Abstract
Background
Strains with increased alkali tolerance have a broad application in industrial, especially for bioremediation, biodegradation, biocontrol and production of bio-based chemicals. A novel synthetic chromosome recombination and modification by LoxP-mediated evolution (SCRaMbLE) system has been introduced in the synthetic yeast genome (Sc 2.0), which enables generation of a yeast library with massive structural variations and potentially drives phenotypic evolution. The structural variations including deletion, inversion and duplication have been detected within synthetic yeast chromosomes.
Results
Haploid yeast strains harboring either one (synV) or two (synV and synX) synthetic chromosomes were subjected to SCRaMbLE. Seven of evolved strains with increased alkali tolerance at pH 8.0 were generated through multiple independent SCRaMbLE experiments. Various of structural variations were detected in evolved yeast strains by PCRTag analysis and whole genome sequencing including two complex structural variations. One possessed an inversion of 20,743 base pairs within which YEL060C (PRB1) was deleted simultaneously, while another contained a duplication region of 9091 base pairs in length with a deletion aside. Moreover, a common deletion region with length of 11,448 base pairs was mapped in four of the alkali-tolerant strains. We further validated that the deletion of YER161C (SPT2) within the deleted region could increase alkali tolerance in Saccharomyces cerevisiae.
Conclusions
SCRaMbLE system provides a simple and efficient way to generate evolved yeast strains with enhanced alkali tolerance. Deletion of YER161C (SPT2) mapped by SCRaMbLE can improve alkali tolerance in S. cerevisiae. This study enriches our understanding of alkali tolerance in yeast and provides a standard workflow for the application of SCRaMbLE system to generate various phenotypes that may be interesting for industry and extend understanding of phenotype-genotype relationship.
Electronic supplementary material
The online version of this article (10.1186/s12934-019-1102-4) contains supplementary material, which is available to authorized users.
Keywords: SCRaMbLE, Alkali tolerance, Synthetic biology, Saccharomyces cerevisiae, SPT2
Background
Genomic variation drives phenotypic diversification in populations and evolutionary changes among different species [1–3]. Genome evolution in nature is a long-term process with accumulation of DNA variation that mainly involves single nucleotide polymorphisms (SNP), insertion-deletion (InDel) and structural variation (SV) [4–6]. Accordingly, techniques of manipulating varied length of DNA are constantly being developed and updated to engineer biological systems. Traditional techniques such as physical and chemical mutagenesis and error-prone PCR, could generate mutations in vivo and in vitro at the level of single nucleotide polymorphisms or insertion-deletion [7, 8]. Genome-editing technique mediated by TALEN, ZFN or CRISPR-Cas9 has made it possible to edit one or multiple targets on the genome rationally and precisely [9–11]. However, very few techniques related to large-scale genome rearrangement are reported to generate a structural variation library. The synthetic yeast genome project (Sc 2.0 project) is designed to encode an inducible genome rearrangement system in the chemically synthesized yeast genome by thousands of loxPsym sites inserted in the 3′ untranslated region (UTR) of nonessential genes [12–18]. With the expression of Cre recombinase in vivo, the synthetic chromosome recombination and modification by LoxP-mediated evolution (SCRaMbLE) system could drive generation of a yeast library with massive structural variations. The structural variations including deletion, inversion and duplication have been detected within synthetic yeast chromosomes and ring synthetic yeast chromosomes after SCRaMbLEing [19, 20]. Moreover, some evolved yeast strains with enhanced phenotype (i.e., temperature tolerance, caffeine tolerance, production of carotenoids) have been generated by SCRaMbLE system, showing promising application of this technology [21–27].
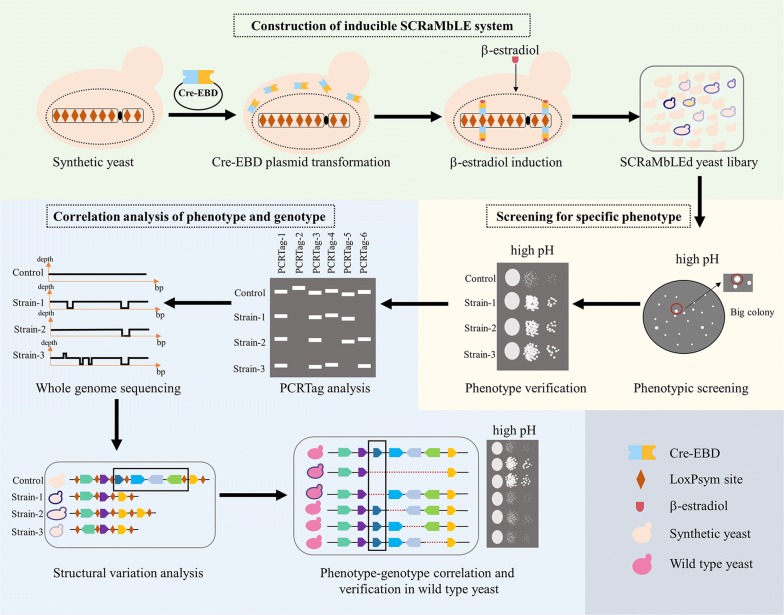
Extracellular pH, as an important environmental condition, has a significant influence on the survival and growth of cells. As a model organism, S. cerevisiae grows more rapidly in acidic than neutral or alkaline pH [28, 29]. It is important to study this underling mechanism for the application of industrial fermentation in alkaline environments. The discoveries of genes related to alkali tolerance in previous studies have been reported via random mutation, long-term adaptive evolution, deletion or overexpression of a dubious gene [29–32]. The transcriptional response to alkaline stress in S. cerevisiae was also studied by a short-term exposure in alkaline pH [33]. However, these methods are non-rational designed and make it complicated to uncover phenotype-genotype correlations. In this study, we accelerated the evolution of synthetic yeast strains in alkaline pH using SCRaMbLE system. Two synthetic yeast strains synV and synV&X, which harbor one synthetic yeast chromosome V and two synthetic yeast chromosomes V and X respectively, were used as initial strains [12, 13]. Through multiple independent SCRaMbLE experiments, seven of evolved strains with increased alkali tolerance at pH 8.0 were obtained. Many structural variations were detected in the evolved yeast strains by PCRTag analysis and whole genome sequencing. A common deletion region with length of 11,448 base pairs has been mapped in four of the alkali-tolerant strains. We further validated that the deletion of YER161C (SPT2) in this region could lead to the enhanced phenotype under alkaline stress. The SCRaMbLE method provides an efficient way to generate evolved strains with increased alkali tolerance and a straightforward way to dissect the correlation of phenotype and genotype (Fig. 1).
Fig. 1.
The workflow of using SCRaMbLE to improve and analyze alkali tolerance in yeast. Three sections in varied colors indicated three sequential steps of the whole process including construction of inducible SCRaMbLE system, screening for specific phenotype, correlation analysis of phenotype and genotype
Results and discussion
Construction and characterization of SCRaMbLE system
Synthetic yeast chromosome V (synV) is 536,024 base pairs long encoding 176 loxPsym sites [13]. Synthetic yeast chromosome X (synX) is 707,459 base pairs in length encoding 245 loxPsym sites [12]. Haploid strains bearing one (synV) or two (synV and synX) synthetic chromosomes were subjected to SCRaMbLE in this study. Cre recombinase fused with estrogen-binding domain (EBD) has been used to control the SCRaMbLE system in previous studies [19, 22]. Two Cre-EBD plasmids pYW079 (pRS416-pSCW11-Cre-EBD) and pYW180 (pRS416-pCLB2-Cre-EBD) with different promoters were transformed to strains yXZX846 (synV) and yYW169 (synV&X). pCLB2 is a G2/M-specific promoter which could lead cells to expose to Cre recombinase continually in each cell cycle [34], while pSCW11 is a strong promoter which only initiates transcription in daughter cells [35]. β-Estradiol was added in medium to enable the fusion protein Cre-EBD to transfer into cell nucleus and bind to the loxPsym sites in the synthetic chromosomes, resulting an activated SCRaMbLE system.
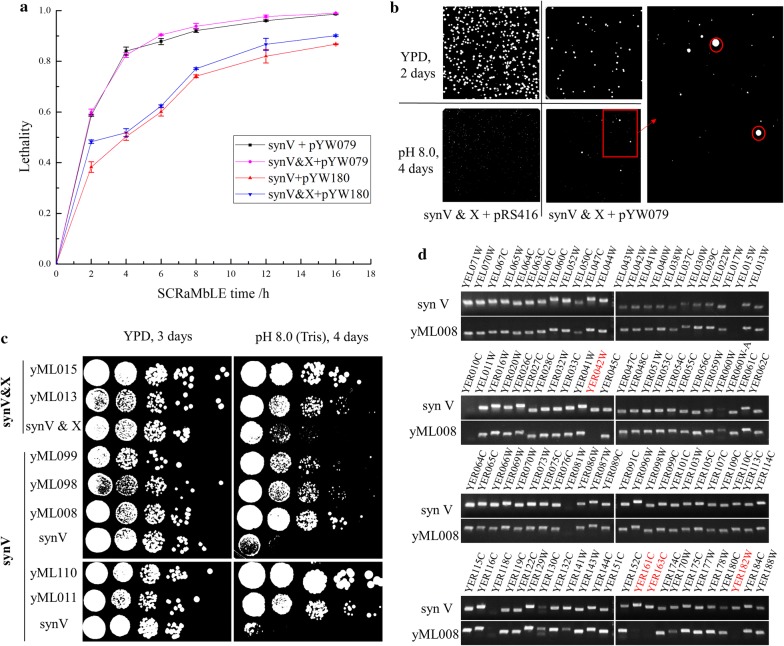
Although the loxPsym sites were inserted in the 3′ UTR of non-essential genes, essential genes might be deleted by Cre/loxPsym reaction across these genes, resulting the loss of viability for haploid synthetic yeasts. We characterized the lethality caused by SCRaMbLE firstly. As shown in Fig. 2a, lethality of strains carrying pYW079 was higher than strains carrying pYW180 due to the enhanced expression of Cre recombinase by a stronger promoter. And lethality of synV&X strains was slightly higher than synV strain. This may be caused by larger number of loxPsym sites within two synthetic chromosomes in synV&X strains. After 8 h’ SCRaMbLE, more than 70% lethality was detected in strains carried pYW180 and more than 90% lethality in strains carried pYW079. Then 8 h’ SCRaMbLE was considered as an appropriate intensity of genome evolution and chosen for the following SCRaMbLE experiments.
Fig. 2.
Characterization of SCRaMbLEd strains with increased alkali tolerance. a Characterization of lethality for SCRaMbLE system. Four strains (synV strain carrying pYW079, synV carrying pYW180, synV&X carrying pYW079 and synV&X carrying pYW079) were cultured in SC-Ura medium and exposed in β-estradiol for varied time (2 h, 4 h, 6 h, 8 h, 12 h, 16 h), and then diluted on YPD plates. SynV and synV&X strains containing empty vector pRS416 were used as control strains. b SCRaMbLEd synV&X strains on YPD medium and selective medium (YPD medium at pH 8.0). Strains on YPD medium were cultured 2 days and strains on selective medium were cultured 4 days in 30 °C before photographed. SynV&X strain containing empty vector pRS416 was used as a control strain. c Phenotype verification of SCRaMbLEd strains. SynV and synV&X strains were used as control strains. d PCRTag analysis. PCRTags of genes YER042W, YER161C, YER163C and YER182W were labeled in red, indicating deletions of these regions in strain yML008. None amplification of PCRTags of YEL017W, YER010C, YER081W, YER118C in both synV and yML008 strains was caused by nonspecific primers. SynV strain was used as a control strain. All PCRTag primers were listed in Additional file 1: Table S1
SCRaMbLE generates evolved yeasts with increased alkali tolerance
YPD media with different pH were tested to select an appropriate alkaline condition for screening of SCRaMbLEd strains. pH 8.0 was chosen as the selective condition as showing enough alkaline pressure while keeping a reasonable culture time. Several bigger colonies from the SCRaMbLEd pool were detected though visual inspection on the selective plates after cultured at 30 °C for 4 days, while very few big colonies were grown on the selective plates from unSCRaMbLE pool (Fig. 2b). To avoid the possible leaky expression of Cre recombinase on candidate strains, the Cre-EBD plasmids were lost via serially culturing in YPD liquid medium. A total of seven strains (yML008, yML011, yML013, yML015, yML098, yML099 and yML110) generated through multiple independent SCRaMbLE experiments were verified with increased tolerance to alkali on YPD medium at pH 8.0 (Fig. 2c). Interestingly, SCRaMbLEd strains showed varied growth fitness under various stressful conditions (Additional file 1: Figure S1). Although all showed improved alkali tolerance, these strains were displayed with varied growth rate on the selective medium at pH 8.0 (i.e., yML008 grew faster than yML098 and yML099). For strain yML098, decreased growth fitness was detected on YPD medium at 30 °C, YP medium with 20 g L−1 xylose, and YPD medium with 1.5 M sorbitol. Meanwhile, some of SCRaMbLEd strains were displayed with increased growth fitness (Additional file 1: Figure S1, i.e., yML008 grown better than initial strain synV at 39 °C). These might be caused by diverse structural variations among the SCRaMbLEd strains.
The SCRaMbLEd genomes were preliminarily investigated by PCRTag method. The synthetic yeast chromosome V was divided into 177 segments by 176 loxPsym sites. 96 of distributed PCRTags at different loci were chosen to cover as many segments as possible (Additional file 1: Table S1). All PCRTags in segments encoding essential genes were deprecated considering low probability of deletion of these regions in haploid strains. A total of seven SCRaMbLEd strains with enhanced alkali tolerance were analyzed using the 96 PCRTags in the synthetic yeast chromosome V (Fig. 2d, Additional file 1: Figure S2). At least one PCRTag missing was found in yML008, yML013, yML015, yML098 and yML099 when compared to initial strain synV, indicating deletion events induced by SCRaMbLE. As shown in Fig. 2d, PCRTag analysis of strain yML008 indicated deletions of four genes including YER042W (MXR1), YER161C (SPT2), YER163C (GCG1), and YER182W (FMP10). However, other structural variations such as inversion, transposition, duplication and more complex genome rearrangements may not be detected by PCRTag assay directly.
Complex structural variations generated by SCRaMbLE
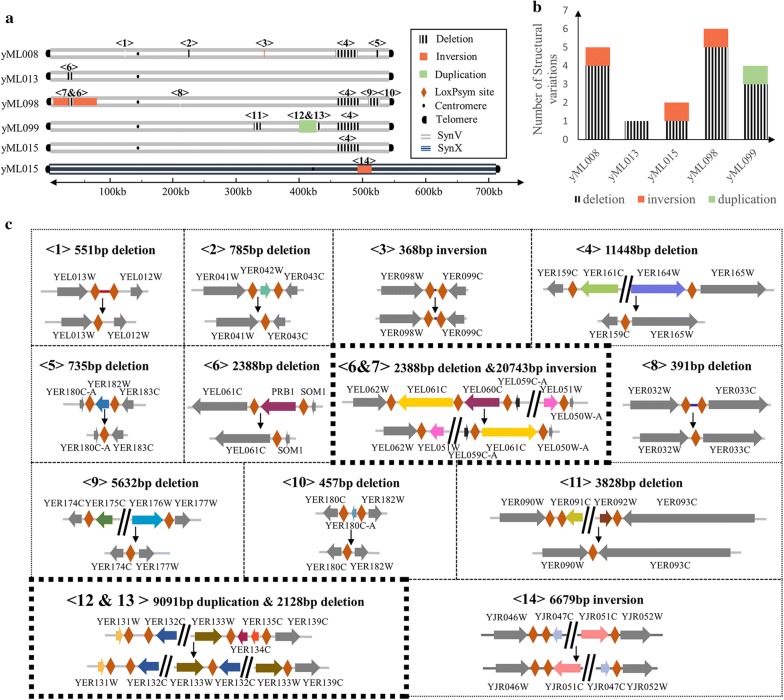
All the seven SCRaMbLEd strains were further analyzed using whole genome sequencing to determine genomic variations driven by SCRaMbLE. Different types of structural variations including deletion, inversion, and duplication were detected in the SCRaMbLEd strains (Fig. 3a). Coverage maps of sequenced strains showed absence of reads in corresponding regions (Additional file 1: Figures S3–S9). There were at least four different rearranged events in strains yML008, yML098, and yML099 respectively. A maximum of six of rearranged events was generated in strain yML098 including five deletions and one inversion. Strains yML013 and yML015 generated from initial strain synV&X were detected with one or two structural variations. Statistical analysis of structural variations in SCRaMbLEd strains were shown in Fig. 3b. We speculated that strain synV&X providing more recombinational sites may result higher frequency of lethality caused by deletion of essential genes or synthetic lethal genetic interactions especially under the pressure of high pH.
Fig. 3.
Analysis of structural variations in SCRaMbLEd strains. a Structural variations in five of the SCRaMbLEd genomes detected by whole genome sequencing. b Statistical analysis of structural variations in SCRaMbLEd genomes. c Detailed information of 14 varied rearrangement events. Two complex structural variations in section <6 and 7> and <12 and 13> were indicated with thick borders
We focused on novel junctions in the SCRaMbLEd genome based on data of sequence alignments. The sequencing result of strain yML098 revealed a complex structural variation which possessed an inversion of the region between YEL061C (CIN8) and YEL051W (VMA8) within which YEL060C (PRB1) was deleted simultaneously (Fig. 3a, c, region <6 and 7>). The total length of fragments deleted in strain yML098 reaches up to 19,965 base pairs, which is about 4% of the total length of synthetic yeast chromosome V. Another complex structural variation was also observed in the SCRaMbLEd genome of strain yML099 which contained a duplication region of 9091 base pairs with a deletion aside (Fig. 3a, c, region <12 and 13>). Besides, five of deletions (Fig. 3a, c, region <2>, <4>, <6>, <11> and <13>) were observed to be adjacent to an essential gene, indicating that essential genes may limit complex rearrangements across large fragments.
Deletion of YE161C (SPT2) increases the alkali tolerance
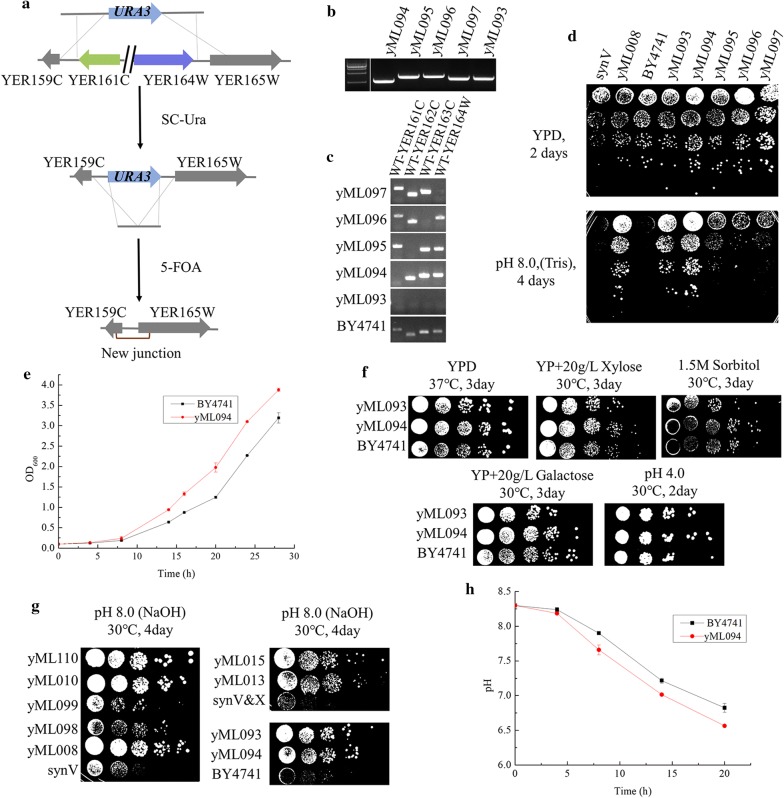
One of the most obvious features among all structural variations observed in the SCRaMbLEd genome is the deletion of a region from YER161C to YER164W. This was commonly found in strains yML008, yML015, yML098 and yML099. The deleted region involved four genes with 11,448 base pairs in length. Therefore, we speculate that this region might be responsible for the enhanced phenotype of SCRaMbLEd strains under alkaline stress. To test the hypothesis above, we knocked out the relevant regions in wild type strain BY4741 which was the initial strain for both synthetic strains synV and synV&X. We constructed the knockout strains using the strategy shown in Fig. 4a. Strain yML093 with YER161C–YER164W deleted was verified by PCR (Fig. 4b, c). Compared to initial strain BY4741, yML093 had an enhanced phenotype at pH 8.0 which was similar to the SCRaMbLEd strains (Fig. 4d). We concluded that the deletion of the region YER161C–YER164W increased alkali tolerance of yeast strain BY4741 and was responsible for the enhanced phenotype of SCRaMbLEd strains yML008, yML015, yML098 and yML099 under alkaline pH. The varied degree of alkali tolerance among these SCRaMbLEd strains may be caused by other rearranged events in the SCRaMbLEd genomes. For instance, there were another four deletion regions in strain yML098. Among them, ECM32 encodes a DNA helicase involving in modulating translation termination, and the deletion of ECM32 results a decreased competitive fitness [36, 37].
Fig. 4.
Deletion of YER161C (SPT2) increases the alkali tolerance in yeast. a Construction of seamless knockout yeast strains. With two rounds of genome integration, transformants were selected on SC-Ura and 5-FOA medium, respectively. b Verification of targeted deletions by junction PCR. All junction primers were listed in Additional file 1: Table S1. c Verification of individual gene deletions. All primers of individual genes were listed in Additional file 1: Table S1. d Characterization of the knockout yeast strains on YPD medium at pH 8.0. Strains on selective medium were cultured 4 days in 30 °C before photographed. e Growth curves of yML094 and BY4741 at pH 8.3. f Characterization of SPT2 knockout strains under various stressful conditions. g Growth fitness of SCRaMbLEd strains and SPT2 knockout strains at pH 8.0 using NaOH as alkali source. h pH changes of yML094 and BY4741 in liquid YPD medium at pH 8.3
To further narrow down the region responsible for the enhanced phenotype under alkali stress, we knocked out all four genes YER161C (SPT2), YER162C (RAD4), YER163C (GCG1) and YER164W (CHD1) within this region individually in strain BY4741. All four knockout strains were characterized on YPD medium at pH 8.0. Among them, strain yML094 with YER161C (SPT2) deleted showed a similar phenotype with the YER161C–YER164W deleted strain yML093, while the deletion of other 3 genes had no obvious influence on growth fitness of these strains under alkaline stress (Fig. 4d). Moreover, we quantified the tolerance to alkali condition by measuring growth curves of strains on YPD liquid medium at pH 8.3. Strain yML094 grows faster than BY4741 under alkali environment (Fig. 4e). These results reveal that YER161C (SPT2) might be a target gene responsible for increased alkali tolerance.
To further explore the mechanism of alkali tolerance caused by deletion of YER161C (SPT2), we tested growth fitness of the SPT2 knockout strains on various stressful conditions. As shown in Fig. 4f, the SPT2 deleted strains were displayed with similar growth fitness as control strain BY4741 under stressful conditions tested. It suggests that the mechanism of alkali tolerance caused by the deletion of SPT2 is not suitable for other types of tolerance. The growth fitness of SCRaMbLEd strains and SPT2 knockout strains were further evaluated at pH 8.0 using NaOH as alkali source (Fig. 4g). And the result is consistent with pervious data using Tris as alkali source (Figs. 2g and 4d). It suggests that the deletion of SPT2 could enhance alkali tolerance specifically and not due to tolerance to Tris as a compound. We also tested the pH changes of cultured strains during the growth process with initial pH at 8.3 (Fig. 4h). The data indicates that SPT2 knockout strains could accelerate the neutralization of the medium when compared with control strain BY4741. On the basis of previous studies, Spt2p is a DNA binding protein with HMG-like domains, and is required for RNA polyadenylation and involved in negative regulation of transcription [38–41]. Another study had found that the SER3 mRNA level could be dramatically increased due to the deletion of SPT2 [39]. Deletion of SPT2 may change content of certain metabolites in cells or in the medium which may improve the growth fitness of yeast cell under alkaline environment. Although the exact underlying mechanism is not clear yet, deletion of YER161C (SPT2) enhanced the alkali tolerance indeed.
For other evolved strains without deletion of SPT2 (yML011, yML013 and yML110), results of genome sequencing revealed very few structural variations (Additional file 1: Figures S4, S5, S9). However, a few single nucleotide polymorphisms (SNPs) were detected in the evolved genomes (i.e. 78 for yML110, 141 for yML011, 71 for yML013). These point mutations might be responsible for increased alkali tolerance of the evolved strains.
Our understanding of complex phenotypes (i.e., growth, tolerance) is limit mainly due to the absence of efficient multiplex engineering tools in genome scale [31, 42]. The strategy of using SCRaMbLE system to generate diverse and combinational rearrangements in the synthetic yeast genome provides a powerful platform to dissect phenotype-genotype relationship.
Conclusions
The SCRaMbLE system enables generation of a yeast structural variation library in a short time with the expression of Cre recombinase. In this study, alkali tolerant strains were obtained via one round of SCRaMbLE in synthetic yeast strains synV and synV&X. Various combinations of deletion, inversion and duplication were detected in the SCRaMbLEd genome of strains with alkali tolerance. Through comparative analysis of structural variations in the SCRaMbLEd genome, a deletion region with 11,448 base pairs in length was mapped to be responsible for the enhanced phenotype. Furthermore, we validated the deletion of SPT2 gene within the mapped region could improve alkali tolerance in S. cerevisiae. This study extends our knowledge of alkali tolerance in yeast and provides a standard workflow for the application of SCRaMbLE system to generate various phenotypes that may be interesting for industry and extend understanding of phenotype-genotype relationship.
Methods
Strains, plasmids and media
Plasmids and yeast strains used in this study were listed in Table 1. pRS416-pSCW11-Cre-EBD and pRS416-pCLB2-Cre-EBD are available from Addgene. Synthetic strains synV (MATa, his3Δ1, leu2Δ0, met15Δ0, ura3Δ0, LYS2) and synV&X (MATa, his3Δ1, leu2Δ0, met15Δ0, ura3Δ0, HO::tR(ccu)J, lys::NAT) were subjected to SCRaMbLE experiments. Wild type strain BY4741 (MATa, his3Δ1, leu2Δ0, met15Δ0, ura3Δ0) was used to test targets of alkali tolerance mapped by SCRaMbLE in synthetic yeast. Yeast strains were grown in YPD medium containing 20 g L−1 glucose, 20 g L−1 peptone and 10 g L−1 yeast extract. SC-Ura (synthetic media lacking uracil) medium with 1 μM β-estradiol (Sigma-Aldrich) was used to induce SCRaMbLE. Selective medium for alkali tolerance was YPD medium at pH 8.0. 1 M Tris buffer or 2 M NaOH was used to adjust the pH, and glucose was added after sterilization at high temperature. SC medium contained 1 g L−1 5-FOA (Sigma-Aldrich) was used to screen strains without URA3 marker. All yeast solid media were added with 20 g L−1 agar. Escherichia coli DH5α purchased from BEIJING Biomed Co., Ltd were used for plasmids transformation. Escherichia coli were cultivated at 37 °C in LB medium with 10 g L−1 tryptone, 5 g L−1 yeast extract, and 10 g L−1 NaCl. 100 μg mL−1 ampicillin or kanamycin were added for selection. LB solid medium were added with 15 g L−1 agar.
Table 1.
Strains and plasmids used in this study
| Strain and plasmid | Description |
|---|---|
| yXZX846 | Haploid strain synV |
| yYW169 | Haploid strain SynV&X |
| yML008 | SCRaMbLEd strain with enhanced alkali tolerance from yXZX846 |
| yML011 | SCRaMbLEd strain with enhanced alkali tolerance from yXZX846 |
| yML013 | SCRaMbLEd strain with enhanced alkali tolerance from yYW169 |
| yML015 | SCRaMbLEd strain with enhanced alkali tolerance from yYW169 |
| yML098 | SCRaMbLEd strain with enhanced alkali tolerance from yXZX846 |
| yML099 | SCRaMbLEd strain with enhanced alkali tolerance from yXZX846 |
| yML110 | SCRaMbLEd strain with enhanced alkali tolerance from yXZX846 |
| yML092 | yYW0169 with YER161C-164W deleted |
| yML093 | BY4741 with YER161C-164W deleted |
| yML094 | BY4741 with YER161C deleted |
| yML095 | BY4741 with YER162C deleted |
| yML096 | BY4741 with YER163C deleted |
| yML097 | BY4741 with YER164W deleted |
| pRS416 | CEN/ARS with URA3 marker |
| pYW079 | pRS416-SCW11-Cre-EBD |
| pYW180 | pRS416-CLB2-Cre-EBD |
pH tolerance test of the synV and synV&X strains
To identify a suitable pH selective condition that synthetic strains synV and synV&X could survive but have a limited growth speed, we used the serial dilution assay. According to a previous study [30], YPD, YPD at pH 7.4, YPD at pH 7.6, YPD at pH 7.8, YPD at pH 8.0 and YPD at pH 8.2 were used for the test.
Yeast transformation
LiAc/SS carrier method was used for yeast transformation. Strains grown overnight in YPD were diluted to OD600 of 0.1 in fresh YPD and cultured to exponential phase (5–8 h) at 30 °C. Cells were washed once with ddH2O, resuspended in 0.1 M LiAc and put on ice until needed. Yeast transformation system contained 620 μL 50% polyethylene glycol (PEG-3350), 40 μL salmon sperm DNA (100 mg mL−1), 90 μL 1 M LiAc, and 150 μL mixture of plasmids or fragments and cells. The system needed to be incubated at 30 °C for 30 min. 90 μL DMSO was added followed by heat-shocked at 42 °C for 18 min. Centrifuged and resuspended cells with 5 mM CaCl2, plated on selective medium. For plasmid, 100 ng was enough for transformation. Both pYW079 (pRS416-SCW11-Cre-EBD) and pYW180 (pRS416-pCLB2-Cre-EBD) plasmids were transformed to haploid strains synV and synV&X for SCRaMbLE. After culturing for 72 h at 30 °C, correct colonies could be selected on SC-Ura plates. As for fragments integrated to genome, at least 300 ng was required.
SCRaMbLE
First, single colony was cultured in 5 mL SC-Ura at 30 °C overnight. Then cultures were diluted to OD600 of 0.1 and inoculated to 5 mL fresh SC-Ura. 1 μM β-estradiol was added to induce SCRaMbLE. After incubated at 30 °C for 8 h, cells were washed twice with ddH2O to wash out β-estradiol. Finally, cells were diluted to spot onto both YPD plates and selective plates. The number of colonies on YPD was counted to figure out the lethality. Selective plates were incubated at 30 °C for 4–5 days.
Screening and verification of alkali tolerance strains
For preliminary screening, big colonies were selected on the selective media at pH 8.0. Then the candidate colonies were serially cultured in liquid YPD to lose the Cre-EBD plasmid. After that, candidate strains were phenotypically verified on YPD media at pH 8.0 using tenfold serial dilution assay.
Extraction of the yeast genomic DNA
Strains were cultured overnight to saturation. Centrifuged at 12,000 rpm to harvest cells. 200 μL STES buffer (500 mM L−1 NaCl, 200 mM L−1 Tris–HCl, 100 mM L−1 EDTA, 1% SDS), 200 μL silica sand and 200 μL phenol/chloroform/isoamyl alcohol (25:24:1) were added to cells. Disrupted cells by vortex mixer for 10 min. Then added 1 mL ethanol to the supernatant, mixed and centrifuged at 4 °C for 10 min. Precipitate was washed with 75% ethanol and dried at 42 °C. 100 μL ddH2O was added to dissolve the yeast genome DNA. Stored the genome DNA at − 20 °C.
PCRTagging analysis
Fifteen microlitre PCR reaction system contained 7.5 μL 2X rapid Taq master mix (Vazyme), 0.4 μL forward primer (10 μM), 0.4 μL reverse primer (10 μM), 1 μL genome DNA, and 5.7 μL ddH2O. The procedure: 95 °C/1 min, 30 cycles of (95 °C/20 s, 53 °C/20 s, 72 °C/15 s), and 72 °C/5 min. Agarose gel electrophoresis was used for PCRTag analysis. Primers involved in this study were listed Additional file 1: Table S1.
Whole genome sequencing
Cells were harvested at exponential phase and sent to BGI (the Beijing Genomics Institute) or BIOMARKER TECHNOLOGIES for whole genome sequencing. Libraries were prepared and performed on Illumina (HiSeq X-Ten). The original data obtained were filtered. SOAPaligner were used for average depth analysis. Through comparing with reference sequences, SNP, InDel and SV could be detected. The SVs including insertion, deletion, inversion, intra-chromosomal translocation, and inter-chromosomal translocation were analyzed.
Deletion of target genes
Genes were deleted by homologous recombination. The deletion of gene YER161C (SPT2) was used as an example to explain the strategy. Homologous arms of upstream and downstream of YER161C (SPT2) gene were amplified from the genome of BY4741. Selective marker URA3 was amplified from pRS416. Then three parts were joined together by overlap PCR and 300 ng of gel purified DNA fragments were directly transformed into BY4741 on SC-Ura plates. DNA fragment with homologous arms of gene YER161C (SPT2) was used to replace the marker URA3 by the same assay to avoid the effect of URA3 gene on the phenotypes of alkali tolerance. Correct transformants could be selected on 5-FOA plates. Other genes (YER162C, YER163C, YER164W, YER161C–YER164W) were deleted in the same way.
Growth curve assay
Single colony was cultured to saturation in 5 mL YPD medium at 30 °C. The cultures were inoculated into a 250 mL shake flask containing 50 mL of YPD medium at pH 8.3 (34 mM Tris L−1) with initial OD600 at 0.1, and cultured at 30 °C, 220 rpm. The OD value was measured at appropriate intervals. Growth curves were plotted using Origin software.
Additional file
Additional file 1: Figure S1. Stress tolerance of SCRaMbLEd strains. SCRaMbLEd strains were tested under various stressful conditions (YPD medium at 30 °C, YPD medium at 37 °C, YPD medium at 39 °C, YP medium with 20 g/L Xylose, YPD medium with 1.5 M Sorbitol and YP medium with 20 g/L Galactose). The growth of SCRaMbLEd strains was evaluated based on serial dilution. Two independent experiments were performed. Figure S2. PCRTag analysis of SCRaMbLEd strains. PCRTag analysis indicated deletion of YEL060C in strain yML013, deletions of YER161C and YER163C in strain yML015, deletions of YEL060C, YER161C, YER163C and YER175C in strain yML077, deletions of YER091C, YER161C and YER163C in yML099. No PCRTags were deleted in yML011 and yML110. SynV strain was used as a control strain. All PCRTag primers were listed in Table S1. Figure S3. Sequencing depth of synthetic chromosome V in yML008. Deep sequencing coverage of yML008 revealed four deletions (an intergenic sequence between YEL013W and YEL012W, YER042W, YER161C-YER164W and YER182W). Figure S4. Sequencing depth of synthetic chromosome V in yML011. Deep sequencing coverage revealed no synthetic fragments deleted in yML011. Figure S5. Sequencing depth of synthetic chromosomes in yML013. a Deep sequencing coverage of synthetic chromosome V in yML013 revealed a deletion of YEL060C. b Deep sequencing coverage revealed no synthetic fragments deleted in synthetic chromosome X in yML013. Figure S6. Sequencing depth of synthetic chromosomes in yML015. a Deep sequencing coverage of synthetic chromosome V in yML015 revealed a deletion of YER161C-YER164W. b Deep sequencing coverage revealed no synthetic fragments deleted in synthetic chromosome X in yML015. Figure S7. Sequencing depth of synthetic chromosome V in yML098. Deep sequencing coverage of yML098 revealed five deletions (YEL060C, an intergenic sequence between YER032W and YEL033C, YER161C-YER164W, YER175C-YER176W, and YER180C-A). Figure S8. Sequencing depth of synthetic chromosome V in yML099. Deep sequencing coverage of yML099 revealed three deletions (YER091C-YER092W, YER134C-YER135C, YER161C-YER164W) and one duplication (YER132C-YER133W). Figure S9. Sequencing depth of synthetic chromosome V in yML110. Deep sequencing coverage revealed no synthetic fragments deleted in yML110. Table S1. Primers used in this study.
Authors’ contributions
LM, YW, MZD and YJY designed the experiments. LM, YXL and XYC performed the experiments. LM and YW wrote the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors are grateful for the financial support from the National Natural Science Foundation of China (21750001 and 21621004) and Young Elite Scientist Sponsorship Program by CAST (YESS) (2018QNRC001).
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
All data generated or analyzed during this study are included in this article.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
This work was funded by the National Natural Science Foundation of China (21750001 and 21621004), and Young Elite Scientist Sponsorship Program by CAST (YESS) (2018QNRC001).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- SCRaMbLE
synthetic chromosome recombination and modification by LoxP-mediated evolution
- Sc 2.0 project
synthetic yeast genome project
- 3′UTR
3′ untranslated region
- EBD
estrogen-binding domain
Contributor Information
Lu Ma, Email: malu@tju.edu.cn.
Yunxiang Li, Email: liyunxiangcool@163.com.
Xinyu Chen, Email: ccchenxy@tju.edu.cn.
Mingzhu Ding, Email: mzding@tju.edu.cn.
Yi Wu, Email: yi.wu@tju.edu.cn.
Ying-Jin Yuan, Email: yjyuan@tju.edu.cn.
References
- 1.Peter J, Chiara MD, Friedrich A, Yue JX, Pflieger D, Bergström A, et al. Genome evolution across 1,011 Saccharomyces cerevisiae isolates. Nature. 2018;556:339–344. doi: 10.1038/s41586-018-0030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dujon B, Sherman D, Fischer G, Durrens P, Casaregola S, Lafontaine I, et al. Genome evolution in yeasts. Nature. 2004;430:35. doi: 10.1038/nature02579. [DOI] [PubMed] [Google Scholar]
- 3.Dujon B. Yeast evolutionary genomics. Nat Rev Genet. 2010;11:512. doi: 10.1038/nrg2811. [DOI] [PubMed] [Google Scholar]
- 4.Radke DW, Lee C. Adaptive potential of genomic structural variation in human and mammalian evolution. Brief Funct Genom. 2015;14:358–368. doi: 10.1093/bfgp/elv019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shastry BS. SNP alleles in human disease and evolution. J Hum Genet. 2002;47:561–566. doi: 10.1007/s100380200086. [DOI] [PubMed] [Google Scholar]
- 6.Mills RE. An initial map of insertion and deletion (INDEL) variation in the human genome. Genome Res. 2006;16:1182–1190. doi: 10.1101/gr.4565806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellis HM, Yu D, DiTizio T, Court DL. High efficiency mutagenesis, repair, and engineering of chromosomal DNA using single-stranded oligonucleotides. P Natl Acad Sci USA. 2001;98:6742–6746. doi: 10.1073/pnas.121164898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loo BV, Spelberg JHL, Kingma J, Sonke T, Wubbolts MG, Janssen DB. Directed evolution of epoxide hydrolase from A. radiobacter toward higher enantioselectivity by error-prone PCR and DNA shuffling. Chem Biol. 2004;11:981–990. doi: 10.1016/j.chembiol.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 9.Gaj T, Gersbach CA, Barbas CR. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013;31:397–405. doi: 10.1016/j.tibtech.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li W, Teng F, Li TD, Zhou Q. Simultaneous generation and germline transmission of multiple gene mutations in rat using CRISPR-Cas systems. Nat Biotechnol. 2013;31:684–686. doi: 10.1038/nbt.2652. [DOI] [PubMed] [Google Scholar]
- 11.Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157:1262–1278. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Y, Li BZ, Zhao M, Mitchell LA, Xie ZX, Lin QH, et al. Bug mapping and fitness testing of chemically synthesized chromosome X. Science. 2017;355:eaaf4706. doi: 10.1126/science.aaf4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie ZX, Li BZ, Mitchell LA, Wu Y, Qi X, Jin Z, et al. “Perfect” designer chromosome V and behavior of a ring derivative. Science. 2017;355:eaaf4704. doi: 10.1126/science.aaf4704. [DOI] [PubMed] [Google Scholar]
- 14.Mercy G, Mozziconacci J, Scolari VF, Yang K, Zhao G, Thierry A, et al. 3D organization of synthetic and scrambled chromosomes. Science. 2017;355:eaaf4597. doi: 10.1126/science.aaf4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen Y, Wang Y, Chen T, Gao F, Gong J, Abramczyk D, et al. Deep functional analysis of synII, a 770-kilobase synthetic yeast chromosome. Science. 2017;355:eaaf4791. doi: 10.1126/science.aaf4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchell LA, Wang A, Stracquadanio G, Kuang Z, Wang X, Yang K, et al. Synthesis, debugging, and effects of synthetic chromosome consolidation: synVI and beyond. Science. 2017;355:eaaf4831. doi: 10.1126/science.aaf4831. [DOI] [PubMed] [Google Scholar]
- 17.Zhang W, Zhao G, Luo Z, Lin Y, Wang L, Guo Y, et al. Engineering the ribosomal DNA in a megabase synthetic chromosome. Science. 2017;355:eaaf3981. doi: 10.1126/science.aaf3981. [DOI] [PubMed] [Google Scholar]
- 18.Richardson SM, Mitchell LA, Stracquadanio G, Yang K, Dymond JS, DiCarlo JE, et al. Design of a synthetic yeast genome. Science. 2017;355:1040–1044. doi: 10.1126/science.aaf4557. [DOI] [PubMed] [Google Scholar]
- 19.Shen Y, Stracquadanio G, Wang Y, Yang K, Mitchell LA, Xue Y, et al. SCRaMbLE generates designed combinatorial stochastic diversity in synthetic chromosomes. Genome Res. 2016;26:36–49. doi: 10.1101/gr.193433.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, Xie ZX, Ma Y, Li BZ, Yuan YJ. Ring synthetic chromosome V SCRaMbLE. Nat Commun. 2018;9:3783. doi: 10.1038/s41467-018-06216-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jia B, Wu Y, Li BZ, Mitchell LA, Liu H, Pan S, et al. Precise control of SCRaMbLE in synthetic haploid and diploid yeast. Nat Commun. 2018;9:1933. doi: 10.1038/s41467-018-03084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen MJ, Wu Y, Yang K, Li YX, Xu H, Zhang HR, et al. Heterozygous diploid and interspecies SCRaMbLEing. Nat Commun. 2018;9:1934. doi: 10.1038/s41467-018-04157-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Y, Zhu RY, Mitchell LA, Ma L, Liu R, Zhao M, et al. In vitro DNA SCRaMbLE. Nat Commun. 2018;9:1935. doi: 10.1038/s41467-018-03743-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu W, Luo ZQ, Wang Y, Pham NT, Tuck L, Pérez-Pi I, et al. Rapid pathway prototyping and engineering using in vitro and in vivo synthetic genome SCRaMbLE-in methods. Nat Commun. 2018;9:1936. doi: 10.1038/s41467-018-04254-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blount BA, Gowers GF, Ho JCH, Ledesma-Amaro R, Jovicevic D, McKiernan RM, et al. Rapid host strain improvement by in vivo rearrangement of a synthetic yeast chromosome. Nat Commun. 2018;9:1932. doi: 10.1038/s41467-018-03143-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo ZQ, Wang LH, Wang Y, Zhang WM, Guo YK, Shen Y, et al. Identifying and characterizing SCRaMbLEd synthetic yeast using ReSCuES. Nat Commun. 2018;9:1930. doi: 10.1038/s41467-017-00806-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J, Jia B, Xie ZX, Li YX, Yuan YJ. Improving prodeoxyviolacein production via multiplex SCRaMbLE iterative cycles. Front Chem Sci Eng. 2018 doi: 10.1007/s11705-018-1739-2. [DOI] [Google Scholar]
- 28.Lamb TM, Xu W, Diamond A, Mitchell AP. Alkaline response genes of Saccharomyces cerevisiae and their relationship to the RIM101 pathway. J Biol Chem. 2001;276:1850–1856. doi: 10.1074/jbc.M008381200. [DOI] [PubMed] [Google Scholar]
- 29.Serrano R, Martín H, Casamayor A, Ariño J. Signaling alkaline pH stress in the yeast Saccharomyces cerevisiae through the Wsc1 cell surface sensor and the Slt2 MAPK pathway. J Biol Chem. 2006;281:39785–39795. doi: 10.1074/jbc.M604497200. [DOI] [PubMed] [Google Scholar]
- 30.Romano GH, Gurvich Y, Lavi O, Ulitsky I, Shamir R, Kupiec M. Different sets of QTLs influence fitness variation in yeast. Mol Syst Biol. 2010 doi: 10.1038/msb.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Storms RK, Ni L, Herman Z, Davis K, Entian K, André B, et al. Functional profiling of the Saccharomyces cerevisiae genome. Nature. 2002;418:387–391. doi: 10.1038/nature00935. [DOI] [PubMed] [Google Scholar]
- 32.Serrano R, Bernal D, Simón E, Ariño J. Copper and iron are the limiting factors for growth of the yeast Saccharomyces cerevisiae in an alkaline environment. J Biol Chem. 2004;279:19698–19704. doi: 10.1074/jbc.M313746200. [DOI] [PubMed] [Google Scholar]
- 33.Serrano R, Ruiz A, Bernal D, Chambers JR, Ariño J. The transcriptional response to alkaline pH in Saccharomyces cerevisiae: evidence for calcium-mediated signalling. Mol Microbiol. 2002;46:1319–1333. doi: 10.1046/j.1365-2958.2002.03246.x. [DOI] [PubMed] [Google Scholar]
- 34.Veis J, Klug H, Koranda M, Ammerer G. Activation of the G2/M-specific gene CLB2 requires multiple cell cycle signals. Mol Cell Biol. 2007;27:8364–8373. doi: 10.1128/MCB.01253-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindstrom DL, Gottschling DE. The mother enrichment program: a genetic system for facile replicative life span analysis in Saccharomyces cerevisiae. Genetics. 2009;183:413–422. doi: 10.1534/genetics.109.106229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bean DW, Matson SW. Identification of the gene encoding scHelI, a DNA helicase from Saccharomyces cerevisiae. Yeast. 1997;13:1465. doi: 10.1002/(SICI)1097-0061(199712)13:15<1465::AID-YEA193>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 37.Biswas EE, Chen PH, Leszyk J, Biswas SB. Biochemical and genetic characterization of a replication protein A-dependent DNA helicase from the yeast, Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1995;206:850–856. doi: 10.1006/bbrc.1995.1121. [DOI] [PubMed] [Google Scholar]
- 38.Nourani A, Robert F, Winston F. Evidence that Spt2/Sin1, an HMG-like factor, plays roles in transcription elongation, chromatin structure, and genome stability in Saccharomyces cerevisiae. Mol Cell Biol. 2006;26:1496–1509. doi: 10.1128/MCB.26.4.1496-1509.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thebault P, Boutin G, Bhat W, Rufiange A, Martens J, Nourani A. Transcription regulation by the noncoding RNA SRG1 requires Spt2-dependent chromatin deposition in the wake of RNA polymerase II. Mol Cell Biol. 2011;31:1288–1300. doi: 10.1128/MCB.01083-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhat W, Boutin G, Rufiange A, Nourani A. Casein kinase 2 associates with the yeast chromatin reassembly factor Spt2/Sin1 to regulate its function in the repression of spurious transcription. Mol Cell Biol. 2013;33:4198–4211. doi: 10.1128/MCB.00525-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hershkovits G, Bangio H, Cohen R, Katcoff DJ. Recruitment of mRNA cleavage/polyadenylation machinery by the yeast chromatin protein Sin1p/Spt2p. P Natl Acad Sci USA. 2006;103:9808–9813. doi: 10.1073/pnas.0602014103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hampsey M. A review of phenotypes in Saccharomyces cerevisiae. Yeast. 1997;13:1099–1133. doi: 10.1002/(SICI)1097-0061(19970930)13:12<1099::AID-YEA177>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Stress tolerance of SCRaMbLEd strains. SCRaMbLEd strains were tested under various stressful conditions (YPD medium at 30 °C, YPD medium at 37 °C, YPD medium at 39 °C, YP medium with 20 g/L Xylose, YPD medium with 1.5 M Sorbitol and YP medium with 20 g/L Galactose). The growth of SCRaMbLEd strains was evaluated based on serial dilution. Two independent experiments were performed. Figure S2. PCRTag analysis of SCRaMbLEd strains. PCRTag analysis indicated deletion of YEL060C in strain yML013, deletions of YER161C and YER163C in strain yML015, deletions of YEL060C, YER161C, YER163C and YER175C in strain yML077, deletions of YER091C, YER161C and YER163C in yML099. No PCRTags were deleted in yML011 and yML110. SynV strain was used as a control strain. All PCRTag primers were listed in Table S1. Figure S3. Sequencing depth of synthetic chromosome V in yML008. Deep sequencing coverage of yML008 revealed four deletions (an intergenic sequence between YEL013W and YEL012W, YER042W, YER161C-YER164W and YER182W). Figure S4. Sequencing depth of synthetic chromosome V in yML011. Deep sequencing coverage revealed no synthetic fragments deleted in yML011. Figure S5. Sequencing depth of synthetic chromosomes in yML013. a Deep sequencing coverage of synthetic chromosome V in yML013 revealed a deletion of YEL060C. b Deep sequencing coverage revealed no synthetic fragments deleted in synthetic chromosome X in yML013. Figure S6. Sequencing depth of synthetic chromosomes in yML015. a Deep sequencing coverage of synthetic chromosome V in yML015 revealed a deletion of YER161C-YER164W. b Deep sequencing coverage revealed no synthetic fragments deleted in synthetic chromosome X in yML015. Figure S7. Sequencing depth of synthetic chromosome V in yML098. Deep sequencing coverage of yML098 revealed five deletions (YEL060C, an intergenic sequence between YER032W and YEL033C, YER161C-YER164W, YER175C-YER176W, and YER180C-A). Figure S8. Sequencing depth of synthetic chromosome V in yML099. Deep sequencing coverage of yML099 revealed three deletions (YER091C-YER092W, YER134C-YER135C, YER161C-YER164W) and one duplication (YER132C-YER133W). Figure S9. Sequencing depth of synthetic chromosome V in yML110. Deep sequencing coverage revealed no synthetic fragments deleted in yML110. Table S1. Primers used in this study.
Data Availability Statement
All data generated or analyzed during this study are included in this article.