Abstract
The unusual temperature dependence of exciton emission decay in CsPbX3 perovskite nanocrystals (NCs) attracts considerable attention. Upon cooling, extremely short (sub-ns) lifetimes were observed and were explained by an inverted bright–dark state splitting. Here, we report temperature-dependent exciton lifetimes for CsPbCl3 NCs doped with 0–41% Mn2+. The exciton emission lifetime increases upon cooling from 300 to 75 K. Upon further cooling, a strong and fast sub-ns decay component develops. However, the decay is strongly biexponential and also a weak, slow decay component is observed with a ∼40–50 ns lifetime below 20 K. The slow component has a ∼5–10 times stronger relative intensity in Mn-doped NCs compared to that in undoped CsPbCl3 NCs. The temperature dependence of the slow component resembles that of CdSe and PbSe quantum dots with an activation energy of ∼19 meV for the dark–bright state splitting. Based on our observations, we propose an alternative explanation for the short, sub-ns exciton decay time in CsPbX3 NCs. Slow bright–dark state relaxation at cryogenic temperatures gives rise to almost exclusively bright state emission. Incorporation of Mn2+ or high magnetic fields enhances the bright–dark state relaxation and allows for the observation of the long-lived dark state emission at cryogenic temperatures.
Introduction
The discovery of CsPbX3 (X = Cl, Br, I) nanocrystals (NCs) with unique optical properties has initiated a worldwide increase in research aimed at providing fundamental understanding of their peculiar properties and realizing applications of these NCs, e.g., in lighting and solar cells.1−4 The optical properties of the CsPbX3 NCs resemble those of Cd- and Pb-chalcogenide quantum dots (QDs). Bulk CsPbX3 is a semiconductor. For NCs that are typically ∼10–15 nm, the exciton is confined. The NC size is however larger than the exciton Bohr radius (varying from 5 nm in CsPbCl3 to 12 nm in CsPbI3), giving rise to weak confinement and only small shifts of the exciton emission wavelength.5 Tuning of the emission color is therefore not realized by size variation but by changing the chemical composition. The emission color of CsPbX3 NCs can span the full visible region through anion exchange. Replacement of Cl– by Br– shifts the emission from violet to green, and subsequent replacement by I– shifts the emission further to the deep red spectral region. Additional color tuning can be realized by doping with luminescent ions (typically 3d transition metals and 4f rare earth ions) and energy transfer of the exciton to the luminescent dopant.6−9 For applications of the perovskite NCs in light-emitting devices, not only the high quantum yield (>90%) and color tunability but also the short emission lifetime are advantageous, which is typically in the ns range at room temperature. The faster emission decay of the perovskite NCs in comparison to that of, e.g., CdSe and InP QDs has advantages in high-brightness applications. The limited stability of the perovskite NCs is a point of concern that is addressed by passivation strategies but at present hampers their application.10,11
In recent papers, the temperature dependence of the exciton emission lifetime was investigated for CsPbX3 NCs. Both for ensemble and individual NCs, an unexpected temperature dependence was observed: upon cooling to cryogenic temperatures, the already short decay time decreased even further to sub-ns lifetimes.12,13 This behavior is markedly different from that of the traditional Cd- and Pb-chalcogenide QDs. Typically, upon cooling, the exciton lifetime lengthens because of the energy level structure of the emitting exciton states, where the lowest energy state is a dark state that has a forbidden transition to the exciton ground state.14−16 In well-passivated QDs, the emission from the dark state is still efficient (so the state is not really dark) but the forbidden nature causes the emission lifetime to lengthen when the system is frozen into this lowest excitonic state. For CsPbX3 perovskite NCs, the opposite behavior was observed and was explained by inversion of the dark–bright state splitting induced by the Rashba effect.12 As a result, fast bright state emission is observed at 5 K. Theoretical calculations were able to explain the short sub-ns lifetime. However, alternative observations were also reported. Luminescence decay measurements for CsPbBr3, FAPbBr3, and FAPbI3, also in high magnetic fields, revealed a composition-dependent dark–bright state splitting with a lowest exciton dark state, and for CsPbBr3, dark exciton emission in high magnetic fields was reported.17−19
Here, we report temperature-dependent luminescence lifetime measurements for exciton emission in Mn-doped CsPbCl3 NCs. For different Mn-doping concentrations, we observe a clear lengthening of the exciton decay time upon cooling. Below 200 K, a rise time is observed for the exciton emission, which indicates that relaxation rates from higher exciton levels to the emitting levels slow down upon cooling. Upon further cooling, below 50 K, a biexponential decay is observed with a strong fast component (<1 ns) and a slow component develops that becomes longer-lived upon lowering the temperature. The temperature dependence of this slow component shows the typical behavior expected for a dark–bright state splitting with a lower-energy dark state. As an alternative to an inverted dark–bright splitting model, we propose that inhibited relaxation from the bright to the dark state explains the observation of fast bright state emission in CsPbX3 NCs at cryogenic temperatures.
Methods
Synthesis
All of the chemicals used were obtained from Sigma-Aldrich. These include cesium carbonate (Cs2CO3), lead chloride (PbCl2), manganese chloride (MnCl2·4H2O), octadecene (ODE), oleic acid (OA), oleylamine (OM), and hexane (anhydrous). For the preparation of the cesium precursor, 0.2 g of Cs2CO3 was mixed with 7.5 mL of ODE and 0.625 mL of OA and loaded into a 50 mL flask; then, the reaction mixture was degassed, dried under 120 °C for 30 min under vacuum, and then heated at 150 °C under a N2 atmosphere for additional 30 min to complete the reaction between Cs2CO3 and OA. The resulting solution was kept at 150 °C for further use. Undoped CsPbCl3 NCs and CsPbCl3:Mn2+ NCs with different dopant concentrations were synthesized at a temperature of 190 °C with various Mn/Pb feeding ratios (see Table 1).
Table 1. Precursor Composition for the Synthesis of CsPbCl3:Mn2+ NCs with Various Mn-Doping Concentrations and Mn Concentrations Incorporated in CsPbCl3 NCs.
| Mn/Pb molar ratio | 0 | 0.5 | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|---|
| MnCl2·4H2O (g) | 0 | 0.037 | 0.055 | 0.07 | 0.079 | 0.085 | 0.089 |
| PbCl2 (g) | 0.15 | 0.10 | 0.078 | 0.05 | 0.037 | 0.03 | 0.025 |
| Mn concentration in sample (atom %) | 0 | 1 | 3 | 9 | 20 | 32 | 41 |
For a typical synthesis, 0.1 g of PbCl2 and 0.037 g of MnCl2·4H2O were loaded into a 50 mL three-neck flask with 10 mL of ODE. The flask was then transferred to a Schlenk line and dried under vacuum at 120 °C for 1 h. Dried OA (1 mL) and 1 mL of OM were subsequently injected and then heated to 190 °C under a N2 atmosphere. After reaching 190 °C, 0.9 mL of the hot Cs precursor was injected swiftly. The reaction was quenched 5 s later by immersion of the reaction container in an ice–water bath. The product was separated by centrifugation, washed once with acetone/hexane, and finally dissolved in 5 mL of hexane.
Characterization
Transmission electron microscopy (TEM) images were obtained with FEI Tecnai T20, operating at 200 kV. The samples for TEM imaging were prepared by dipping a carbon-coated copper mesh TEM grid into a hexane solution of NCs. The excess liquid was evaporated under vacuum. Luminescence (emission and excitation) spectra and photoluminescence (PL) decay curves were measured using an Edinburgh Instruments FLS920 spectrofluorometer equipped with a 450 W xenon lamp as an excitation source and a 0.22 m double grating monochromator for excitation (Bentham DTMS300, 1200 lines/mm grating, blazed at 300 nm for excitation). Emission spectra (380–700 nm) were recorded with a single 0.22 m monochromator (500 nm blazed grating), and the emitted light was detected by a Hamamatsu R928 photomultiplier tube (PMT). The fast decay profiles of the exciton emission were recorded using an Edinburgh EPL375 pulsed diode laser (λex = 376.8 nm and pulse width: 65 ps) with a fast Hamamatsu H74422-40 PMT to detect the emission. An Oxford Instruments liquid helium flow cryostat was used to measure the PL properties at low temperatures (down to 4.2 K). The samples for room temperature optical analysis were prepared by dissolving the crude NCs mixture in hexane and transferring the solution to a quartz cuvette. For low-temperature measurements, the NC solution was loaded in a sealed quartz cuvette.
Results and Discussion
The synthesis of the Mn-doped and undoped CsPbCl3 NCs yielded cubic NCs of ∼12 nm, as shown in the TEM images in Figure 1. The actual amount of Mn incorporated in the CsPbCl3 NCs was determined with energy-dispersive X-ray spectroscopy (EDX, see Table 1) and was always much lower than the amount of Mn present in the reaction mixture. After thorough washing with hexane, the Mn concentration in the NCs was determined using EDX. The samples investigated contained 0, 1, 3, 9, 20, 32, and 41% of Mn2+. The NCs show bright luminescence with a sharp excitonic emission line at around 400 nm and a broad Mn2+ emission band at around 600 nm. The emission spectra in Figure 1 show that the relative intensity of the Mn2+ emission band increases and shifts to longer wavelengths for higher Mn2+ concentrations. Upon cooling, the relative intensity of the Mn2+ emission strongly decreases from 300 to 150 K. Below 75 K, the relative Mn-emission intensity increases again, but it remains much lower than that at 300 K (Supporting Information, Figures S1 and S2). This temperature behavior is consistent with reports in the literature and reflects the temperature dependence of the exciton-to-Mn2+ energy transfer rate relative to processes giving rise to exciton emission.20,21
Figure 1.
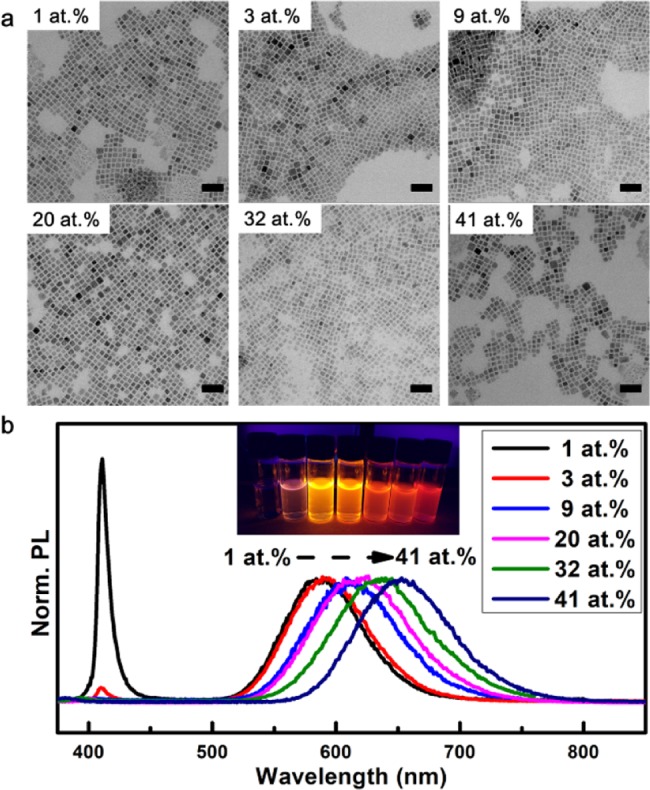
Morphology and optical properties of CsPbCl3:Mn2+ (1–41 atom %) NCs. (a) TEM images of CsPbCl3:Mn2+ NCs with different Mn2+ concentrations (1–41 atom %, scale bar: 50 nm). (b) Emission spectra of CsPbCl3:Mn2+ NCs with different Mn2+ concentrations (1–41 atom %, λex = 355 nm, inset: sample under 365 nm excitation provided by a handheld UV lamp).
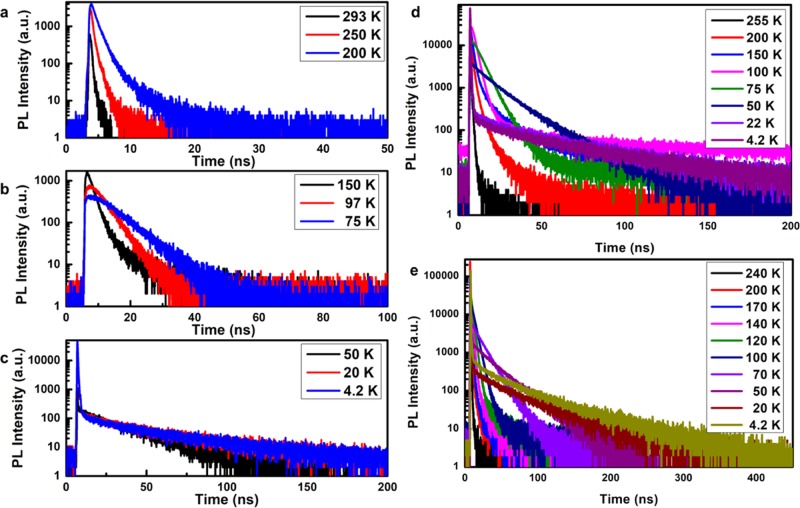
Luminescence decay curves were recorded for both the exciton and Mn2+ emission between 4.2 and 300 K for CsPbCl3 NCs doped with 0, 1, 3, 9, 20, 32, and 41% of Mn2+. Here, we focus on the exciton emission decay. As an example, Figure 2 displays the luminescence decay curves for the 20% Mn-doped CsPbCl3 NCs. Three temperature regimes are depicted. Between 293 and 200 K (Figure 2a), the decay curves are close to single exponential. Upon cooling from 293 to 200 K, the luminescence decay time lengthens from 0.1 ns at 293 K to 0.4 ns at 200 K and the intensity increases. This can be explained by less-efficient exciton to Mn2+ energy transfer and is consistent with the strong decrease in the relative Mn2+ emission intensity between 293 and 200 K. Upon further cooling to 75 K, the lengthening of the decay time continues (note the change in time scales from Figure 2a–c). In addition, the decay curves show a clear rise with a rise time that increases from 1.8 ns at 150 K to 3.2 ns at 75 K. The observation of a rise indicates that relaxation from the higher excited states (initially populated under pulsed excitation at 376 nm) to the emitting exciton states at around 400 nm slows down at cryogenic temperatures. Usually, fast (10–100 ps) relaxation is observed for relaxation to band edge states in semiconductors, but in the CsPbCl3 NCs, phonon relaxation is relatively slow and the phonon population relaxation rates decrease to the ns regime upon freezing.
Figure 2.
Decay curves of CsPbCl3:Mn2+ (20 atom %) NCs (a–c), CsPbCl3:Mn2+ (9 atom %) NCs (d), and CsPbCl3:Mn2+ (32 atom %) NCs (e) at different temperatures plotted on a semilogarithmic scale (λex = 376.8 nm, pulse width: 65 ps).
Upon further cooling from 75 to 4 K, the luminescence decay curves become strongly biexponential with a fast sub-nanosecond component and a slow component that lengthens as the temperature decreases from 75 to 4 K. The relative contribution of the fast component increases at lower temperatures, and the decay of the slow component slows down and stabilizes below ∼20 K. A similar behavior is observed for other high Mn concentrations. As an example Figure 2d,e shows the luminescence decay curves for the exciton emission in CsPbCl3:Mn2+ with 9 and 32 atom % Mn2+. An overview of the temperature-dependent decay behavior of all CsPbCl3:Mn2+ (1–41 atom %) samples is given in the Supporting Information (Figures S3–S6). These results show that for low doping concentrations (1 or 3% Mn2+) the influence of the Mn doping is not as pronounced as for Mn2+ concentrations of 9% and higher. For all higher (9–41%) Mn2+ concentrations, the relative intensity of the long-lived component is about 5–10 times stronger than that for undoped CsPbCl3.
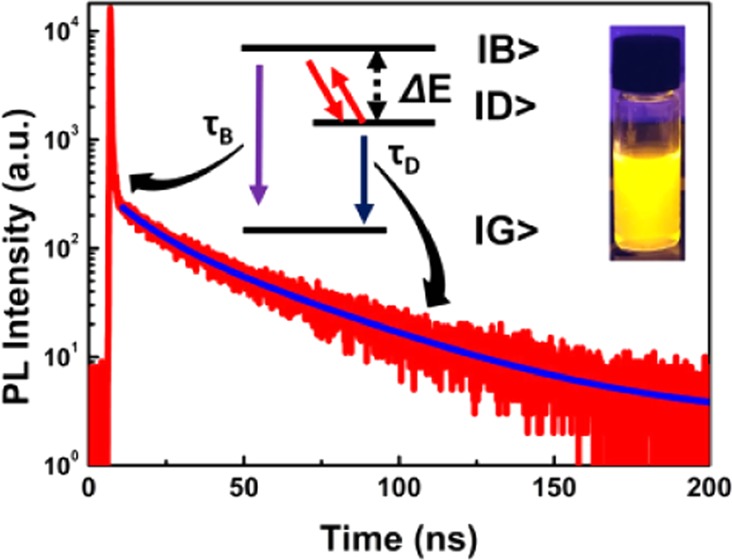
The present observations resemble those for the exciton emission in CsPbBr3 in a magnetic field where also a fast initial decay component at low temperatures was followed by a weak and slow decay component.19 The fast decay component was assigned to bright state exciton emission and the slow component to dark exciton emission. Slow relaxation from the bright to the dark state exciton at low temperatures can explain the observation of short-lived bright state emission decay prior to relaxation to the dark state. From the temperature dependence of the dark state emission decay time, a dark–bright state splitting of ∼8 meV was calculated for CsPbBr3. Also, for CdSe QDs, an initial fast decay related to the bright state emission prior to relaxation to the dark state has been reported at temperatures below 10 K.15 The temperature-dependent decay behavior of the exciton emission for CsPbX3 NCs in a magnetic field and the present observations for Mn-doped CsPbCl3 NCs can be explained by a slow bright–dark state relaxation at cryogenic temperatures. The situation is similar to that for Cd- and Pb-chalcogenide QDs but with a significant slower relaxation between excitonic states, which gives rise to the observation of the intense and fast (sub-ns) bright state emission, which dominates at cryogenic temperatures.
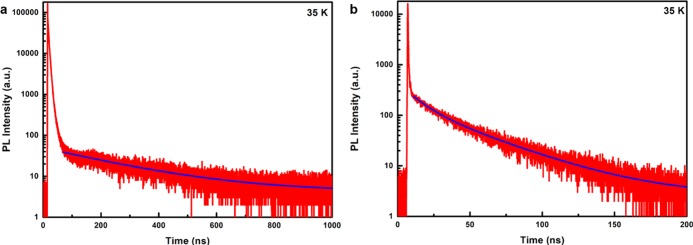
To test the presence of dark state emission in pure CsPbCl3 NCs, we also measured luminescence decay curves as a function of temperature for undoped NCs. The temperature-dependent behavior is similar to that observed for the Mn-doped NCs, but the relative intensity of the dark state emission is much lower. As an example, Figure 3 shows the 35 K decay curves for the exciton emission in CsPbCl3 NCs and CsPbCl3:Mn2+ (20 atom %) NCs. For both systems, a fast and strong initial sub-ns decay is followed by a slow temperature-dependent decay component. The amplitude for the slow component is more than 3 orders of magnitude smaller than that for the fast component. For CsPbCl3 doped with 20% Mn2+, the decay behavior is similar but the amplitude of the slow dark state emission component is about 2 orders of magnitude lower. This can be explained by a faster bright–dark state relaxation induced by the Mn2+ dopants. Similar observations can be found in refs (18) and (19) where an increase in the relative intensity is observed for the slow decay component of the exciton emission from CsPbBr3 NCs upon increasing the external magnetic field to 1019 or 30 T.18 Incorporation of magnetic Mn2+ can generate a magnetic field, which has a similar effect to that of an externally applied magnetic field. This indicates that the presence of magnetic ions (Mn2+) or an external magnetic field induces faster relaxation between the dark and bright states. The role of the magnetic field in the enhanced spin-relaxation between states with different magnetic moments may be mixing of spin states, which can also explain the shorter decay times observed for dark exciton emission in magnetic fields or upon Mn2+ doping.19,22 In single (undoped) dot experiments, the low count rate and more than 3 orders of magnitude higher amplitude of the bright state emission prevents the observation of the slow dark state emission. Indeed, inspection of the single CsPbX3 NC exciton decay curves in ref (12) shows that the noise level starts above 10–3 in the decay curves with a maximum scaled to 100, thus making it impossible to observe the slow decay component of the exciton emission.
Figure 3.
Exciton emission decay curves of undoped CsPbCl3 NCs (a) and CsPbCl3:Mn2+ (20 atom %) NCs (b) at 35 K plotted on a semilogarithmic scale (λex = 376.8 nm, pulse width: 65 ps).
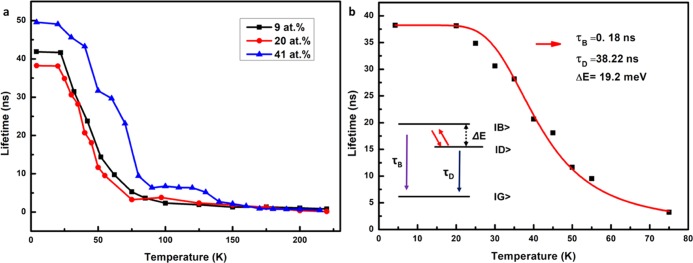
To estimate the dark–bright state splitting, the temperature dependence of the slow decay component was analyzed for CsPbCl3 NCs with 9, 20, and 32% of Mn2+. The nearly single exponential decay in the long-time regime was fitted to exponential decay (see the Supporting Information for details, Figures S7–S9). The choice of the time interval for the fitting is somewhat arbitrary and introduces an uncertainty in the decay times. The time interval chosen is evident from the drawn lines in Figure 3 (and also in Figures S7–S9), which represent a time interval where the slow decay is close to single exponential. In Figure 4, the temperature dependence of the decay times is depicted. For all three samples, a similar behavior is observed. The decay time is constant at around 40–50 ns between 4 and 20 K and decreases strongly between 20 and 75 K. A fit of the temperature dependence to a three-level model
where τObs is the measured decay time, τD is the decay time of the dark state, and τB is the decay time of the bright state, gives an energy difference of ∼19 meV for the dark–bright state splitting. This value is in good agreement with the ∼14 meV splitting for CsPbCl3 NCs calculated from the influence of magnetic-field-induced mixing of dark and bright states, which influences the decay rate of the fast component of the exciton emission.19
Figure 4.
Exciton emission lifetime (slow component) for CsPbCl3:Mn2+ (9, 20, and 32 atom %) NCs as a function of temperature. (a) Evolution of lifetime of CsPbCl3:Mn2+ (9, 20, and 32 atom %) NCs with temperature. (b) Three-level fitting of the long decay component of CsPbCl3:Mn2+ (20 atom %) NCs exciton decay versus temperature.
The present results provide evidence for a normal splitting of the exciton state in CsPbCl3 and can explain earlier observations that led to a model with an inverted dark–bright state splitting in CsPbX3 NCs. To obtain conclusive evidence, it will be interesting to conduct temperature-dependent lifetime measurements on single Mn2+-doped CsPbCl3 NCs and search for the weak, temperature-dependent slow dark state emission in single Mn-doped NCs. Here, the origin of the anomalous exciton decay behavior is assigned to unusually slow phonon relaxation between excitonic states in CsPbCl3 NCs. It will be interesting to verify this slow relaxation by other techniques, e.g., pump–probe experiments, and by theoretical calculations to better understand why phonon relaxation is hampered in halide perovskite NCs.
Conclusions
In summary, we have investigated temperature dependence of exciton decay dynamics in Mn-doped CsPbCl3 NCs. Upon cooling, the exciton decay time initially lengthens. A rise time is observed below 150 K, indicating slow phonon relaxation dynamics feeding the exciton state. At temperatures below 75 K, the decay becomes strongly biexponential with a fast decay component that is assigned to the bright state emission and a weak temperature-dependent slow component that is assigned to the dark state emission. The results are explained by a normal exciton energy level scheme with a higher-bright and lower-energy dark state combined with an extremely slow bright–dark state relaxation at cryogenic temperatures. In Mn-doped CsPbCl3 NCs, the relaxation is an order of magnitude faster than that in undoped CsPbCl3, which explains why the dark state emission, albeit weak, is more easily observed in Mn-doped CsPbCl3 NCs.
Acknowledgments
This work is supported by the China Scholarship Council-Utrecht University Ph.D. Program (201404910557).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jpcc.8b12035.
Additional emission spectra and decay curves: Figure S1, normalized emission spectra of CsPbCl3:xMn2+ NCs; Figure S2, optical properties of CsPbCl3:xMn2+; and Figures S3–S9, exciton emission decay curve of pure CsPbCl3, CsPbCl3:Mn2+ (1 atom %) NCs, CsPbCl3:Mn2+ (3 atom %), CsPbCl3:Mn2+ (41 atom %), CsPbCl3:Mn2+ (9 atom %) NCs; Figure S8, exciton emission decay curves of CsPbCl3:Mn2+ (20 atom %), and CsPbCl3:Mn2+ (32 atom %) NCs, respectively (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Kovalenko M. V.; Protesescu L.; Bodnarchuk M. I. Properties and Potential Optoelectronic Applications of Lead Halide Perovskite Nanocrystals. Science 2017, 358, 745–750. 10.1126/science.aam7093. [DOI] [PubMed] [Google Scholar]
- Stranks S. D.; Snaith H. J. Metal-halide Perovskites for Photovoltaic and Light-emitting Devices. Nat. Nanotechnol. 2015, 10, 391–402. 10.1038/nnano.2015.90. [DOI] [PubMed] [Google Scholar]
- Akkerman Q. A.; Rainò G.; Kovalenko M. V.; Manna L. Genesis, Challenges and Opportunities for Colloidal Lead Halide Perovskite Nanocrystals. Nat. Mater. 2018, 17, 394–405. 10.1038/s41563-018-0018-4. [DOI] [PubMed] [Google Scholar]
- Veldhuis S. A.; Boix P. P.; Yantara N.; Li M.; Sum T. C.; Mathews N.; Mhaisalkar S. G. Perovskite Materials for Light-Emitting Diodes and Lasers. Adv. Mater. 2016, 28, 6804–6834. 10.1002/adma.201600669. [DOI] [PubMed] [Google Scholar]
- Protesescu L.; Yakunin S.; Bodnarchuk M. I.; Krieg F.; Caputo R.; Hendon C. H.; Yang R.; Walsh A.; Kovalenko M. V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. 10.1021/nl5048779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W.; Lin Q.; Li H.; Wu K.; Robel I.; Pietryga J. M.; Klimov V. I. Mn2+-Doped Lead Halide Perovskite Nanocrystals with Dual-Color Emission Controlled by Halide Content. J. Am. Chem. Soc. 2016, 138, 14954–14961. 10.1021/jacs.6b08085. [DOI] [PubMed] [Google Scholar]
- Xu K.; Lin C. C.; Xie X.; Meijerink A. Efficient and Stable Luminescence from Mn2+ in Core and Core–Isocrystalline Shell CsPbCl3 Perovskite Nanocrystals. Chem. Mater. 2017, 29, 4265–4272. 10.1021/acs.chemmater.7b00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein T. J.; Kroupa D. M.; Gamelin D. R. Picosecond Quantum Cutting Generates Photoluminescence Quantum Yields Over 100% in Ytterbium-Doped CsPbCl3 Nanocrystals. Nano Lett. 2018, 18, 3792–3799. 10.1021/acs.nanolett.8b01066. [DOI] [PubMed] [Google Scholar]
- Pan G.; Bai X.; Yang D.; Chen X.; Jing P.; Qu S.; Zhang L.; Zhou D.; Zhu J.; Xu W.; et al. Doping Lanthanide into Perovskite Nanocrystals: Highly Improved and Expanded Optical Properties. Nano Lett. 2017, 17, 8005–8011. 10.1021/acs.nanolett.7b04575. [DOI] [PubMed] [Google Scholar]
- Diroll B. T.; Nedelcu G.; Kovalenko M. V.; Schaller R. D. High-Temperature Photoluminescence of CsPbX3 (X = Cl, Br, I) Nanocrystals. Adv. Funct. Mater. 2017, 27, 1606750–1606756. 10.1002/adfm.201606750. [DOI] [Google Scholar]
- Chen D.; Fang G.; Chen X. Silica-Coated Mn-Doped CsPb(Cl/Br)3 Inorganic Perovskite Quantum Dots: Exciton-to-Mn Energy Transfer and Blue-Excitable Solid-State Lighting. ACS Appl. Mater. Interfaces 2017, 9, 40477–40487. 10.1021/acsami.7b14471. [DOI] [PubMed] [Google Scholar]
- Becker M. A.; Vaxenburg R.; Nedelcu G.; Sercel P. C.; Shabaev A.; Mehl M. J.; Michopoulos J. G.; Lambrakos S. G.; Bernstein N.; Lyons J. L.; et al. Bright Triplet Excitons in Caesium Lead Halide Perovskites. Nature 2018, 553, 189–193. 10.1038/nature25147. [DOI] [PubMed] [Google Scholar]
- Rainò G.; Nedelcu G.; Protesescu L.; Bodnarchuk M. I.; Kovalenko M. V.; Mahrt R. F.; Stöferle T. Single Cesium Lead Halide Perovskite Nanocrystals at Low Temperature: Fast Single-Photon Emission, Reduced Blinking, and Exciton Fine Structure. ACS Nano 2016, 10, 2485–2490. 10.1021/acsnano.5b07328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirmal M.; Norris D. J.; Kuno M.; Bawendi M. G.; Efros AlL.; Rosen M. Observation of the “Dark Exciton” in CdSe Quantum Dots. Phys. Rev. Lett. 1995, 75, 3728–3731. 10.1103/PhysRevLett.75.3728. [DOI] [PubMed] [Google Scholar]
- de Mello Donegá C.; Bode M.; Meijerink A. Size- and Temperature-dependence of Exciton Lifetimes in CdSe Quantum Dots. Phys. Rev. B 2006, 74, 085320 10.1103/PhysRevB.74.085320. [DOI] [Google Scholar]
- Efros A.; Rosen M. The Electronic Structure of Semiconductor Nanocrystals. Annu. Rev. Mater. Sci. 2000, 30, 475–521. 10.1146/annurev.matsci.30.1.475. [DOI] [Google Scholar]
- Fu M.; Tamarat P.; Trebbia J.; Bodnarchuk M. I.; Kovalenko M. V.; Even J.; Lounis B. Unraveling Exciton–Phonon Coupling in Individual FAPbI3 Nanocrystals Emitting Near-Infrared Single Photons. Nat. Commun. 2018, 9, 3318 10.1038/s41467-018-05876-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canneson D.; Shornikova E. V.; Yakovlev D. R.; Rogge T.; Mitioglu A. A.; Ballottin M. V.; Christianen P. C. M.; Lhuillier E.; Bayer M.; Biadala L. Negatively Charged and Dark Excitons in CsPbBr3 Perovskite Nanocrystals Revealed by High Magnetic Fields. Nano Lett. 2017, 17, 6177–6183. 10.1021/acs.nanolett.7b02827. [DOI] [PubMed] [Google Scholar]
- Chen L.; Li B.; Zhang C.; Huang X.; Wang X.; Xiao M. Composition-Dependent Energy Splitting between Bright and Dark Excitons in Lead Halide Perovskite Nanocrystals. Nano Lett. 2018, 18, 2074–2080. 10.1021/acs.nanolett.8b00184. [DOI] [PubMed] [Google Scholar]
- Yuan X.; Ji S.; De Siena M. C.; Fei L.; Zhao Z.; Wang Y.; Li H.; Zhao J.; Gamelin D. R. Photoluminescence Temperature Dependence, Dynamics, and Quantum Efficiencies in Mn2+-Doped CsPbCl3 Perovskite Nanocrystals with Varied Dopant Concentration. Chem. Mater. 2017, 29, 8003–8011. 10.1021/acs.chemmater.7b03311. [DOI] [Google Scholar]
- Xu K.; Meijerink A. Tuning Exciton–Mn2+ Energy Transfer in Mixed Halide Perovskite Nanocrystals. Chem. Mater. 2018, 30, 5346–5352. 10.1021/acs.chemmater.8b02157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granados del Águila A.; Pettinari G.; Groeneveld E.; de Mello Donegá C.; Vanmaekelbergh D.; Maan J. C.; Christianen P. C. M. Optical Spectroscopy of Dark and Bright Excitons in CdSe Nanocrystals in High Magnetic Fields. J. Phys. Chem. C 2017, 121, 23693–23704. 10.1021/acs.jpcc.7b06170. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.