Abstract
Background:
Solid tumor chemotherapy regimens pose a risk for hepatitis B virus (HBV) reactivation, but screening and antiviral prophylaxis remains controversial because of insufficient evidence.
Purpose:
To determine the risk for HBV reactivation with and without antiviral prophylaxis and the effectiveness of prophylaxis in adults with solid tumors and chronic or resolved HBV infection.
Data Sources:
MEDLINE through 1 July 2015 and Web of Science, Cochrane Central Register of Controlled Trials, TOXNET, and Scopus through 1 March 2015.
Study Selection:
26 English-language observational studies and randomized, controlled trials in patients with chronic or resolved HBV receiving chemotherapy for solid tumors.
Data Extraction:
Study characteristics, quality, and risk of bias were assessed by 1 researcher and verified by another independent researcher.
Data Synthesis:
Random-effects model meta-analyses were used to estimate the risk and odds ratio (OR) of reactivation with versus without antiviral prophylaxis. Reactivation in chronic HBV without prophylaxis ranged from 4% to 68% (median, 25%) with substantial heterogeneity. Prophylaxis reduced the risk for HBV reactivation (OR, 0.12 [95% CI, 0.06 to 0.22]), HBV-related hepatitis (OR, 0.18 [CI, 0.10 to 0.32]), and chemotherapy interruption (OR, 0.10 [CI, 0.04 to 0.27]). In 3 studies of patients with resolved HBV infection, none received HBV prophylaxis and reactivation risk ranged from 0.3% to 9.0%.
Limitations:
Significant heterogeneity in underlying study populations and treatment regimens, incomplete baseline data, possibility of publication bias, and limited study quality. Most studies were observational and from Asia.
Conclusion:
In patients with chronic HBV receiving solid tumor chemotherapy, the risk for HBV reactivation is similar to the risk with other types of immunosuppressive therapy. Results support HBV screening and antiviral prophylaxis before initiation of chemotherapy for solid tumors.
Primary Funding Source:
National Center for Advancing Translational Sciences and National Institutes of Health.
More than 350 million persons worldwide have hepatitis B virus (HBV) infection (1, 2) and are at risk for virus reactivation when given immunosuppressive therapy for various diseases (3, 4). In oncology, reported reactivation rates range from 30% to 80% depending on the chemotherapy regimen and HBV serologic status (3). Although reactivation can be asymptomatic, it can also delay chemotherapy and lead to severe hepatitis, liver failure, or death (5). Multiple studies (5–10) have shown that antiviral prophylaxis before initiation of immunosuppressive treatment can markedly decrease the risk for HBV reactivation.
With increasing recognition of reactivation risk and the availability of effective prophylactic treatment, interest in appropriate HBV screening before chemotherapy initiation has grown (3, 11). Current national guidelines, however, disagree on which populations to screen and which tests to use (12–14). Hepatitis B virus screening is recommended in patients receiving rituximab chemotherapy and hematopoietic stem cell transplantation (14, 15). However, despite the risk for reactivation (3, 16), oncologic guidelines do not recommend universal screening for patients receiving chemotherapy for solid tumors because of insufficient evidence (14).
Recent meta-analyses (17, 18) have reported the risk for HBV reactivation with rituximab therapy for hematologic tumors, but none have examined HBV reactivation with chemotherapy for solid tumors. Therefore, the purpose of this study was to determine the absolute risk for HBV reactivation with and without antiviral prophylaxis and the effectiveness of prophylaxis in reducing the risk for reactivation in patients with chronic or resolved HBV infection across solid tumors.
Methods
All steps of the systematic review and meta-analysis were conducted using standard methods in accordance with the MOOSE (Meta-analysis of Observational Studies in Epidemiology) guidelines (19). We developed and followed an unregistered protocol.
Data Sources and Searches
We searched MEDLINE through 1 July 2015 and Web of Science, Cochrane Central Register of Controlled Trials, TOXNET, and Scopus through 31 March 2015. Three index search terms for hepatitis B virus, virus reactivation, and cancer chemotherapy were combined (Appendix Table 1, available at www.annals.org). The search was limited to English-language articles, and conference abstracts were excluded. References from relevant review articles were examined to identify other potential studies. Two investigators (S.P. and A.S.) independently reviewed all articles for study inclusion. Discrepancies were resolved by consensus or by a third investigator (J.B.W.).
Study Selection
We included published studies of patients with HBV receiving chemotherapy for any solid tumor. Hepatitis B virus was defined serologically (before chemotherapy initiation) as either chronic HBV infection (positive surface antigen [HBsAg], positive core antibody [HBcAb], and negative surface antibody [HBsAb] with various HBV DNA levels) or resolved infection (negative HBsAg, positive HBcAb, variable HBsAb, and negative HBV DNA). Please see the Glossary for more details. We included randomized, controlled trials (RCTs) and observational studies and required at least 5 patients per group with a minimum 1-month follow-up after chemotherapy initiation. Case series; review articles; and studies involving pediatric populations (aged <18 years), autoimmune conditions, HIV, hepatitis C, or hepatocellular carcinoma were excluded.
We included studies that used chemotherapy for solid tumors with or without concomitant HBV prophylactic therapy. Antiviral therapy included lamivudine, telbivudine, adefovir, tenofovir, or entecavir. Patients could receive long-term antiviral treatment or prophylaxis before chemotherapy initiation. The comparator of interest, although not required, was chemotherapy without antiviral prophylaxis.
Our primary outcome was HBV reactivation as defined by a greater than 10-fold increase in HBV DNA levels from baseline or an absolute increase greater than 105 copies/mL (in chronic HBV infection) or the reemergence of HBsAg when previously negative (in resolved HBV infection). Secondary outcomes included HBV-related hepatitis, interrupted or delayed chemotherapy, acute liver failure (with coagulopathy and hepatic encephalopathy), and death. Although each study defined the degree of alanine aminotransferase (ALT) elevation needed for HBV-related hepatitis, virologic breakthrough was required. Withdrawal hepatitis, which refers to increased ALT or HBV DNA levels after antiviral cessation, was not included.
Data Extraction and Quality Assessment
All data were extracted by 1 researcher (S.P.) and verified by another independent researcher (A.S.) and included study author, publication date, country, patient age and sex, baseline HBV serology, baseline laboratory ALT and total bilirubin levels, type of tumor, chemotherapy, HBV prophylaxis given, presence of lamivudine resistance, study follow-up period, and the risk for each study outcome. If a study involved both solid and hematologic tumors, only the solid tumor data were extracted. Discrepancies were resolved through discussion or by a third investigator (J.B.W.). Attempts were made to contact authors of included studies to clarify or collect additional data.
Two independent investigators (S.P. and A.S.) assessed study quality using the Newcastle–Ottawa Scale (NOS) for observational studies (20). For RCTs, we applied the Cochrane Collaboration’s tool for assessing risk of bias (21) in sequence generation, allocation concealment, blinding, outcome data, and selective outcome reporting.
Data Synthesis and Analysis
All meta-analyses were performed using random-effects models, and results were pooled using the maximum likelihood estimation. The arcsine transformation was used to estimate the absolute risk for HBV reactivation and 95% CI with and without antiviral prophylaxis for each study. Because of the small number of events, the arcsine transformation was applied to the risk estimates to stabilize the variance (22). We also estimated the pooled odds ratio (OR) with 95% CI of HBV reactivation with versus without prophylaxis. Because of sparse event data and imbalanced study groups, the treatment group continuity correction (the reciprocal of the opposite treatment group size) was added when no events were observed in 1 group of a study (23).
Study heterogeneity was assessed using the Cochrane I2 statistic (24). Data were not pooled if the I2 statistic was greater than 40%. Sensitivity analyses explored the effect of low-quality data, various outcome definitions, and lamivudine resistance. A statistical sensitivity analysis was performed by using a Mantel–Haenszel analysis without a continuity correction factor. When heterogeneity was low and the number of studies was greater than 10, the relation between study precision and treatment effect was assessed with funnel plots and the Rücker test (25). The Rücker test was used because it avoids false-positive results in the presence of substantial between-study heterogeneity or a substantial intervention effect (26). Statistical analyses were performed with OpenMetaAnalyst (27) and the meta and metafor packages in R, version 3.2.1 (R Foundation for Statistical Computing) (28).
Role of the Funding Source
This project was supported by the National Center for Advancing Translational Sciences and National Institutes of Health. Dr. Paul received grant support through the 2013 to 2014 Bristol-Myers Squibb Virology Research Training Program. The funding sources had no role in data abstraction, analysis, or interpretation; synthesizing of conclusions; or manuscript preparation.
Results
We identified 2192 citations though database searches, and 26 original reports met eligibility criteria (Appendix Figure 1, available at www.annals.org): 23 involved chronic infection (29–51), and 3 involved resolved infection (52–54). Nine studies were not funded, 8 had government or hospital funding, 3 were supported by industry, and 6 mentioned no funding sources (Appendix Table 2, available at www.annals.org).
Study and Patient Characteristics
Appendix Table 2 summarizes baseline study characteristics for patients with chronic and resolved HBV infection. We noted 13 double-group comparative studies, 9 single-group studies, 1 RCT of patients with chronic HBV, and 3 single-group studies of patients with resolved HBV. The 26 studies included 22 from Asia and 4 from Europe and involved cancer of the gastrointestinal tract, breast, lung, head and neck, and other sites. Chemotherapy regimens and corticosteroid use varied across studies. There were 1751 patients (range, 6 to 258) with chronic HBV infection, and 328 (range, 14 to 291) with resolved HBV. When reported, the median patient age across studies was 47 years (range, 20 to 80 years), with 357 men (24%) and 937 women. Further, 774 patients with chronic HBV received HBV prophylaxis (mostly lamivudine). None of the patients with resolved HBV infection received HBV prophylaxis. Baseline serum HBV DNA levels were inconsistently reported. The HBsAb status was reported in only 1 study (53) of resolved infection in which all 24 patients had positive HBsAb.
Study quality is reported in Appendix Table 3 (available at www.annals.org). All observational studies scored well on patient selection, with variable quality with respect to cohort comparability and outcome. In the 1 RCT, complete outcome data were reported but there was no allocation concealment or selective outcome reporting. None of the studies had blind assessment of outcomes.
Absolute Risk for HBV Reactivation in Patients With Chronic HBV Across All Solid Tumors
The risk for HBV reactivation in patients with chronic HBV receiving chemotherapy for solid tumors without antiviral prophylaxis ranged from 4% to 68% (median, 25%) (Figure 1) in 19 studies. Given the substantial between-study heterogeneity (I2 = 78%), the estimates were not pooled. Although each study had adequate patient selection, 6 did not have adequate cohort follow-up (Appendix Table 3).
Figure 1.
Absolute risk for HBV reactivation without antiviral prophylaxis in patients with chronic HBV infection.
With antiviral prophylaxis, the risk was much lower, ranging from 0.9% to 31.4% (median, 4.1%) in 18 studies. Although heterogeneity was significant in the analysis (I2 = 60%), this was mostly explained by 1 study (30) in which the reactivation rates were higher than anticipated and were speculated to be secondary to medication nonadherence. After removing this study, the I2 statistic decreased from 60% to 0%, with a reactivation risk of 3.5% (95% CI, 2.3% to 5.0%).
Absolute Risk for HBV Reactivation in Chronic HBV, by Tumor and Chemotherapy Subtype
When examining HBV reactivation without prophylaxis by solid tumor subtype, Figure 2 shows a variable but appreciable risk among cancer of the gastrointestinal tract, breast, lung, and head and neck ranging from 5% to 68%. Appendix Figure 2 (available at www.annals.org) shows HBV reactivation without antiviral prophylaxis by the type of chemotherapy administered. (See Appendix Table 4, available at www.annals.org, for a description of the chemotherapies analyzed.) Reactivation risk varied from 3.0% in the taxane group to 88% in 1 anthracycline-based study. Most studies (15 of 16) had a reactivation risk greater than 10%. The studies using anthracycline-based chemotherapy had a median reactivation risk of 29% (range, 14% to 88%) with considerable between-group heterogeneity (I2 = 90%). All but 1 anthracycline-based study (43) involved patients with breast cancer. The platinum-based studies had a median reactivation risk of 25% (range, 24% to 49%) and involved cancer of the head and neck, lung, and breast and neuroendocrine tumors. The FOLFOX-/FOLFIRI-based (folinic acid, fluorouracil, and oxaliplatin/folinic acid, fluorouracil, and irinotecan hydrochloride) chemotherapy regimens involved patients with mainly colonic or gastric tumors, with the reactivation risk ranging from 19% to 25% (median, 25%). Antimetabolites used to treat colon and breast cancer had a reactivation risk of 17% to 23% (median, 20%). The single study of patients treated with taxane involved gastric, esophageal, and breast cancer, with a 3% reactivation risk.
Figure 2.
Absolute risk for HBV reactivation without antiviral prophylaxis in patients with chronic HBV infection, by tumor subtype.
Of the 9 studies that reported specific chemotherapy regimens, only 4 reported concomitant corticosteroid use. Of the 94 patients who received corticosteroids, 19 (20%) had HBV reactivation. Fifty-six patients did not receive corticosteroids, and 8 had reactivation events (14%). No patients receiving prophylaxis in either group had HBV reactivation. Four studies (37, 39, 42, 43) used steroids but did not report who had HBV reactivation, and 1 study (40) did not record steroid use.
Absolute Risk for Secondary Outcomes in Patients With Chronic HBV
Appendix Figure 3 (available at www.annals.org) displays the risk for each secondary outcome without antiviral prophylaxis. Without prophylaxis, there was a 2% to 60% risk (median, 23%) for HBV-related hepatitis and chemotherapy interruption. The risks for HBV-related acute liver failure (median, 2%; range, 1% to 20%) and death (median, 2.3%; range, 0.4% to 20%) were lower but still appreciable.
The definition of HBV-related hepatitis varied across studies. Most studies used a common definition of a greater than 3-fold increase in ALT level that exceeded the upper limit of normal or an absolute increase in ALT level to more than 100 U/L compared with baseline. Two studies (30, 38) defined it as an ALT level twice the upper limit of normal. Four studies (34, 47, 48, 50) did not predefine hepatitis in their methods section. A sensitivity analysis removing these 4 studies revealed similar results (median risk, 21%; range, 2.3% to 54%).
Odds Ratio Comparing Antiviral Prophylaxis With No Prophylaxis in Chronic HBV
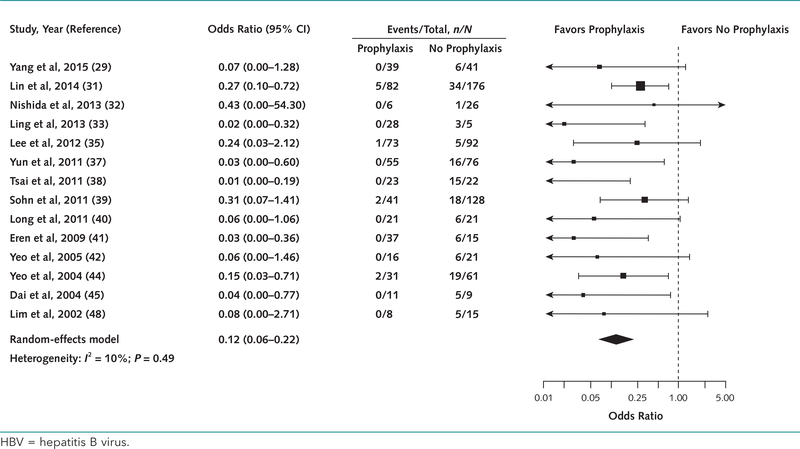
Among the 13 studies comparing reactivation risk in patients receiving antiviral prophylaxis versus no prophylaxis, 6 had excellent cohort comparability (29, 31, 39, 42, 45, 48) and 4 had adequate cohort comparability (33, 37, 38, 44). Three studies (32, 33, 41) did not compare the groups and were classified as low quality. The pooled OR was 0.12 (CI, 0.06 to 0.22; I2 = 10%), indicating a significant reduction in HBV reactivation with prophylaxis (Figure 3). Excluding the 3 low-quality studies yielded similar results (Appendix Table 5, available at www.annals.org). The regression test for funnel plot asymmetry was significant (P = 0.002).
Figure 3.
Odds ratio for HBV reactivation with and without antiviral prophylaxis in patients with chronic HBV infection.
Of note, 29 patients in 5 studies (30, 35, 36, 39, 51) were tested for lamivudine resistance as a cause of their HBV reactivation. Eighteen tested positive and were included in the initial analysis. When we excluded them from the analysis, the OR for HBV reactivation did not change (OR, 0.12 [CI, 0.07 to 0.23]; I2 = 10%).
Figure 4 shows the ORs for each secondary outcome. Antiviral prophylaxis was found to be significantly beneficial in preventing HBV-related hepatitis and chemotherapy interruption (OR, 0.18 [CI, 0.10 to 0.32] and 0.10 [CI, 0.04 to 0.27], respectively). Although the ORs favored prophylaxis in reducing the risk for acute liver failure (OR, 0.31 [CI, 0.09 to 1.02]) and death (OR, 0.43 [CI, 0.15 to 1.20]), the results were not statistically significant. Regression testing for funnel plot asymmetry for hepatitis and death were not significant (P = 0.15 and 0.39, respectively). Not enough studies were available to test funnel plot asymmetry for the other secondary outcomes.
Figure 4.
Odds ratio for secondary outcomes with and without antiviral prophylaxis in patients with chronic HBV infection.
Sensitivity analyses are discussed in Appendix Table 5. The results remained consistent after removing the 3 low-quality studies and the 4 studies with only adequate cohort comparability. We performed a Mantel–Haenszel meta-analysis without a continuity correction factor and found a statistically significant effect for acute liver failure (OR, 0.06 [CI, 0 to 0.70]) and death (OR 0.12 [CI, 0.02 to 0.87]).
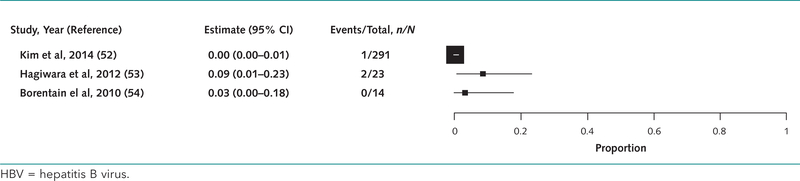
Absolute Risk for HBV Reactivation in Patients With Resolved HBV
In 3 studies (52–54) of 328 patients with resolved HBV infection, the risk for reactivation without antiviral prophylaxis ranged from 0.3% to 9% (median, 3%) (Appendix Figure 4, available at www.annals.org). Two of the 3 studies had inadequate follow-up but otherwise were representative of the cohort with no prophylaxis. The 3 patients who had HBV reactivation received platinum-based chemotherapy regimens. Most patients had lung cancer (186), 2 of whom had HBV reactivation (52, 53). Seven patients had head and neck cancer, 1 of whom had HBV reactivation (54).
Discussion
We found that patients receiving chemotherapy for solid tumors who did not receive antiviral prophylaxis had a reactivation risk ranging from 4% to 68% (median, 25%) in chronic HBV infection and 0.3% to 9% (median, 3%) in resolved HBV infection. In chronic HBV, antiviral prophylaxis significantly reduced the risk for HBV reactivation (OR, 0.12 [CI, 0.06 to 0.22]), HBV-related hepatitis (OR, 0.18 [CI, 0.10 to 0.32]), and chemotherapy interruption (OR, 0.10 [CI, 0.04 to 0.27]). The results were not statistically significant for acute liver failure or death.
Our results are similar to those of previous systematic reviews (6–8, 55) in patients with tumors and chronic HBV (searched in MEDLINE through 1 July 2015) that have found reactivation risks ranging from 33% to 67% among those who did not receive prophylaxis and 0% to 20% among those who received prophylaxis (mainly lamivudine). However, these studies combined solid and liquid tumors and did not report reactivation rates separately. In patients with solid tumors, meta-analyses (9, 10) have focused solely on the use of lamivudine prophylaxis in patients with breast cancer, with relative measures favoring prophylactic lamivudine (RR, 0.23 [CI, 0.13 to 0.39]; OR, 0.09 [CI, 0.03 to 0.26]), similar to our findings.
Recently, the American Gastroenterological Association performed a technical review and published guidelines on the management of HBV reactivation during immunosuppressive treatment (56). The review included biological agents, traditional immunosuppressive agents (for example, 6-mercaptopurine and methotrexate), corticosteroid use, and commonly used oncologic therapies. It used stricter study inclusion criteria and consequently included fewer cohort studies than our analysis. Further, it defined high-risk patients as those with an HBV reactivation risk exceeding 10% or those with positive HBsAg who were receiving anthracycline-based chemotherapy. Although we could not pool estimates with regard to chemotherapy classes because of substantial heterogeneity and small sample sizes, most studies (15 of 16) examined by chemotherapy class had a risk for HBV reactivation greater than 10%. On the basis of this definition, our results would also classify patients receiving platinum-, FOLFOX-/FOLFIRI-, or antimetabolite-based chemotherapy regimens to be at high risk for HBV reactivation and merit screening before chemotherapy. This can also be extended to the breast, gastrointestinal, lung, and head and neck tumor subtypes in which most studies had a reactivation risk exceeding 10%.
We found no meta-analyses and limited studies examining reactivation risk in patients with resolved HBV in the literature on solid tumors. Our review found a small risk for reactivation ranging from 0.3% to 9% (median, 3.0%) in a limited number of patients. Although some data have indicated that HBsAb can protect against reactivation in patients with lymphoma and resolved HBV infection (57), we could not comment on the utility of HBsAb because not all studies included this information. Given this low reactivation risk without antiviral prophylaxis, periodic monitoring of liver enzymes and HBV DNA levels is probably warranted, but optimal management for patients with resolved HBV and solid tumors remains uncertain.
In 2008, the Centers for Disease Control and Prevention recommended universal HBV screening for all patients awaiting chemotherapy (12). Specialty societies, including the American Association for the Study of Liver Diseases (13), Asian Pacific Association for the Study of the Liver (58), and the European Association for the Study of the Liver (59), recommended screening all patients receiving immunosuppressive therapy. However, the American Society of Clinical Oncology (14) recommended screening persons at high risk for HBV infection (based on birth country, blood transfusion history, history of intravenous drug use, and other risk factors) and those receiving “highly suppressive chemotherapy regimens,” including hematopoietic stem cell transplant and rituximab therapy, before starting any form of chemotherapy.
Previous studies (17, 60, 61) in patients with resolved HBV receiving rituximab-based chemotherapy have reported that the reactivation risk ranges from 6% to 10%. Our study finds a greater reactivation risk in patients with chronic HBV infection receiving solid tumor chemotherapy. In addition, several studies (62–64) have shown that risk-based screening methods are often ineffective because patients and health care providers may be unaware of the need or may forget to screen for identifiable risk factors. These implementation issues coupled with the high risk for reactivation in patients receiving solid tumor chemotherapy support universal HBV screening for all patients before initiating chemotherapy.
It is interesting that patients with a history of resolved HBV infection are still at risk for reactivation in the setting of immunosuppressive therapies. This is related to the immunologic mechanism underlying HBV reactivation. Once infected with HBV, hepatocytes contain covalently closed circular HBV DNA replicative intermediates that persist even after clearance of the virus (3) and are responsible for reactivation in the setting of host immunosuppression. As a result, even patients with presumed immunity to HBV with circulating HBsAb and negative HBV DNA serum levels are at risk for reactivation. In addition, HBsAg titers may be undetectable over time or patients may have mutant HBsAg leading to false-negative results (3). This underscores the importance of screening all patients before solid tumor chemotherapy for HBV with not only HBsAg but also HBcAb. The Centers for Disease Control and Prevention, American Association for the Study of Liver Diseases, and others have also recommended testing for HBsAb in those receiving immunosuppressive therapy.
The issue of cost-effectiveness has often been raised when discussing universal HBV screening, especially in low-prevalence countries, such as the United States. One study (65) found universal HBV screening in patients with lymphoma receiving rituximab to be cost-effective, but an Australian study (66) did not find screening to be cost-effective in patients with breast cancer. Future studies should examine the cost-effectiveness of HBV screening before initiating chemotherapy to determine optimal screening strategies across different types of cancer and populations of HBV infection.
Our systematic review fills an important knowledge gap about the risk for HBV reactivation in patients with chronic HBV infection receiving chemotherapy for solid tumors and places this risk in context across different types of tumors, chemotherapies, and HBV serologies. Our results support universal HBV screening before chemotherapy for patients with solid tumors and antiviral prophylaxis for those with chronic HBV infection. The analysis also highlights the lack of published data about HBV reactivation in those with resolved HBV infection and solid tumors.
Our review also has several limitations. First, there was substantial heterogeneity seen in the risk for HBV reactivation without prophylaxis, which did not allow us to meta-analyze the data. Such heterogeneity is not surprising given differences in underlying types of cancer, treatment regimens, HBV genotypes, baseline serum HBV DNA levels, and dose and duration of both antiviral prophylaxis and chemotherapy. Given the aggregate nature of the studies, it was impossible to determine and quantify the degree of chemotherapeutic immunosuppression for each patient in each study. An individual patient–level meta-analysis would have been preferable to explore unexplained sources of heterogeneity.
Second, most studies were observational and retrospective, with only 1 RCT. Several studies were only single-group descriptive studies, and many had missing demographic and clinical information. As a result, known risk factors for reactivation, such as male sex, HBV e antigen seropositivity, younger age, increased HBV DNA levels, and longer chemotherapy duration (31), could not be examined. Further, sex bias may have occurred because many of the included patients were women (937 of 1294 patients with recorded sex status). This may limit the generalizability of the findings because male sex has previously been found to be a risk factor for HBV reactivation (49, 67). Of note, few studies commented on tumor response, cancer-related death, or all-cause mortality, which are clinically important and meaningful end points. Such inconsistencies call for more uniform reporting of data.
Third, quality was also of concern because none of the studies had blind assessment of outcomes. Patients in the exposed cohort seemed to be representative of the average patient with cancer and HBV, and the nonexposed cohort was selected from the same population. These studies were done in Asia, however, where the prevalence of HBV is high, and this may limit the generalizability of the results to less prevalent areas. Further, comparability of cohorts was questionable because only 5 of the studies controlled for the type or duration of chemotherapy. However, sensitivity analysis after excluding lower-quality studies did not alter the findings.
Finally, the funnel plot asymmetry test showed a statistically significant small-study effect in the meta-analysis of prophylaxis for reactivation. That is, the smaller, less precise studies showed a greater effect of prophylaxis than the larger studies, but even the larger studies showed benefit. Publication bias is a potential source of the funnel plot asymmetry, and there may have been studies that did not show evidence of HBV reactivation and were not retrieved because of publication in non–English-language journals or conference proceedings. Other potential explanations of the asymmetry include selective outcome reporting, clinical heterogeneity, and variation in study quality.
In conclusion, this systematic review and meta-analysis examined the risk for HBV reactivation and the benefits of antiviral prophylaxis in patients receiving solid tumor chemotherapy. Our results indicate that in patients with chronic HBV infection, the risk for reactivation is similar to or exceeds the risk from other types of immunosuppressive therapy in which screening and treatment are currently recommended. In addition, antiviral prophylaxis can significantly reduce HBV reactivation in this patient population. Our study supports universal HBV screening with HBsAg and HBcAb before initiation of chemotherapy for solid tumors and antiviral prophylaxis for patients with chronic infection. Future studies should further examine the risk for HBV reactivation in those with resolved infection and evaluate the cost-effectiveness of universal screening and treatment in these patient populations.
Acknowledgments
Grant Support: By the National Center for Advancing Translational Sciences and National Institutes of Health (grants UL1 TR001064 and UL1 TR000073). Dr. Paul received grant support through the 2013 to 2014 Bristol-Myers Squibb Virology Research Training Program.
Glossary
- Hepatitis B surface antigen (HBsAg)
HBsAg tests for the presence of the virus and is the serological hallmark of infection. In patients who recover from HBV infection, HBsAg is usually undetectable after about 4 to 6 months.
- Hepatitis B surface antibody (HBsAb)
In patients who clear hepatitis B infection, HBsAg disappears and HBsAb appears, which is the hallmark of recovery. Although most will have HBsAb for life (with lifelong immunity), there are some patients whose antibody titers decrease over time.
- Hepatitis B core antibody (HBcAb)
HBcAb (IgG) is an antibody that detects for the presence of hepatitis B core antigen (HBcAg). It is present throughout the course of infection (both current and resolved). Further, HBcAb (IgM) is found in acute infection only and can sometimes be found in HBV reactivation.
- HBV serologic screening tests and interpretation
Chronic HBV infection: HBsAg-positive, HBcAb (IgG)-positive, HBsAB-negative, and variable HBV DNA (Positive HBV DNA indicates chronic infection. Negative HBV DNA with a positive HBsAg and HBcAb is referred to as a carrier state. For the purposes of this paper, we have defined chronic HBV infection as those with variable HBV DNA levels.)
- Resolved/cleared HBV infection
HBsAg negative, HBcAb (IgG) positive, HBsAb positive, and HBV DNA negative.
- Occult HBV infection:
HBsAg negative, HBcAb (IgG) positive, HBsAb negative, and HBV DNA negative/positive.
- HBV reactivation
HBsAg positive, HBcAb (IgM) positive, HBsAb negative/positive, and HBV DNA positive.
- Vaccination
HBsAg negative, HBcAb negative, HBsAb positive, and HBV DNA negative.
Appendix
Appendix Figure 1.
Summary of evidence search and selection.
Appendix Figure 2.
Absolute risk for HBV reactivation without antiviral prophylaxis in patients with chronic HBV, by chemotherapy subtype.
Appendix Figure 3.
Absolute risk for secondary outcomes without antiviral prophylaxis in patients with solid tumors and chronic HBV infection.
Appendix Figure 4.
Absolute risk for HBV reactivation without antiviral prophylaxis in patients with resolved HBV infection.
Appendix Table 1.
MEDLINE Search Strategy
| 1. | exp Hepatitis B/ |
| 2. | exp Hepatitis B virus/ |
| 3. | HBV.mp. |
| 4. | Hep$ B.mp. |
| 5. | Hep$ B virus$.mp. |
| 6. | 1 or 2 or 3 or 4 or 5 |
| 7. | exp Virus Activation/ |
| 8. | reactivate$.mp. |
| 9. | 7 or 8 |
| 10. | 6 or 9 |
| 11. | limit 10 to "topic reviews (cochrane)" |
| 12. | exp Antineoplastic Agents/ |
| 13. | exp Drug Therapy |
| 14. | exp Neoplasms/ |
| 15. | 13 and 14 |
| 16. | 12 or 15 |
| 17. | 10 and 16 |
Appendix Table 2.
Characteristics of Studies Involving Solid Tumors and Chronic or Resolved HBV Infection
| Study, Year (Reference) |
Country | Study Design |
Disease | Total, n | Viral PPX* | Group | Men/ Women, n/N |
Median Age (Range), y |
Median Baseline ALT Level (Range), IU/L |
Median Baseline Bilirubin Level (Range), mg/dL |
Baseline HBV DNA Level |
Median Follow-up (Range) |
Study Funding Source |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chronic HBV infection (HBsAg positive, HBcAb positive, HBsAb negative) | |||||||||||||
| Yang et al, 2015(29) | China | R, double | Gl | 80 | L | PPX | 27/12 | 51 (21–66) | NR | NR | Known; 16 patients >104 copies/mL | NR | National Basic Research Program of China |
| No PPX | 26/15 | 52 (35–68) | |||||||||||
| Ho et al, 2015 (30) | China | R, single | Colon, head/neck,lung, breast, Gl, sarcoma | 51 | L (24), A (27) | NR | NR | NR | NR | Mean 3.27 (1.5–11.0 log10 copies/mL) | 10.1 mo (1.0–21.8) | Gilead Sciences | |
| Chen etal, 2015(51) | Taiwan | R, single | Breast, lung, Gl, head/neck, gynecologic,GU | 173 | L (128),E (45) | 83/90 | 56.3 (SD 10.3) | 35.5 (SD 21.1) | I NR | 65 patients had >2000 lU/mL | 14.9 (6.7) | None | |
| Lin etal, 2014(31) | China | R, double | Non-small cell lung cancer | 258 | L (82) | PPX | 49/33 | 61.5 (34–77) | 26.5 (1 1–67) | 12.7 (μmol/L), (4.8–32.4) | 167 patients had >104copies/rnL | NR | None |
| No PPX | 107/69 | 59 (30–79) | 31 (9–88) | 12.3 (μmol/L), (5.2–30.5) | |||||||||
| Nishida etal, 2013(32) | Japan | R, double | Breast, Gl, heme, lung, renal, prostate, qynecoloqic, others | 32 | E (6) | Total | 8/24 | 59 (36–79) | NR | NR | Known in 14, 8 patients average 3.1 log copies/mL; negative in 6 | 21 mo (NR) | NR |
| Ling etal, 2013(33) | Singapore | R, double | Breast, colon, lung | 33 | L (24)or E (4) | Total | NR | NR | NR | NR | Mean (SD): 1.46 (5.77) × 107 lU/mL | NR | None |
| Xu etal, 2012(34) | China | R, single | Gastric | 44 | NA | Total | 28/16 | 56 (35–73) | NR | NR | <5 pg/mL† | NR | Chinese National Basic Research Program |
| Lee etal, 2014 (35) | Korea | R, double | Breast | 165 | L (73) | PPX | 0/73 | 46 (29–72) | 20 (6–50) | 0.6 (0.3–2.1) | Known in 48 patients | 49.7 mo (16.1–121.3) | Korean Ministry of Health and Welfare |
| No PPX | 0/93 | 45 (29–72) | 19(6–40) | 0.6 (0.3–2) | 74 mo (23.5–140.6) | ||||||||
| Kim etal, 2012(36) | Korea | R, single | Breast, Gl, lung, head/neck, others | 73 | L (73) | NR | NR | NR | NR | 40 patients >2000 lU/mL; 61 patients <2000 lU/mL; NR 9 | 22 mo (1.2–83.6) | None | |
| Yun etal, 2011 (37) | Korea | R, double | Breast | 131 | L (55) | PPX | 0/55 | 48 (30–68) | 25 (mean, NR) | 0.6 (mean, NR) | NR | 6 mo after che motherapy | Korean Ministry of Health and Welfare |
| No PPX | 0/76 | 46 (30–69) | 25 (mean, NR) | 0.5 (mean, NR) | |||||||||
| Tsai etal, 2011 (38) | Taiwan | P, double | Breast | 45 | L (23) | PPX | 0/23 | 46.7 (9.2) | NR | NR | Mean (SD): 2.59 (1.64) log copies/mL | 3 mo after chemotherapy | Taiwan National Science Council |
| No PPX | 0/22 | 50.4 (7.7) | |||||||||||
| Sohn etal, 2011 (39) | Korea | R, double | Breast | 169 | L (41) | PPX | 0/128 | 46 (23–75) | 18(5–64) | 0.7 (0.3–2.2) | NR | NR | None |
| No PPX | 0/41 | 48(29–66) | 18(5–81) | 0.8 (0.4–1.9) | |||||||||
| Longetal, 2011 (40) | China | RCT | Breast | 42 | L (21) | PPX | 0/21 | 45 (29–64) | 22.3 (7–96) | 13.6 μmol/L (5.6–21.6) | 14 patients >1.0 × 103 copies/mL | NR | None |
| No PPX | 0/21 | 43 (20–62) | 15(6–27) | 16.7 μmol/L (6.4–44.1) | |||||||||
| Eren etal, 2009(41) | Turkey | R, double | Lung, Gl, breast, others | 52 | L (37) | NR | NR | NR | NR | NR | 1.5 mo after chemotherapy | NR | |
| Yeoetal, 2005(42) | United Kingdom | R, double | Nasopharyngeal | 37 | L (16) | PPX | 14/2 | 46.5 (30–58) | 44.4(19–96) | 8 μmol/L (3–45) | NR | 2 mo after chemotherapy | Glaxo Welcome Ltd. (Hong Kong) |
| No PPX | 15/6 | 46 (40–65) | 29 (12–84) | 6 μmol/L (3–20) | |||||||||
| Yeoetal, 2004(44) | Hong Kong | R, double | Breast | 92 | L (31) | PPX No | 0/31 | 46 (31–68) | 28 (13–137) | 7 μmol/L (3–13) | NR | 2 mo after chemotherapy | Glaxo Welcome Ltd. (Hong Kong) |
| PPX | 0/61 | 46 (31–71) | 27 (10–98) | 6 μmol/L (1 –16) | |||||||||
| Yeo etal, 2004 (43) | Hong Kong | P, single | Breast, Gl, head/neck, lung | 116 | NA | NR | NR | NR | NR | 82 patients detectable, 5 patients undetectable | 2 mo after chemotherapy | NR | |
| Daietal, 2004 (46) | Taiwan | P, single | Breast | 6 | L (6) | Total | 0/6 | 46 (44–55) | NR | NR | 5 patients positive HBV DNA | 6 mo after chemotherapy | NR |
| Daietal, 2004 (45) | Taiwan | P, double | Breast | 20 | L (9) | Total | 0/9 0/11 | 42 (27–55) 45 (36–58) | 24 (6–54) 29 (12–31) | NR | All PCR+ at baseline | NR | Tri-Service General Hospital |
| Cheng et a 1, 2004(47) | Taiwan | R, single | Gastric | 11 | NA | NR | NR | NR | NR | NR | 4 mo after chemotherapy | National Science Council, Taiwan University Hospital | |
| Lim etal, 2002 (48) | Singapore | R, double | Solids, not otherwise specified | 23 | L (8) | NR | NR | NR | NR | NR | PPX: 11.5 mo No PPX: 12 mo | None | |
| Yeoetai, 2000(49) | Hong Kong | P, single | Gl, lung, breast, others | 62 | NA | NR | NR | NR | NR | NR | 12 mo (NR) | None | |
| Alexopoulos et al, 1999 (50) | Greece | P, single | Lung, breast, Gl, urothelial, head/neck, ovary | 36 | NA | NR | NR | NR | NR | NR | 12 mo (NR) | NR | |
| Resolved HBV infection (HBsAg negative, HBcAb positive, HBsAb positive or negative) | |||||||||||||
| Kim et al, 2014 (52) | Korea | R, single | Pancreatic, gastric, breast, colon, other | 291 | NA | NR | NR | NR | NR | NR | NR | NR | |
| Hagiwara et al, 2012 (53) | Japan | R, single | Lung, Gl, breast, head/neck, | 23 | NA | 20/7 | 66 (47–80) | NR | NR | Negative (<1.5 log copies/mL) | 6.3 mo (1–32.5) | National Cancer Center R&D Fund | |
| Borentain etal, 2010(54) | France | R, single | Lung, Gl, breast, kidney | 14 | NA | NR | NR | NR | NR | NR | NR | None | |
ALT = alanine aminotransferase; double = double group study; E = entecavir; Gl = gastrointestinal; GU = genitourinary; HBcAb = hepatitis B core antibody; HBsAb = hepatitis B surface antibody; HBsAg = hepatitis B surface antigen; HBV = hepatitis B virus; L = lamivudine; NA = not applicable; NR = not reported; P = prospective cohort; PCR = polymerase chain reaction; PPX = prophylaxis; R = retrospective cohort; RCT = randomized, controlled trial; single = single group study.
The number of patients who received each type of viral PPX is reported in parentheses
1 pg/mL = 2.83 × 105 copies/mL.
Appendix Table 3.
Quality Assessment for Cohort Studies Using the Newcastle-Ottawa Scale
| Study, Year (Reference) | Selection* |
Comparability† |
Outcome‡ |
|||||
|---|---|---|---|---|---|---|---|---|
| Representativeness of Exposed Cohort |
Selection of Nonexposed Cohort |
Ascertainment of Exposure |
Demonstration Outcome Not Present at Study Start |
Comparability of Cohorts |
Assessment of Outcome |
Follow-up Enough for Outcomes to Occur |
Adequacy of Follow-up |
|
| Chronic HBV infection | ||||||||
| Yang et al, 2015 (29) | • | • | • | • | •• | • | • | 엯 |
| Ho etal, 2015 (30) | • | NA | • | • | NA | • | • | • |
| Chen etal, 2015(51) | • | NA | • | • | NA | • | • | • |
| Lin etal, 2014(31) | • | • | • | • | •• | • | • | 엯 |
| Nishida etal, 2013(32) | • | • | • | • | 엯엯 | • | • | • |
| Ling etal, 2013 (33) | • | • | • | • | 엯엯 | • | • | 엯 |
| Xu etal, 2012 (34) | NA | • | • | • | NA | • | • | 엯 |
| Lee etal, 2014(35) | • | • | • | • | • | • | • | • |
| Kim etal, 2012 (36) | • | NA | • | • | NA | • | • | • |
| Yun etal, 2011 (37) | • | • | • | • | • | • | • | • |
| Tsai etal, 2011 (38) | • | • | • | • | • | • | • | • |
| Sohn etal, 2011 (39) | • | • | • | • | •• | • | • | 엯 |
| Eren etal, 2009 (41) | • | • | • | • | 엯엯 | • | • | 엯 |
| Yeo etal, 2005(42) | • | • | • | • | •• | • | • | • |
| Yeo etal, 2004(44) | • | • | • | • | • | • | • | • |
| Yeo etal, 2004(43) | NA | • | • | • | NA | • | • | • |
| Dai etal, 2004(46) | • | NA | • | • | NA | • | • | • |
| Dai etal, 2004(45) | • | • | • | • | •• | • | • | • |
| Cheng etal, 2004(47) | NA | • | • | • | NA | • | • | 엯 |
| Lim etal, 2002 (48) | • | • | • | • | •• | • | • | • |
| Yeo etal, 2000(49) | NA | • | • | • | NA | • | • | • |
| Alexopoulos et al, 1 999 (50) | NA | • | • | • | NA | • | • | • |
| Resolved HBV infection | ||||||||
| Kim etal, 2014(52) | • | NA | • | • | NA | • | • | 엯 |
| Hagiwara et al, 2012 (53) | • | NA | • | • | NA | • | • | • |
| Borentain et al, 2010 (54) | • | NA | • | • | NA | • | • | 엯 |
• = study fulfilled listed criteria; 엯 = study did not fulfill listed criteria; HBV = hepatitis B virus; NA = criteria not applicable to the study.
Representativeness of exposed cohort: • given if the cohort was representative of the average patient with cancer and HBV infection in the community; 엯 given if the cohort was selected based on convenience (i.e., volunteers) or if there was no description of the derivation of the cohort. Selection of nonexposed cohort: • given if the nonexposed cohort was drawn from the same community as the exposed cohort; 엯 was given if it was drawn from a different source or there was no description of the cohort derivation. Exposure ascertainment: • given if obtained from secure record (hospital chart); 엯 was given if from a written self-report or no description given. • given if outcome of interest was not present at start of study.
• given if study controlled for or adjusted for type or duration of chemotherapy used; additional • if controlled for age or sex.
Assessment of outcome: • given if independent blind assessment; additional • given if evidence of record linkage (i.e., through medical records); 엯 given if through self-report or no description. • given if follow-up was long enough for outcomes to occur. Adequacy of follow-up of cohorts: • given if complete follow-up and all participants accounted for or if loss to follow-up was small and unlikely to introduce bias (follow-up rate >90% or description provided of those lost); 엯 given if follow-up rate <90%, no description of those lost, or no statement
Appendix Table 4.
Description of Chemotherapy Subtypes*
| Chemotherapy Subtype | Mechanism of Action | Examples |
|---|---|---|
| Anthracycline | Antitumor antibiotics that interfere with enzymes involved in DNA replication | Daunorubicin, doxorubicin, epirubicin, idarubicin |
| Platinum | Directly damage DNA, preventing DNA replication (similar to alkylating agents) | Cisplatin, carboplatin, oxaliplatin |
| Antimetabolites | Interfere with DNA and RNA growth during S phase of the cell cycle | 5-Fluorouracil, capecitabine, cytarabine, floxuridine, fludarabine, gemcitabine, hydroxyurea |
| Taxane | Mitotic inhibitor | Paclitaxel and docetaxel |
| FOLFOX (folinic acid, fluorouracil, oxaliplatin)/ FOLFIRI (folinic acid, fluorouracil, irinotecan hydrochloride) | Several | Regimen used to treat advanced colon, gastric, and pancreatic cancer |
Data from American Cancer Society. Chemotherapy principles: an in-depth discussion of the techniques and its role in cancer treatment. 2010. Accessed at www.cancer.org/acs/groups/cid/documents/webcontent/002995-pdf.pdf on 3 August 2015.
Appendix Table 5.
Sensitivity Analyses of the Risk for HBV Reactivation and Secondary Outcomes in Patients With Chronic HBV Infection
| Outcome | OR (95% CI);
I2 |
|||
|---|---|---|---|---|
| Random Effects, Maximum Likelihood (With Treatment Group Continuity Correction) | Exclusion of 3 Low-Quality Studies | Exclusion of 4 Adequate-Quality Studies and 3 Low-Quality Studies | Fixed Effects, Mantel-Haenszel (Without Continuity Correction) | |
| HBV reactivation | 0.12 (0.06–0.22); 10% | 0.15 (0.08–0.28); 0% | 0.19 (0.09–0.38); 0% | 0.10 (0.05–0.18); 0% |
| HBV-related hepatitis | 0.18 (0.10–0.32); 0% | 0.22 (0.12–0.39); 0% | 0.24 (0.11–0.49); 0% | 0.14 (0.08–0.25); 0% |
| Interrupted chemotherapy | 0.10 (0.04–0.27); 0% | 0.15 (0.05–0.45); 0% | 0.20 (0.04–0.99); 0% | 0.05 (0.01–0.20); 0% |
| Acute liver failure | 0.31 (0.09–1.02); 0% | 0.40 (0.09–1.84); 0% | 0.31 (0.05–2.0); 0% | 0.06 (0.001–0.70); 0% |
| Mortality | 0.43 (0.15–1.20); 0% | 0.58 (0.17–1.98); 0% | 0.55 (0.12–2.45); 0% | 0.12 (0.02–0.87); 0% |
HBV = hepatitis B virus; OR = odds ratio.
Footnotes
Disclosures: Dr. Paul reports grants from National Center for Advancing Translational Sciences, National Institutes of Health, and the 2013 to 2014 Bristol-Myers Squibb Virology Research Training Program during the conduct of the study. Dr. Wong reports nonfinancial support from the American Association for the Study of Liver Diseases (as a member of systematic review writing group for an HBV practice guideline) outside the submitted work. Authors not named here have disclosed no conflicts of interest. Disclosures can also be viewedatwww.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M15–1121.
Tufts Medical Center, Massachusetts General Hospital, and Boston Medical Center, Boston, Massachusetts, and Brown University School of Public Health, Providence, Rhode Island.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Reproducible Research Statement: Study protocol, statistical code, and data set: Available from Dr. Paul upon request (sonali.paul2@gmail.com).
Current author addresses and author contributions are available at www.annals.org.
Contributor Information
Dr. Sonali Paul, Massachusetts General Hospital Gastroenterology Associates, 55 Fruit Street, Blake 4, Boston, MA 02114.
Dr. Akriti Saxena, Boston Medical Center, Gastroenterology, Moakley Building, 2nd Floor, 830 Harrison Avenue, Boston, MA 02118.
Dr. Norma Terrin, Institute for Clinical Research and Health Policy Studies, Tufts Medical Center, 35 Kneeland Street, 9th Floor, Boston, MA 02111.
Dr. Kathleen Viveiros, Division of Gastroenterology and Hepatology, Tufts Medical Center, 800 Washington Street, Box 233, Boston, MA 02111..
Dr. Ethan M. Balk, Center for Evidence-Based Medicine, Brown University School of Public Health, Box G-S121-8, Providence, RI 02912.
Dr. John B. Wong, Division of Clinical Decision Making, Tufts Medical Center, 35 Kneeland Street, 7th Floor, Boston, MA 02111..
References
- 1.Alter MJ. Epidemiology of hepatitis B in Europe and worldwide. J Hepatol. 2003;39 Suppl 1:S64–9. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 2.Wasley A, Kruszon-Moran D, Kuhnert W, Simard EP, Finelli L, McQuillan G, et al. The prevalence of hepatitis B virus infection in the United States in the era of vaccination. J Infect Dis. 2010;202:192–201. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 3.Hwang JP, Vierling JM, Zelenetz AD, Lackey SC, Loomba R. Hepatitis B virus management to prevent reactivation after chemotherapy: a review. Support Care Cancer. 2012;20:2999–3008. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lok AS, Ward JW, Perrillo RP, McMahon BJ, Liang TJ. Reactivation of hepatitis B during immunosuppressive therapy: potentially fatal yet preventable. Ann Intern Med. 2012;156:743–5. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shouval D, Shibolet O. Immunosuppression and HBV reactivation. Semin Liver Dis. 2013;33:167–77. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 6.Loomba R, Rowley A, Wesley R, Liang TJ, Hoofnagle JH, Pucino F, et al. Systematic review: the effect of preventive lamivudine on hepatitis B reactivation during chemotherapy. Ann Intern Med. 2008; 148:519–28. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martyak LA, Taqavi E, Saab S. Lamivudine prophylaxis is effective in reducing hepatitis B reactivation and reactivation-related mortality in chemotherapy patients: a meta-analysis. Liver Int. 2008;28:28–38. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 8.Katz LH, Fraser A, Gafter-Gvili A, Leibovici L, Tur-Kaspa R. Lamivudine prevents reactivation of hepatitis B and reduces mortality in immunosuppressed patients: systematic review and meta-analysis. J Viral Hepat. 2008;15:89–102. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 9.Zheng Y, Zhang S, Tan Grahn HM, Ye C, Gong Z, Zhang Q. Prophylactic Lamivudine to Improve the Outcome of Breast Cancer Patients With HBsAg Positive During Chemotherapy: A Meta-Analysis. Hepat Mon. 2013;13:e6496 [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu JY, Sheng YJ, Ding XC, Tang H, Tong SW, Zhang DZ, et al. The efficacy of lamivudine prophylaxis against hepatitis B reactivation in breast cancer patients undergoing chemotherapy: a meta-analysis. J Formos Med Assoc. 2015;114:164–73. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 11.Perrillo RP, Martin P, Lok AS. Preventing hepatitis B reactivation due to immunosuppressive drug treatments. JAMA. 2015;313: 1617–8. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 12.Weinbaum CM, Williams I, Mast EE, Wang SA, Finelli L, Wasley A, et al. ; Centers for Disease Control and Prevention (CDC). Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep. 2008;57:1–20. [PMID: ] [PubMed] [Google Scholar]
- 13.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–2. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 14.Artz AS, Somerfield MR, Feld JJ, Giusti AF, Kramer BS, Sabichi AL, et al. American Society of Clinical Oncology provisional clinical opinion: chronic hepatitis B virus infection screening in patients receiving cytotoxic chemotherapy for treatment of malignant diseases. J Clin Oncol. 2010;28:3199–202. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 15.Day FL, Link E, Thursky K, Rischin D. Current hepatitis B screening practices and clinical experience of reactivation in patients undergoing chemotherapy for solid tumors: a nationwide survey of medical oncologists. J Oncol Pract. 2011;7:141–7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yeo W, Chan PK, Hui P, Ho WM, Lam KC, Kwan WH, et al. Hepatitis B virus reactivation in breast cancer patients receiving cytotoxic chemotherapy: a prospective study. J Med Virol. 2003;70:553–61. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 17.Dong HJ, Ni LN, Sheng GF, Song HL, Xu JZ, Ling Y. Risk of hepatitis B virus (HBV) reactivation in non-Hodgkin lymphoma patients receiving rituximab-chemotherapy: a meta-analysis. J Clin Virol. 2013;57:209–14. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 18.Evens AM, Jovanovic BD, Su YC, Raisch DW, Ganger D, Belknap SM, et al. Rituximab-associated hepatitis B virus (HBV) reactivation in lymphoproliferative diseases: meta-analysis and examination of FDA safety reports. Ann Oncol. 2011;22:1170–80. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 20.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Accessed at www.ohri.ca/programs/clinical_epidemiology/oxford.asp on 5 November 2015.
- 21.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. ; Cochrane Bias Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011; 343:d5928 [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rücker G, Schwarzer G, Carpenter J, Olkin I. Why add anything to nothing? The arcsine difference as a measure of treatment effect in meta-analysis with zero cells. Stat Med. 2009;28:721–38. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 23.Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med. 2004;23:1351–75. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 24.Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration; 2011. Accessed at www.cochrane-handbook.org on 20 August 2015. [Google Scholar]
- 25.Rücker G, Schwarzer G, Carpenter J. Arcsine test for publication bias in meta-analyses with binary outcomes. Stat Med. 2008;27:746–63. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 26.Sterne JAC, Egger M, Moher D, eds. Chapter 10: Addressing reporting biases In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 The Cochrane Collaboration; 2011. Accessed at http://community.cochrane.org/sites/default/files/Handbook510pdf_Ch10_ReportingBias.pdf on 11 November 2015. [Google Scholar]
- 27.Wallace BC, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, Schmid CH. Closing the gap between methodologists and end-users: R as a computational back-end. J Stat Softw. 2012;49:1–15. [Google Scholar]
- 28.Viechtbauer W Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48. Accessed at www.jstatsoft.org/v36/i03 on 20 August 2015. [Google Scholar]
- 29.Yang Y, Du Y, Luo WX, Li C, Chen Y, Cheng K, et al. Hepatitis B virus reactivation and hepatitis in gastrointestinal cancer patients after chemotherapy. Cancer Chemother Pharmacol. 2015;75:783–90. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 30.Ho EY, Yau T, Rousseau F, Heathcote EJ, Lau GK. Preemptive adefovir versus lamivudine for prevention of hepatitis B reactivation in chronic hepatitis B patients undergoing chemotherapy. Hepatol Int. 2015;9:224–30. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 31.Lin GN, Peng JW, Xiao JJ, Liu DY, Xia ZJ. Hepatitis B virus reactivation in hepatitis B surface antigen seropositive patients with metastatic non-small cell lung cancer receiving cytotoxic chemotherapy: the efficacy of preemptive lamivudine and identification of risk factors. Med Oncol. 2014;31:119 [PMID: ] [DOI] [PubMed] [Google Scholar]
- 32.Nishida T, Hiramatsu N, Mizuki M, Nagatomo I, Kida H, Tazumi K, et al. Managing hepatitis B virus carriers with systemic chemotherapy or biologic therapy in the outpatient clinic. Hepatol Res. 2013;43: 339–46. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 33.Ling WH, Soe PP, Pang AS, Lee SC. Hepatitis B virus reactivation risk varies with different chemotherapy regimens commonly used in solid tumours. Br J Cancer. 2013;108:1931–5. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu J, Zhu H, Zhao Y, Wang X, Shen Y, Wang W, et al. Factors associated with hepatic dysfunction in hepatitis B-positive patients with postgastrectomy adenocarcinoma. Oncol Lett. 2012;4:471–476. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee HJ, Kim DY, Keam B, Lee JH, Han SW, Oh DY, et al. Lamivudine prophylaxis for hepatitis B virus carrier patients with breast cancer during adjuvant chemotherapy. Breast Cancer. 2014;21:387–93. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 36.Kim IK, Kim BG, Kim W, Kim D, Kim YJ, Yoon JH, et al. Clinical prediction of failure of Lamivudine prophylaxis for hepatitis B virusinfected patients undergoing cytotoxic chemotherapy for malignancy. Antimicrob Agents Chemother. 2012;56:5511–9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yun J, Kim KH, Kang ES, Gwak GY, Choi MS, Lee JE, et al. Prophylactic use of lamivudine for hepatitis B exacerbation in postoperative breast cancer patients receiving anthracycline-based adjuvant chemotherapy. Br J Cancer. 2011;104:559–63. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsai SH, Dai MS, Yu JC, Ho CL, Chen YC, Wu YY, et al. Preventing chemotherapy-induced hepatitis B reactivation in breast cancer patients: a prospective comparison of prophylactic versus deferred preemptive lamivudine. Support Care Cancer. 2011;19:1779–87. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 39.Sohn BS, Ahn JH, Jung KH, Ahn SH, Son BH, Gong G, et al. Updated longitudinal data on acute exacerbation of chronic hepatitis B in patients with breast cancer receiving anthracycline-based adjuvant chemotherapy: therapeutic vs. pre-emptive use of lamivudine. Jpn J Clin Oncol. 2011;41:1059–66. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 40.Long M, Jia W, Li S, Jin L, Wu J, Rao N, et al. A single-center, prospective and randomized controlled study: Can the prophylactic use of lamivudine prevent hepatitis B virus reactivation in hepatitis B s-antigen seropositive breast cancer patients during chemotherapy? Breast Cancer Res Treat. 2011;127:705–12. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 41.Eren OO, Artac M, Boruban MC, Yavas O, Arslan U, Basaranoglu M. Chemotherapy-induced Hepatitis B virus reactivation in HbsAg positive cancer patients: a single center experience. Med Oncol. 2009;26:386–92. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 42.Yeo W, Hui EP, Chan AT, Ho WM, Lam KC, Chan PK, et al. Prevention of hepatitis B virus reactivation in patients with nasopharyngeal carcinoma with lamivudine. Am J Clin Oncol. 2005;28:379–84. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 43.Yeo W, Zee B, Zhong S, Chan PK, Wong WL, Ho WM, et al. Comprehensive analysis of risk factors associating with Hepatitis B virus (HBV) reactivation in cancer patients undergoing cytotoxic chemotherapy. Br J Cancer. 2004;90:1306–11. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yeo W, Ho WM, Hui P, Chan PK, Lam KC, Lee JJ, et al. Use of lamivudine to prevent hepatitis B virus reactivation during chemotherapy in breast cancer patients. Breast Cancer Res Treat. 2004;88: 209–15. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 45.Dai MS, Wu PF, Shyu RY, Lu JJ, Chao TY. Hepatitis B virus reactivation in breast cancer patients undergoing cytotoxic chemotherapy and the role of preemptive lamivudine administration. Liver Int. 2004;24:540–6. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 46.Dai MS, Wu PF, Lu JJ, Shyu RY, Chao TY. Preemptive use of lamivudine in breast cancer patients carrying hepatitis B virus undergoing cytotoxic chemotherapy: a longitudinal study. Support Care Cancer. 2004;12:191–6. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 47.Cheng JC, Liu MC, Tsai SY, Fang WT, Jer-Min Jian J, Sung JL. Unexpectedly frequent hepatitis B reactivation by chemoradiation in postgastrectomy patients. Cancer. 2004;101:2126–33. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 48.Lim LL, Wai CT, Lee YM, Kong HL, Lim R, Koay E, et al. Prophylactic lamivudine prevents hepatitis B reactivation in chemotherapy patients. Aliment Pharmacol Ther. 2002;16:1939–44. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 49.Yeo W, Chan PK, Zhong S, Ho WM, Steinberg JL, Tam JS, et al. Frequency of hepatitis B virus reactivation in cancer patients undergoing cytotoxic chemotherapy: a prospective study of 626 patients with identification of risk factors. J Med Virol. 2000;62:299–307. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 50.Alexopoulos CG, Vaslamatzis M, Hatzidimitriou G. Prevalence of hepatitis B virus marker positivity and evolution of hepatitis B virus profile, during chemotherapy, in patients with solid tumours. Br J Cancer. 1999;81:69–74. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen WC, Cheng JS, Chiang PH, Tsay FW, Chan HH, Chang HW, et al. A Comparison of Entecavir and Lamivudine for the Prophylaxis of Hepatitis B Virus Reactivation in Solid Tumor Patients Undergoing Systemic Cytotoxic Chemotherapy. PLoS One. 2015;10:e0131545 [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim E, Yune S, Ha JM, Lee WJ, Hwang JW, Paik YH, et al. Hepatitis B virus reactivation during anti-cancer chemotherapy in patients with past hepatitis B virus infection. Hepatogastroenterology. 2014; 61:1704–11. [PMID: ] [PubMed] [Google Scholar]
- 53.Hagiwara S, Sakurai T, Nishina S, Tanaka K, Ikeda M, Ueshima K, et al. Characteristic pattern of reactivation of hepatitis B virus during chemotherapy for solid cancers. Dig Dis. 2012;30:541–6. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 54.Borentain P, Colson P, Coso D, Bories E, Charbonnier A, Stoppa AM, et al. Clinical and virological factors associated with hepatitis B virus reactivation in HBsAg-negative and anti-HBc antibodies-positive patients undergoing chemotherapy and/or autologous stem cell transplantation for cancer. J Viral Hepat. 2010;17:807–15. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 55.Kohrt HE, Ouyang DL, Keeffe EB. Systematic review: lamivudine prophylaxis for chemotherapy-induced reactivation of chronic hepatitis B virus infection. Aliment Pharmacol Ther. 2006;24:1003–16. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 56.Reddy KR, Beavers KL, Hammond SP, Lim JK, Falck-Ytter YT; American Gastroenterological Association Institute. American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148:215–9; quiz e16–7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 57.Seto WK, Chan TS, Hwang YY, Wong DK, Fung J, Liu KS, et al. Hepatitis B reactivation in patients with previous hepatitis B virus exposure undergoing rituximab-containing chemotherapy for lymphoma: a prospective study. J Clin Oncol. 2014;32:3736–43. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 58.Liaw YF, Kao JH, Piratvisuth T, Chan HL, Chien RN, Liu CJ, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int. 2012;6:531–61. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 59.European Association For The Study Of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–85. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 60.Chen KL, Chen J, Rao HL, Guo Y, Huang HQ, Zhang L, et al. Hepatitis B virus reactivation and hepatitis in diffuse large B-cell lymphoma patients with resolved hepatitis B receiving rituximab-containing chemotherapy: risk factors and survival. Chin J Cancer. 2015;34:225–34. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kusumoto S, Tanaka Y, Suzuki R, Watanabe T, Nakata M, Takasaki H, et al. Monitoring of Hepatitis B Virus (HBV) DNA and Risk of HBV Reactivation in B-Cell Lymphoma: A Prospective Observational Study. Clin Infect Dis. 2015;61:719–29. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 62.Centers for Disease Control (CDC). Prevention of perinatal transmission of hepatitis B virus: prenatal screening of all pregnant women for hepatitis B surface antigen. MMWR Morb Mortal Wkly Rep. 1988;37:341–6, 351. [PMID: ] [PubMed] [Google Scholar]
- 63.Lin SY, Chang ET, So SK. Why we should routinely screen Asian American adults for hepatitis B: a cross-sectional study of Asians in California. Hepatology. 2007;46:1034–40. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 64.Hwang JP, Mohseni M, Gor BJ, Wen S, Guerrero H, Vierling JM. Hepatitis B and hepatitis C prevalence and treatment referral among Asian Americans undergoing community-based hepatitis screening. Am J Public Health. 2010;100 Suppl 1:S118–24. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zurawska U, Hicks LK, Woo G, Bell CM, Krahn M, Chan KK, et al. Hepatitis B virus screening before chemotherapy for lymphoma: a cost-effectiveness analysis. J Clin Oncol. 2012;30:3167–73. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 66.Day FL, Karnon J, Rischin D. Cost-effectiveness of universal hepatitis B virus screening in patients beginning chemotherapy for solid tumors. J Clin Oncol. 2011;29:3270–7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 67.Lok AS, Liang RH, Chiu EK, Wong KL, Chan TK, Todd D. Reactivation of hepatitis B virus replication in patients receiving cytotoxic therapy. Report of a prospective study. Gastroenterology. 1991;100: 182–8. [PMID: ] [DOI] [PubMed] [Google Scholar]