Abstract
Background: Recent models of eating disorders (EDs) have proposed social and emotional difficulties as key factors in the development and maintenance of the illness. While a number of studies have demonstrated difficulties in theory of mind and emotion recognition, little is known about empathic abilities in those with EDs. Further, few studies have examined the cognitive-affective empathy profile in EDs. The aim of this systematic review and meta-analysis was to provide a synthesis of empathy studies in EDs, and examine whether those with EDs differ from healthy controls (HC) on self-reported total, cognitive, and affective empathy.
Methods: Electronic databases were systematically searched for studies using self-report measures of empathy in ED populations. In total, 17 studies were identified, 14 of which could be included in the total empathy meta-analysis. Eight of the 14 studies were included in the cognitive and affective empathy meta-analyses.
Results: Meta-analyses showed that while total empathy and affective empathy scores did not differ between those with anorexia nervosa (AN) and HC, those with AN had significantly lower cognitive empathy scores compared to HCs (small effect size). Meta-analyses of Interpersonal Reactivity Index sub-scores revealed that AN had significantly lower Fantasy scores than HC (small effect size), indicating that those with AN have more difficulty in identifying themselves with fictional characters. Only 3 studies examined empathy in those with bulimia nervosa (BN) or binge eating disorder (BED).
Conclusions: The lowered cognitive empathy and intact affective empathy profile found in AN is similar to that found in other psychiatric and neurodevelopmental conditions, such as autism spectrum disorder (ASD). These findings add to the literature characterizing the socio-emotional phenotype in EDs. Future research should examine the influence of comorbid psychopathology on empathy in EDs.
Keywords: empathy, eating disorders, anorexia nervosa, autism, self-report, insight
Introduction
Rationale
Empathy refers to our ability to understand and identify the mental states of others, as well as our ability to share the feelings of others (1). It is considered a key component of social cognition, cooperation, and prosocial behavior, as it allows us to make sense of and respond appropriately to other people's behavior (2). Empathy can be separated into two major facets. Cognitive empathy refers to the ability to recognize and understand another's mental state (part of theory of mind (ToM) or mentalising) while affective empathy is the ability to share the feelings of others, without any direct emotional stimulation to oneself (3). As an illustrative example, sharing the excitement of a close friend's job offer is fundamentally different from understanding that your friend must be having thoughts and feelings, and what these feelings might be. These two aspects of empathy rely on different brain structures and take different developmental pathways, with affective empathy developing much earlier than cognitive empathy (1).
Differences in empathic abilities have been observed in a number of psychiatric disorders including schizophrenia (4, 5), autism spectrum disorder [ASD; (6, 7)], borderline personality disorder [BPD; (8)], and depression (9). Importantly, far from there being a universal deficit in empathic abilities, research in these psychiatric disorders shows that there is often a difficulty in a specific aspect of empathy, while other empathic abilities remain intact. For example, it has been found that those with ASD have problems with cognitive empathy, but do not differ from neurotypical controls in affective empathy (10). Reduced attention to informative social information may provide one explanation for the problems in cognitive empathy seen in those with ASD. For example, it is reported that individuals with ASD pay less attention to faces, and especially eyes (11), and this is associated with poorer emotion recognition and ToM ability (12–14), as well as lower social competence (15). Similarly, while healthy controls (HCs) show significantly higher levels of cognitive empathy compared to affective empathy, those with BPD show significantly poorer cognitive empathy than HCs, and slightly increased levels of affective empathy (16). In bipolar disorder (BD), this cognitive/affective empathy distinction is further complicated by clinical state. In both manic and depressive phases of illness, there is an impairment in cognitive empathy compared to HCs. However, during the manic phase, affective empathy is significantly higher than in HCs and patients in the depression phase of BD, who did not differ from one another (17). Increased affective empathy in BPD and BD may be related to disturbances in emotion inhibition.
Recent models of eating disorders (EDs) have put forward social and emotional difficulties as key factors in the development and maintenance of the illness (18, 19). However, relatively little is known about the specific empathy profile in those with EDs. Based on longitudinal research in a community sample from Sweden, Gillberg et al. published a number of papers reporting a subgroup of AN patients with “empathy disorders”—those that had severe problems in social understanding and communication, consistent with ASD (20). Poorer outcomes were found in this group (21, 22). Since then, a growing body of evidence has documented overlap between symptoms in ASD and AN. For example, both groups show high levels of social anxiety (23, 24) and alexithymia (25, 26), differences in social attention (11, 27, 28), and poorer emotion recognition (29, 30) and ToM ability (31, 32). Reduced social networks have been documented in AN and bulimia nervosa (BN) (33, 34), as well as difficulties in understanding the concept of friendship (35). It is possible that reduced empathic abilities, along with communication difficulties, may contribute to the diminished social networks and isolation that characterize EDs. Given that interpersonal difficulties are associated with more severe ED psychopathology (36, 37), understanding mechanisms that may contribute to these problems may be helpful in improving outcomes in those with these EDs.
Objectives
The aim of this systematic review and meta-analysis is to provide a synthesis of empathy research in EDs. Previous reviews on social processes in EDs have ascribed relatively little attention to the topic, and focus on emotion recognition rather than other aspects of empathy such as affect sharing [e.g., (31)]. In addition, new studies have been published in the intervening years. An additional aim is to examine potential differences between those with EDs and HCs in the specific types of empathy (self-reported cognitive and affective empathy), to permit better comparisons with other psychiatric populations. Self-reported empathy measures will be the focus of this review, in order to elicit patients' views and self-assessment of their skills.
Research Questions
The research questions are as follows: (1) do levels of self-reported empathy differ in those with EDs compared to HCs? (2) do levels of cognitive and affective empathy differ between EDs and HCs? (3) are empathy levels associated with any psychopathological or clinical variables?
Methods
Systematic Review Protocol
The review and meta-analysis was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (38).
Eligibility Criteria
Studies using a self-report measure of empathy were included. Inclusion criteria were: (1) means and standard deviations reported for empathy scores in at least one clinical ED group and a HC group (2) the clinical ED group met criteria for any ED diagnosis, according to DSM or ICD criteria (3) full article available in English (4) published in a peer reviewed journal. Articles that examined disordered eating samples rather than a clinical ED were not included.
Data Sources and Search Strategy
The electronic databases SCOPUS, Web of Science, PsycInfo, and PubMed were searched for papers up to September 2018. The following search terms were used: anorexia nervosa OR bulimia nervosa OR eating disorder AND empathy OR emotional empathy OR empathic concern OR interpersonal reactivity. No other search limits were applied, with the exception of Web of Science, where results were filtered by the ED term for relevance. Reference lists were also searched for relevant papers.
Study Selection
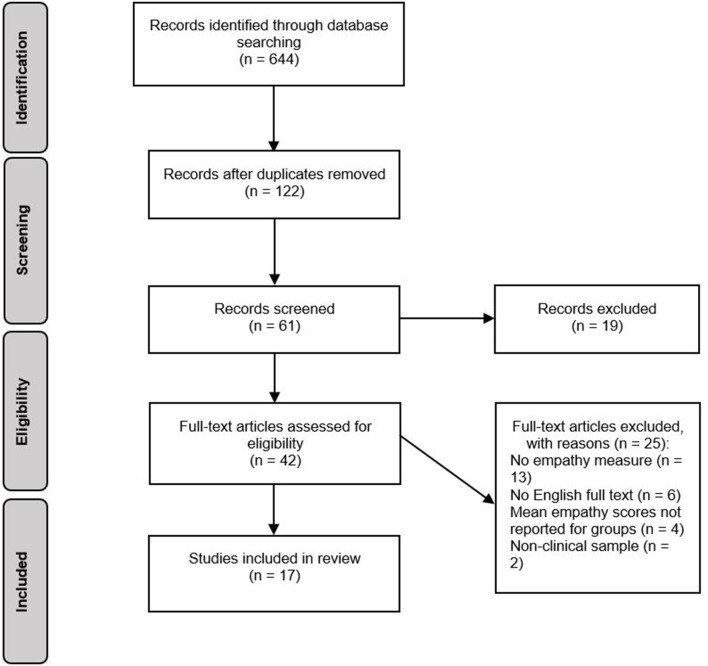
The selection process for studies is displayed in Figure 1. In total, the search generated 644 records. After removing duplicates, 122 records were assessed for relevance based on article titles. If titles were ambiguous or potentially relevant, records were retained and their abstracts screened against the eligibility criteria. This resulted in 61 abstracts being screened, 19 of which were excluded as they did not meet eligibility criteria. After screening of abstracts, 42 potentially eligible full-text articles were identified. One study included a sample of participants with BN, however at the time of publication, BN was not yet included in the DSM. The study was included in the review as participants had a clinical diagnosis of BN. If means and standard deviations for individual groups were not reported, study authors were contacted. If no response was received, studies were excluded. Evaluation of these full texts resulted in 25 studies being excluded, and 17 studies being included in the review.
Figure 1.
Systematic review search process.
Data Extraction
The following data was extracted from each paper that met all eligibility criteria: number of participants in each group, mean age, mean body mass index (BMI), percentage of female participants, empathy measure used, mean empathy scores, and any subscale scores, if they were reported. Where studies reported sub-scale scores only, total, cognitive, and affective empathy scores were calculated so that studies could be included in meta-analyses.
Data Analysis
All analyses were performed using R Studio (39) using the metafor package (40). Cohen's d was used to estimate effect sizes and is reported with 95% confidence intervals (CIs). Effect sizes are interpreted using Cohen's (41) definitions of small (0.2), medium (0.5), and large (0.8). Negative effect sizes indicate lower empathy scores in the ED group compared to HC. Separate meta-analyses were performed for different components of empathy. Where two measures of empathy were used in the same study (and therefore on the same group of participants), a multivariate meta-analysis was performed using the rma.mv command. Between-study heterogeneity was calculated using Cochran's Q test. Where heterogeneity was found (p < 0.05), meta-regressions were performed using age and empathy measure as moderators.
Risk of Bias
Publication bias was assessed through visual inspection of funnel plots, where the absence of studies in the bottom right corner indicates publication bias. The symmetry of the funnel plots was formally assessed using Begg's rank correlation test (42). Publication bias was also assessed using Rosenthal's fail-safe N (43), which estimates the number of unpublished studies required to change the significant effect size into a non-significant one.
Risk of bias in individual studies was assessed using the Clinical Appraisal Skills Programme checklist for case–control studies (44). The checklist considers how methodological features of studies may have impacted the results, e.g., exclusion and inclusion criteria, recruitment sources, and whether potential confounding variables were included in analyses. Studies can receive a maximum score of 17.
Results
Study Characteristics
Study characteristics are shown in Table 1. Fourteen of the included studies compared AN and HC groups. Of these studies, one study also included a recovered AN group, two included an ASD group, and one included a group with BPD. Two studies compared those with binge eating disorder (BED) to HC, and one study compared participants with BN to HC.
Table 1.
Characteristics of studies.
| References | Group | Mean age (SD) | Mean BMI (SD) | % female | Empathy measure | Mean (SD) total empathy | Subscales reported? | |
|---|---|---|---|---|---|---|---|---|
| Mean (SD) cognitive empathy | Mean (SD) affective empathy | |||||||
| Adenzato et al. (45) | 30 AN | 19.73 (6.06) | 15.06 (1.74) | 100 | EQ | 44.17 (11.47) | NR | NR |
| 32 HC | 20.47 (2.72) | 20.21 (1.45) | 100 | 50.72 (8.35) | ||||
| Aloi et al. (46) | 22 BED | 43.8 (10.7) | 36.9 (4.2) | 81.4 | EQ | 41.8 (14.9) | NR | NR |
| 16 sub-threshold BED | 42.5 (11.3) | 37.5 (4.5) | 68.8 | 50.5 (11.6) | ||||
| 20 obese controls | 50.6 (8.6) | 38.2 (6.5) | 45 | 50.1 (12.4) | ||||
| Baron Cohen et al. (47) | 66 AN | 17.85 (0.39) | NR | 100 | EQ (adult and adolescent versions)† |
Younger: 44.7 (16.4) Older: 49.6 (9.7) |
NR | NR |
| 1609 HC | 18.56 (3.99) | NR | 100 |
Younger: 51.2 (14.3) Older: 48.0 (11.3) |
||||
| Butler and Montgomery (48) | 15 AN | 27.9 (9.9) | NR | 100 | I7 | 15.40 (2.61) | NA | NA |
| 16 HC | 28.4 (8.3) | 22.75 | 100 | 14.19 (2.74) | ||||
| Calderoni et al. (49) | 32 AN | 14.78 (1.75) | 15.07 (1.54) | 100 | IRI | 5.13 (6.98) | 0.44 (6.87) | 4.69 (7.08) |
| 41 HC | 14.02 (1.69) | NR | 100 | 9.44 (5.66) | 5.24 (6.45) | 4.20 (4.75) | ||
| Courty et al. (50) | 15 AN | 23.9 (4.7) | 16.4 (1.7) | 93.33 | EQ-short | 23.0 (6.8) | NR | NR |
| IRI | 70.8 (4.83) | 34.1 (4.85) | 36.7 (4.8) | |||||
| 15 HC | 24.0 (4.9) | 21.0 (1.8) | 93.33 | EQ-short | 21.1 (7.4) | NR | NR | |
| IRI | 73.09 (3.79) | 38.3 (3.31) | 35.6 (4.22) | |||||
| 15 ASD | 28.1 (7.5) | 23.2 (5.0) | 13.33 | EQ-short | 10.1 (5.7) | NR | NR | |
| IRI | 65.5 (4.77) | 32.6 (4.73) | 32.9 (4.81) | |||||
| 15 HC | 28.1 (7.3) | 22.2 (3.0) | 13.33 | EQ-short | 19.9(3.4) | NR | NR | |
| IRI | 67.0 (3.39) | 34.7 (2.75) | 32.3 (3.93) | |||||
| Duchesne et al. (51) | 60 BED | NR | 38.1 | 100 | IRI | 81.4 (4.04) | 42.4 (4.40) | 39.0 (3.65) |
| 60 obese controls | NR | 37.9 | 100 | 81.3 (3.95) | 42.7 (3.36) | 38.6 (4.46) | ||
| 54 HC | NR | 21.4 (1.6) | 100 | 80.6 (4.02) | 43.4 (4.30) | 37.2 (3.72) | ||
| Feldman and Eysenck (52) | 45 BN | 25.13 (6.59) | NR | 100 | I7 | 14.73 (3.17) | NA | NA |
| 761 HC | NR | NR | 100 | 14.39 (2.87) | ||||
| Gramaglia et al. (53) | 39 AN | 30.59 (3.0) | 16.3 | NR | IRI | 82.93 (3.81) | 41.19 (4.48) | 41.74 (2.99) |
| 48 HC | 33.19 (3.37) | 21.82 | 100 | 80.48 (3.78) | 41.9 (4.15) | 38.58 (3.37) | ||
| Guttman and Laporte (54) | 28 AN | 22 | NR | 100 | IRI | 72.7 (5.60) | 35.1 (5.51) | 37.6 (5.69) |
| 26 BPD | 32 | NR | 100 | 78.9 (5.45) | 34.7 (5.56) | 44.2 (5.35) | ||
| 27 HC | 21 | NR | 100 | 71.9 (4.83) | 35.9 (4.61) | 36 (5.04) | ||
| Hambrook et al. (55) | 22 AN | 26.73 (4.77) | 15.27 (1.22) | 100 | EQ | 45.9 (12.5) | NR | NR |
| 45 HC | 32.51 (9.63) | 23.36 (3.76) | 100 | 46.2 (11.1) | ||||
| Jermakow and Brzezicka (56) | 11 AN | 26.80 (4.3) | NR | 100 | EQ | 44.60 (8.58) | NR | NR |
| IRI | 63.10 (3.39) | 34.9 (6.22) | 28.2 (4.46) | |||||
| 33 female HC | 21.33 (1.4) | NR | 100 | EQ | 42.42 (9.84) | NR | NR | |
| IRI | 70.03 (2.13) | 38.52 (4.40) | 31.52 (4.40) | |||||
| 10 ASD | 28.30 (9.5) | NR | 0 | EQ | 30.00 (5.05) | NR | NR | |
| IRI | 57.90 (2.20) | 33.5 (5.59) | 24.4 (3.64) | |||||
| 27 male HC | 21.76 (2.0) | NR | 0 | EQ | 32.63 (9.97) | NR | NR | |
| IRI | 62.70 (2.33) | 33.38 (5.60) | 29.33 (5.21) | |||||
| Lule et al. (57) | 15 AN | 16.2 (1.26) | 17.07 (1.44) | 100 | IRI | 121.14 (11.25) | NR | NR |
| 15 HC | 16.5 (1.09) | 21.06 (1.57) | 100 | 118.50 (10.20) | ||||
| Morris et al. (58) | 28 AN | 26.3 (7.9) | 15.5 (1.3) | 100 | SEQ | 18.8 (2.5) | NA | NA |
| 25 AN-REC | 29.5 (9.2) | 20.1 (1.9) | 100 | 19.8 (3.0) | ||||
| 54 HC | 29.4 (9.6) | 23.1 (3.9) | 100 | 20.4 (2.4) | ||||
| Nandrino et al. (59) | 23 AN | 19.64 (1.82) | 15.2 (1.07) | 100 | BES | 79.57 (6.70) | 35.57 (3.45) | 44.00 (5.44) |
| 23 HC | 20.65 (1.90) | 21.05 (1.78) | 100 | 80.78 (6.04) | 36.78 (3.19) | 44.00 (4.93) | ||
| Peres et al. (60) | 41 AN | 16.2 (1.4) | 79.78 (8.71) %IBW | 100 | IRI | 74.44 (4.30) | 35.5 (6.99) | 39.0 (6.45) |
| 38 HC | 15.84 (1.83) | 100.5 (11.71) %IBW | 100 | 73.1 (4.1) | 37.6 (7.18) | 35.6 (5.21) | ||
| Redondo and Herrero-Fernandez(61) | 38 AN | 21.9 (5.30) | NR | 100 | EQ-short | 23.42 (7.25) | 11.26 (4.84) | 7.11 (2.68) |
| IRI†† | NR | NR | NR | |||||
| 321 HC | NR | NR | 100 | EQ-short | 25.79 (7.21) | 11.03 (4.63) | 7.55 (2.35) | |
| IRI†† | NR | NR | NR | |||||
Significant differences between ED and HCs are indicated in bold. Italics indicate where scores were not reported in the study, but could be calculated from subscale scores. Potential significant differences could therefore not be reported for calculated scores. AN, anorexia nervosa; AN-REC, recovered anorexia nervosa; ASD, autism spectrum disorder; BED, binge eating disorder; BES, Basic Empathy Scale; BMI, body mass index; BN, bulimia nervosa; BPD, borderline personality disorder; EQ, Empathy Quotient; HC, healthy control; I7, Impulsiveness, Venturesomeness, and Empathy questionnaire; IRI, interpersonal reactivity index; NA, not applicable; NR, not reported; SD, standard deviation; SEQ, Socio-Emotional Questionnaire.
Groups were split into groups depending on age and EQ version used.
Only the PT subscale of the IRI was used.
In total, 6 different self-report measures were used across studies, with the Interpersonal Reactivity Index [IRI; (62)] being used most often (9 studies). The IRI comprises of four subscales: perspective taking (PT; the tendency to spontaneously adopt the psychological viewpoint of others), fantasy (FS; the tendency to identify oneself with fictional characters in books, plays and movies), empathic concern (EC; assesses “other-oriented” feelings of sympathy and concern for others), and personal distress (PD; assesses “self-oriented” feelings of anxiety and unease in tense interpersonal settings). Cognitive and affective empathy scores can be calculated by taking the sum of PT and FS, and EC and PD respectively. The Empathy Quotient [EQ; (6)], and the EQ-short (63) were used in seven studies, and both have three subscales: cognitive empathy, affective empathy, and social skills. Other measures used were: the empathy subscale of the Impulsiveness, Venturesomeness, and Empathy questionnaire (I7; (64) (2 studies), the empathy subscale of the Socio-Emotional Questionnaire [SEQ; (65)] (1 study), and the Basic Empathy Scale [BES; (66)] (1 study). One study used two different versions of the EQ depending on participants' age; the parent reported version for younger adolescents, and the self-report version for older adolescents (47). Only the self-report scores are included in the meta-analysis, as this was the focus of the present review.
Methodological quality of the studies varied considerably (range: 7–16). None of the studies reported a power calculation, and sample sizes were generally small (ranging from 11 to 66 in ED groups). All but one study (46) matched participants on at least one characteristic, most often sex. The mean age of participants ranged from 14.02 to 50.60 years, although three studies did not report the mean age of at least one participant group (51, 52, 61). Seven studies did not report mean BMI or percentage IBW in at least one participant group (47–49, 52, 54, 56, 61). Most studies used exclusively female samples, however three studies included male participants (46, 50, 56).
Synthesized Findings
Only studies comparing AN and HC could be included in meta-analyses, due to too few studies with other ED groups (2 BED, 1 BN). The number of studies in each meta-analysis is displayed in Figure 2.
Figure 2.
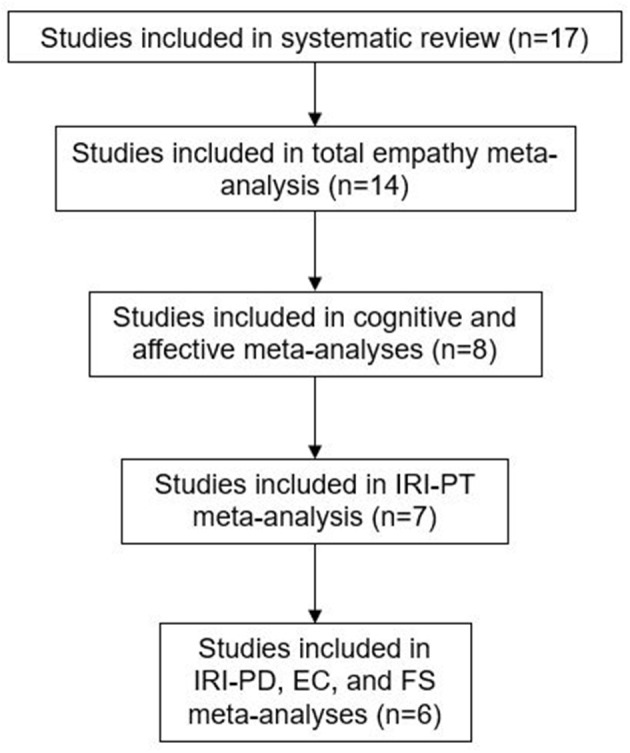
Studies included in the review and meta-analyses.
Total Empathy
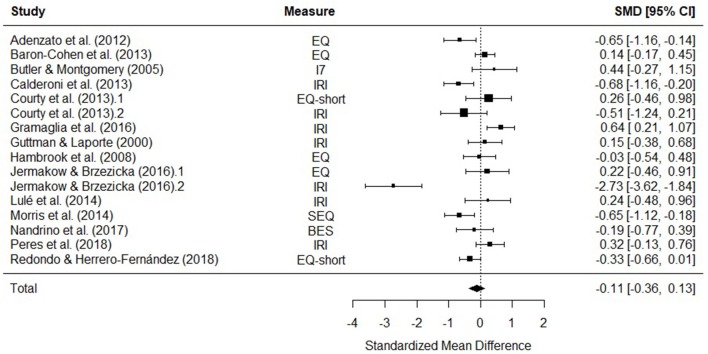
Fourteen studies were included in a meta-analysis comparing total empathy scores in AN and HCs. The random effects model with a total sample size of 2165 participants (AN = 379, HC = 1746) revealed that total empathy scores in AN did not differ from those of HCs [d = −0.11, (95% CI −0.36, 0.13) z = −0.92, p = 0.36] (Figure 3).
Figure 3.
Forest plot of standardized mean effect size for differences (SMD) between anorexia nervosa (AN) and healthy controls (HC) on total empathy scores. Negative effect sizes indicate lower empathy scores in the AN group. BES, Basic Empathy Scale; CI, confidence interval; EQ, empathy quotient; I7, Impulsiveness, Venturesomeness, and Empathy questionnaire; IRI, interpersonal reactivity index; SEQ, Socio-Emotional Questionnaire.
There was evidence of significant heterogeneity across studies [Q(15) = 79.61, p < 0.001], therefore meta-regressions with age and empathy measure as moderator variables were performed. The moderators explained a significant amount of the variance [QM(6) = 27.88, p = < 0.001], however no single factor had a significant influence on the size of the effect. The test for residual heterogeneity was significant [QE(8) = 65.08, p = < 0.001].
Cognitive Empathy
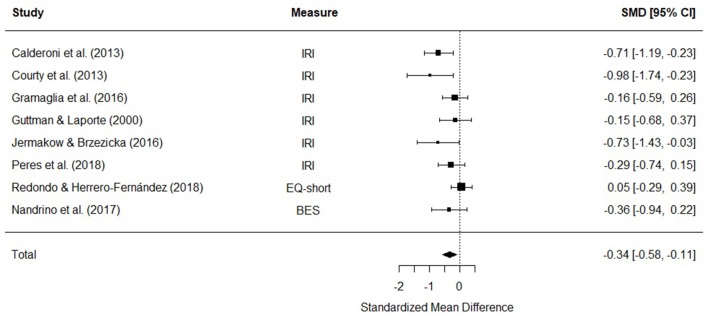
Eight studies were included in a meta-analysis comparing cognitive empathy scores in AN and HC. The random effects model with at total sample size of 773 participants (AN = 227, HC = 546) revealed that cognitive empathy scores in AN were significantly lower than HCs [d = −0.34, (95% CI −0.58, −0.11) z = −2.86, p = 0.004] (Figure 4). There was no evidence of significant heterogeneity [Q(7) = 12.27, p = 0.09].
Figure 4.
Forest plot of standardized mean effect size for differences (SMD) between anorexia nervosa (AN) and healthy controls (HC) on cognitive empathy scores. Negative effect sizes indicate lower empathy scores in the AN group. BES, Basic Empathy Scale; CI, confidence interval; EQ, empathy quotient; IRI, interpersonal reactivity index.
Affective Empathy
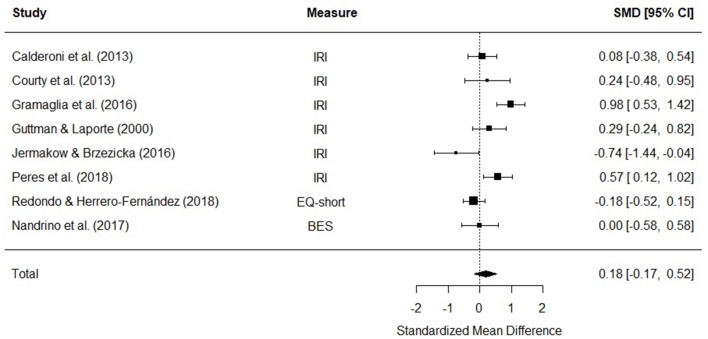
Eight studies were included in a meta-analysis comparing affective empathy scores in AN and HC. The random effects model with a total sample size of 773 participants (AN = 227, HC = 546) revealed that affective empathy scores in AN did not differ from those of HCs [d = 0.18, (95% CI −0.17, 0.52) z = 1.01, p = 0.31] (Figure 5).
Figure 5.
Forest plot of standardized mean effect size for differences (SMD) between anorexia nervosa (AN) and healthy controls (HC) on affective empathy scores. Negative effect sizes indicate lower empathy scores in the AN group. BES, Basic Empathy Scale; CI, confidence interval; EQ, empathy quotient; IRI, interpersonal reactivity index.
There was evidence of significant heterogeneity across studies [Q(7) = 26.99, p < 0.001], therefore meta-regressions with age and empathy measure as moderator variables were performed. The moderators did not explain a significant amount of the variance [QM(3) = 0.64, p = 0.88], and the test for residual heterogeneity was significant [Q(4) = 17.6, p = 0.002].
Risk of Bias
The funnel plots for total empathy, cognitive empathy, and affective empathy scores are displayed in Figures 6–8. There was no evidence of publication bias in the total empathy meta-analysis (Begg's test p = 0.45), however there was evidence of publication bias in the studies included in the cognitive empathy meta-analysis (Begg's test p = 0.03, Rosenthal's fail safe N = 38). Studies included in the affective empathy meta-analysis did not show any evidence of publication bias (Begg's test p = 0.40).
Figure 6.
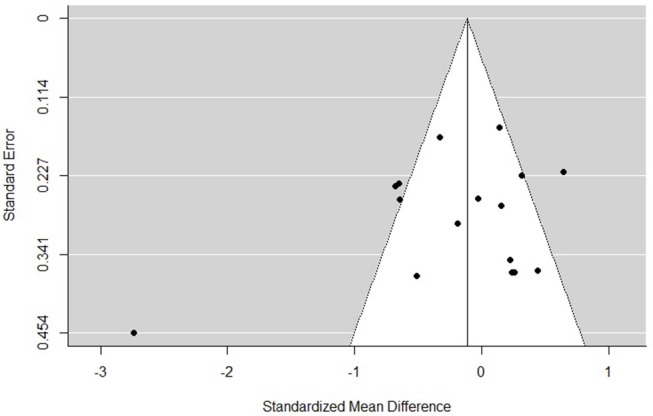
Funnel plot of studies included in the total empathy meta-analysis.
Figure 8.
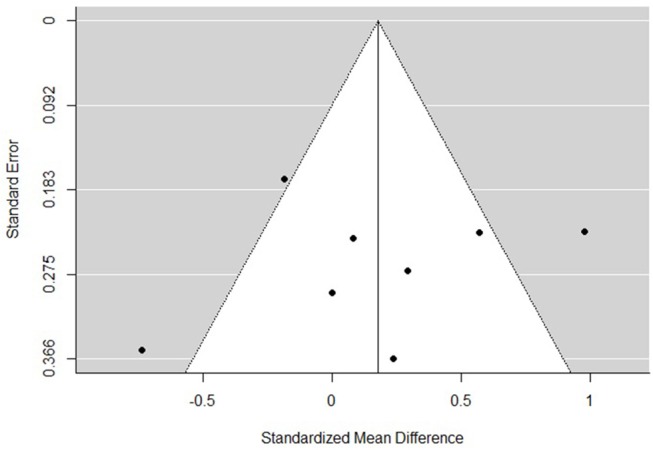
Funnel plot of studies included in the total affective meta-analysis.
Figure 7.
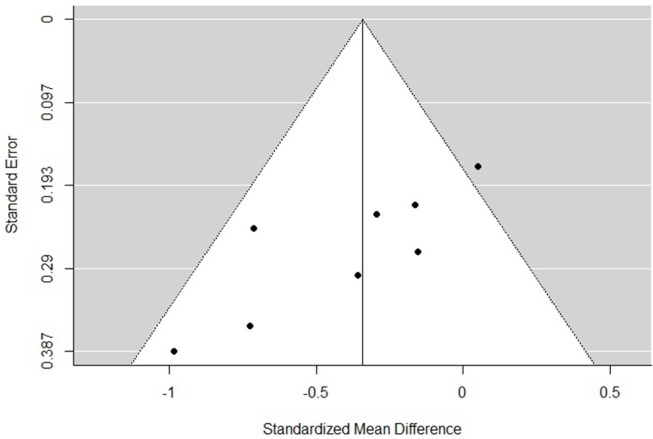
Funnel plot of studies included in the cognitive empathy meta-analysis.
Additional Analyses
Because several studies reported on the PT, FS, EC, and PD subscales of the IRI, additional meta-analyses were performed to test for differences between AN and HC. Six studies reported scores for all four subscales, while one additional study reported PT scores only. The results are shown in Table 2. AN had significantly lower FS scores compared to HC, however there were no significant differences in the other sub-scales. There was no evidence of significant heterogeneity in any of the subscale meta-analyses, nor was there significant evidence of publication bias (Begg's test all p > 0.05) (see Supplementary Material for subscale forest and funnel plots).
Table 2.
Statistical outcomes for meta-analyses of the four IRI subscales.
| IRI subscale | N studies | Pooled AN sample N | Pooled HC sample N | Cohen's d | 95% CI | Z | p |
|---|---|---|---|---|---|---|---|
| Perspective taking | 7 | 204 | 523 | −0.2 | −0.44, 0.05 | −1.59 | 0.11 |
| Fantasy | 6 | 166 | 202 | −0.41 | −0.62, −0.20 | 3.83 | >0.001 |
| Empathic concern | 6 | 166 | 202 | 0.01 | −0.20, 0.22 | 1.1 | 0.92 |
| Personal distress | 6 | 166 | 202 | 0.3 | −0.13, 0.74 | 1.36 | 0.17 |
Significant differences between AN and HCs are indicated in bold. AN, anorexia nervosa; CI, confidence intervals; HC, healthy control; IRI, Interpersonal Reactivity Index.
Qualitative Findings
Studies in AN
Studies using the EQ or the EQ-short reported very mixed findings. Adenzato et al. (45) found that those with AN had significantly lower total EQ scores compared to HCs. In adolescents, this was only found to be true for those aged 12–15years, using the parent report version of the EQ (47). The older AN group did not differ from age-matched HC on the self-report EQ. Redondo and Herrero-Fernández (61) found that while total EQ-short scores in those with AN and HCs did not differ, those with AN scored significantly lower than HCs on the social skills subscale. Three studies found no differences in EQ scores between AN and HC, however both groups scored significantly higher than those with ASD (50, 55, 56).
Results from studies using the IRI were similarly mixed. Only two studies tested for group differences in total IRI scores, with one reporting significantly lower scores in those with AN than HCs (56) and the other reporting no differences (57). Two studies tested for group differences in cognitive and affective empathy sub-scores of the IRI. Cognitive empathy scores are calculated by summing the F and PT subscale scores together, while the EC and PD subscale scores are summed to calculate affective empathy scores. Calderoni et al. (49) found that those with AN had significantly lower cognitive empathy scores, whereas Peres et al. (60) reported significantly higher emotional empathy scores in AN compared to HC.
Six studies reported on group differences between AN and HCs on IRI EC, PD, FS, and PT (with one additional study included the PT subscale only). Regarding EC, there were no significant differences between AN and HC across all six studies (49, 50, 53, 54, 56, 60). However, those with AN had significantly higher EC scores compared to those with ASD (50), and significantly lower scores than women with BPD (54). Two studies found that those with AN scored higher on PD than HC (53, 60), while one reported that AN and ASD groups had lower scores than HCs (56). Three studies reported no differences in PD scores between AN and HC, however those with BPD had higher scores than both AN and HC groups (49, 50, 54). Regarding the FS subscale, it was found that those with AN had significantly lower scores than HC, similar to those with ASD (49, 50). However, four studies did not find significant differences between groups (53, 54, 56, 60). Calderoni et al. (49) and Redondo and Herrero-Fernandez (61) reported that AN had significantly lower PT scores compared to HCs, however the remaining five studies found no significant differences (50, 53, 54, 56, 60).
The remaining AN studies used the I7, the empathy subscale of the SEQ, and the BES. Morris et al. (58) found that AN scored significantly lower on the SEQ than HC. Scores in the recovered AN group did not differ from either group, lying between the two. The remaining two studies found no significant differences between AN and HCs (48, 59). However, both studies were limited in their sample sizes (15 and 23 participants in the clinical groups respectively), and therefore there may not be sufficient power to detect group differences.
Studies in Other EDs
Only three studies involved participants with BED or BN. Feldman and Eysenck (52) reported no differences in empathy scores between women with BN and HCs. However, this study had the poorest methodological quality rating of all studies include in the review, mainly because it included little information about the HC group, and did not control for any confounding variables. In BED, total empathy scores did not significantly differ across those with BED, subthreshold BED, and HCs (46). However, 51 reported that women with BED scored significantly higher than obese and HC women on the PD subscale of the IRI. Further, a logistic regression revealed that lower PT and higher PD scores were associated with BED. Unfortunately, this study did not control for confounding variables such as depression, which has been found to be associated with PD (9).
Associations With Psychopathology and Clinical Variables
Few studies examined associations between empathy and clinical variables or other measures of psychopathology. In BED and AN, negative correlations were found between EQ and alexithymia scores on the twenty-item Toronto Alexithymia Scale [TAS-20; (67)], such that lower levels of empathy were associated with higher alexithymia (45, 46). The latter study also found that higher EQ scores were associated with more social support in AN, as measured by the Multidimensional Scale of Perceived Social Support [MSPSS; (68)]. Only two studies examined whether empathy was associated with ED psychopathology and illness severity in AN. Baron-Cohen et al. (47) reported that EQ scores were not associated with scores on the Eating Disorder Examination Questionnaire [EDEQ; (69)], and Calderoni et al. (49) found that cognitive empathy scores were not associated with BMI, disease duration, or general psychopathology in AN. Finally, Peres et al. (60) reported that IRI, AE and PD subscale scores were positively associated with anxiety, but not depression, as measured by the Hospital Anxiety and Depression Scale [HADS; (70)]. However, linear regressions revealed that anxiety did not explain the differences in empathy between AN and HC better than group membership.
Discussion
Summary of Main Findings
The aim of this review was to examine group differences in empathy in those with EDs compared to HC, and provide a qualitative synthesis of the literature. Meta-analyses were run for total empathy, cognitive empathy, affective empathy, and four further sub-components of empathy: PT, FS, EC, and PD. There were no significant differences between those with AN and HC in overall empathy (14 studies) or affective empathy scores (8 studies). However, it was found that those with AN had significantly lower cognitive empathy scores compared to HC (8 studies), with a small effect size. Further, it those with AN had significantly lower FS scores than HC (6 studies), with a small effect size, but did not significantly differ from HC on any of the other IRI subscores.
The finding that AN have lower cognitive empathy abilities compared to HC is in accordance with studies examining related, performance-based measures of empathy, such as ToM (32), emotion recognition (31), and emotional intelligence (71). Affective empathy has been less well-studied in EDs, although it appears from this review, and a few experimental studies, that individuals with ED are not impaired in affective empathy. For example, one study found that those with BN reported higher levels of sadness than restrained eaters and HCs in response to video clip, during which they were asked to identify themselves with the protagonist whose boyfriend leaves them for an attractive woman (72). Another study examined individuals' own emotional reactions to video clips depicting an individual displaying emotion, finding that the intensity of the emotions experienced by those with EDs (AN and BN) did not differ from HC (73). However, those with EDs did show less facial expressivity while watching the clips—a component of empathy that has been termed “motor empathy” (74). Studies that utilize physiological measurements of empathy, such as facial electromyographic activity (EMG), skin conductance, and heart rate may be useful in further understanding affective empathy in EDs.
There are a number of possible explanations for the dissociation between cognitive and affective empathic abilities found here. Distinct brain systems for cognitive and affective empathy have been described: the ventromedial prefrontal cortex is involved in cognitive empathy, while the inferior frontal gyrus is involved in affective empathy (75). Neuroimaging studies have reported differences in the ventromedial prefrontal cortex in those with AN (76, 77), thus providing a possible explanation for lowered cognitive empathy abilities. fMRI studies utilizing performance-based measures of empathy could be useful in testing this hypothesis. Relatedly, difficulties in executive functioning are reported in those with AN and BN (78). Since executive functions contribute to the development of cognitive empathy (79), it would be of interest to determine whether there is a relation between empathy abilities and executive functioning in those with EDs. Relatedly, it might be that reduced attention to faces and eyes found in AN (28, 80, 81) leads to decreased cognitive empathy abilities.
There was evidence of significant heterogeneity in the overall empathy and affective empathy studies. While age and empathy measurement did explain some of the variance in total empathy scores, no single factor had a significant influence on the size of the effect. Due to a lack of studies reporting on factors such as BMI and illness duration, it was not possible to include these indicators of illness severity as moderators. The two studies that did examine potential associations between ED severity and empathy did not find any significant relationships (47, 49). Research examining the relationship between illness severity and constructs related to empathy such as mentalizing (the ability to understand the mental states of oneself or others, and how such states might influence behavior) have been mixed. While some have reported independence from BMI and illness length (82), a meta-analysis found that poorer performance on the RMET was associated with longer illness duration (83). Examining whether cognitive or affective empathy are state or trait variables will be important in characterizing the socio-emotional phenotype proposed for EDs (84).
Relatedly, it would be of interest to examine whether other psychopathological variables may have influenced the effect sizes reported in this review. One candidate is ASD symptoms. Support for this idea comes from a longitudinal population-based study which examined mentalizing abilities in those with AN and HCs (21), in which 29% of the AN group also met criteria for a diagnosis of ASD. They found that when mentalizing ability was compared between AN+ASD, AN only, and HCs, only the AN+ASD group had significantly lower scores than HC. Thus, it is possible that there is a sub-group of individuals with AN who display the most severe difficulties in socio-emotional measures, whose difficulties are missed when assessing group differences. While ASD symptoms could not be included as moderators in the meta-analyses presented here, it would be important to ascertain whether reduced empathy in AN is a characteristic of the ED, or some other comorbid psychopathology.
Alternatively, it could be the case that the heterogenous results in AN might be explained by alexithymia. Indeed, a few studies included in this review found that lower levels of empathy in AN and BED were associated with higher alexithymia (45, 46). Alexithymia is a subclinical phenomenon characterized by difficulties in describing and recognizing one's own emotions, and distinguishing feelings from bodily sensations of emotional arousal. “Shared network” models of empathy propose that the networks in the brain responsible for processing one's own emotions are the same networks used to represent the emotions of others (85–87). Thus, it is possible that the high levels of alexithymia experienced by those with AN might be responsible for lower levels of empathy compared to HCs. In support of this hypothesis, an fMRI study in ASD showed that the strength of empathic brain responses in the left anterior insula were predictive of degree of alexithymia in both ASD and HCs, but did not vary as a function of group (88). The potential contribution of alexithymia to reduced empathy, and indeed other aspects of socio-emotional functioning in EDs, should be explored.
Only two studies examined empathy in BED, finding no difference in total empathy scores, but significantly higher PD scores compared to HCs (46, 51). The finding that those with BED experience more stress and unease in tense social settings is consistent with literature documenting emotion regulation difficulties in those with BED, and it is hypothesized that binge eating may be a strategy to deal with increased negative emotions (89). It would therefore be of interest to examine whether higher PD scores in BED are associated with more severe ED psychopathology. The only study that measured empathy in BN found no significant differences in empathy compared to HCs (52). This study used the I7 to measure empathy, and therefore no study has yet examined cognitive and affective components of empathy in BN. Clearly, the lack of studies in BN and BED prevent any conclusions being made regarding empathy in these groups. Given that problems with interpersonal functioning are a prominent feature in BN (18, 90), research using multidimensional measures of empathy in this population are needed.
The findings from the current review have implications for treatment of AN. Socio-communicative and interpersonal problems are associated with poorer outcomes (20, 21, 82, 91, 92) and more severe ED psychopathology (36, 37), therefore socio-emotional functioning may be a potential target for the development of new, more holistic treatment approaches. For example, group social skills interventions are effective in improving communication, social anxiety, and social functioning in those with ASD (93, 94). There is also evidence to suggest that Cognitive Remediation and Emotion Skills Training (CREST), an intervention designed to improve emotion processing, is effective in decreasing alexithymia and social anhedonia, while increasing motivation in those with AN (95, 96). Recently, there has also been interest in exploring the effect of oxytocin, a hormone implicated in prosocial behavior, on socio-emotional functioning (97, 98). In ASD, administration of intranasal oxytocin has been found to increase interactions with socially cooperative peers, and enhance feelings of trust (99). Oxytocin also increased participants' attention to the eyes of pictures of faces, avoidance of which is a core feature of ASD (100). A few studies have examined the effect of oxytocin on socio-emotional cognition in those with EDs. One study found intranasal oxytocin increased emotion recognition and decreased calorie consumption in those with BN, however no effects were seen in AN (101). Another found no effect of oxytocin on RMET performance in AN (102). However, whether oxytocin has an effect on real-life social behavior in those with EDs has yet to be examined.
Limitations
Several limitations of this review should be noted. Firstly, many studies did not report empathy subscale scores, and therefore could not be included in affective and cognitive empathy meta-analyses. Secondly, although this method has been employed in previous reviews of this type (103, 104), it could be questioned whether it is appropriate to compare different scales that purport to measure the same empathy constructs. For example, the affective subscales of the IRI have been criticized as more closely reflecting sympathy, as they focus on reactions to others, rather than emotion matching (105). However, studies in this review generally included the most widely used measures of empathy (e.g., the EQ and the IRI), and as previously noted, empathy measure did not significantly influence effect sizes in moderator analyses.
It is also important to note the limitations of self-report empathy measures generally. Socially desirable responding may be an issue with self-report measures, as they do not objectively measure empathic abilities, but rather how empathetic individuals perceive themselves to be. In other psychiatric disorders, a discrepancy between performance-based empathy tasks and self-report measures has been reported. For example, a meta-analysis found that people with schizophrenia display greater affective empathy deficits in performance-based tasks than on self-report measures (103). If affective empathy partly relies on one's ability to report on their own emotional reactions, this might be especially difficult in populations with high levels of alexithymia, such as AN (106).
The number of studies in other EDs, such as BN and BED, was greatly lacking. Therefore, meta-analyses for group differences between these groups and HCs could not be carried out. Furthermore, only three studies included males with EDs, thus the results from this review cannot be generalized to this population. Interestingly, it is reported that while males with EDs (AN, BN, or eating disorder not otherwise specified) show the same difficulties in cognitive flexibility and weak central coherence often found in women with EDs, they do not differ from HC men in terms of ToM performance or sensitivity to social threat (107). Future work should therefore examine performance in a broader range of socio-emotional tasks in order to understand possible similarities and differences in the male and female presentations of EDs.
Finally, there was evidence of publication bias in the cognitive empathy meta-analysis, indicating that studies with non-significant results may have been missing from analyses. However, the fact that the affective empathy meta-analysis, which included the same studies as the cognitive meta-analysis, did not show any evidence of publication bias and showed a non-significant result, perhaps lends support to the validity of our findings. Nonetheless, the results should be interpreted with caution.
Conclusions
Although there is an extensive literature documenting difficulties in ToM and emotion recognition in those with EDs, relatively little is known about empathic abilities in this population. This systematic review and meta-analysis aimed to examine whether those with EDs differed from HCs on several dimensions of self-reported empathy, and provide a qualitative synthesis of the literature. While those with AN did not differ from HCs in overall empathy, a meta-analysis of 8 studies found that AN had significantly lower levels of cognitive empathy compared to HC, with a small effect size. It was also found that AN had significantly lower levels of fantasy, a subdivision of cognitive empathy. AN did not differ from HC in affective empathy. This profile of intact affective empathy and lowered cognitive empathy mirrors that of those with ASD, a disorder that shares a number of neuropsychological and socio-cognitive traits with AN. Conclusions regarding the empathic profiles of those with other EDs are not possible, given the lack of studies in these groups. Future research should investigate empathic abilities in other EDs, and examine the influence of comorbid psychopathological traits.
Author Contributions
JK-G performed the search, data extraction, and wrote the manuscript. KT leads the research group within which this work was conducted and is JK-G lead supervisor for Ph.D. KT and AH edited the manuscript before submission.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
JK-G is supported by the Economic and Social Research Council (ESRC). KT would like to acknowledge support from MRC and MRF Child and Young Adult Mental Health (Grant no: MR/R004595/1) and Prof Tracey Wade and Norman Munn Distinguished Visiting Scholar Award 2017/2018 from Flinders University South Australia. AH would like to acknowledge support from the MRC.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2019.00102/full#supplementary-material
References
- 1.Singer T. The neuronal basis and ontogeny of empathy and mind reading: Review of literature and implications for future research. Neurosci Biobehav Rev. (2006) 30:855–63. 10.1016/j.neubiorev.2006.06.011 [DOI] [PubMed] [Google Scholar]
- 2.Decety J, Bartal IBA, Uzefovsky F, Knafo-Noam A. Empathy as a driver of prosocial behaviour: highly conserved neurobehavioural mechanisms across species. Philos Transact R Soc B Biol Sci. (2016) 371:20150077. 10.1098/rstb.2015.0077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warrier V, Toro R, Chakrabarti B, Børglum AD, Grove J, Agee M, et al. Genome-wide analyses of self-reported empathy: correlations with autism, schizophrenia, and anorexia nervosa. Transl Psychiatry. (2018) 8:35. 10.1038/s41398-017-0082-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonfils KA, Lysaker PH, Minor KS, Salyers MP. Empathy in schizophrenia: a meta-analysis of the interpersonal reactivity index. Psychiatry Res. (2017) 249:293–303. 10.1016/j.psychres.2016.12.033 [DOI] [PubMed] [Google Scholar]
- 5.Lee J, Zaki J, Harvey P-O, Ochsner K, Green MF. Schizophrenia patients are impaired in empathic accuracy. Psychol Med. (2011) 41:2297–304. 10.1017/S0033291711000614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baron-Cohen S, Wheelwright S. The empathy quotient: an investigation of adults with asperger syndrome or high functioning autism, and normal sex differences. J Autism Dev Disord. (2004) 34:163–75. 10.1023/B:JADD.0000022607.19833.00 [DOI] [PubMed] [Google Scholar]
- 7.Kok FM, Groen Y, Becke M, Fuermaier ABM, Tucha O. Self-reported empathy in adult women with autism spectrum disorders – a systematic mini review. PLoS ONE. (2016) 11:e0151568. 10.1371/journal.pone.0151568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dziobek I, Preißler S, Grozdanovic Z, Heuser I, Heekeren HR, Roepke S. Neuronal correlates of altered empathy and social cognition in borderline personality disorder. Neuroimage. (2011) 57:539–48. 10.1016/j.neuroimage.2011.05.005 [DOI] [PubMed] [Google Scholar]
- 9.Schreiter S, Pijnenborg GHM, Aan Het Rot M. Empathy in adults with clinical or subclinical depressive symptoms. J Affect Disord. (2013) 150:1–16. 10.1016/J.JAD.2013.03.009 [DOI] [PubMed] [Google Scholar]
- 10.Dziobek I, Rogers K, Fleck S, Bahnemann M, Heekeren HR, Wolf OT, et al. Dissociation of cognitive and emotional empathy in adults with Asperger syndrome using the Multifaceted Empathy Test (MET). J Autism Dev Disord. (2008) 38:464–73. 10.1007/s10803-007-0486-x [DOI] [PubMed] [Google Scholar]
- 11.Chita-Tegmark M. Social attention in ASD: a review and meta-analysis of eye-tracking studies. Res Dev Disabil. (2016) 48:79–93. 10.1016/j.ridd.2015.10.011 [DOI] [PubMed] [Google Scholar]
- 12.Corden B, Chilvers R, Skuse D. Avoidance of emotionally arousing stimuli predicts social–perceptual impairment in Asperger's syndrome. Neuropsychologia. (2008) 46:137–47. 10.1016/j.neuropsychologia.2007.08.005 [DOI] [PubMed] [Google Scholar]
- 13.Falkmer M, Bjällmark A, Larsson M, Falkmer T. Recognition of facially expressed emotions and visual search strategies in adults with Asperger syndrome. Res Autism Spectr Disord. (2011) 5:210–7. 10.1016/j.rasd.2010.03.013 [DOI] [Google Scholar]
- 14.Müller N, Baumeister S, Dziobek I, Banaschewski T, Poustka L. Validation of the movie for the assessment of social cognition in adolescents with ASD: Fixation duration and pupil dilation as predictors of performance. J Autism Develop Dis. (2016) 46:2831–44. 10.1007/s10803-016-2828-z [DOI] [PubMed] [Google Scholar]
- 15.Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Arch Gen Psychiatry. (2002) 59:809. 10.1001/archpsyc.59.9.809 [DOI] [PubMed] [Google Scholar]
- 16.Harari H, Shamay-Tsoory SG, Ravid M, Levkovitz Y. Double dissociation between cognitive and affective empathy in borderline personality disorder. Psychiatry Res. (2010) 175:277–9. 10.1016/j.psychres.2009.03.002 [DOI] [PubMed] [Google Scholar]
- 17.Bodnar A, Rybakowski JK. Mentalization deficit in bipolar patients during an acute depressive and manic episode: association with cognitive functions. Int J Bipolar Disord. (2017) 5:38. 10.1186/s40345-017-0107-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arcelus J, Haslam M, Farrow C, Meyer C. The role of interpersonal functioning in the maintenance of eating psychopathology: A systematic review and testable model. Clin Psychol Rev. (2013) 33:156–67. 10.1016/j.cpr.2012.10.009 [DOI] [PubMed] [Google Scholar]
- 19.Cardi V, Tchanturia K, Treasure J. Premorbid and illness-related social difficulties in eating disorders: an overview of the literature and treatment developments. Curr Neuropharmacol. (2018) 16:1122–30. 10.2174/1570159X16666180118100028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gillberg IC, Råstam M, Gillberg C. Anorexia nervosa outcome: six-year controlled longitudinal study of 51 cases including a population cohort. J Am Acad Child Adolesc Psychiatry. (1994) 33:729–39. 10.1097/00004583-199406000-00014 [DOI] [PubMed] [Google Scholar]
- 21.Anckarsäter H, Hofvander B, Billstedt E, Gillberg IC, Gillberg C, Wentz E, et al. The sociocommunicative deficit subgroup in anorexia nervosa: autism spectrum disorders and neurocognition in a community-based, longitudinal study. Psychol Med. (2012) 42:1957–67. 10.1017/S0033291711002881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wentz E, Gillberg IC, Anckarsäter H, Gillberg C, Råstam M. Adolescent-onset anorexia nervosa: 18-year outcome. Br J Psychiatry. (2009) 194:168–74. 10.1192/bjp.bp.107.048686 [DOI] [PubMed] [Google Scholar]
- 23.Kerr-gaffney J, Harrison A, Tchanturia K. Social anxiety in the eating disorders: a systematic review and meta-analysis. Psychol Med. (2018) 48:2477–91. 10.1017/S0033291718000752 [DOI] [PubMed] [Google Scholar]
- 24.Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry. (2008) 47:921–9. 10.1097/CHI.0B013E318179964F [DOI] [PubMed] [Google Scholar]
- 25.Bird G, Cook R. Mixed emotions: The contribution of alexithymia to the emotional symptoms of autism. Transl Psychiatry. (2013) 3:e285. 10.1038/tp.2013.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Westwood H, Kerr-Gaffney J, Stahl D, Tchanturia K. Alexithymia in eating disorders: systematic review and meta-analyses of studies using the toronto alexithymia scale. J Psychosom Res. (2017) 99:66–81. 10.1016/j.jpsychores.2017.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrison A, Sullivan S, Tchanturia K, Treasure J. Emotional functioning in eating disorders: attentional bias, emotion recognition and emotion regulation. Psychol Med. (2010) 40:1887–97. 10.1017/S0033291710000036 [DOI] [PubMed] [Google Scholar]
- 28.Watson KK, Werling DM, Zucker NL, Platt ML. Altered social reward and attention in anorexia nervosa. Front Psychol. (2010) 1:36. 10.3389/fpsyg.2010.00036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bal E, Harden E, Lamb D, Van Hecke AV, Denver JW, Porges SW. Emotion recognition in children with autism spectrum disorders: relations to eye gaze and autonomic state. J Autism Dev Disord. (2010) 40:358–70. 10.1007/s10803-009-0884-3 [DOI] [PubMed] [Google Scholar]
- 30.Oldershaw A, Hambrook D, Tchanturia K, Treasure J, Schmidt U. Emotional theory of mind and emotional awareness in recovered anorexia nervosa patients. Psychosom Med. (2010) 72:73–9. 10.1097/PSY.0b013e3181c6c7ca [DOI] [PubMed] [Google Scholar]
- 31.Caglar-Nazali HP, Corfield F, Cardi V, Ambwani S, Leppanen J, Olabintan O, et al. A systematic review and meta-analysis of “systems for social processes” in eating disorders. Neurosci Biobehav Rev. (2014) 42:55–92. 10.1016/j.neubiorev.2013.12.002 [DOI] [PubMed] [Google Scholar]
- 32.Leppanen J, Sedgewick F, Treasure JL, Tchanturia K. Differences in the Theory of Mind profiles of patients with anorexia nervosa and individuals on the autism spectrum : a meta-analytic review. Neurosci Biobehav Rev. (2018) 90:146–63. 10.1016/j.neubiorev.2018.04.009 [DOI] [PubMed] [Google Scholar]
- 33.Tiller JM, Sloane G, Schmidt U, Troop N, Power M, Treasure JL. Social support in patients with anorexia nervosa and bulimia nervosa. Int J Eat Disord. (1997) 21:31–8. [DOI] [PubMed] [Google Scholar]
- 34.Westwood H, Lawrence V, Fleming C, Tchanturia K. Exploration of friendship experiences, before and after illness onset in females with anorexia nervosa: a qualitative study. PLoS ONE. (2016) 11:e0163528. 10.1371/journal.pone.0163528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doris E, Westwood H, Mandy W, Tchanturia K. A qualitative study of friendship in patients with anorexia nervosa and possible autism spectrum disorder. Psychology. (2014) 5:13381349 10.4236/psych.2014.511144 [DOI] [Google Scholar]
- 36.Illing V, Tasca GA, Balfour L, Bissada H. Attachment insecurity predicts eating disorder symptoms and treatment outcomes in a clinical sample of women. J Nerv Ment Dis. (2010) 198:653–9. 10.1097/NMD.0b013e3181ef34b2 [DOI] [PubMed] [Google Scholar]
- 37.Tasca GA, Presniak MD, Demidenko N, Balfour L, Krysanski V, Trinneer A, et al. Testing a maintenance model for eating disorders in a sample seeking treatment at a tertiary care center: a structural equation modeling approach. Compr. Psychiatry. (2011) 52:678–87. 10.1016/J.COMPPSYCH.2010.12.010 [DOI] [PubMed] [Google Scholar]
- 38.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. (2009) 6:e1000100. 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.R Core Team R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna. (2017). Available online at: https://www.R-project.org/
- 40.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. (2010) 36:3 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 41.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. London: Laurence Erlbaum Associates; (1988). [Google Scholar]
- 42.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. [PubMed] [Google Scholar]
- 43.Rosenthal R. The “file drawer problem” and tolerance for null results. Psychol Bull. (1979) 86:638–41. 10.1037/0033-2909.86.3.638 [DOI] [Google Scholar]
- 44.CASP Programme. CASP Case Control Study Checklist. (2013). Available online at: https://casp-uk.net/casp-tools-checklists/
- 45.Adenzato M, Todisco P, Ardito RB. Social cognition in anorexia nervosa: evidence of preserved theory of mind and impaired emotional functioning. PLoS ONE. (2012) 7:3–10. 10.1371/journal.pone.0044414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aloi M, Rania M, Caroleo M, De Fazio P, Segura-Garcia C. Social cognition and emotional functioning in patients with binge eating disorder. Eur Eat Disord Rev. (2017) 25:172–8. 10.1002/erv.2504 [DOI] [PubMed] [Google Scholar]
- 47.Baron-Cohen S, Jaffa T, Davies S, Auyeung B, Allison C, Wheelwright S. Do girls with anorexia nervosa have elevated autistic traits? Mol. Autism. (2013) 4:24. 10.1186/2040-2392-4-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Butler GKL, Montgomery AMJ. Subjective self-control and behavioural impulsivity coexist in anorexia nervosa. Eat Behav. (2005) 6:221–7. 10.1016/j.eatbeh.2004.11.002 [DOI] [PubMed] [Google Scholar]
- 49.Calderoni S, Fantozzi P, Maestro S, Brunori E, Narzisi A, Balboni G, et al. Selective cognitive empathy deficit in adolescents with restrictive anorexia nervosa. Neuropsychiatr Dis Treat. (2013) 9:1583–9. 10.2147/NDT.S50214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Courty A, Maria AS, Lalanne C, Ringuenet D, Vindreau C, Chevallier C, et al. Levels of autistic traits in anorexia nervosa: a comparative psychometric study. BMC Psychiatry. (2013) 13:222. 10.1186/1471-244X-13-222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duchesne M, De Oliveira Falcone EM, De Freitas SR, D'Augustin JF, Marinho V, Appolinario JC. Assessment of interpersonal skills in obese women with binge eating disorder. J Health Psychol. (2011) 17:1065–75. 10.1177/1359105311432326 [DOI] [PubMed] [Google Scholar]
- 52.Feldman J, Eysenck S. Addictive personality traits in bulimic patients. Pers Individ Dif. (1986) 7:923–6. 10.1016/0191-8869(86)90097-8 [DOI] [Google Scholar]
- 53.Gramaglia C, Ressico F, Gambaro E, Palazzolo A, Mazzarino M, Bert F, et al. Alexithymia, empathy, emotion identification and social inference in anorexia nervosa: a case-control study. Eat Behav. (2016) 22:46–50. 10.1016/j.eatbeh.2016.03.028 [DOI] [PubMed] [Google Scholar]
- 54.Guttman HA, Laporte L. Empathy in families of women with borderline personality disorder, anorexia nervosa, and a control group. Fam. Process. (2000) 39:345–58. 10.1111/j.1545-5300.2000.39306.x [DOI] [PubMed] [Google Scholar]
- 55.Hambrook D, Tchanturia K, Schmidt U, Russell T, Treasure J. Empathy, systemizing, and autistic traits in anorexia nervosa: a pilot study. Br J Clin Psychol. (2008) 47:335–9. 10.1348/014466507X272475 [DOI] [PubMed] [Google Scholar]
- 56.Jermakow N, Brzezicka A. How autistic are anorectic females? Similarities and differences between anorexia nervosa and autism spectrum disorders. Clin Neuropsychiatry. (2016) 13:53–8. [Google Scholar]
- 57.Lulé D, Schulze UME, Bauer K, Schöll F, Müller S, Fladung AK, et al. Anorexia nervosa and its relation to depression, anxiety, alexithymia and emotional processing deficits. Eat Weight Disord. (2014) 19:209–16. 10.1007/s40519-014-0101-z [DOI] [PubMed] [Google Scholar]
- 58.Morris R, Bramham J, Smith E, Tchanturia K. Empathy and social functioning in anorexia nervosa before and after recovery. Cogn. Neuropsychiatry. (2014) 19:47–57. 10.1080/13546805.2013.794723 [DOI] [PubMed] [Google Scholar]
- 59.Nandrino JL, Ducro C, Iachini T, Coello Y. Perception of peripersonal and interpersonal space in patients with restrictive-type anorexia. Eur Eat Disord Rev. (2017) 25:179–87. 10.1002/erv.2506 [DOI] [PubMed] [Google Scholar]
- 60.Peres V, Corcos M, Robin M, Pham-Scottez A. Emotional intelligence, empathy and alexithymia in anorexia nervosa during adolescence. Eat Weight Disord. (2018) [Epub ahead of print]. 10.1007/s40519-018-0482-5 [DOI] [PubMed] [Google Scholar]
- 61.Redondo I, Herrero-Fernández D. Validation of the reading the mind in the eyes test in a healthy spanish sample and women with anorexia nervosa. Cogn Neuropsychiatry. (2018) 23:201–17. 10.1080/13546805.2018.1461618 [DOI] [PubMed] [Google Scholar]
- 62.Davis MH. Measuring individual differences in empathy: evidence for a multidimensional approach. J Pers Soc Psychol. (1983) 44:113–26. 10.1037/0022-3514.44.1.113 [DOI] [Google Scholar]
- 63.Wakabayashi A, Baron-Cohen S, Wheelwright S, Goldenfeld N, Delaney J, Fine D, et al. Development of short forms of the Empathy Quotient (EQ-Short) and the Systemizing Quotient (SQ-Short). Pers Individ Dif. (2006) 41:929–40. 10.1016/J.PAID.2006.03.017 [DOI] [Google Scholar]
- 64.Eysenck SBG, Pearson PR, Easting G, Allsopp JF. Age norms for impulsiveness, venturesomeness and empathy in adults. Pers Individ Dif. (1985) 6:613–9. 10.1016/0191-8869(85)90011-X [DOI] [Google Scholar]
- 65.Bramham J, Morris RG, Hornak J, Bullock P, Polkey CE. Social and emotional functioning following bilateral and unilateral neurosurgical prefrontal cortex lesions. J Neuropsychol. (2009) 3:125–43. 10.1348/174866408X293994 [DOI] [PubMed] [Google Scholar]
- 66.Jolliffe D, Farrington DP. Development and validation of the basic empathy scale. J Adolesc. (2006) 29:589–611. 10.1016/j.adolescence.2005.08.010 [DOI] [PubMed] [Google Scholar]
- 67.Bagby RM, Parker JDA, Taylor GJ. The twenty-item toronto alexithymia scale—i. item selection and cross-validation of the factor structure. J Psychosom Res. (1994) 38:23–32. 10.1016/0022-3999(94)90005-1 [DOI] [PubMed] [Google Scholar]
- 68.Dahlem NW, Zimet GD, Walker RR. The multidimensional scale of perceived social support: a confirmation study. J Clin Psychol. (1991) 47:756–61. [DOI] [PubMed] [Google Scholar]
- 69.Fairburn CG, Beglin SJ. Assessment of eating disorders: interview or self-report questionnaire? Int J Eat Disord. (1994) 16:363–70. [DOI] [PubMed] [Google Scholar]
- 70.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr. Scand. (1983) 67:361–70. 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 71.Hambrook D, Brown G, Tchanturia K. Emotional intelligence in anorexia nervosa: Is anxiety a missing piece of the puzzle? Psychiatry Res. (2012) 200:12–9. 10.1016/j.psychres.2012.05.017 [DOI] [PubMed] [Google Scholar]
- 72.Tuschen-Caffier B, Vögele C. Psychological and physiological reactivity to stress: an experimental study on bulimic patients, restrained eaters and controls. Psychother Psychosom. (1999) 68:333–40. 10.1159/000012352 [DOI] [PubMed] [Google Scholar]
- 73.Cardi V, Corfield F, Leppanen J, Rhind C, Deriziotis S, Hadjimichalis A, et al. Emotional processing, recognition, empathy and evoked facial expression in eating disorders: an experimental study to map deficits in social cognition. PLoS ONE. (2015) 10:e0133827. 10.1371/journal.pone.0133827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Blair RJR. Responding to the emotions of others: dissociating forms of empathy through the study of typical and psychiatric populations. Conscious Cogn. (2005) 14:698–718. 10.1016/j.concog.2005.06.004 [DOI] [PubMed] [Google Scholar]
- 75.Decety J, Meyer M. From emotion resonance to empathic understanding: a social developmental neuroscience account. Dev Psychopathol. (2008) 20:1053–80. 10.1017/S0954579408000503 [DOI] [PubMed] [Google Scholar]
- 76.Uher R, Brammer MJ, Murphy T, Campbell IC, Ng VW, Williams SC, et al. Recovery and chronicity in anorexia nervosa: brain activity associated with differential outcomes. Biol. Psychiatry. (2003) 54:934–42. 10.1016/S0006-3223(03)00172-0 [DOI] [PubMed] [Google Scholar]
- 77.Uher R, Murphy T, Brammer MJ, Dalgleish T, Phillips ML, Andrew CM, et al. Medial prefrontal cortex activity associated with symptom provocation in eating disorders. Am J Psychiatry. (2004) 161:1238–46. 10.1176/appi.ajp.161.7.1238 [DOI] [PubMed] [Google Scholar]
- 78.Hirst RB, Beard CL, Colby KA, Quittner Z, Mills BM, Lavender JS. Anorexia nervosa and bulimia nervosa: a meta-analysis of executive functioning. Neurosci Biobehav Rev. (2017) 83:678–90. 10.1016/j.neubiorev.2017.08.011 [DOI] [PubMed] [Google Scholar]
- 79.Decety J, Jackson PL. The functional architecture of human empathy. Behav Cogn Neurosci Rev. (2004) 3:71–100. 10.1177/1534582304267187 [DOI] [PubMed] [Google Scholar]
- 80.Fujiwara E, Kube VL, Rochman D, Macrae-Korobkov AK, Peynenburg V. Visual attention to ambiguous emotional faces in eating disorders: role of alexithymia. Eur Eat Disord Rev. (2017) 25:451–60. 10.1002/erv.2535 [DOI] [PubMed] [Google Scholar]
- 81.Harrison A, Watterson SV, Bennett SD. An experimental investigation into the use of eye-contact in social interactions in women in the acute and recovered stages of anorexia nervosa. Int J Eat Disord. (2018)52: 61–70. 10.1002/eat.22993 [DOI] [PubMed] [Google Scholar]
- 82.Gillberg IC, Billstedt E, Wentz E, Anckarsäter H, Råstam M, Gillberg C. Attention, executive functions, and mentalizing in anorexia nervosa eighteen years after onset of eating disorder. J Clin Exp Neuropsychol. (2010) 32:358–65. 10.1080/13803390903066857 [DOI] [PubMed] [Google Scholar]
- 83.Bora E, Kose S. Meta-analysis of theory of mind in anorexia nervosa and bulimia nervosa: a specific impairment of cognitive perspective taking in anorexia nervosa? Int J Eat Disord. (2016) 49:739–40. 10.1002/eat.22572 [DOI] [PubMed] [Google Scholar]
- 84.Treasure J, Schmidt U. The cognitive-interpersonal maintenance model of anorexia nervosa revisited: a summary of the evidence for cognitive, socio-emotional and interpersonal predisposing and perpetuating factors. J Eat Disord. (2013) 1:13. 10.1186/2050-2974-1-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Carr L, Iacoboni M, Dubeau M, Mazziotta JC, Lenzi GL. Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. PNAS. (2003) 100:5497–502. 10.1073/pnas.0935845100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jackson PL, Brunet E, Meltzoff AN, Decety J. Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain. Neuropsychologia. (2006) 44:752–61. 10.1016/j.neuropsychologia.2005.07.015 [DOI] [PubMed] [Google Scholar]
- 87.Jackson PL, Meltzoff AN, Decety J. How do we perceive the pain of others? A window into the neural processes involved in empathy. NeuroImage. (2005) 24:771–9. 10.1016/j.neuroimage.2004.09.006 [DOI] [PubMed] [Google Scholar]
- 88.Bird G, Silani G, Brindley R, White S, Frith U, Singer T. Empathic brain responses in insula are modulated by levels of alexithymia but not autism. Brain. (2010) 133:1515–25. 10.1093/brain/awq060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gianini LM, White MA, Masheb RM. Eating pathology, emotion regulation, and emotional overeating in obese adults with binge eating disorder. Eat Behav. (2013) 14:309–13. 10.1016/j.eatbeh.2013.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fairburn CG, Cooper Z, Shafran R. Cognitive behaviour therapy for eating disorders: a “transdiagnostic” theory and treatment. Behav Res Ther. (2003) 41:509–28. 10.1016/S0005-7967(02)00088-8 [DOI] [PubMed] [Google Scholar]
- 91.Jones A, Lindekilde N, Lübeck M, Clausen L. The association between interpersonal problems and treatment outcome in the eating disorders: a systematic review. Nord J Psychiatry. (2015) 69:563–73. 10.3109/08039488.2015.1019924 [DOI] [PubMed] [Google Scholar]
- 92.Zipfel S, Löwe B, Reas DL, Deter H-C, Herzog W. Long-term prognosis in anorexia nervosa: lessons from a 21-year follow-up study. Lancet. (2000) 355:721–2. 10.1016/S0140-6736(99)05363-5 [DOI] [PubMed] [Google Scholar]
- 93.Spain D, Blainey SH. Group social skills interventions for adults with high-functioning autism spectrum disorders: a systematic review. Autism. (2015) 19:874–86. 10.1177/1362361315587659 [DOI] [PubMed] [Google Scholar]
- 94.Spain D, Blainey SH, Vaillancourt K. Group cognitive behaviour therapy (CBT) for social interaction anxiety in adults with autism spectrum disorders (ASD). Res Autism Spectr Disord. (2017) 41–2:20–30. 10.1016/J.RASD.2017.07.005 [DOI] [Google Scholar]
- 95.Adamson J, Leppanen J, Murin M, Tchanturia K. Effectiveness of emotional skills training for patients with anorexia nervosa with autistic symptoms in group and individual format. Eur Eat Disord Rev. (2018) 26:367–75. 10.1002/erv.2594 [DOI] [PubMed] [Google Scholar]
- 96.Tchanturia K, Doris E, Mountford V, Fleming C. Cognitive remediation and emotion skills training (CREST) for anorexia nervosa in individual format: self-reported outcomes. BMC Psychiatry. (2015) 15:53. 10.1186/s12888-015-0434-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Leppanen J, Ng KW, Tchanturia K, Treasure J. Meta-analysis of the effects of intranasal oxytocin on interpretation and expression of emotions. Neurosci Biobehav Rev. (2017) 78:125–44. 10.1016/j.neubiorev.2017.04.010 [DOI] [PubMed] [Google Scholar]
- 98.Van IJzendoorn MH, Bakermans-Kranenburg MJ. A sniff of trust: Meta-analysis of the effects of intranasal oxytocin administration on face recognition, trust to in-group, and trust to out-group. Psycho Neuroendocrinol. (2012) 37:438–43. 10.1016/j.psyneuen.2011.07.008 [DOI] [PubMed] [Google Scholar]
- 99.Andari E, Duhamel JR, Zalla T, Herbrecht E, Leboyer M, Sirigu A. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc Natl Acad Sci USA. (2010) 107:4389–94. 10.1073/pnas.0910249107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Frazier TW, Strauss M, Klingemier EW, Zetzer EE, Hardan AY, Eng C, et al. A meta-analysis of gaze differences to social and nonsocial information between individuals with and without autism. J Am Acad Child Adolesc Psychiatry. (2017) 56:546–55. 10.1016/j.jaac.2017.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim YR, Eom JS, Yang JW, Kang J, Treasure J. The impact of oxytocin on food intake and emotion recognition in patients with eating disorders: a double blind single dose within-subject cross-over design. PLoS ONE. (2015) 10:e0137514. 10.1371/journal.pone.0137514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Leppanen J, Cardi V, Ng KW, Paloyelis Y, Stein D, Tchanturia K, et al. Effects of intranasal oxytocin on interpretation and expression of emotions in anorexia nervosa. J Neuroendocrinol. (2017) 29:125–44. 10.1111/jne.12458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bonfils KA, Lysaker PH, Minor KS, Salyers MP. Affective empathy in schizophrenia: A meta-analysis. Schizophr Res. (2016) 175:109–17. 10.1016/j.schres.2016.03.037 [DOI] [PubMed] [Google Scholar]
- 104.Jolliffe D, Farrington DP. Empathy and offending: a systematic review and meta-analysis. Aggress Viol Behav. (2004) 9:441–76. 10.1016/j.avb.2003.03.001 [DOI] [Google Scholar]
- 105.Michaels TM, Horan WP, Ginger EJ, Martinovich Z, Pinkham AE, Smith MJ. Cognitive empathy contributes to poor social functioning in schizophrenia: evidence from a new self-report measure of cognitive and affective empathy. Psychiatry Res. (2014) 220:803–10. 10.1016/j.psychres.2014.08.054 [DOI] [PubMed] [Google Scholar]
- 106.Parling T, Mortazavi M, Ghaderi A. Alexithymia and emotional awareness in anorexia nervosa: time for a shift in the measurement of the concept? Eat Behav. (2010) 11:205–10. 10.1016/j.eatbeh.2010.04.001 [DOI] [PubMed] [Google Scholar]
- 107.Goddard E, Carral-Fernández L, Denneny E, Campbell IC, Treasure J. Cognitive flexibility, central coherence and social emotional processing in males with an eating disorder. World J Biol Psychiatry. (2014) 15:317–26. 10.3109/15622975.2012.750014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.