.1039/c2ee22608a. [DOI] [Google Scholar]
- Tokunaga S.; Kato H.; Kudo A. Selective Preparation of Monoclinic and Tetragonal BiVO4 with Scheelite Structure and Their Photocatalytic Properties. Chem. Mater. 2001, 13, 4624. 10.1021/cm0103390. [DOI] [Google Scholar]
- Abdi F. F.; Firet N.; van de Krol R. Efficient BiVO4 Thin Film Photoanodes Modified with Cobalt Phosphate Catalyst and W-doping. ChemCatChem 2013, 5, 490. 10.1002/cctc.201200472. [DOI] [Google Scholar]
- Doscher H.; Geisz J. F.; Deutsch T. G.; Turner J. A. Sunlight absorption in water - efficiency and design implications for photoelectrochemical devices. Energy Environ. Sci. 2014, 7, 2951. 10.1039/C4EE01753F. [DOI] [Google Scholar]
- Bolton J. R.; Strickler S. J.; Connolly J. S. Limiting and realizable efficiencies of solar photolysis of water. Nature 1985, 316, 495. 10.1038/316495a0. [DOI] [Google Scholar]
- Abdi F. F.; Savenije T. J.; May M. M.; Dam B.; van de Krol R. The Origin of Slow Carrier Transport in BiVO4 Thin Film Photoanodes: A Time-Resolved Microwave Conductivity Study. J. Phys. Chem. Lett. 2013, 4, 2752. 10.1021/jz4013257. [DOI] [Google Scholar]
- Pattengale B.; Huang J. The effect of Mo doping on the charge separation dynamics and photocurrent performance of BiVO4 photoanodes. Phys. Chem. Chem. Phys. 2016, 18, 32820. 10.1039/C6CP06407H. [DOI] [PubMed] [Google Scholar]
- Luo W.; Yang Z.; Li Z.; Zhang J.; Liu J.; Zhao Z.; Wang Z.; Yan S.; Yu T.; Zou Z. Solar hydrogen generation from seawater with a modified BiVO4 photoanode. Energy Environ. Sci. 2011, 4, 4046. 10.1039/c1ee01812d. [DOI] [Google Scholar]
- Yao W.; Iwai H.; Ye J. Effects of molybdenum substitution on the photocatalytic behavior of BiVO4. Dalton Transactions 2008, 1426. 10.1039/b713338c. [DOI] [PubMed] [Google Scholar]
- Abdi F. F.; Han L.; Smets A. H. M.; Zeman M.; Dam B.; van de Krol R., Efficient solar water splitting by enhanced charge separation in a bismuth vanadate-silicon tandem photoelectrode, Nat. Commun. 2013, 4, 10.1038/ncomms3195. [DOI] [PubMed] [Google Scholar]
- Kim T. W.; Ping Y.; Galli G. A.; Choi K.-S.. Simultaneous enhancements in photon absorption and charge transport of bismuth vanadate photoanodes for solar water splitting, Nat. Commun. 2015, 6, 10.1038/ncomms9769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan J.; Lu X.; Rajeeva B. B.; Menz R.; Tong Y.; Zheng Y. Efficient Photoelectrochemical Water Oxidation over Hydrogen-Reduced Nanoporous BiVO4 with Ni–Bi Electrocatalyst. ChemElectroChem 2015, 2, 1385. 10.1002/celc.201500091. [DOI] [Google Scholar]
- Cooper J. K.; Scott S. B.; Ling Y.; Yang J.; Hao S.; Li Y.; Toma F. M.; Stutzmann M.; Lakshmi K. V.; Sharp I. D. Role of Hydrogen in Defining the n-Type Character of BiVO4 Photoanodes. Chem. Mater. 2016, 28, 5761. 10.1021/acs.chemmater.6b01994. [DOI] [Google Scholar]
- Kim T. W.; Choi K.-S. Nanoporous BiVO4 Photoanodes with Dual-Layer Oxygen Evolution Catalysts for Solar Water Splitting. Science 2014, 343, 990. 10.1126/science.1246913. [DOI] [PubMed] [Google Scholar]
- Hong S. J.; Lee S.; Jang J. S.; Lee J. S. Heterojunction BiVO4/WO3 electrodes for enhanced photoactivity of water oxidation. Energy Environ. Sci. 2011, 4, 1781. 10.1039/c0ee00743a. [DOI] [Google Scholar]
- Pihosh Y.; Turkevych I.; Mawatari K.; Asai T.; Hisatomi T.; Uemura J.; Tosa M.; Shimamura K.; Kubota J.; Domen K.; Kitamori T. Nanostructured WO3/BiVO4 Photoanodes for Efficient Photoelectrochemical Water Splitting. Small 2014, 10, 3692. 10.1002/smll.201400276. [DOI] [PubMed] [Google Scholar]
- Pihosh Y.; Turkevych I.; Mawatari K.; Uemura J.; Kazoe Y.; Kosar S.; Makita K.; Sugaya T.; Matsui T.; Fujita D.; Tosa M.; Kondo M.; Kitamori T. Photocatalytic generation of hydrogen by core-shell WO3/ BiVO4 nanorods with ultimate water splitting efficiency. Sci. Rep. 2015, 5, 11141. 10.1038/srep11141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao P. M.; Cai L.; Liu C.; Cho I. S.; Lee C. H.; Weisse J. M.; Yang P.; Zheng X. Simultaneously Efficient Light Absorption and Charge Separation in WO3/ BiVO4 Core/Shell Nanowire Photoanode for Photoelectrochemical Water Oxidation. Nano Lett. 2014, 14, 1099. 10.1021/nl500022z. [DOI] [PubMed] [Google Scholar]
- Pilli S. K.; Janarthanan R.; Deutsch T. G.; Furtak T. E.; Brown L. D.; Turner J. A.; Herring A. M. Efficient photoelectrochemical water oxidation over cobalt-phosphate (Co-Pi) catalyst modified BiVO4/1D-WO3 heterojunction electrodes. Phys. Chem. Chem. Phys. 2013, 15, 14723. 10.1039/c3cp52401a. [DOI] [PubMed] [Google Scholar]
- Su J.; Guo L.; Bao N.; Grimes C. A. Nanostructured WO3/BiVO4 Heterojunction Films for Efficient Photoelectrochemical Water Splitting. Nano Lett. 2011, 11, 1928. 10.1021/nl2000743. [DOI] [PubMed] [Google Scholar]
- Chen C.-J.; Chen P.-T.; Basu M.; Yang K.-C.; Lu Y.-R.; Dong C.-L.; Ma C.-G.; Shen C.-C.; Hu S.-F.; Liu R.-S. An integrated cobalt disulfide (CoS2) co-catalyst passivation layer on silicon microwires for photoelectrochemical hydrogen evolution. J. Mater. Chem. A 2015, 3, 23466. 10.1039/C5TA06202K. [DOI] [Google Scholar]
- Garnett E.; Yang P. Light Trapping in Silicon Nanowire Solar Cells. Nano Lett. 2010, 10, 1082. 10.1021/nl100161z. [DOI] [PubMed] [Google Scholar]
- Kelzenberg M. D.; Boettcher S. W.; Petykiewicz J. A.; Turner-Evans D. B.; Putnam M. C.; Warren E. L.; Spurgeon J. M.; Briggs R. M.; Lewis N. S.; Atwater H. A. Enhanced absorption and carrier collection in Si wire arrays for photovoltaic applications. Nat. Mater. 2010, 9, 239. 10.1038/nmat2635. [DOI] [PubMed] [Google Scholar]
- Zhu J.; Yu Z.; Burkhard G. F.; Hsu C.-M.; Connor S. T.; Xu Y.; Wang Q.; McGehee M.; Fan S.; Cui Y. Optical Absorption Enhancement in Amorphous Silicon Nanowire and Nanocone Arrays. Nano Lett. 2009, 9, 279. 10.1021/nl802886y. [DOI] [PubMed] [Google Scholar]
- Warren E. L.; Atwater H. A.; Lewis N. S. Silicon Microwire Arrays for Solar Energy-Conversion Applications. J. Phys. Chem. C 2014, 118, 747. 10.1021/jp406280x. [DOI] [Google Scholar]
- Liang Y.; Tsubota T.; Mooij L. P. A.; van de Krol R. Highly Improved Quantum Efficiencies for Thin Film BiVO4 Photoanodes. J. Phys. Chem. C 2011, 115, 17594. 10.1021/jp203004v. [DOI] [Google Scholar]
- Abdi F. F.; van de Krol R. Nature and Light Dependence of Bulk Recombination in Co-Pi-Catalyzed BiVO4 Photoanodes. J. Phys. Chem. C 2012, 116, 9398. 10.1021/jp3007552. [DOI] [Google Scholar]
- Neves M. C.; Trindade T. Chemical bath deposition of BiVO4. Thin Solid Films 2002, 406, 93. 10.1016/S0040-6090(01)01787-4. [DOI] [Google Scholar]
- Alarcon-Llado E.; Chen L.; Hettick M.; Mashouf N.; Lin Y.; Javey A.; Ager J. W. BiVO4 thin film photoanodes grown by chemical vapor deposition. Phys. Chem. Chem. Phys. 2014, 16, 1651. 10.1039/C3CP53904K. [DOI] [PubMed] [Google Scholar]
- Rettie A. J. E.; Mozaffari S.; McDaniel M. D.; Pearson K. N.; Ekerdt J. G.; Markert J. T.; Mullins C. B. Pulsed Laser Deposition of Epitaxial and Polycrystalline Bismuth Vanadate Thin Films. J. Phys. Chem. C 2014, 118, 26543. 10.1021/jp5082824. [DOI] [Google Scholar]
- Yu J.; Kudo A. Hydrothermal Synthesis of Nanofibrous Bismuth Vanadate. Chem. Lett. 2005, 34, 850. 10.1246/cl.2005.850. [DOI] [Google Scholar]
- Seabold J. A.; Choi K.-S. Efficient and Stable Photo-Oxidation of Water by a Bismuth Vanadate Photoanode Coupled with an Iron Oxyhydroxide Oxygen Evolution Catalyst. J. Am. Chem. Soc. 2012, 134, 2186. 10.1021/ja209001d. [DOI] [PubMed] [Google Scholar]
- Dall’Antonia L. H.; de Tacconi N. R.; Chanmanee W.; Timmaji H.; Myung N.; Rajeshwar K. Electrosynthesis of Bismuth Vanadate Photoelectrodes. Electrochem. Solid-State Lett. 2010, 13, D29. 10.1149/1.3322641. [DOI] [Google Scholar]
- Coridan R. H.; Shaner M.; Wiggenhorn C.; Brunschwig B. S.; Lewis N. S. Electrical and Photoelectrochemical Properties of WO3/Si Tandem Photoelectrodes. J. Phys. Chem. C 2013, 117, 6949. 10.1021/jp311947x. [DOI] [Google Scholar]
- Milbrat A.; Elbersen R.; Kas R.; Tiggelaar R. M.; Gardeniers H.; Mul G.; Huskens J. Spatioselective Electrochemical and Photoelectrochemical Functionalization of Silicon Microwires with Axial p/n Junctions. Adv. Mater. 2016, 28, 1400. 10.1002/adma.201504609. [DOI] [PubMed] [Google Scholar]
- Shaner M. R.; Fountaine K. T.; Ardo S.; Coridan R. H.; Atwater H. A.; Lewis N. S. Photoelectrochemistry of core-shell tandem junction n-p+-Si/n-WO3 microwire array photoelectrodes. Energy Environ. Sci. 2014, 7, 779. 10.1039/C3EE43048K. [DOI] [Google Scholar]
- Frost R. L.; Henry D. A.; Weier M. L.; Martens W. Raman spectroscopy of three polymorphs of BiVO4: clinobisvanite, dreyerite and pucherite, with comparisons to (VO4)3-bearing minerals: namibite, pottsite and schumacherite. J. Raman Spectrosc. 2006, 37, 722. 10.1002/jrs.1499. [DOI] [Google Scholar]
- Sleight A. W.; Chen H. y.; Ferretti A.; Cox D. E. Crystal growth and structure of BiVO4. Mater. Res. Bull. 1979, 14, 1571. 10.1016/0025-5408(72)90227-9. [DOI] [Google Scholar]
- Chen L.; Toma F. M.; Cooper J. K.; Lyon A.; Lin Y.; Sharp I. D.; Ager J. W. Mo-Doped BiVO4 Photoanodes Synthesized by Reactive Sputtering. ChemSusChem 2015, 8, 1066. 10.1002/cssc.201402984. [DOI] [PubMed] [Google Scholar]
- Berglund S. P.; Rettie A. J. E.; Hoang S.; Mullins C. B. Incorporation of Mo and W into nanostructured BiVO4 films for efficient photoelectrochemical water oxidation. Phys. Chem. Chem. Phys. 2012, 14, 7065. 10.1039/c2cp40807d. [DOI] [PubMed] [Google Scholar]
- Merupo V. I.; Velumani S.; Kassiba A.; García-Sánchez M. A. In 2014 11th International Conference on Electrical Engineering, Computing Science and Automatic Control (CCE) 2014; p 1. [Google Scholar]
- Sandhya Kumari L.; Prabhakar Rao P.; Narayana Pillai Radhakrishnan A.; James V.; Sameera S.; Koshy P. Brilliant yellow color and enhanced NIR reflectance of monoclinic BiVO4 through distortion in VO43– tetrahedra. Sol. Energy Mater. Sol. Cells 2013, 112, 134. 10.1016/j.solmat.2013.01.022. [DOI] [Google Scholar]
- Park H. S.; Kweon K. E.; Ye H.; Paek E.; Hwang G. S.; Bard A. J. Factors in the Metal Doping of BiVO4 for Improved Photoelectrocatalytic Activity as Studied by Scanning Electrochemical Microscopy and First-Principles Density-Functional Calculation. J. Phys. Chem. C 2011, 115, 17870. 10.1021/jp204492r. [DOI] [Google Scholar]
- Park Y.; Kang D.; Choi K.-S. Marked enhancement in electron-hole separation achieved in the low bias region using electrochemically prepared Mo-doped BiVO4 photoanodes. Phys. Chem. Chem. Phys. 2014, 16, 1238. 10.1039/C3CP53649A. [DOI] [PubMed] [Google Scholar]
- Elbersen R.; Tiggelaar R. M.; Milbrat A.; Mul G.; Gardeniers H.; Huskens J. Controlled Doping Methods for Radial p/n Junctions in Silicon. Adv. Energy Mater. 2015, 5, 1401745. 10.1002/aenm.201401745. [DOI] [Google Scholar]
- Elbersen R.; Vijselaar W.; Tiggelaar R. M.; Gardeniers H.; Huskens J. Effects of Pillar Height and Junction Depth on the Performance of Radially Doped Silicon Pillar Arrays for Solar Energy Applications. Adv. Energy Mater. 2016, 6, 1501728. 10.1002/aenm.201501728. [DOI] [Google Scholar]
- Chakthranont P.; Hellstern T. R.; McEnaney J. M.; Jaramillo T. F. Design and Fabrication of a Precious Metal-Free Tandem Core–Shell p+n Si/W-Doped BiVO4 Photoanode for Unassisted Water Splitting. Adv. Energy Mater. 2017, 7, 1701515. 10.1002/aenm.201701515. [DOI] [Google Scholar]
- Boettcher S. W.; Warren E. L.; Putnam M. C.; Santori E. A.; Turner-Evans D.; Kelzenberg M. D.; Walter M. G.; McKone J. R.; Brunschwig B. S.; Atwater H. A.; Lewis N. S. Photoelectrochemical Hydrogen Evolution Using Si Microwire Arrays. J. Am. Chem. Soc. 2011, 133, 1216. 10.1021/ja108801m. [DOI] [PubMed] [Google Scholar]
- Boettcher S. W.; Spurgeon J. M.; Putnam M. C.; Warren E. L.; Turner-Evans D. B.; Kelzenberg M. D.; Maiolo J. R.; Atwater H. A.; Lewis N. S. Energy-Conversion Properties of Vapor-Liquid-Solid–Grown Silicon Wire-Array Photocathodes. Science 2010, 327, 185. 10.1126/science.1180783. [DOI] [PubMed] [Google Scholar]
- Roske C. W.; Popczun E. J.; Seger B.; Read C. G.; Pedersen T.; Hansen O.; Vesborg P. C. K.; Brunschwig B. S.; Schaak R. E.; Chorkendorff I.; Gray H. B.; Lewis N. S. Comparison of the Performance of CoP-Coated and Pt-Coated Radial Junction n+p-Silicon Microwire-Array Photocathodes for the Sunlight-Driven Reduction of Water to H2(g). J. Phys. Chem. Lett. 2015, 6, 1679. 10.1021/acs.jpclett.5b00495. [DOI] [PubMed] [Google Scholar]
- Xiang C.; Meng A. C.; Lewis N. S. Evaluation and optimization of mass transport of redox species in silicon microwire-array photoelectrodes. Proc. Natl. Acad. Sci. U. S. A. 2012, 109, 15622. 10.1073/pnas.1118338109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKone J. R.; Lewis N. S. In Photoelectrochemical Water Splitting: Materials, Processes and Architectures; Lewerenz H.-J., Ed.; The Royal Society of Chemistry, 2013. [Google Scholar]
- Vijselaar W.; Westerik P.; Veerbeek J.; Tiggelaar R. M.; Berenschot E.; Tas N. R.; Gardeniers H.; Huskens J. Spatial decoupling of light absorption and catalytic activity of Ni–Mo-loaded high-aspect-ratio silicon microwire photocathodes. Nature Energy 2018, 3, 185. 10.1038/s41560-017-0068-x. [DOI] [Google Scholar]
- Chen Z. B.; Forman A. J.; Jaramillo T. F. Bridging the Gap Between Bulk and Nanostructured Photoelectrodes: The Impact of Surface States on the Electrocatalytic and Photoelectrochemical Properties of MoS2. J. Phys. Chem. C 2013, 117, 9713. 10.1021/jp311375k. [DOI] [Google Scholar]
- Resasco J.; Zhang H.; Kornienko N.; Becknell N.; Lee H.; Guo J. H.; Briseno A. L.; Yang P. D. TiO2/BiVO4 Nanowire Heterostructure Photoanodes Based on Type II Band Alignment. ACS Cent. Sci. 2016, 2, 80. 10.1021/acscentsci.5b00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.